Abstract
Aspergillus fumigatus biofilms still present a challenge for effective treatment in clinical settings. While mild heat stress has been introduced as a treatment for infectious diseases, the effectiveness of mild heat stress on A. fumigatus biofilm formation and antifungal susceptibility is still unknown. In the present study, confocal laser scanning microscopy (CLSM) was used to image and quantify Aspergillus fumigatus biofilm formation under three different regimens of continuous mild heat stress: at 37, 39, and 41°C. Furthermore, fungal growth has been investigated under the above conditions in combination with antifungal drugs (amphotericin B [AMB], micafungin [MCF], and voriconazole [VOC]) at early and late stages. CLSM analysis showed that higher temperatures induce earlier germination and greater hyphal elongation but poorer polar growth and reduced biofilm thickness. In the early stage of biofilm formation, the combination of treatment at 39 or 41°C with MCF or VOC produced no visible difference in biomass formation from similar treatments at 37°C with the same drug. Interestingly, AMB treatment at 37°C inhibited early stage biofilm formation to a much greater extent than at 39 and 41°C. At the late stage of biofilm formation, the mild heat treatments at 39 and 41°C with AMB, MCF, and VOC inhibited biomass formation compared to that at 37°C. The present data show that mild heat stress has a negative regulatory effect on biofilm formation in vitro, and antifungal drug improvement with mild heat treatment at late-stage biofilm formation provides useful indications of possible effective strategies for clinical management of aspergillosis.
INTRODUCTION
The frequency of systemic mycoses has dramatically increased mainly because of the increased use of invasive medical devices, immunosuppressive therapy, and transplantation. Aspergillus fumigatus is the second most common invasive fungal pathogen after Candida albicans (1). In some patient populations, A. fumigatus is associated with mortality rates ranging from 30% to 90% (2–5). The significance of biofilm formation in fungal infections caused by Aspergillus spp. has been noted in bronchopulmonary lavage samples under microscopic examination (6, 7).
Biofilms are structured microbial communities attached to surfaces and encased within a matrix of extracellular polymeric substances (8, 9). The Centers for Disease Control and Prevention reported that 65% of human infections are related to biofilms (10). Compared with planktonic morphologies, this lower-dimensional morphology has increased resistance to antimicrobial agents (11, 12) and enhanced pathogenicity for the host (13, 14). The biofilm morphology of A. fumigatus has been demonstrated in vitro (9, 15), and its susceptibility to azoles, polyenes, and echinocandins according to the CLSI M38-A methodology is almost 1,000 times lower than that of planktonic cells (16–18). Combinations of some antifungal drugs themselves or new potential antifungal agents against A. fumigatus biofilms in vitro have shown that synergistic antifungal activity occurred for most common antifungal drugs, but the susceptibility was lower than that of planktonic cell morphologies (19–21). With the greater prevalence of antifungal agent-resistant strains, the treatment of invasive A. fumigatus has become more difficult. Therefore, new therapeutic strategies against biofilm-associated mycoses are urgently needed.
Continuous mild heat stress has been introduced in the treatment of muscle injury (22) and infectious diseases (23, 24). Cowen and Lindquist (25) found that the susceptibility of planktonic C. albicans cells from clinical isolates to fluconazole is increased by mild heat stress. Cho (26) believes that continuous mild heat stress increases the susceptibility of C. albicans biofilm to antifungal drugs, such as fluconazole, micafungin (MCF), and amphotericin B (AMB). Given the fact that A. fumigatus differs greatly from C. albicans with regard to genotype and phenotype, leading to its superior adaptability and more favorable entry, it is reasonable to determine whether antifungal drug susceptibilities are affected by mild heat stress in this pathogenic fungus. To address such a matter, we used confocal laser scanning microscopy (CLSM) to analyze the effectiveness of mild heat stress on biofilm formation in A. fumigatus in the present study. The particular significance of temperature adaptation for drug effectiveness in A. fumigatus was further investigated in vitro by testing antifungal drug susceptibility at early and late stages of biofilm formation after mild heat stress treatment. Our results demonstrate that the antifungal drug susceptibilities at either stage differ with the application of persistent mild heat treatment. The increased susceptibility to AMB, MCF, and voriconazole (VOC) at the late stage under high temperature suggests a potentially novel strategy of combining mild heat stress with antifungal agents.
MATERIALS AND METHODS
Strains and conidial preparation.
A. fumigatus Af293 (ATCC MYA-4609, CBS 101355) was used throughout this study. The isolate was maintained at 4°C on Sabouraud dextrose agar (SDA) slopes and grown on an SDA plate at 37°C for 72 h. The cell suspension at 1 × 105 conidia ml−1 in RPMI 1640 was prepared according to the method previously described (11). All manipulations of cells were carried out in an Esco purifier biological safety cabinet.
Antifungal drug preparations.
All antifungal agents were purchased in powder form: voriconazole (VOC) and AMB from Sigma and MCF from Fujisawa. VOC and AMB were diluted in 100% dimethyl sulfoxide (DMSO) to make a stock solution of 1,280 μg/ml. MCF was diluted in sterile distilled water as a 1,280-μg/ml stock solution. Each drug was tested for antifungal susceptibility in RPMI 1640 medium, buffered to pH 7.0 with 0.165 M MOPS (morpholinepropanesulfonic acid), and serially diluted 2-fold into final concentrations ranging from 0.06 to 2.0 μg/ml. Cultures without antifungal agents at each temperature in RPMI 1640 medium were used as controls.
Biofilm visualization and thickness measurement.
A. fumigatus Af293 biofilms were formed on a coverslip in a polystyrene, flat-bottom, 24-well tissue culture plate (Corning). Biofilm phenotypes observed at 39 and 41°C, respectively, are compared to the phenotypes at 37°C. A 13-mm-diameter sterile plastic cell culture coverslip (Fisher) was used as a growth surface for the biofilm formation and was inserted into the 24-well tissue culture plate. Approximately 500 μl of the A. fumigatus cell suspension (total of 1.2 × 105 cells) described above was added to each well. Subsequently, the plates were incubated at 37, 39, and 41°C. The biofilms were checked at the time points 2, 4, 6, 8, 10, 16, 18, 24, 48, and 72 h. The temperature stabilization of the culture was guaranteed by continuous monitoring of the incubator. The biofilms on the coverslips were washed by phosphate-buffered saline (PBS) at each indicated time point and then stained with FUN-1 (Invitrogen), thereby binding the intravacuolar structures of the fungal cells according to the manufacturers' instructions. For biofilm visualization, 200 μl of FUN-1 solution at a concentration of 25 μmol/liter was added to the surface of each biofilm and then incubated at 37°C in dark for an additional 20 min. The biofilm was washed again with PBS and mounted on a slide. CLSM (Olympus FV1000) was used to examine the fluorescent filamentous biomass. An excitation wavelength of 488 nm using an argon ion laser at a magnification of ×200 was used to examine the biofilms. The hyphal length was measured by means of software provided by the manufacturer. The thickness of the biofilm was measured at 1-μm intervals along the z axis of the sections taken parallel to the x-y plane. Three-dimensional images were assembled using software (Olympus FluoView version 3.1 viewer) provided by Olympus. All experiments were conducted in triplicate.
Drug susceptibility at the early stage of biofilm formation.
A. fumigatus Af293 biofilm was created according to a partially modified method by Pierce et al. (27). Approximately, 200 μl of A. fumigatus cell suspension (4.8 × 104 cells in total) was added to the 96-well tissue culture plates. The cells were allowed to adhere for 4 h at 37°C. After 4 h, the medium was removed, and the wells were washed three times with PBS. The RPMI 1640 medium, with and without antifungal agents (AMB, MCF, and VOC), was added to the wells at concentrations ranging from 0.06 to 2.00 μg/ml. The plates were then incubated at three temperatures (37, 39, and 41°C) for 24 h individually. The temperature was monitored consistently. After 24 h of incubation, biofilm biomass was assessed by XTT [2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide] assay and cellular viability (CV) assay as previously described by Pierce et al. (27) and Li et al. (28). The optical densities (OD) measured by micro-enzyme-linked immunosorbent assay (micro-ELISA) plate reader at 490 nm for the XTT assay and 570 nm for the CV assay were recorded. All experiments were conducted in quintuplicate.
Drug susceptibility at the late stage of biofilm formation.
Drug susceptibility testing of A. fumigatus Af293 biofilm was carried out following the method of Pierce et al. (27). About 200 μl of cell suspension of A. fumigatus (4.5 × 104 cells in total) was added to 96-well tissue culture plates (Corning), and then all of the plates were incubated at 37°C for 24 h. After 24 h, the medium was removed and the biofilms were washed three times with sterile PBS without touching the biofilms. Fresh RPMI 1640 medium with and without the antifungal agents was added as described above. The plates were then incubated at 37, 39, and 41°C, respectively, for an additional 48 h. The medium then was discarded, and the plates were washed three times with PBS. Biofilm biomass was assessed by the XTT assay and CV assay as described above. All experiments were conducted in quintuplicate.
Statistical analysis.
All assays were performed on at least three independent occasions. Results of statistical analyses are presented as means ± standard deviations (SD). Differences between groups were tested using Student's t test, with P values of <0.05 considered statistically significant.
RESULTS
The dynamics of biofilm production under different temperatures.
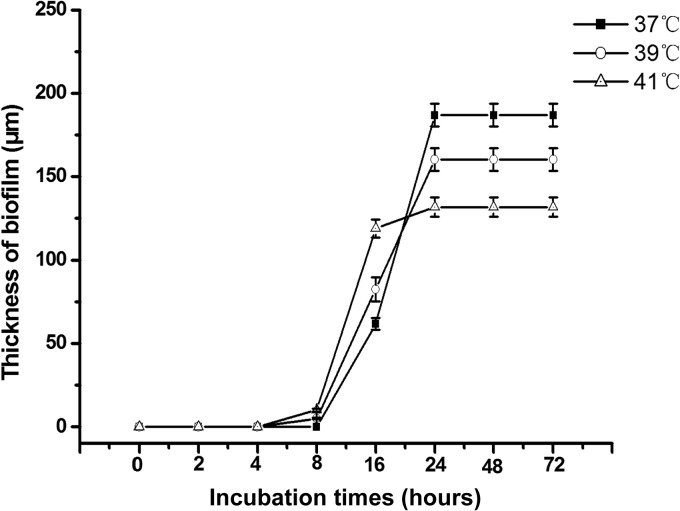
To better understand whether mild heat stress has the potential to affect biofilm formation and interfere with drug susceptibility in A. fumigatus, we first monitored the process of biofilm formation at three different temperatures. A host temperature of 37°C was assigned as the control for entire study, and a persistent mild heat treatment was set up at 39 or 41°C. The thickness of biofilm formed by A. fumigatus was measured quantitatively by CLSM after staining with FUN-1. As shown in Fig. 1, the thickness of biofilm produced at the higher temperatures exceeded that at the control temperature, with 82.4 ± 7.1 μm (P < 0.05) at 39°C and 118.8 ± 3.5 μm (P < 0.05) at 41°C after 16 h. The difference in levels of biofilm production between 39 and 41°C was also significant (P < 0.05) at 16 h (Fig. 1). Conversely, in the time period from 24 h to 72 h, the thickness of biofilm produced at higher temperatures lagged behind that formed at 37°C, with 160 ± 6.7 μm (P < 0.05) at 39°C and 131 ± 5.8 μm (P < 0.05) at 41°C (Fig. 1). Moreover, the difference in biofilm thicknesses between 39 and 41°C was statistically significant (P < 0.05) (Fig. 1). These results indicate that mild heat stress accelerates biofilm production at early stages (<16 h), but this early faster growth will not remain at 24 h. Therefore, while 37°C is the optimal temperature for maximal biofilm development, the mild heat treatment alleviated this process effectively at 24 h.
FIG 1.
The thickness of biofilm under different mild heat stress conditions at 37, 39, and 41°C. The biofilm thickness of A. fumigatus was measured by confocal laser-scanning microscopy (CLSM) at different time points, as indicated in the graph. Means of thickness from three independent experiments are shown. Error bars represent standard deviations (SD).
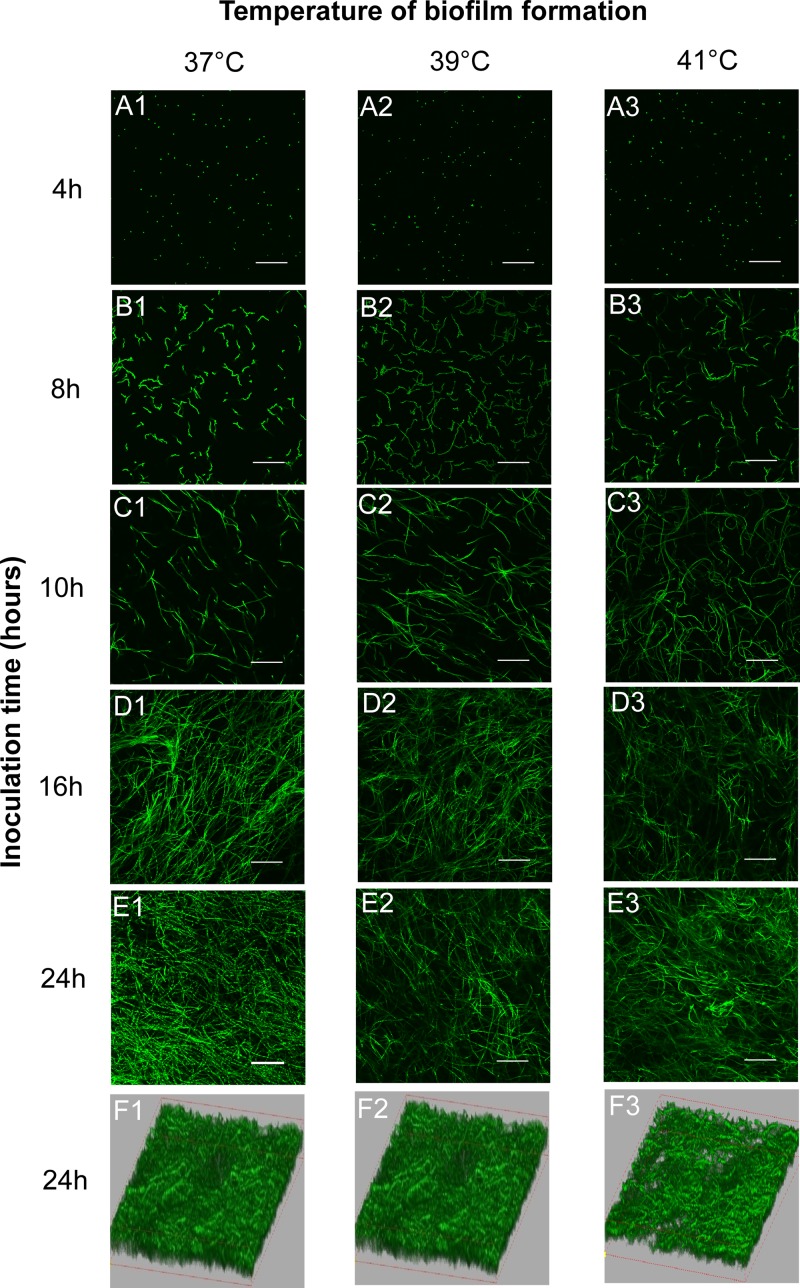
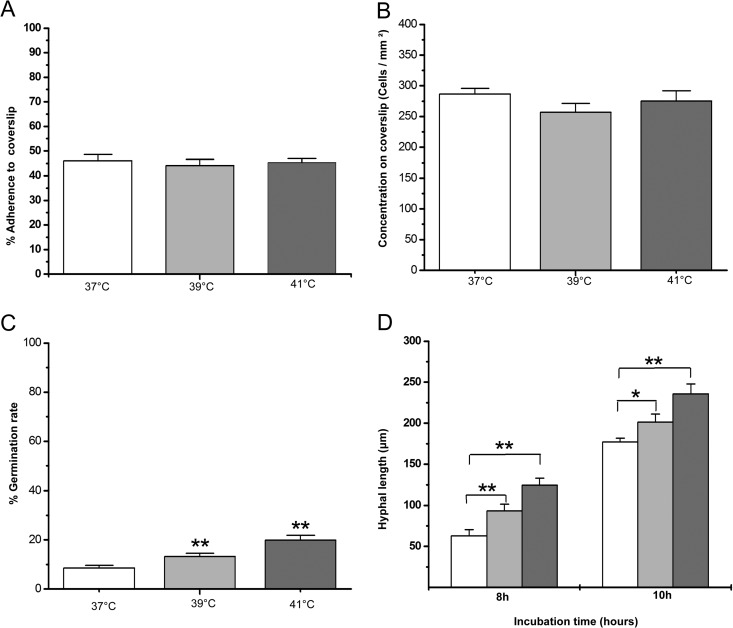
Apparently, the dynamics of biofilm formation in A. fumigatus were affected differently at different temperatures in CLSM studies, as observed above, especially in the time period from 16 to 24 h. To understand roles of the early events on the biofilm development, we then examined the adherence rate and morphological features at early stage of biofilm formation. At 4 h, we found that the conidia began to adhere to the supporter regardless of the set temperature, as shown in Fig. 2A. Compared to 37°C, the adhesion rate of conidia to a coverslip at 39 or 41°C is not significantly different from a statistical point of view (P > 0.05) (Fig. 3A). Compared to 37°C, the conidial concentration on a coverslip at 39 or 41°C is not significantly different from a statistical point of view (P > 0.05) (Fig. 3B). At 8 h, adhered conidia began to germinate at all 3 temperatures. However, the germination rates of the conidia were increased if the temperature was elevated. In Fig. 3C, the germination rates at 8 h are shown as 8.56% ± 1.04%, 13.2% ± 1.4%, and 19.88% ± 1.9% for 37, 39, and 41°C, respectively. In same period (from 8 h to 10 h), the hyphal length formed at 39 or at 41°C in Fig. 3D was much longer than the one at 37°C. The higher germination rate and longer germination tube under mild heat treatment at the early stage of biofilm development are consistent with a thicker biofilm at 16 h, as we mentioned above. However, after 10 h (Fig. 2C), the polar growth of hyphal was clearly disrupted by mild heat stress (39 or at 41°C), which correlated to a less compact biofilm structure after 24 h of observation in Fig. 2F. Taken together, these data indicate that the polar growth of conidia at 37°C optimizes biofilm thickness and density, although the higher temperature leads to faster germination rates and adherence at an earlier stage of biofilm formation.
FIG 2.
Images of A. fumigatus Af293 biofilm formation at 37, 39, and 41°C obtained by CLSM. The cells were stained with FUN-1. Three-dimensional images (row F) depict the differences of biofilm thickness with different heat stress treatments (37, 39, and 41°C). The original magnification was ×200, and the scale bar is 100 μm.
FIG 3.
The dynamics of A. fumigatus cells in biofilm formation at different mild heat stresses. The results were quantified in triplicate by FUN-1 staining. The values that are significantly different by Student's t test are indicated by asterisks: *, P < 0.05;**, P < 0.01. Light bars represent 37°C, light-gray bars 39°C, and dark-gray bars 41°C. (A) Adherence rates. The rates were also normalized with the cell number observed in CLSM by the following formula: amount of cells adhered to coverslips/total cells inoculated in tested well. (B) The concentration of cells on the coverslips was calculated by the number of cells in CLSM assay by the following formula: amount of cells in photographed area/area size. (C) The germination rate of A. fumigatus cells was quantified with CLSM by the following formula: amount of germination cells/total in area. (D) The hyphal lengths of each experimental condition at 8 and 10 h were measured by CLSM.
The early stage of biofilm is resistant to AMB at 39 and 41°C.
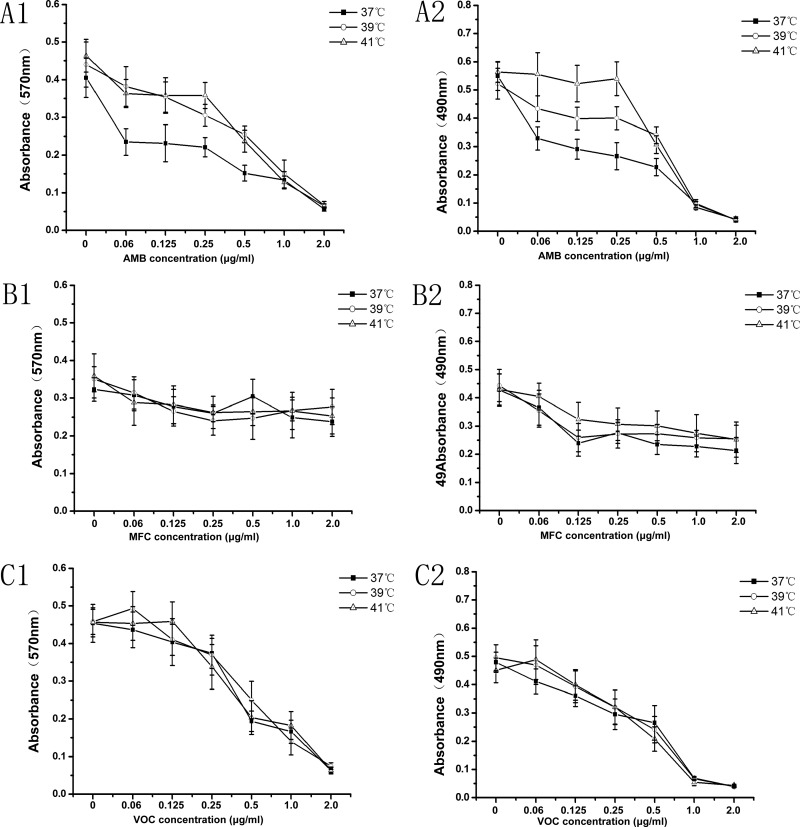
Our next focus was on drug susceptibility in the biofilms developed at different temperatures. For the study of the early stages, AMB, MCF, and VOC at concentrations from 0.06 to 2.0 μg/ml were introduced at the early stage of biofilm formation (4 h) at all 3 set temperatures. Growth inhibition under drug treatment was measured by both XTT assay and CV assay for each drug. As shown in Fig. 4, mild heat stress at 39 or 41°C shows no effect on biofilm formation with the antifungal drugs MCF or VOC within the range of concentrations tested (0.06 to 2.00 μg/m) in the CV assay and XTT assay: P > 0.05 compared to 37°C (Fig. 4B1, B2, C1, and C2). There was also no statistical difference (P > 0.05) in levels of biofilm formation in the presence of MCF or VOC between 39 and 41°C. However, the biomasses of cells experiencing AMB concentrations ranging from 0.06 to 0.50 μg/ml at 39 or 41°C were not reduced in either the CV or the XTT assay, demonstrating statistical significance (P < 0.05) compared to the 37°C results in Fig. 4A1 and A2. Within the same range of AMB concentrations, mild heat stress at 39°C produced a greater reduction in biofilm formation than at 41°C in the XTT assay (P < 0.05) but not in the CV assay (P > 0.05). The different effects seen with the two assay methods here may be due to their difference in detection principles (29). The XTT assay detects living cells only, and the CV assay stains both living and dead cells. Therefore, these subtle differences may come from the dead cells in the CV assay.
FIG 4.
Antifungal drug susceptibilities at an early stage of biofilm formation. The antifungal activities of AMB, MCF, and VOC against the early stage of biofilm at different mild heat stresses (37, 39, and 41°C) were tested in RPMI 1640. The results were obtained by crystal violet assay at OD570 (left panels) and XTT assay at OD490 (right panels). Error bars represent standard deviations. The data are shown as means ± SD for 5 samples.
With the higher concentrations of the antifungal drug AMB (1.0 to 2 μg/ml), mild heat stress at 39 or 41°C shows no effects on biofilm formation compared to those at 37°C in both the CV and XTT assays (P > 0.05). The AMB tolerance seen in the early stages of biofilm formation at 39 or 41°C occurred only with the lower concentrations (0.06 to 0.50 μg/ml) of the drug.
Late-stage biofilm is sensitive to AMB, MCF, and VOC at 39 and 41°C.
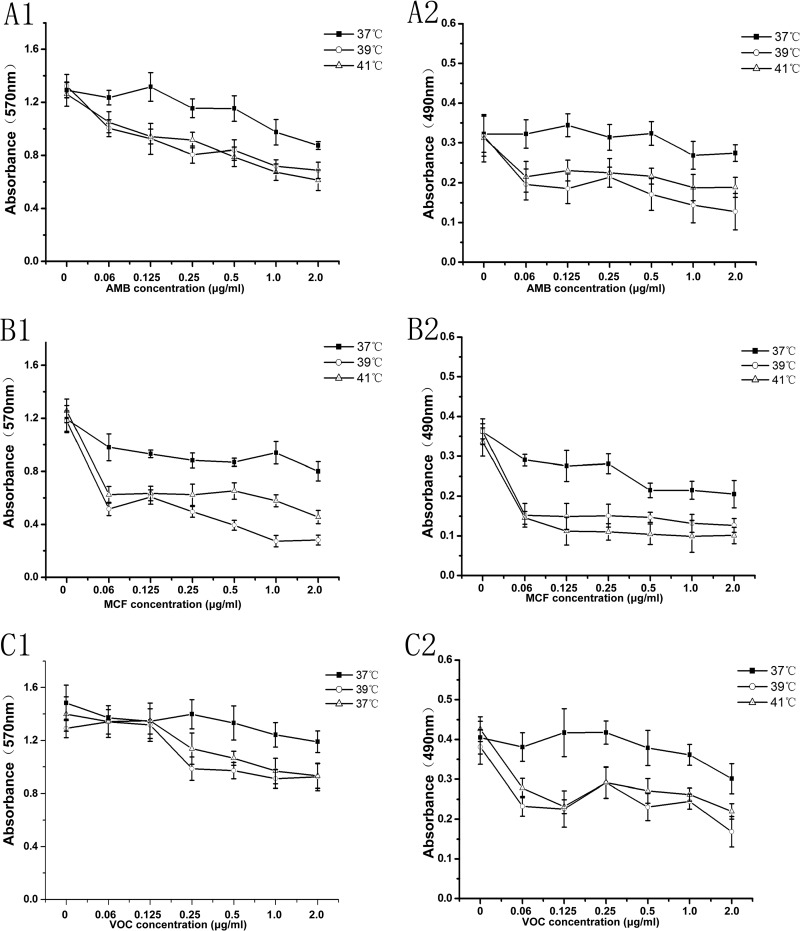
The drug sensitivities of AMB, MCF, and VOC were also tested on late-stage biofilm under mild heat circumstances. As shown in Fig. 5, the A. fumigatus biofilm formed after 24 h of being pregrown at 37°C displayed different drug inhibitory patterns when subjected to mild heat environments (39 and 41°C) after formation. Compared to persistence at 37°C, the inhibitory effects of AMB and MCF in the range of 0.06 to 2.0 μg/ml were significantly higher in both the CV assay (Fig. 5A1 and B1) and the XTT assay (Fig. 5A2 and B2) when tested at 39 or 41°C (P < 0.05). This relationship of hypersusceptibility to mild heat treatment was also observed with VOC in the XTT assay (Fig. 5C2) in a broad range of drug concentrations (0.06 to 2.0 μg/ml) but was only seen in a high range of drug concentrations (0.25 to 2.0 μg/ml) with the CV assay for VOC testing (Fig. 5C1). These results indicate that mild heat stress at 39 or 41°C increases the sensitivity of late-stage biofilm to the antifungal drugs AMB, MCF, and VOC.
FIG 5.
Antifungal drug susceptibilities at the late stage of biofilm formation. The antifungal activities of AMB, MCF, and VOC against later stages of biofilm formation at different mild heat stresses (37, 39, and 41°C) were tested in RPMI 1640. The results were obtained by crystal violet assay at OD570 (left panels) and XTT assay at OD490 (right panels). Error bars represent standard deviations. The data are shown as means ± SD for 5 samples.
DISCUSSION
A. fumigatus is a ubiquitous opportunistic fungus that causes the majority of human aspergillosis cases. A. fumigatus infections have undergone a dramatic upsurge recently. Although new antifungal drugs have been introduced, the mortality of A. fumigatus infections is still high. Thus, new alternatives to increase the antifungal susceptibility of A. fumigatus biofilms are urgently needed. Mild heat stress achieves satisfactory efficacy in some diseases. This study tests whether this satisfactory effect can occur in vitro. Our study is the first to determine the effects of mild heat stress on A. fumigatus biofilms and is also the first to relate those effects to antifungal susceptibility of biofilms in vitro.
Biofilm formation consists of initial adherence, conidial germination, filamentous growth, hyphal intertwining, and matrix growth. Our results show that the adherence phase proceeds similarly no matter what temperature is used, indicating similar adherence rates at 37, 39, and 41°C; however, the conidia behave differently at the phase of morphogenetic conversion at the three temperatures, when conidia switches to the filamentous phase. The germination times were much earlier and the germination rates higher at 39 and 41°C than those at the control temperature of 37°C. This result is similar to that described by Cho et al. in their study of C. albicans biofilm formation (26). We also find that polar growth (i.e., coherent directional growth) was noticeable only at 37°C. While it is clear that this coherent polar growth was somehow disturbed at higher temperatures, suggesting that it may reduce the pathogenicity of Aspergillus fumigatus, the reason is still unclear. Polarized growth is found mostly in filamentous fungi (30) and is highly correlated with the pathogenicity of fungi (31). The conidia undergo a brief period of symmetric growth after germination and continue to grow in this manner until they form the hyphae and mycelia. Although much progress in investigating the mechanisms for polar growth has been made, the mechanisms for the complex process of cell wall and cell membrane synthesis for polar growth are still not very clear. Among them, some researchers found that maintaining polar growth had a certain relationship with temperature. Zhang (32) reported that the temperature-sensitive A. fumigatus ΔAfcwh41 strain showed abnormalities of polarity comparing to the wild-type Aspergillus fumigatus YJ-407 strain when incubated at 37°C for 24 h. Similar results were found by Castillo-Lluva (33) for a temperature-sensitive cdk5 mutant from Ustilago maydis in his study and by Meyer (34) for a temperature-sensitive apically branching ramosa-1 mutant from Aspergillus nidulans. However, Chen and Song (35) have found that polar growth at 42°C was similar to polar growth at 37°C on an Aspergillus nidulans green fluorescent protein-calmodulin promoter (GFP-cam) strain. This result is quite different from ours. One explanation is that as an environmental fungus, Aspergillus nidulans must retain improved temperature adaptability, which is not needed for the human-pathogenic fungus A. fumigatus.
Somewhat related to the polar growth status (since more coherent growth can be expected to lead to denser formations), we found that the biofilm was thicker at 37°C than those at 39°C and at 41°C after a 24-h period. Momany (36) claimed that highly polar growth allowed A. fumigates to invade blood vessels and tissue. Scheffer (31) also found that polar growth was essential for the ergot fungus Claviceps purpurea to penetrate the host. Given these results, it would appear that polar growth may have better growth in the vertical direction. Therefore, it can form a thicker biofilm.
With regard to drug interference in biofilm formation, our results from this study differ from those of Cho (26) when drug resistance was tested at the earliest stage of biofilm formation of 4 h (after spores adhering to the supporter). Cho concluded that mild heat stress increased the effectiveness of antifungal agents at low concentrations against C. albicans biofilm formation at 1.5 h after spore application (37, 38). However, in our study, mild heat stress did not significantly influence the biomass and metabolism of the biofilm formation when either MCF or VOC was used at concentrations ranging from 0.06 to 2.00 μg/ml. Ji and Yang (39) reported that mild heat stress increased AMB resistance but reduced VOC resistance and had no influence on echinocandin susceptibility in planktonic A. fumigatus cells. This AMB resistance was also seen with our higher-temperature treatment at an early stage of biofilm formation when AMB concentrations were in the range of 0.06 to 0.50 μg/ml. However, this correlation of AMB resistance with high temperature was not evident when AMB concentrations were higher than 1.00 μg/ml.
The observation of Ji and Yang (39) with planktonic A. fumigatus cells may be due to the precise antifungal mechanism of AMB. At low concentrations, AMB prompts fungal cells to increase production of DNA and RNA (40) and therefore may indirectly promote biofilm production. Increasing the drug concentration to 1.00 μg/ml increased the AMB antifungal effect sufficiently to become lethal against biofilms, suggesting that we may need to avoid situations in clinical settings where the drug dose is low and the host is suffering from fever. The antifungal effects of AMB, MCF, and VOC were improved with higher-temperature treatment once the biofilm has been formed after 24 h in this study, indicating that the mild heat stress response of A. fumigatus favors drug susceptibility on well-formed biofilm.
The activation of calcineurin and the high-osmolarity glycerol (HOG) mitogen-activated protein kinase (MAPK) cascade (39, 41) have also been associated with a mild heat stress response. Both pathways promote FKS2 expression (42), which is known to reduce susceptibility to MCF in planktonic A. fumigatus cells (43, 44). However, the precise mechanism for drug susceptibility of A. fumigatus biofilm under mild heat stress remains unclear. The growth of biofilm extracellular matrix (ECM) could be one explanation. At 24 h, the biofilm reaches its maximum thickness and then synthesizes the matrix in succeeding 24-h periods (45). The extracellular polymeric substances (EPS) of A. fumigatus biofilms usually contain glucose (74%), mannitol (18%), glycerol (5%), trehalose (3%), melanin, and proteins (2%) (46). Extracellular DNA (eDNA) accounts for less than 1% of the ECM composition of Pseudomonas aeruginosa biofilms. It plays an important role in biofilm structural integrity and antifungal resistance (47–50), which can also be found in A. fumigatus biofilms (47, 48). The release of eDNA (which is genomic DNA coming from autolysis) of ECM in A. fumigatus biofilms was lower with higher-temperature treatment but phase dependent (release at 8 h < at 12 h < at 24 h < at 48 h) in another study (47). The reason for this situation may be that long-term mild heat stress will force chitinase, a regulator of autolysis (51), to lose its activity (52). The evidence shown that only 75% ± 4%, 34% ± 1%, and 0% chitinase activities were retained, respectively, after 30 min at 40, 50, and 60°C, although the activity can reach its maximum at 55 to 65°C (52). Thus, we presume that increasing the temperature would decrease the activity of chitinase and the release of eDNA, which may increase susceptibility of A. fumigatus biofilms to antifungal drugs.
Almost all of the results of the XTT assay agreed with the results of the CV biomass assay, and both methods showed high repeatability in our investigation. In antifungal tests on mature biofilm, the CV biomass assay encountered difficulty in distinguishing live from dead cells and the matrix. However, even with these drawbacks, the CV biomass assay method is still able to reveal the effect of mild heat stress on the biomass under the influence of antifungal drugs.
In summary, we conclude that mild heat stress affects A. fumigatus biofilm independently of any of the antifungal treatments studied here and also affects the antifungal susceptibility of mature biofilm. More animal tests and a larger amount of clinical experience are needed to determine whether this effect can be used in clinical treatment.
ACKNOWLEDGMENT
This work was support by grants from the Major National Science and Technology Projects (2012ZX09301002005001005). The funders had no role in the design of the study, data analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published ahead of print 16 December 2013
REFERENCES
- 1.Ellis M, Richardson M, de Pauw B. 2000. Epidemiology. Hosp. Med. 61:605–609 [DOI] [PubMed] [Google Scholar]
- 2.Denning DW, Marinus A, Cohen J, Spence D, Herbrecht R, Pagano L, Kibbler C, Kcrmery V, Offner F, Cordonnier C, Jehn U, Ellis M, Collette L, Sylvester R. 1998. An EORTC multicentre prospective survey of invasive aspergillosis in haematological patients: diagnosis and therapeutic outcome. EORTC Invasive Fungal Infections Cooperative Group. J. Infect. 37:173–180 [DOI] [PubMed] [Google Scholar]
- 3.Brakhage AA. 2005. Systemic fungal infections caused by Aspergillus species: epidemiology, infection process and virulence determinants. Curr. Drug Targets 6:875–886. 10.2174/138945005774912717 [DOI] [PubMed] [Google Scholar]
- 4.Herbrecht R, Denning DW, Patterson TF, Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, Kern WV, Marr KA, Ribaud P, Lortholary O, Sylvester R, Rubin RH, Wingard JR, Stark P, Durand C, Caillot D, Thiel E, Chandrasekar PH, Hodges MR, Schlamm HT, Troke PF, de Pauw B. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408–415. 10.1056/NEJMoa020191 [DOI] [PubMed] [Google Scholar]
- 5.Maschmeyer G, Haas A, Cornely OA. 2007. Invasive aspergillosis: epidemiology, diagnosis and management in immunocompromised patients. Drugs 67:1567–1601. 10.2165/00003495-200767110-00004 [DOI] [PubMed] [Google Scholar]
- 6.Muller FM, Seidler M, Beauvais A. 2011. Aspergillus fumigatus biofilms in the clinical setting. Med. Mycol. 49(Suppl 1):S96–S100. 10.3109/13693786.2010.502190 [DOI] [PubMed] [Google Scholar]
- 7.Loussert C, Schmitt C, Prevost MC, Fadel E, Philippe B, Kauffmann-Lacroix C, Latgé JP, Beauvais A. 2010. In vivo biofilm composition of Aspergillus fumigatus. Cell. Microbiol. 12:405–410. 10.1111/j.1462-5822.2009.01409.x [DOI] [PubMed] [Google Scholar]
- 8.Donlan RM. 2002. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 8:881–890. 10.3201/eid0809.020063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muszkieta L, Beauvais A, Pähtz V, Gibbons JG, Anton Leberre V, Beau R, Shibuya K, Rokas A, Francois JM, Kniemeyer O, Brakhage AA, Latgé JP. 2013. Investigation of Aspergillus fumigatus biofilm formation by various “omics” approaches. Front. Microbiol. 4:13. 10.3389/fmicb.2013.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potera C. 1999. Forging a link between biofilms and disease. Science 283:1837-1839. 10.1126/science.283.5409.1837 [DOI] [PubMed] [Google Scholar]
- 11.Mowat E, Butcher J, Lang S, Williams C, Ramage G. 2007. Development of a simple model for studying the effects of antifungal agents on multicellular communities of Aspergillus fumigatus. J. Med. Microbiol. 56:1205–1212. 10.1099/jmm.0.47247-0 [DOI] [PubMed] [Google Scholar]
- 12.Hawser SP, Douglas LJ. 1995. Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrob. Agents Chemother. 39:2128–2131. 10.1128/AAC.39.9.2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suh JD, Ramakrishnan V, Palmer JN. 2010. Biofilms. Otolaryngol. Clin. North Am. 43:521–530. 10.1016/j.otc.2010.02.010 [DOI] [PubMed] [Google Scholar]
- 14.Psaltis AJ, Weitzel EK, Ha KR, Wormald PJ. 2008. The effect of bacterial biofilms on post-sinus surgical outcomes. Am. J. Rhinol. 22:1–6. 10.2500/ajr.2008.22.3119 [DOI] [PubMed] [Google Scholar]
- 15.Papi M, Maiorana A, Bugli F, Torelli R, Posteraro B, Maulucci G, De Spirito M, Sanguinetti M. 2012. Detection of biofilm-grown Aspergillus fumigatus by means of atomic force spectroscopy: ultrastructural effects of alginate lyase. Microsc. Microanal. 18:1088–1094. 10.1017/S1431927612001067 [DOI] [PubMed] [Google Scholar]
- 16.Mowat E, Lang S, Williams C, McCulloch E, Jones B, Ramage G. 2008. Phase-dependent antifungal activity against Aspergillus fumigatus developing multicellular filamentous biofilms. J. Antimicrob. Chemother. 62:1281–1284. 10.1093/jac/dkn402 [DOI] [PubMed] [Google Scholar]
- 17.Seidler MJ, Salvenmoser S, Muller FM. 2008. Aspergillus fumigatus forms biofilms with reduced antifungal drug susceptibility on bronchial epithelial cells. Antimicrob. Agents Chemother. 52:4130–4136. 10.1128/AAC.00234-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiori B, Posteraro B, Torelli R, Tumbarello M, Perlin DS, Fadda G, Sanguinetti M. 2011. In vitro activities of anidulafungin and other antifungal agents against biofilms formed by clinical isolates of different Candida and Aspergillus species. Antimicrob. Agents Chemother. 55:3031–3035. 10.1128/AAC.01569-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu W, Li L, Sun Y, Chen W, Wan Z, Li R, Liu W. 2012. Interaction of the echinocandin caspofungin with amphotericin B or voriconazole against Aspergillus biofilms in vitro. Antimicrob. Agents Chemother. 56:6414–6416. 10.1128/AAC.00687-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bugli F, Posteraro B, Papi M, Torelli R, Maiorana A, Paroni Sterbini F, Posteraro P, Sanguinetti M, De Spirito M. 2013. In vitro interaction between alginate lyase and amphotericin B against Aspergillus fumigatus biofilm determined by different methods. Antimicrob. Agents Chemother. 57:1275–1282. 10.1128/AAC.01875-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajendran R, Mowat E, McCulloch E, Lappin DF, Jones B, Lang S, Majithiya JB, Warn P, Williams C, Ramage G. 2011. Azole resistance of Aspergillus fumigatus biofilms is partly associated with efflux pump activity. Antimicrob. Agents Chemother. 55:2092–2097. 10.1128/AAC.01189-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petering RC, Webb C. 2011. Treatment options for low back pain in athletes. Sports Health 3:550–555. 10.1177/1941738111416446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasday JD, Fairchild KD, Shanholtz C. 2000. The role of fever in the infected host. Microbes Infect. 2:1891–1904. 10.1016/S1286-4579(00)01337-X [DOI] [PubMed] [Google Scholar]
- 24.Jiang Q, Cross AS, Singh IS, Chen TT, Viscardi RM, Hasday JD. 2000. Febrile core temperature is essential for optimal host defense in bacterial peritonitis. Infect. Immun. 68:1265–1270. 10.1128/IAI.68.3.1265-1270.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cowen LE, Lindquist S. 2005. Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi. Science 309:2185–2189. 10.1126/science.1118370 [DOI] [PubMed] [Google Scholar]
- 26.Cho T, Nagao J, Imayoshi R, Kodama J, Morotomi T, Kaminishi H. 2012. In vitro efficacy of continuous mild heat stress on the antifungal susceptibility of Candida albicans biofilm formation. Biol. Pharm. Bull. 35:1371–1373. 10.1248/bpb.b12-00228 [DOI] [PubMed] [Google Scholar]
- 27.Pierce CG, Uppuluri P, Tristan AR, Wormley FL, Jr, Mowat E, Ramage G, Lopez-Ribot JL. 2008. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 3:1494–1500. 10.1038/nport.2008.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Yan Z, Xu J. 2003. Quantitative variation of biofilms among strains in natural populations of Candida albicans. Microbiology 149:353–362. 10.1099/mic.0.25932-0 [DOI] [PubMed] [Google Scholar]
- 29.Peeters E, Nelis HJ, Coenye T. 2008. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J. Microbiol. Methods 72:157–165. 10.1016/j.mimet.2007.11.010 [DOI] [PubMed] [Google Scholar]
- 30.Harris SD, Momany M. 2004. Polarity in filamentous fungi: moving beyond the yeast paradigm. Fungal Genet. Biol. 41:391–400. 10.1016/j.fgb.2003.11.007 [DOI] [PubMed] [Google Scholar]
- 31.Scheffer J, Ziv C, Yarden O, Tudzynski P. 2005. The COT1 homologue CPCOT1 regulates polar growth and branching and is essential for pathogenicity in Claviceps purpurea. Fungal Genet. Biol. 42:107–118. 10.1016/j.fgb.2004.10.005 [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Feng D, Fang W, Ouyang H, Luo Y, Du T, Jin C. 2009. Comparative proteomic analysis of an Aspergillus fumigatus mutant deficient in glucosidase I (AfCwh41). Microbiology 155:2157–2167. 10.1099/mic.0.027490-0 [DOI] [PubMed] [Google Scholar]
- 33.Castillo-Lluva S, Alvarez-Tabares I, Weber I, Steinberg G, Perez-Martin J. 2007. Sustained cell polarity and virulence in the phytopathogenic fungus Ustilago maydis depends on an essential cyclin-dependent kinase from the Cdk5/Pho85 family. J. Cell Sci. 120:1584–1595. 10.1242/jcs.005314 [DOI] [PubMed] [Google Scholar]
- 34.Meyer V, Arentshorst M, Flitter SJ, Nitsche BM, Kwon MJ, Reynaga-Peña CG, Bartnicki-Garcia S, van den Hondel CA, Ram AF. 2009. Reconstruction of signaling networks regulating fungal morphogenesis by transcriptomics. Eukaryot. Cell 8:1677–1691. 10.1128/EC.00050-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen S, Song Y, Cao J, Wang G, Wei H, Xu X, Lu L. 2010. Localization and function of calmodulin in live-cells of Aspergillus nidulans. Fungal Genet. Biol. 47:268–278. 10.1016/j.fgb.2009.12.008 [DOI] [PubMed] [Google Scholar]
- 36.Momany M. 2005. Growth control and polarization. Med. Mycol. 43(Suppl 1):S23–S25. 10.1080/13693780400024263 [DOI] [PubMed] [Google Scholar]
- 37.Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. 2001. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J. Bacteriol. 183:5385–5394. 10.1128/JB.183.18.5385-5394.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramage G, Vandewalle K, Wickes BL, Lopez-Ribot JL. 2001. Characteristics of biofilm formation by Candida albicans. Rev. Iberoam. Micol. 18:163–170 [PubMed] [Google Scholar]
- 39.Ji Y, Yang F, Ma D, Zhang J, Wan Z, Liu W, Li R. 2012. HOG-MAPK signaling regulates the adaptive responses of Aspergillus fumigatus to thermal stress and other related stress. Mycopathologia 174:273–282. 10.1007/s11046-012-9557-4 [DOI] [PubMed] [Google Scholar]
- 40.Brajtburg J, Powderly WG, Kobayashi GS, Medoff G. 1990. Amphotericin B: current understanding of mechanisms of action. Antimicrob. Agents Chemother. 34:183–188. 10.1128/AAC.34.2.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winkler A, Arkind C, Mattison CP, Burkholder A, Knoche K, Ota I. 2002. Heat stress activates the yeast high-osmolarity glycerol mitogen-activated protein kinase pathway, and protein tyrosine phosphatases are essential under heat stress. Eukaryot. Cell 1:163–173. 10.1128/EC.1.2.163-173.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao C, Jung US, Garrett-Engele P, Roe T, Cyert MS, Levin DE. 1998. Temperature-induced expression of yeast FKS2 is under the dual control of protein kinase C and calcineurin. Mol. Cell. Biol. 18:1013–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arendrup MC, Perkhofer S, Howard SJ, Garcia-Effron G, Vishukumar A, Perlin D, Lass-Flörl C. 2008. Establishing in vitro-in vivo correlations for Aspergillus fumigatus: the challenge of azoles versus echinocandins. Antimicrob. Agents Chemother. 52:3504–3511. 10.1128/AAC.00190-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gardiner RE, Souteropoulos P, Park S, Perlin DS. 2005. Characterization of Aspergillus fumigatus mutants with reduced susceptibility to caspofungin. Med. Mycol. 43(Suppl 1):S299–S305 [DOI] [PubMed] [Google Scholar]
- 45.Mowat E, Williams C, Jones B, McChlery S, Ramage G. 2009. The characteristics of Aspergillus fumigatus mycetoma development: is this a biofilm? Med. Mycol. 47(Suppl 1):S120–S126. 10.1080/13693780802238834 [DOI] [PubMed] [Google Scholar]
- 46.Beauvais A, Schmidt C, Guadagnini S, Roux P, Perret E, Henry C, Paris S, Mallet A, Prévost MC, Latgé JP. 2007. An extracellular matrix glues together the aerial-grown hyphae of Aspergillus fumigatus. Cell. Microbiol. 9:1588–1600. 10.1111/j.1462-5822.2007.00895.x [DOI] [PubMed] [Google Scholar]
- 47.Rajendran R, Williams C, Lappin DF, Millington O, Martins M, Ramage G. 2013. Extracellular DNA release acts as an antifungal resistance mechanism in mature Aspergillus fumigatus biofilms. Eukaryot. Cell 12:420–429. 10.1128/EC.00287-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shopova I, Bruns S, Thywissen A, Kniemeyer O, Brakhage AA, Hillmann F. 2013. Extrinsic extracellular DNA leads to biofilm formation and colocalizes with matrix polysaccharides in the human pathogenic fungus Aspergillus fumigatus. Front. Microbiol. 4:141. 10.3389/fmicb.2013.00141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martins M, Uppuluri P, Thomas DP, Cleary IA, Henriques M, Lopez-Ribot JL, Oliveira R. 2010. Presence of extracellular DNA in the Candida albicans biofilm matrix and its contribution to biofilms. Mycopathologia 169:323–331. 10.1007/s11046-009-9264-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martins M, Henriques M, Lopez-Ribot JL, Oliveira R. 2012. Addition of DNase improves the in vitro activity of antifungal drugs against Candida albicans biofilms. Mycoses 55:80–85. 10.1111/j.1439-0507.2011.02047.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alcazar-Fuoli L, Clavaud C, Lamarre C, Aimanianda V, Seidl-Seiboth V, Mellado E, Latgé JP. 2011. Functional analysis of the fungal/plant class chitinase family in Aspergillus fumigatus. Fungal Genet. Biol. 48:418–429. 10.1016/j.fgb.2010.12.007 [DOI] [PubMed] [Google Scholar]
- 52.van Munster JM, van der Kaaij RM, Dijkhuizen L, van der Maarel MJ. 2012. Biochemical characterization of Aspergillus niger CfcI, a glycoside hydrolase family 18 chitinase that releases monomers during substrate hydrolysis. Microbiology 158:2168–2179. 10.1099/mic.0.054650-0 [DOI] [PubMed] [Google Scholar]