Abstract
Fosfomycin may be a treatment option for multiresistant Gram-negative bacteria. This study compared susceptibility methods using 94 multiresistant clinical isolates. With agar dilution (AD), susceptibilities were 81%, 7%, 96%, and 100% (CLSI) and 0%, 0%, 96%, and 30% (EUCAST), respectively, for Acinetobacter baumannii, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Enterobacter spp. Categorical agreement between Etest and AD for Enterobacteriaceae and A. baumannii was ≥80%. Disk diffusion was adequate only for Enterobacter. CLSI criteria for urine may be adequate for systemic infections.
TEXT
Multidrug Gram-negative bacilli are a worldwide threat (1, 2). Furthermore, the availability of new antimicrobials has not matched new patterns of resistance (3). Tigecycline and aminoglycosides are treatment options; however, tigecycline has low plasma and urinary levels and aminoglycosides have significant adverse effects. Moreover, resistance to these antibiotics has increased (4–6).
Fosfomycin, derived from phosphonic acid (7, 8), is chemically unrelated to other antimicrobials and used to treat community-acquired urinary tract infections (8–10). With the spread of multiresistance, fosfomycin is a potential option, although experience with intravenous fosfomycin is scarce (11, 12).
In addition, breakpoints are not well defined by American (CLSI) or European (EUCAST) standards. By CLSI guidelines, only disk diffusion (DD) and agar dilution (AD) for urinary isolates of Escherichia coli and Enterococcus faecalis are approved methods and broth microdilution should not be used (13). EUCAST recommends agar dilution or broth microdilution (14).
Our study compared methods to evaluate the susceptibility to fosfomycin of Gram-negative multiresistant clinical isolates.
Blood, urine, and respiratory isolates of hospitalized patients were obtained from 2 teaching hospitals in Brazil (2010 to 2013). One isolate per patient was included: there were 42 Acinetobacter baumannii isolates, 15 Pseudomonas aeruginosa isolates, 27 Klebsiella pneumoniae isolates, and 10 Enterobacter species isolates, all resistant to carbapenems by microdilution.
A. baumannii strains were previously studied for resistance genes: all studied strains carried blaOXA-51 and carO, 25 carried blaOXA-148, 7 carried blaOXA-23 and blaOXA-143, 2 carried blaIMP, 1 carried blaIMP and blaOXA-143, and 1 carried only blaOXA-23 (15). All K. pneumoniae strains carried blaKPC (16).
Three susceptibility methods were used, and these were DD, AD, and Etest. AD used fosfomycin disodium salt (Sigma-Aldrich Laboratories, St. Louis, MO, USA) and Mueller-Hinton agar (MHA) containing 25 μg/ml of glucose-6-phosphate (G6P) (13). DD used MHA with 50 μg of G6P with 50-μg (Oxoid, Basingstoke, United Kingdom) and 200-μg (Cefar, São Paulo, Brazil) fosfomycin disks. Fosfomycin Etest strips (supplemented with G6P) (bioMérieux, Hazelwood, MO, USA) were used for determination of MICs. E. coli ATCC 25922 and Staphylococcus aureus ATCC 25923 were controls (13). Experiments were performed in duplicate on separate days. Results were read by two observers plus a third, in case of nonagreement.
Isolates were classified according to CLSI breakpoints (susceptible, MIC of ≤64 μg/ml or zone of ≥16 mm [200-μg disk]; intermediate, MIC of 128 μg/ml or zone of 13 to 15 mm; resistant, MIC of ≥256 μg/ml or zone of ≤12 mm) and EUCAST breakpoints (no criteria for DD; susceptible, MIC of ≤32 μg/ml; resistant, MIC of >32 μg/ml) (13, 14). DD and Etest results were compared with AD results. Categorical agreement was defined as results within the same susceptibility category (17). Errors were ranked as very major errors, major errors, and minor errors (17, 18).
AD and Etest results and interpretation according to EUCAST and CLSI standards are shown in Table 1.
TABLE 1.
Antimicrobial activity of fosfomycin against 94 multiresistant Gram-negative bacillus clinical isolatesa
| Microorganism | No. of isolates | Method | MIC (μg/ml) |
% of isolates |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CLSI |
EUCAST |
|||||||||
| MIC50 | MIC90 | Range | S | I | R | S | R | |||
| A. baumannii | 42 | AD | 64 | 128 | 64–128 | 81 | 19 | 0 | 0 | 100 |
| ET | 48 | 96 | 32->1,024 | 90 | 2 | 8 | 31 | 69 | ||
| P. aeruginosa | 15 | AD | 256 | 256 | 64–256 | 7 | 13 | 80 | 0 | 100 |
| ET | 128 | 128 | 48–256 | 33 | 60 | 7 | 0 | 100 | ||
| K. pneumoniae | 27 | AD | 16 | 32 | 2–256 | 96 | 0 | 4 | 96 | 4 |
| ET | 48 | 256 | 0.5->1,024 | 85 | 4 | 11 | 48 | 52 | ||
| Enterobacter spp. | 10 | AD | 64 | 64 | 8–64 | 100 | 0 | 0 | 30 | 70 |
| ET | 12 | 128 | 8–128 | 80 | 20 | 0 | 70 | 30 | ||
AD, agar dilution; ET, Etest; S, susceptible; I, intermediate susceptibility; R, resistant; MIC50, MIC that inhibited 50% of isolates; MIC90, MIC that inhibited 90% of isolates; CLSI, Clinical and Laboratory Standards Institute interpretative criteria (susceptible, MIC of ≤64 μg/ml; intermediate, MIC of 128 μg/ml; resistant, MIC of ≥256 μg/ml); EUCAST, European Committee on Antimicrobial Susceptibility interpretative criteria (susceptible, MIC of ≤32 μg/ml; resistant, MIC of >32 μg/ml).
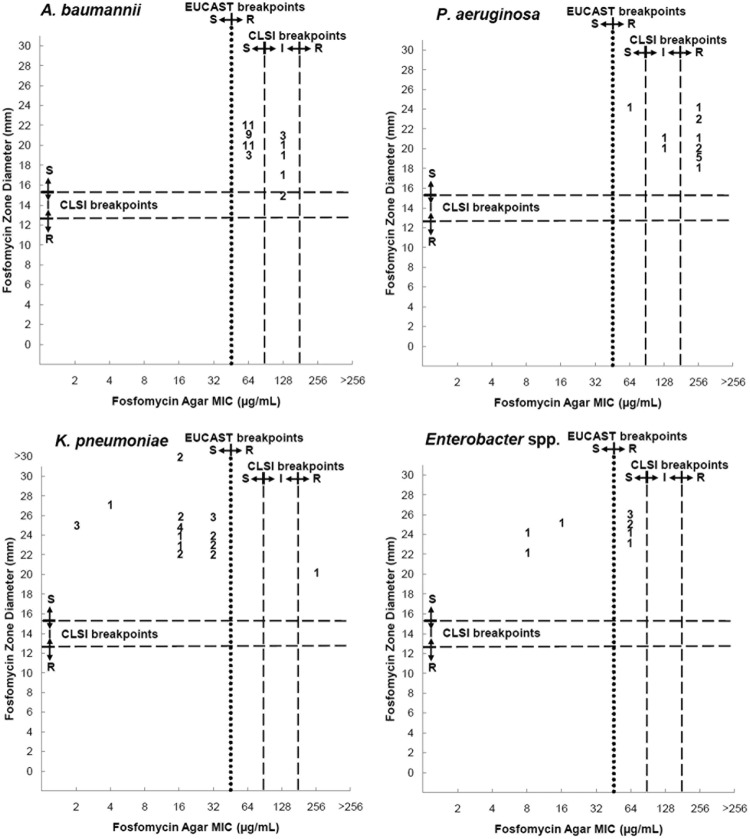
Median zone diameters using the 200-μg disk were 21 mm, 20 mm, 25 mm, and 25 mm for A. baumannii, P. aeruginosa, K. pneumoniae, and Enterobacter, respectively. For the 50-μg disk, median diameters were 11 mm, 3 mm, 15 mm, and 18 mm, respectively. Using CLSI criteria, susceptibility to A. baumannii was 95% (5% intermediate). All P. aeruginosa, K. pneumoniae, and Enterobacter isolates were considered susceptible.
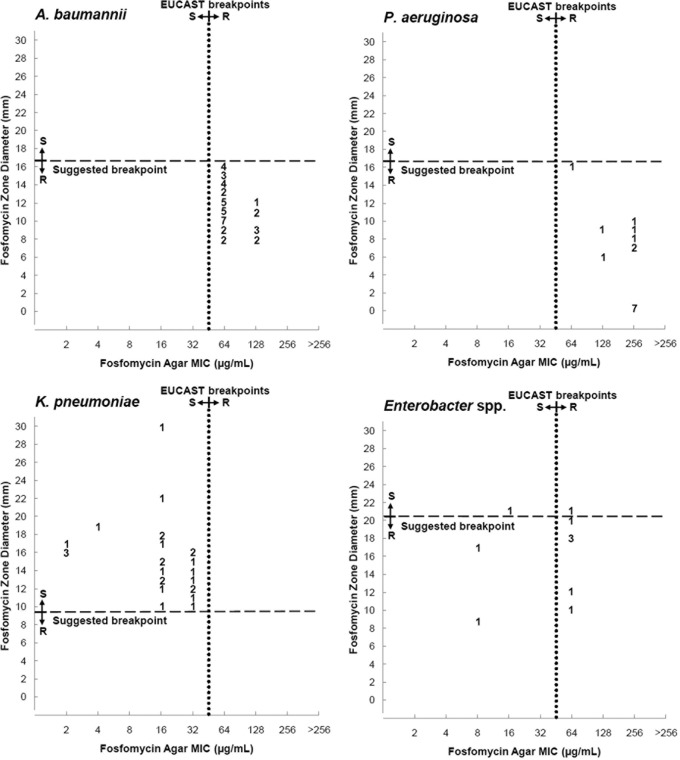
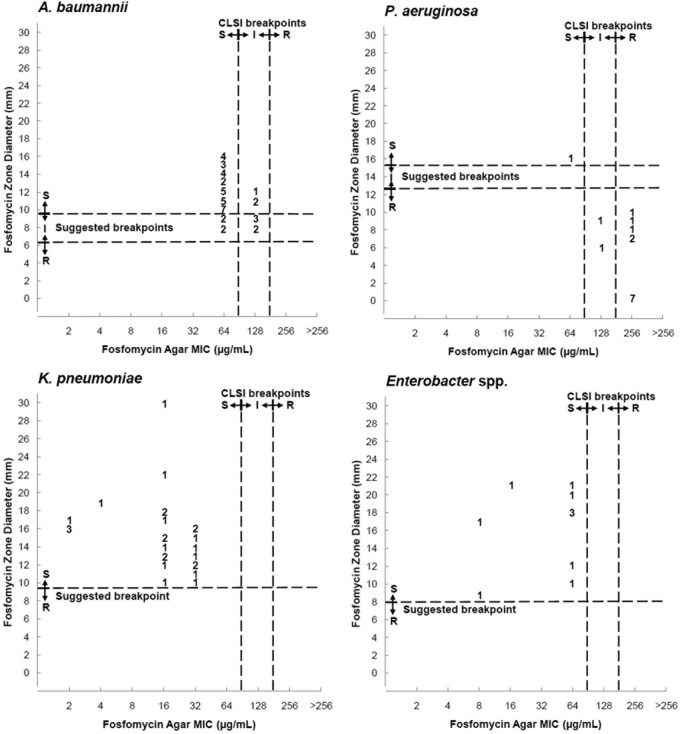
Table 2 and Fig. 1 to 3 compare results of DD with AD, and in the figures, we suggest breakpoints for the 50-μg DD method.
TABLE 2.
Comparison of disk diffusion using the 200-μg disk with agar dilution against fosfomycin for 94 multiresistant Gram-negative bacillus clinical isolatesa
| Microorganism | No. of isolates | % of isolates with agreement or errors |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| CLSI |
EUCAST |
||||||||
| CA | m | M | VM | CA | m | M | VM | ||
| A. baumannii | 42 | 86 | 14 | 0 | 0 | 0 | 5 | 0 | 95 |
| P. aeruginosa | 15 | 7 | 13 | 0 | 80 | 0 | 0 | 0 | 100 |
| K. pneumoniae | 27 | 96 | 0 | 0 | 4 | 96 | 0 | 0 | 4 |
| Enterobacter spp. | 10 | 100 | 0 | 0 | 0 | 30 | 0 | 0 | 70 |
CA, categorical agreement; m, minor error; M, major error; VM, very major error; CLSI, Clinical and Laboratory Standards Institute interpretative criteria (susceptible, MIC of ≤64μg/ml or zone of ≥16 mm; intermediate, MIC of 128 μg/ml or zone of 13 to 15 mm; resistant, MIC of ≥256 μg/ml or zone of ≤12 mm); EUCAST, European Committee on Antimicrobial Susceptibility interpretative criteria (no criteria for DD; susceptible, MIC of ≤32 μg/ml; resistant, MIC of >32 μg/ml).
FIG 1.
Scattergram results for 200-μg fosfomycin disk zone diameters and agar dilution MICs. S, susceptible; I, intermediate susceptibility; R, resistant.
FIG 3.
Scattergram results for 50-μg fosfomycin disk zone diameters and agar dilution MICs interpreted according to EUCAST breakpoints. We included suggested disk diffusion breakpoints. S, susceptible; I, intermediate susceptibility; R, resistant.
FIG 2.
Scattergram results for 50-μg fosfomycin disk zone diameters and agar dilution MICs interpreted according to CLSI breakpoints. We included suggested disk diffusion breakpoints. S, susceptible; I, intermediate susceptibility; R, resistant.
Agreement (≤2-fold dilutions) between Etest and AD results was high for A. baumannii (98%) and P. aeruginosa (93%). For K. pneumoniae and Enterobacter, differences of >2 dilutions occurred frequently (30% and 50%, respectively).
Table 3 compares Etest and AD. The best performance occurred for P. aeruginosa using EUCAST breakpoints. Very major errors occurred only with EUCAST breakpoints.
TABLE 3.
Categorization of Etest errors when compared with agar dilution method for fosfomycin for 94 multiresistant Gram-negative bacillus clinical isolatesa
| Microorganism | % of isolates with agreement or errors |
|||||||
|---|---|---|---|---|---|---|---|---|
| CLSI |
EUCAST |
|||||||
| CA | m | M | VM | CA | m | M | VM | |
| A. baumannii | 81 | 17 | 2 | 0 | 69 | 0 | 0 | 31 |
| P. aeruginosa | 53 | 34 | 13 | 0 | 100 | 0 | 0 | 0 |
| K. pneumoniae | 89 | 4 | 7 | 0 | 52 | 0 | 48 | 0 |
| Enterobacter spp. | 80 | 20 | 0 | 0 | 40 | 0 | 10 | 50 |
CA, categorical agreement; m, minor error; M, major error; VM, very major error; CLSI, Clinical and Laboratory Standards Institute interpretative criteria; EUCAST, European Committee on Antimicrobial Susceptibility interpretative criteria.
In short, fosfomycin presented activity against carbapenem-resistant Gram-negative bacilli and may be a treatment alternative. Fosfomycin MICs were lower for Enterobacteriaceae than for nonfermenters. In another study, most isolates of Enterobacteriaceae presented MICs of ≤64 μg/ml, similar to our results, but the MICs for P. aeruginosa and A. baumannii were lower than in our findings (19).
Although our MICs may be considered high, we cannot conclude without clinical trials that therapeutic failure is the predictable outcome. In pharmacokinetic studies, peak concentrations varied from 132.1 ± 31.8 mg/liter to 350.2 ± 124.69 mg/liter when 20 to 100 mg/kg (body weight) of fosfomycin was infused (20–23). It has not been established if fosfomycin activity is concentration dependent or time dependent.
Agar dilution is considered the gold standard, but breakpoints are not clear. Our main interest is to use fosfomycin against systemic infections. Based on predicted serum levels of fosfomycin, it would seem that EUCAST breakpoints are very stringent. Probably CLSI breakpoints, defined for urinary tract infections (13), would be applicable to systemic infections.
To consider a susceptibility test adequate, CLSI recommends that it obtain <10% minor error, <3% major error, and <1.5% very major error rates (17, 18). Performance of DD (200-μg disk) was adequate for Enterobacter, although only 10 isolates were tested. For K. pneumoniae, despite high categorical concordance, there was a 4% rate of very major errors, and for A. baumannii, the rate of minor errors was 14%. Performance for P. aeruginosa was very poor. CLSI breakpoints, in another study, were not appropriate for K. pneumoniae, Enterobacter cloacae, and P. aeruginosa (19). We proposed breakpoints for DD using 50-μg disks; however, these were different for each microorganism.
Despite low agreement between Etest and AD for K. pneumoniae and Enterobacter, categorical agreement was frequent. For P. aeruginosa, the opposite occurred and results for A. baumannii were more uniform. Etest performed very poorly for P. aeruginosa, as already reported recently (24). For A. baumannii and Enterobacter, there were unacceptable proportions of minor errors, and for K. pneumoniae, there were unacceptable major errors.
Our study has limitations, including small numbers of isolates, as only carbapenem-resistant clinical isolates were used, and similar MICs, making it difficult to analyze the performance of the methods. On the other hand, the 42 isolates of A. baumannii used in this study were previously evaluated and belonged to 10 different clusters and 21 profiles (15). Moreover, although K. pneumoniae isolates were not typed, isolates that circulate in Brazil belong to several different strains (25). These facts minimize the possibility that our results refer to only one or very few clones.
In conclusion, fosfomycin presented activity against multiresistant microorganisms. It is possible that CLSI interpretative criteria for urine may be adequate for systemic infections. DD using 200 μg presented a medium performance for A. baumannii and Enterobacteriaceae and very poor performance for P. aeruginosa. Etest performed poorly.
ACKNOWLEDGMENTS
None of the authors have conflicts of interest.
No specific funding was received for this study.
Footnotes
Published ahead of print 9 December 2013
REFERENCES
- 1.Fritsche TR, Sader HS, Toleman MA, Walsh TR, Jones RN. 2005. Emerging metallo-beta-lactamase-mediated resistances: a summary report from the worldwide SENTRY antimicrobial surveillance program. Clin. Infect. Dis. 41:276–278 [DOI] [PubMed] [Google Scholar]
- 2.Rice LB. 2012. Mechanisms of resistance and clinical relevance of resistance to β-lactams, glycopeptides, and fluoroquinolones. Mayo Clin. Proc. 87:198–208. 10.1016/j.mayocp.2011.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cantón R, Lumb J. 2011. Emerging resistance in Gram-negative pathogens and implications for clinical practice. Future Microbiol. 6:19–22. 10.2217/fmb.10.150 [DOI] [PubMed] [Google Scholar]
- 4.Cai Y, Wang R, Liang B, Bai N, Liu Y. 2011. Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of infectious disease. Antimicrob. Agents Chemother. 55:1162–1172. 10.1128/AAC.01402-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blot S, De Waele JJ, Vogelaers D. 2012. Essentials for selecting antimicrobial therapy for intra-abdominal infections. Drugs 72:e17–e32. 10.2165/11599800-000000000-00000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagenlehner FM, Wullt B, Perletti G. 2011. Antimicrobials in urogenital infections. Int. J. Antimicrob. Agents 38:3–10. 10.1016/j.ijantimicag.2011.09.004 [DOI] [PubMed] [Google Scholar]
- 7.Michalopoulos AS, Livaditis IG, Gougoutas V. 2011. The revival of fosfomycin. Int. J. Infect. Dis. 11:e732–e739 [DOI] [PubMed] [Google Scholar]
- 8.Falagas ME, Giannopoulou KP, Kokolakis GN, Rafailidis PI. 2008. Fosfomycin: use beyond urinary tract and gastrointestinal infections. Clin. Infect. Dis. 46:1069–1077. 10.1086/527442 [DOI] [PubMed] [Google Scholar]
- 9.Maraki S, Samonis G, Rafailidis PI, Vouloumanou EK, Mavromanolakis E, Falagas ME. 2009. Susceptibility of urinary tract bacteria to fosfomycin. Antimicrob. Agents Chemother. 53:4508–4510. 10.1128/AAC.00721-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuner EA, Sekeres J, Hall GS, van Duin D. 2012. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob. Agents Chemother. 56:5744–5748. 10.1128/AAC.00402-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karageorgopoulos DE, Wang R, Yu W, Falagas ME. 2012. Fosfomycin: evaluation of the published evidence on the emergence of antimicrobial resistance in Gram-negative pathogens. J. Antimicrob. Chemother. 67:255–268. 10.1093/jac/dkr466 [DOI] [PubMed] [Google Scholar]
- 12.Pasteran F, Lucero C, Rapoport M, Guerriero L, Barreiro I, Albornoz E, Veliz O, Corso A. 2012. Tigecycline and intravenous fosfomycin zone breakpoints equivalent to the EUCAST MIC criteria for Enterobacteriaceae. J. Infect. Dev. Ctries. 6:452–456 [DOI] [PubMed] [Google Scholar]
- 13.Clinical and Laboratory Standards Institute 2012. Performance standards for antimicrobial susceptibility testing: 22nd informational supplement M100-S22. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 14.European Committee on Antimicrobial Susceptibility Testing 2012. European Committee on Antimicrobial Susceptibility Testing clinical breakpoints. http://www.eucast.org/clinical_breakpoints Accessed 1 December 2012
- 15.Mostachio AK, Levin AS, Rizek C, Rossi F, Zerbini J, Costa SF. 2012. High prevalence of OXA-143 and alteration of outer membrane proteins in carbapenem-resistant Acinetobacter spp. isolates in Brazil. Int. J. Antimicrob. Agents 39:396–401. 10.1016/j.ijantimicag.2012.01.021 [DOI] [PubMed] [Google Scholar]
- 16.Rasheed JK, Biddle JW, Anderson KF, Washer L, Chenoweth C, Perrin J, Newton DW, Patel JB. 2008. Detection of the Klebsiella pneumoniae carbapenemase type 2 carbapenem-hydrolyzing enzyme in clinical isolates of Citrobacter freundii and K. oxytoca carrying a common plasmid. J. Clin. Microbiol. 46:2066–2069. 10.1128/JCM.02038-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clinical and Laboratory Standards Institute 2008. Development of in vitro susceptibility testing criteria and quality control parameters; approved guidelines M23-A3. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 18.Endimiani A, Patel G, Hujer KM, Swaminathan M, Perez F, Rice LB, Jacobs MR, Bonomo RA. 2010. In vitro activity of fosfomycin against blaKPC-containing Klebsiella pneumoniae isolates, including those nonsusceptible to tigecycline and/or colistin. Antimicrob. Agents Chemother. 54:526–529. 10.1128/AAC.01235-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu CL, Liu CY, Huang YT, Liao CH, Teng LJ, Turnidge JD, Hsueh PR. 2011. Antimicrobial susceptibilities of commonly encountered bacterial isolates to Fosfomycin determined by agar dilution and disk diffusion methods. Antimicrob. Agents Chemother. 55:4295–4301. 10.1128/AAC.00349-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goto M, Sugiyama M, Nakajima S, Yamashina H. 1981. Fosfomycin kinetics after intravenous and oral administration to human volunteers. Antimicrob. Agents Chemother. 20:393–397. 10.1128/AAC.20.3.393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lastra CF, Mariño EL, Barrueco M, Gervós MS, Gil AD. 1984. Disposition of phosphomycin in patients with pleural effusion. Antimicrob. Agents Chemother. 25:458–462. 10.1128/AAC.25.4.458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roussos N, Karageorgopoulos DE, Samonis G, Falagas ME. 2009. Clinical significance of the pharmacokinetic and pharmacodynamic characteristics of fosfomycin for the treatment of patients with systemic infections. Int. J. Antimicrob. Agents 34:506–515. 10.1016/j.ijantimicag.2009.08.013 [DOI] [PubMed] [Google Scholar]
- 23.Schintler MV, Traunmüller F, Metzler J, Kreuzwirt G, Spendel S, Mauric O, Popovic M, Scharnagl E, Joukhadar C. 2009. High fosfomycin concentrations in bone and peripheral soft tissue in diabetic patients presenting with bacterial foot infection. J. Antimicrob. Chemother. 64:574–578. 10.1093/jac/dkp230 [DOI] [PubMed] [Google Scholar]
- 24.Díez-Aguilar M, Morosini MI, Campo R, García-Castillo M, Zamora J, Cantón R. 2013. In vitro activity of fosfomycin against a collection of clinical Pseudomonas aeruginosa isolates from 16 Spanish hospitals: establishing the validity of standard broth microdilution as susceptibility testing method. Antimicrob. Agents Chemother. 57:5701–5703. 10.1128/AAC.00589-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pereira GH, Garcia DO, Mostardeiro M, Ogassavara CT, Levin AS. 2011. Spread of carbapenem-resistant Klebsiella pneumoniae in a tertiary hospital in Sao Paulo, Brazil. J. Hosp. Infect. 79:182–183. 10.1016/j.jhin.2011.05.023 [DOI] [PubMed] [Google Scholar]