Abstract
Combination therapy is recommended for infections with carbapenemase-producing Klebsiella pneumoniae. However, limited data exist on which antibiotic combinations are the most effective. The aim of this study was to find effective antibiotic combinations against metallo-beta-lactamase-producing K. pneumoniae (MBL-KP). Two VIM- and two NDM-producing K. pneumoniae strains, all susceptible to colistin, were exposed to antibiotics at clinically relevant static concentrations during 24-h time-kill experiments. Double- and triple-antibiotic combinations of aztreonam, ciprofloxacin, colistin, daptomycin, fosfomycin, meropenem, rifampin, telavancin, tigecycline, and vancomycin were used. Synergy was defined as a ≥2 log10 decrease in CFU/ml between the combination and its most active drug after 24 h, and bactericidal effect was defined as a ≥3 log10 decrease in CFU/ml after 24 h compared with the starting inoculum. Synergistic or bactericidal activity was demonstrated for aztreonam, fosfomycin, meropenem, and rifampin in double-antibiotic combinations with colistin and also for aztreonam, fosfomycin, and rifampin in triple-antibiotic combinations with meropenem and colistin. Overall, the combination of rifampin-meropenem-colistin was the most effective regimen, demonstrating synergistic and bactericidal effects against all four strains. Meropenem-colistin, meropenem-fosfomycin, and tigecycline-colistin combinations were not bactericidal against the strains used. The findings of this and other studies indicate that there is great potential of antibiotic combinations against carbapenemase-producing K. pneumoniae. However, our results deviate to some extent from those of previous studies, which might be because most studies to date have included KPC-producing rather than MBL-producing strains. More studies addressing MBL-KP are needed.
INTRODUCTION
Carbapenem resistance in Enterobacteriaceae has increased globally during the past decade, particularly in Klebsiella pneumoniae, and is typically caused by carbapenemase production. However, there have been marked geographic differences in the epidemiology of carbapenemases. Serine KPC-type carbapenemases are predominant in the United States and frequently reported in Southern Europe (1). OXA-48, which was first detected in Turkey, has increased particularly in Europe, the Mediterranean, and North Africa (1, 2). VIM-type metallo-beta-lactamases (MBLs) are endemic in some European countries, whereas IMP-type MBLs are predominant in Asia (3–5). In recent years, Enterobacteriaceae producing NDM-1-type MBLs have disseminated rapidly on the Indian subcontinent (1).
Carbapenemase-producing K. pneumoniae strains are of great clinical concern because of frequent coresistance to multiple antibiotic classes (6, 7). Clinical studies have concluded that combination antibiotic therapy is associated with a better outcome than monotherapy for the treatment of severe infections with these strains, even if the isolated bacteria are susceptible in vitro to the individual drugs (8–10). However, inadequate data exist on which antibiotic combinations are the most effective. To date, recommendations are based on a few retrospective clinical studies and in vitro studies with time-kill or checkerboard methods. Clinical studies have reported favorable outcomes for patients treated with combinations of colistin and a carbapenem, tigecycline, fosfomycin, or an aminoglycoside (10–12). Retrospective analyses have supported the use of combinations including a carbapenem for infections with KPC-producing K. pneumoniae when the carbapenem MIC of the causative bacteria is ≤4 mg/liter (11). These combinations have been recommended and used in clinical practice (13). Double- and triple-antibiotic combinations that include colistin, a carbapenem, rifampin, tigecycline, and fosfomycin have proven effective in vitro and been proposed for clinical use (14–18).
Notably, most studies have addressed KPC-producing K. pneumoniae, and antibiotic combinations considered effective in these studies have not been readily evaluated against MBL-producing strains. Because MBL-producing K. pneumoniae are increasing globally, there is an urgent need to evaluate antibiotic regimens for the treatment of infections caused by these strains. In the absence of clinical studies, which are difficult to perform, in vitro studies may give some insight into this issue. The aim of the present study was to find antibiotic combinations with synergistic and bactericidal activity against VIM- and NDM-producing K. pneumoniae isolates in vitro.
MATERIALS AND METHODS
Bacteria and media.
Mueller-Hinton II broth (MHBII) and Mueller-Hinton II agar plates were used for all experiments (Becton, Dickinson & Co., Sparks, MD, USA). Clinical isolates of K. pneumoniae producing VIM-1 and NDM-1 carbapenemases were obtained from the Department of Clinical Microbiology, Karolinska University Hospital, Stockholm, Sweden. The VIM-1-producing K. pneumoniae (VIM-KP) strains T14789 and ÖN-2211 were originally isolated in Scandinavia but derived from patients recently transferred from Greek hospitals, while the NDM-1-producing K. pneumoniae (NDM-KP) isolates IR8 and IR62E originated from Chennai, India. All strains have been described in previous publications (7, 19).
Antibiotic susceptibility testing.
MICs were determined in triplicate with the Etest method according to the manufacturer's instructions (bioMérieux, Marcy l'Etoile, France).
Detection of coproduced beta-lactamases.
The Check-MDR CT101 array (Check-Points, Wageningen, The Netherlands) was used as recommended by the manufacturer to identify the beta-lactamase genes of extended-spectrum beta-lactamases (ESBLs) and plasmid-mediated AmpCs (pAmpCs). Carbapenemase genes had been detected earlier (7, 19).
Antibiotic concentrations.
Antibiotic concentrations used during time-kill experiments represent mean steady-state concentrations of non-protein-bound drug in humans calculated from literature data (based on the area under the antibiotic concentration-time curve in serum or plasma over 24 h divided by 24 h [AUC0–24/24 h]). For rifampin, the mean concentration [(Cmax + Cmin)/2] was used instead due to a lack of other data. The following concentrations were used: 17 mg/liter for aztreonam (20), 1.0 mg/liter for ciprofloxacin (21), 4.0 mg/liter for colistin (22), 1.7 mg/liter for daptomycin (23), 83 mg/liter for fosfomycin (24), 6.8 mg/liter for meropenem (25), 1.7 mg/liter for rifampin (26), 3.3 mg/liter for telavancin (27), 0.1 mg/liter for tigecycline (28), and 9.1 mg/liter for vancomycin (29).
Time-kill experiments.
A single bacterial colony was added to 2 ml MHBII and grown for 15 to 18 h in a 37°C rocking water bath (150 rpm). A total of 20 μl of the overnight culture was added to 2 ml of prewarmed MHBII, further incubated for 1.5 h, and then inoculated in prewarmed MHBII to obtain a starting inoculum of approximately 5 × 106 CFU/ml. Antibiotics were added to the concentrations described above. Samples were taken at 0, 1, 2, 4, 6, and 24 h, serially diluted, spread on plates, and incubated at 37°C. Bacterial colonies were counted after 24 h.
Experimental design.
The VIM-KP strains were exposed to colistin alone and aztreonam, fosfomycin, meropenem, rifampin, ciprofloxacin, tigecycline, daptomycin, telavancin, and vancomycin alone and in combination with colistin in 24-h time-kill experiments. Based on the synergistic and bactericidal effects detected in these experiments and results from previously published studies, we decided to perform further experiments with double- and triple-antibiotic combinations of aztreonam, fosfomycin, meropenem, rifampin, and colistin. Antibiotics that demonstrated synergistic or bactericidal effects when used in combination with other drugs against the VIM-KP strains were selected for further experiments against the NDM-KP strains. Experiments were performed at least in triplicate for the VIM-KP strains and at least in duplicate for the NDM-KP strains. In total, we performed more than 200 time-kill experiments with 24 antibiotic regimens, including 14 antibiotic combinations.
Analysis of time-kill experiments.
The lower limit of bacterial detection was 1.0 log10 CFU/ml. Bacterial concentrations of <1.0 log10 CFU/ml were counted as 1.0 log10 CFU/ml. Synergy was defined as a ≥2 log10 decrease in CFU/ml between the combination and its most active constituent after 24 h. Bactericidal effect was defined as a ≥3 log10 decrease in CFU/ml after 24 h compared with the starting inoculum.
RESULTS
All strains were susceptible to colistin according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints (www.eucast.org), whereas susceptibilities to the other antibiotics varied substantially (Table 1). The following genes encoding ESBLs and pAmpCs were detected: VIM-KP T14789, blaCMY-I and blaMOX; VIM-KP ÖN-2211, blaCTX-M-1 group; NDM-KP IR8, blaCTX-M-1 group; NDM-KP IR62E, blaCMY-II, blaDHA, and blaCTX-M-1 group.
TABLE 1.
Antibiotic susceptibilities of VIM- and NDM-producing K. pneumoniae strains expressed as MIC values and classification according to the clinical breakpoints defined by EUCASTa
| Antibiotic | VIM-KP T14789 |
VIM-KP ÖN-2211 |
NDM-KP IR8 |
NDM-KP IR62E |
||||
|---|---|---|---|---|---|---|---|---|
| MIC (mg/liter) | SIR | MIC (mg/liter) | SIR | MIC (mg/liter) | SIR | MIC (mg/liter) | SIR | |
| Aztreonam | 2 | I | 4 | I | >256 | R | >256 | R |
| Ciprofloxacin | >32 | R | 4 | R | >32 | R | >32 | R |
| Colistin | 0.125 | S | 0.125 | S | 0.125 | S | 0.125 | S |
| Daptomycin | >256 | – | >256 | – | >256 | – | >256 | – |
| Fosfomycin | 32 | S | 4 | S | 256 | R | 64 | R |
| Meropenem | >32 | R | 2 | S | 4 | I | >32 | R |
| Rifampin | >32 | – | >32 | – | 32 | – | >32 | – |
| Telavancin | 256 | – | 256 | – | 256 | – | 256 | – |
| Tigecycline | 2 | I | 1 | S | 2 | I | 3 | R |
| Vancomycin | >256 | – | >256 | – | >256 | – | >256 | – |
SIR, classification according to EUCAST definitions; S, susceptible; I, intermediate; R, resistant; –, not defined.
Colistin, alone and in any combination with other antibiotics, was associated with a >3 log10 decrease in CFU/ml during the first hour of the experiments. However, considerable regrowth occurred for most colistin combinations and during experiments with colistin alone. Synergistic and bactericidal effects after 24 h were found for combinations that included aztreonam, fosfomycin, meropenem, rifampin, and colistin (Tables 2, 3, 4, 5, and 6; see also Fig. S1 to S4 in the supplemental material).
TABLE 2.
Compiled results from time-kill experiments with combinations that include meropenem, colistin, aztreonam, fosfomycin, and rifampin against VIM- and NDM-producing K. pneumoniae
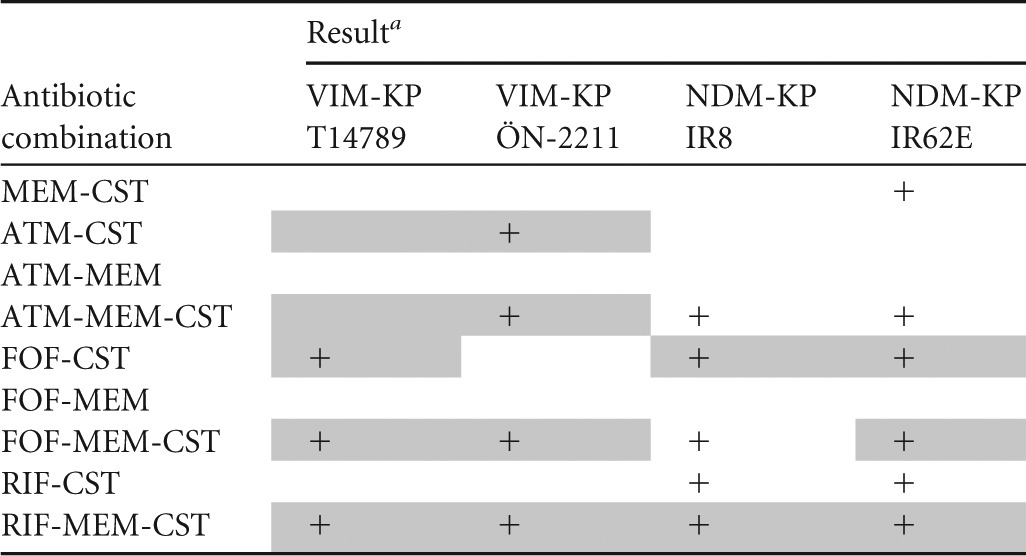
+, synergy; MEM, meropenem; CST, colistin; ATM, aztreonam; FOF, fosfomycin; RIF, rifampin. Synergy (≥2 log10 reduction in CFU/ml after 24 h compared with the most effective individual drug) and bactericidal effect (≥3 log10 decrease in CFU/ml after 24 h compared with the starting inoculum) is depicted. Bactericidal effect is highlighted in gray.
TABLE 3.
Summary of mean bacterial concentrations at 0, 1, and 24 h during time-kill experiments performed against VIM-producing K. pneumoniae strain VIM-KP T14789a
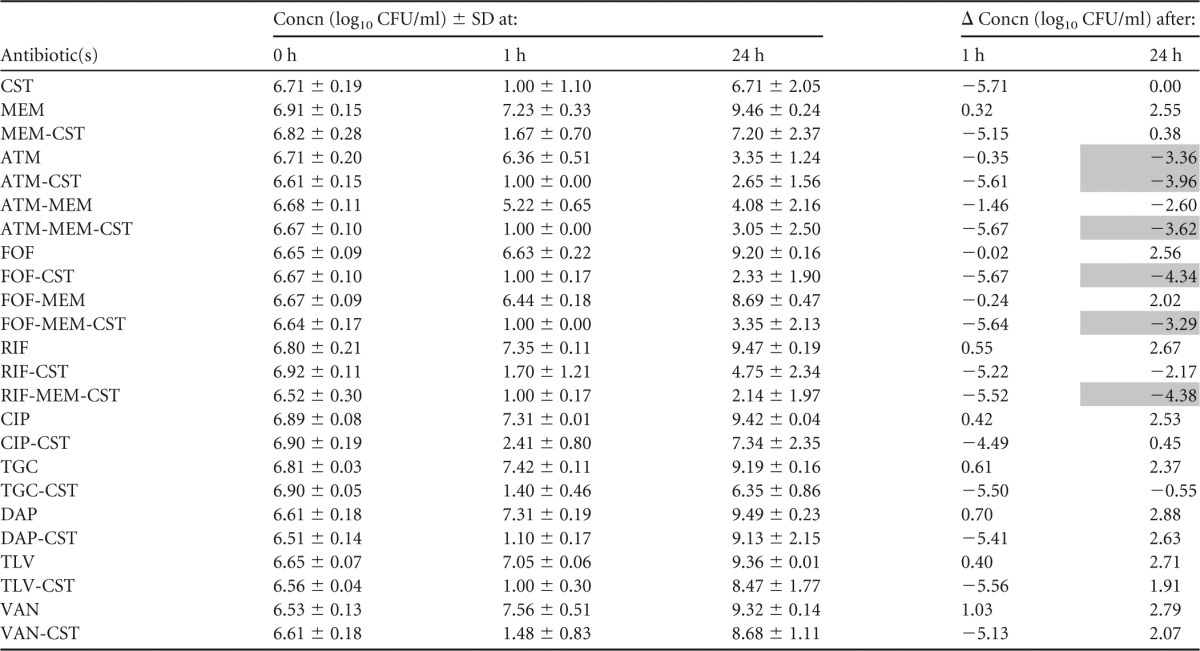
CST, colistin; MEM, meropenem; ATM, aztreonam; FOF, fosfomycin; RIF, rifampin; CIP, ciprofloxacin; TGC, tigecycline; DAP, daptomycin; TLV, telavancin; VAN, vancomycin. The standard deviation (SD) at each time point and change in bacterial concentrations in log10 CFU/ml (Δ) at 1 and 24 h compared with the starting inoculum (0 h) is shown. Bactericidal effect (≥3 log10 reduction in CFU/ml after 24 h) is highlighted in gray.
TABLE 4.
Summary of mean bacterial concentrations at 0, 1, and 24 h during time-kill experiments performed against VIM-producing K. pneumoniae strain VIM-KP ÖN-2211a
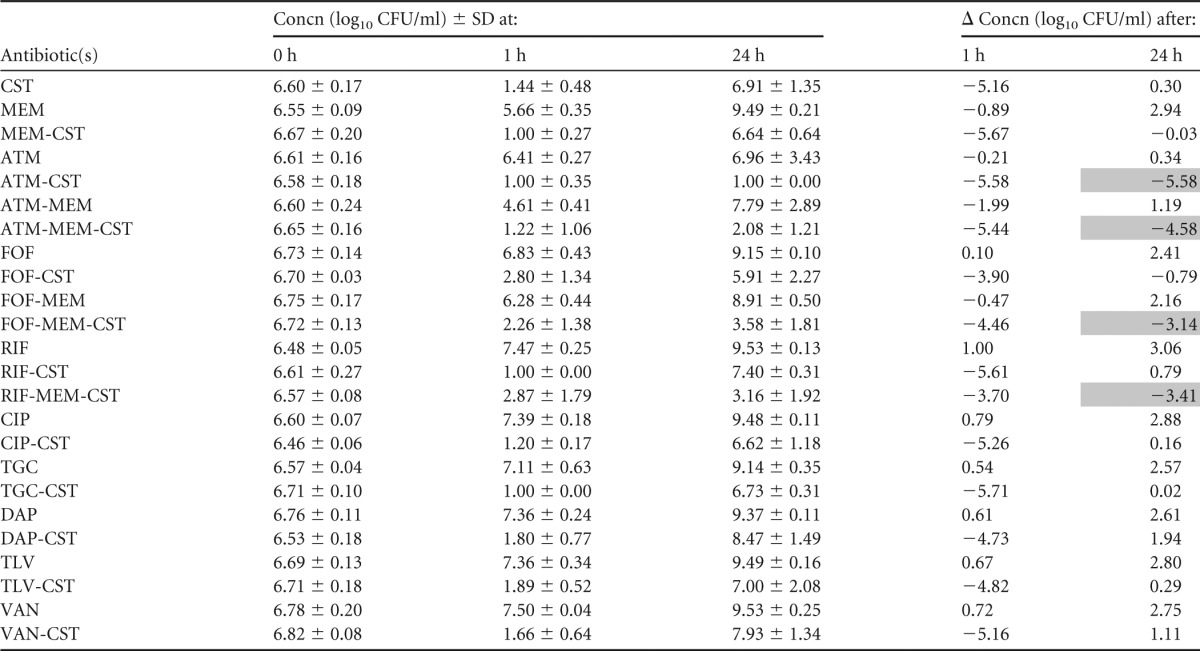
CST, colistin; MEM, meropenem; ATM, aztreonam; FOF, fosfomycin; RIF, rifampin; CIP, ciprofloxacin; TGC, tigecycline; DAP, daptomycin; TLV, telavancin; VAN, vancomycin. The standard deviation (SD) at each time point and change in bacterial concentrations in log10 CFU/ml (Δ) at 1 and 24 h compared with the starting inoculum (0 h) is shown. Bactericidal effect (≥3 log10 reduction in CFU/ml after 24 h) is highlighted in gray.
TABLE 5.
Summary of mean bacterial concentrations at 0, 1, and 24 h during time-kill experiments performed against NDM-producing K. pneumoniae strain NDM-KP IR8a
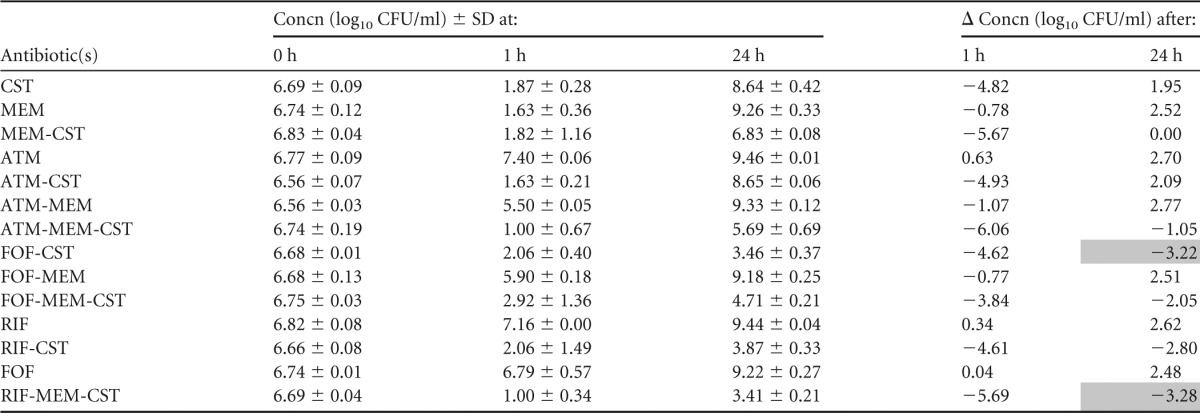
CST, colistin; MEM, meropenem; ATM, aztreonam; FOF, fosfomycin; RIF, rifampin. The standard deviation (SD) at each time point and change in bacterial concentrations in log10 CFU/ml (Δ) at 1 and 24 h compared with the starting inoculum (0 h) is shown. Bactericidal effect (≥3 log10 reduction in CFU/ml after 24 h) is highlighted in gray.
TABLE 6.
Summary of mean bacterial concentrations at 0, 1, and 24 h during time-kill experiments performed against NDM-producing K. pneumoniae strain NDM-KP IR62Ea
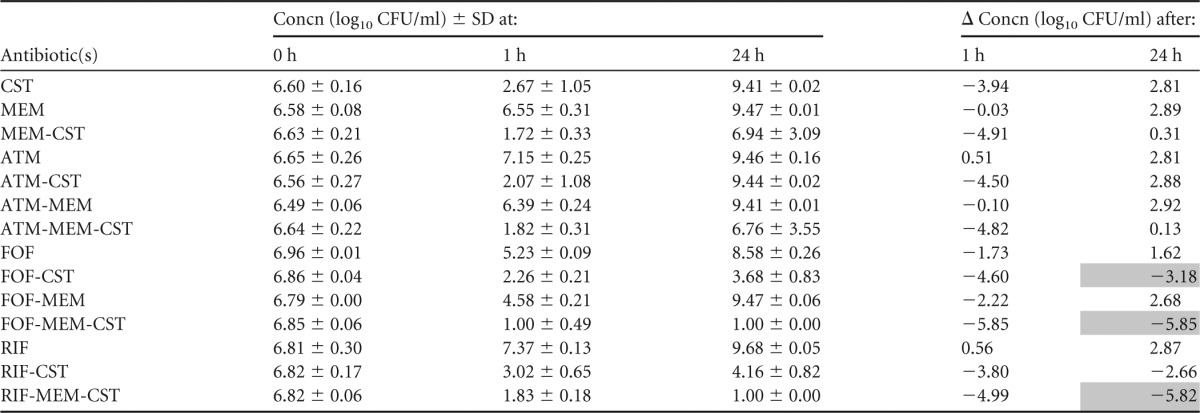
CST, colistin; MEM, meropenem; ATM, aztreonam; FOF, fosfomycin; RIF, rifampin. The standard deviation (SD) at each time point and change in bacterial concentrations in log10 CFU/ml (Δ) at 1 and 24 h compared to the starting inoculum (0 h) is shown. Bactericidal effect (≥3 log10 reduction in CFU/ml after 24 h) is highlighted in gray.
Aztreonam-colistin and aztreonam-meropenem-colistin were bactericidal against the VIM-KP strains, both of which displayed intermediate susceptibility to aztreonam. These combinations resulted in more rapid killing than aztreonam alone and less regrowth than colistin alone and proved a synergistic effect against VIM-KP ÖN-2211 (Tables 3 and 4; see also Fig. S1 and S2a in the supplemental material). Against the NDM-KP strains, aztreonam-meropenem-colistin demonstrated a synergistic effect despite high-level resistance to aztreonam and reduced susceptibility to meropenem. However, the effect was not bactericidal (Tables 5 and 6; see also Fig. S3 and S4a in the supplemental material). Meropenem-colistin had a synergistic effect against NDM-KP IR62E, but the combination was not bactericidal and showed no synergistic or bactericidal effects against the other strains (Tables 3 to 6; see also Fig. S1 to S4d in the supplemental material).
The combination fosfomycin-colistin demonstrated synergy and a bactericidal effect against one of the VIM-KP strains and both NDM-KP strains despite that the latter strains were resistant to fosfomycin (Tables 3 to 6; see also Fig. S1 to S4b in the supplemental material). Fosfomycin-meropenem-colistin showed a synergistic effect against all strains and a bactericidal effect against three of the four strains.
Rifampin-colistin demonstrated synergy and a >2.5 log10 decrease in CFU/ml after 24 h compared with the starting inoculum against both NDM strains (Tables 5 and 6; see also Fig. S3 and S4c in the supplemental material). The triple-antibiotic combination rifampin-meropenem-colistin resulted in synergistic and bactericidal effects against all strains despite high MICs for rifampin in all strains and despite that two of the strains were highly resistant to meropenem alone (Tables 3 to 6; see also Fig. S1 to S4c in the supplemental material).
The antibacterial activities of ciprofloxacin, tigecycline, daptomycin, telavancin, and vancomycin alone and in combination with colistin against the VIM-KP strains were evaluated in time-kill experiments performed in triplicate (Tables 3 and 4; see also Fig. S1 and S2e in the supplemental material). However, no synergistic or bactericidal activity was detected with these antibiotic regimens, and thus they were not further investigated against the NDM-KP strains.
DISCUSSION
In this study, we have used 14 antibiotic combinations against four MBL-producing K. pneumoniae strains and found synergistic or bactericidal activity for seven of these combinations in time-kill experiments at clinically relevant static antibiotic concentrations.
Our results are in part consistent with those of previous studies. Overall, the combination of rifampin-meropenem-colistin was the most effective regimen, demonstrating synergistic and bactericidal effects against all four strains. A similar triple-antibiotic combination of rifampin-doripenem-colistin has been shown to have a bactericidal effect against KPC-producing K. pneumoniae strains in time-kill experiments at antibiotic concentrations of 0.25× MIC (17). Rifampin-colistin was synergistic against both NDM-KP strains in our study and has been effective against the majority of KPC-producing K. pneumoniae isolates in previous time-kill and checkerboard studies (14, 30). Fosfomycin-colistin, which showed synergistic and bactericidal effects against 3 of 4 strains in our study, has previously demonstrated synergy against 2 of 4 NDM-producing Klebsiella spp. in a study with the checkerboard method (15).
Some antibiotic combinations that have been effective against carbapenemase-producing K. pneumoniae in previous studies and recommended for clinical use showed no synergistic or bactericidal effects in our experiments. For example, meropenem-colistin was not bactericidal against any of the strains despite susceptibility to colistin in all strains and susceptibility or intermediate susceptibility to meropenem in two of the strains. The combination of tigecycline-colistin has demonstrated a synergistic and bactericidal effect against KPC-producing K. pneumoniae in previous studies with checkerboard and time-kill methods but was not effective against the VIM-KP isolates used in this study (14, 18). Further, meropenem-fosfomycin, which demonstrated synergistic and bactericidal effects against the majority of KPC-producing K. pneumoniae isolates in a previous time-kill study, was not effective in our experiments (16).
The combination of aztreonam-meropenem-colistin showed a synergistic or bactericidal effect against all strains, despite that the NDM-KP isolates were highly resistant to aztreonam and nonsusceptible to meropenem. One possible explanation for this finding is that aztreonam acts as a competitive MBL inhibitor and meropenem as a competitive inhibitor for other coproduced beta-lactamases (such as ESBLs or AmpCs) that hydrolyze aztreonam but not meropenem. Such competitive inhibitory activities have previously been demonstrated for aztreonam against MBLs and for meropenem against class A, C, and D beta-lactamases (31). The combined inhibitory activities of aztreonam and meropenem were not sufficient to achieve synergy against the MBL-producing K. pneumoniae strains used in this study. However, the addition of colistin resulted in synergistic activity, probably because of increased permeability leading to higher concentrations of aztreonam and meropenem in the periplasmic space with this combination (32).
In summary, we have found several double- and triple-antibiotic combinations of aztreonam, fosfomycin, meropenem, rifampin, and colistin with synergistic and bactericidal effects against MBL-producing K. pneumoniae. Bacterial regrowth at 24 h occurred for most combinations, but the clinical relevance is uncertain, because the experiments were performed at static antibiotic concentrations in vitro, where the effects of the immune system are not taken into account. Our results suggest that rifampin-meropenem-colistin is effective for infections with these strains and that aztreonam-colistin with or without meropenem might be a therapeutic option even when the causative bacteria are not susceptible to aztreonam. More studies are required to confirm the efficacy and evaluate the clinical use of these combinations. Yet our results are encouraging and indicate that there is an antibacterial potential of antibiotic combinations against MBL-producing K. pneumoniae. Effective combinations might include antibiotics to which the bacteria are resistant as well as drugs not usually considered therapeutic options for Gram-negative bacteria. Our results are somewhat inconsistent with those previously reported for carbapenemase-producing K. pneumoniae, which might be explained in part by the fact that most studies to date have addressed KPC-producing rather than MBL-producing strains.
Because clinical studies are not easily performed with patients infected with these bacteria, therapeutic decisions will sometimes depend on the results from in vitro studies. Therefore, we believe that there is an urgent need for more time-kill studies aimed at finding effective antibiotic combinations for the treatment of MBL-producing K. pneumoniae.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by VINNOVA and the Swedish Foundation for Strategic Research.
We thank Charlotte Annerstedt for excellent laboratory assistance.
Footnotes
Published ahead of print 6 January 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00741-13.
REFERENCES
- 1.Nordmann P, Naas T, Poirel L. 2007. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 17:1791–1798. 10.3201/eid1710.110655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cantón R, Akóva M, Carmeli Y, Giske CG, Glupczynski Y, Gniadkowski M, Livermore DM, Miriagou V, Naas T, Rossolini GM, Samuelsen Ø, Seifert H, Woodford N, Nordmann P. 2012. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin. Microbiol. Infect. 18:413–431. 10.1111/j.1469-0691.2012.03821.x [DOI] [PubMed] [Google Scholar]
- 3.Souli M, Galani I, Antoniadou A, Papadomichelakis E, Poulakou G, Panagea T, Vourli S, Zerva L, Armaganidis A, Kanellakopoulou K, Giamarellou H. 2010. An outbreak of infection due to beta-lactamase Klebsiella pneumoniae carbapenemase 2-producing K. pneumoniae in a Greek university hospital: molecular characterization, epidemiology, and outcomes. Clin. Infect. Dis. 50:364–373. 10.1086/649865 [DOI] [PubMed] [Google Scholar]
- 4.Walsh TR. 2008. Clinically significant carbapenemases: an update. Curr. Opin. Infect. Dis. 21:367–371. 10.1097/QCO.0b013e328303670b [DOI] [PubMed] [Google Scholar]
- 5.Cagnacci S, Gualco L, Roveta S, Mannelli S, Borgianni L, Docquier JD, Dodi F, Centanaro M, Debbia E, Marchese A, Rossolini GM. 2008. Bloodstream infections caused by multidrug-resistant Klebsiella pneumoniae producing the carbapenem-hydrolysing VIM-1 metallo-beta-lactamase: first Italian outbreak. J. Antimicrob. Chemother. 61:296–300. 10.1093/jac/dkm471 [DOI] [PubMed] [Google Scholar]
- 6.Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, Chaudhary U, Doumith M, Giske CG, Irfan S, Krishnan P, Kumar AV, Maharjan S, Mushtaq S, Noorie T, Paterson DL, Pearson A, Perry C, Pike R, Rao B, Ray U, Sarma JB, Sharma M, Sheridan E, Thirunarayan MA, Turton J, Upadhyay S, Warner M, Welfare W, Livermore DM, Woodford N. 2010. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect. Dis. 10:597–602. 10.1016/S1473-3099(10)70143-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samuelsen Ø, Toleman MA, Hasseltvedt V, Fuursted K, Leegaard TM, Walsh TR, Sundsfjord A, Giske CG. 2011. Molecular characterization of VIM-producing Klebsiella pneumoniae from Scandinavia reveals genetic relatedness with international clonal complexes encoding transferable multidrug resistance. Clin. Microbiol. Infect. 17:1811–1816. 10.1111/j.1469-0691.2011.03532.x [DOI] [PubMed] [Google Scholar]
- 8.Zarkotou O, Pournaras S, Tselioti P, Dragoumanos V, Pitiriga V, Ranellou K, Prekates A, Themeli-Digalaki K, Tsakris A. 2011. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin. Microbiol. Infect. 17:1798–1803. 10.1111/j.1469-0691.2011.03514.x [DOI] [PubMed] [Google Scholar]
- 9.Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, Polsky B, Adams-Haduch JM, Doi Y. 2012. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob. Agents Chemother. 56:2108-2113. 10.1128/AAC.06268-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirsch EB, Tam VH. 2010. Detection and treatment options for Klebsiella pneumoniae carbapenemases (KPCs): an emerging cause of multidrug-resistant infection. J. Antimicrob. Chemother. 65:1119–1125. 10.1093/jac/dkq108 [DOI] [PubMed] [Google Scholar]
- 11.Daikos GL, Markogiannakis A. 2011. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems? Clin. Microbiol. Infect. 17:1135–1141. 10.1111/j.1469-0691.2011.03553.x [DOI] [PubMed] [Google Scholar]
- 12.Michalopoulos A, Virtzili S, Rafailidis P, Chalevelakis G, Damala M, Falagas ME. 2010. Intravenous fosfomycin for the treatment of nosocomial infections caused by carbapenem-resistant Klebsiella pneumoniae in critically ill patients: a prospective evaluation. Clin. Microbiol. Infect. 16:184–186. 10.1111/j.1469-0691.2009.02921.x [DOI] [PubMed] [Google Scholar]
- 13.Miyakis S, Pefanis A, Tsakris A. 2011. The challenges of antimicrobial drug resistance in Greece. Clin. Infect. Dis. 53:177–184. 10.1093/cid/cir323 [DOI] [PubMed] [Google Scholar]
- 14.Elemam A, Rahimian J, Doymaz M. 2010. In vitro evaluation of antibiotic synergy for polymyxin B-resistant carbapenemase-producing Klebsiella pneumoniae. J. Clin. Microbiol. 48:3558–3562. 10.1128/JCM.01106-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bercot B, Poirel L, Dortet L, Nordmann P. 2011. In vitro evaluation of antibiotic synergy for NDM-1-producing Enterobacteriaceae. J. Antimicrob. Chemother. 66:2295–2297. 10.1093/jac/dkr296 [DOI] [PubMed] [Google Scholar]
- 16.Souli M, Galani I, Boukovalas S, Gourgoulis MG, Chryssouli Z, Kanellakopoulou K, Panagea T, Giamarellou H. 2011. In vitro interactions of antimicrobial combinations with fosfomycin against KPC-2-producing Klebsiella pneumoniae and protection of resistance development. Antimicrob. Agents Chemother. 55:2395–2397. 10.1128/AAC.01086-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urban C, Mariano N, Rahal JJ. 2010. In vitro double and triple bactericidal activities of doripenem, polymyxin B, and rifampin against multidrug-resistant Acinetobacter baumannii, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Escherichia coli. Antimicrob. Agents Chemother. 54:2732–2734. 10.1128/AAC.01768-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pournaras S, Vrioni G, Neou E, Dendrinos J, Dimitroulia E, Poulou A, Tsakris A. 2011. Activity of tigecycline alone and in combination with colistin and meropenem against Klebsiella pneumoniae carbapenemase (KPC)-producing Enterobacteriaceae strains by time-kill assay. Int. J. Antimicrob. Agents 37:244–247. 10.1016/j.ijantimicag.2010.10.031 [DOI] [PubMed] [Google Scholar]
- 19.Giske CG, Fröding I, Hasan CM, Turlej-Rogacka A, Toleman M, Livermore D, Woodford N, Walsh TR. 2012. Diverse sequence types of Klebsiella pneumoniae contribute to the dissemination of blaNDM-1 in India, Sweden, and the United Kingdom. 56:2735–2738. 10.1128/AAC.06142-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyers BR, Wilkinson P, Mendelson MH, Bournazos C, Tejero C, Hirschman SZ. 1993. Pharmacokinetics of aztreonam in healthy elderly and young adult volunteers. J. Clin. Pharmacol. 33:470–474. 10.1002/j.1552-4604.1993.tb04690.x [DOI] [PubMed] [Google Scholar]
- 21.Lettieri JT, Rogge MC, Kaiser L, Echols RM, Heller AH. 1992. Pharmacokinetic profiles of ciprofloxacin after single intravenous and oral doses. Antimicrob. Agents Chemother. 36:993–996. 10.1128/AAC.36.5.993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, Silveira FP, Forrest A, Nation RL. 2011. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob. Agents Chemother. 55:3284–3294. 10.1128/AAC.01733-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dvorchik BH, Brazier D, DeBruin MF, Arbeit RD. 2003. Daptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjects. Antimicrob. Agents Chemother. 47:1318–1323. 10.1128/AAC.47.4.1318-1323.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matzi V, Lindenmann J, Porubsky C, Kugler SA, Maier A, Dittrich P, Smolle-Jüttner FM, Joukhadar C. 2010. Extracellular concentrations of fosfomycin in lung tissue of septic patients. J. Antimicrob. Chemother. 65:995–998. 10.1093/jac/dkq070 [DOI] [PubMed] [Google Scholar]
- 25.Conte JE, Jr, Golden JA, Kelley MG, Zurlinden E. 2005. Intrapulmonary pharmacokinetics and pharmacodynamics of meropenem. Int. J. Antimicrob. Agents. 26:449–456. 10.1016/j.ijantimicag.2005.08.015 [DOI] [PubMed] [Google Scholar]
- 26.Pfizer 2011. Rifampin: package insert. Pfizer Labs, New York, NY [Google Scholar]
- 27.Theravance 2009. Vibativ: package insert. Theravance, San Francisco, CA [Google Scholar]
- 28.Meagher AK, Ambrose PG, Grasela TH, Ellis-Grosse EJ. 2005. Pharmacokinetic/pharmacodynamic profile for tigecycline-a new glycylcycline antimicrobial agent. Diagn. Microbiol. Infect. Dis. 52:165–171. 10.1016/j.diagmicrobio.2005.05.006 [DOI] [PubMed] [Google Scholar]
- 29.James JK, Palmer SM, Levine DP, Rybak MJ. 1996. Comparison of conventional dosing versus continuous-infusion vancomycin therapy for patients with suspected or documented Gram-positive infections. Antimicrob. Agents Chemother. 40:696–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bratu S, Tolaney P, Karumudi U, Quale J, Mooty M, Nichani S, Landman D. 2005. Carbapenemase-producing Klebsiella pneumoniae in Brooklyn, NY: molecular epidemiology and in vitro activity of polymyxin B and other agents. J. Antimicrob. Chemother. 56:128–132. 10.1093/jac/dki175 [DOI] [PubMed] [Google Scholar]
- 31.Drawz SM, Bonomo RA. 2010. Three decades of beta-lactamase inhibitors. Clin. Microbiol. Rev. 23:160–201. 10.1128/CMR.00037-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rahal JJ. 2009. Antimicrobial resistance among and therapeutic options against Gram-negative pathogens. Clin. Infect. Dis. 49(Suppl):S4–S10. 10.1086/599810 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


