Abstract
Advanced-generation cephalosporins are frequently used for empirical coverage of ventilator-associated pneumonia (VAP) due to their activity against a broad spectrum of Gram-positive and Gram-negative aerobic bacteria, including Pseudomonas aeruginosa and Enterobacteriaceae. Providing optimal antibiotic exposure is essential to achieving successful response in patients with VAP. We evaluated exposures of two antipseudomonal cephalosporins, ceftazidime and cefepime, in patients with VAP due to Gram-negative bacilli to identify the pharmacodynamic parameter predictive of microbiological success. Population pharmacokinetic models were used to estimate individual free drug exposures. Pharmacodynamic indices were determined for each patient using the baseline Gram-negative bacilli with the highest drug MIC. Classification and regression tree analysis was utilized to partition exposure breakpoints, and multivariate logistic regression was conducted to identify predictors of microbiological success. A total of 73 patients (18 receiving ceftazidime therapy and 55 receiving cefepime therapy) were included. MICs ranged widely from 0.047 to 96 μg/ml. The microbiological success rate was 58.9%. Predictive breakpoints were identified for all pharmacodynamic parameters, including a serum fT > MIC greater than 53% (P = 0.02). When controlling for APACHE II (odds ratio [OR], 1.01; 95% confidence interval, 0.93 to 1.09; P = 0.85) and combination therapy (OR, 0.74; 95% confidence interval, 0.25 to 2.19; P = 0.59), achieving a greater than 53% fT > MIC remained a significant predictor of success (OR, 10.3; 95% confidence interval, 1.1 to 92.3; P = 0.04). In patients with VAP due to Gram-negative bacilli, serum exposure of greater than 53% fT > MIC was found to be a significant predictor of favorable microbiological response for antipseudomonal cephalosporins. These data are useful when determining dosing regimens for cephalosporin agents under development for pneumonia.
INTRODUCTION
Even with significant enhancements in the management of mechanically ventilated patients, ventilator-associated pneumonia (VAP) remains the most common hospital-acquired infection in intensive care unit (ICU) patients (1, 2). Ceftazidime and cefepime are advanced-generation cephalosporins with activity against a broad spectrum of Gram-positive and Gram-negative aerobic bacteria, including Pseudomonas aeruginosa and Enterobacteriaceae (3, 4, 5, 6, 7). As a result, these agents are routinely prescribed for empirical coverage of many severe infections and are recommended as a backbone empirical therapy in the current VAP guidelines (8). Nevertheless, due to increasing resistance among bacteria often implicated in VAP, new antimicrobial treatments are needed.
Like other β-lactams, cephalosporins exhibit time-dependent bactericidal activity where efficacy is correlated with the percentage of the dosing interval during which free drug concentrations remain above the MIC against the organism (% ƒT > MIC) (9, 10). For cephalosporins, animal infection models have demonstrated that ∼40% and ∼60% to ∼70% ƒT > MICs are necessary for bacteriostatic activity and bactericidal activity, respectively (11). Studies in patients with severe infections have identified similar pharmacodynamic targets in the range of 45% to 60% fT > MIC; however, those studies have had mixed infections or had limited concentration data available for patients when estimating exposure (12, 13).
The paucity of novel agents to treat resistant Gram-negative infections has shifted the focus of development efforts largely toward this public health concern (14, 15). In response, at present, three novel cephalosporins for the treatment of multidrug-resistant (MDR) Gram-negative bacillus infections are under investigation in clinical trials, including plans for study in pneumonia (16). Knowledge of an accurate exposure threshold that would be predictive of microbiological response in VAP would be of significant value when finalizing dosing regimens for study in clinical trials. Here, we evaluated the serum exposure of two antipseudomonal cephalosporins, ceftazidime and cefepime, in patients with VAP due to Gram-negative bacilli to identify the pharmacodynamic parameter predictive of microbiological response.
MATERIALS AND METHODS
Study design.
This study is a retrospective pharmacokinetic/pharmacodynamic analysis. Demographic, pharmacokinetic, and microbiological data from patients treated for VAP with cefepime or ceftazidime were included. Data corresponding to patient demographics (age, gender, and race) and the following clinical parameters were collected from the medical record: height, weight, creatinine clearance (CLCR) on admission, and Acute Physiology and Chronic Health Evaluation (APACHE) II score (17). Pharmacokinetic models were constructed to estimate pharmacodynamic exposure in each included subject. These pharmacodynamic indices were linked to microbiological response to determine the serum drug exposure predictive of a successful response.
Patients.
Patients from previously conducted studies of ceftazidime (18) and cefepime (19) were compiled. The study was approved by the Hartford Hospital Institutional Review Board. A waiver of informed consent was granted because all of the data were already in existence and collected during the conduct of the original studies. For inclusion, adult patients (≥18 years of age) had to have been diagnosed with VAP and to have received ≥3 days of treatment with either ceftazidime or cefepime. Patients were excluded if they had received an antibiotic with Gram-negative activity other than an antipseudomonal fluoroquinolone or aminoglycoside within 24 h before or 72 h after the start of cefepime therapy. Patients receiving hemodialysis were also excluded. Ceftazidime patients were those enrolled in a prospective, open-labeled, randomized controlled study comparing the clinical and microbiological efficacies of continuous versus intermittent ceftazidime administration in ICU patients with VAP (18). Briefly, patients randomly received ceftazidime as either a continuous infusion (CI) or an intermittent infusion (II). For patients with normal renal function (estimated CLCR, ≥50 ml/min), the ceftazidime regimen consisted of either an II of 2 g intravenously (IV) every 8 h over 30 min or a CI of 3 g over 24 h using an infusion pump. Patients receiving the CI regimen were given a one-time loading dose of 1 g ceftazidime over 30 min prior to beginning CI. The CI dose was selected based on pharmacokinetic data and was projected to obtain serum concentrations approximately twice that of the Clinical and Laboratory Standards Institute (CLSI) breakpoint (8 μg/ml) for ceftazidime (20, 21). All patients enrolled in the study had blood samples collected for ceftazidime concentrations; these serum concentrations were used to construct the population pharmacokinetic model. Of these patients, those who had baseline Gram-negative bacilli identified with available ceftazidime MICs were eligible for the pharmacodynamic analysis. Cefepime patients were those included during an observational assessment of a VAP clinical pathway at our institution between July 2004 and August 2007 (19). Cefepime dosing was adjusted based on renal function per the VAP clinical pathway protocol, and many, but not all, of these patients contributed blood samples for cefepime concentrations during previous development of a population pharmacokinetic model (22). Patients in both studies were permitted antibiotic therapy for coverage of methicillin-resistant Staphylococcus aureus, and double coverage for Pseudomonas aeruginosa was encouraged.
Microbiological response.
Microbiological response was the primary outcome endpoint used in this study to link with drug exposure. Microbiological response was categorized as success (eradication or presumed eradication) or failure (persistence or presumed persistence) for each patient. Patients were assessed at the end of treatment or at the time of institutional discharge as follows: (i) eradication—elimination of the original causative organism(s) from the site (e.g., sputum) of isolation during or upon completion of therapy; (ii) presumed eradication—absence of appropriate fluid (e.g., sputum) for culture and evaluation coupled with patient clinical improvement; (iii) persistence—failure to eradicate the original causative organism(s) from the site of infection; and (iv) presumed persistence—absence of an appropriate follow-up culture result coupled with a lack of patient clinical improvement.
Pharmacokinetic analysis. (i) Ceftazidime.
Ceftazidime serum concentrations from included patients were modeled using the nonparametric adaptive grid program (BigNPAG) in the MM-USC*PACK collection (23, 24). Model selection was based on log-likelihood values, Akaike's information criterion, and visual inspection of observed versus predicted scatterplots (25). A two-compartment pharmacokinetic model with zero-order infusion and first-order elimination, utilizing CLCR as a covariate of clearance, was selected. The following patient-specific parameters were determined: volume of distribution in the central compartment (V1 [liters]), intercompartmental transfer constants (K12 and K21 [h−1]), and total body clearance (CL [liters/h]). Total body clearance was determined using the following equation: CL = Cli + Cls × CLCR, where Cli is the intercept, Cls is the slope parameter, and CLCR was calculated using the Cockcroft-Gault equation (26). Model weighting was performed based on the interday assay error variance employing a plot of the assay standard deviations (SD) versus the measured ceftazidime concentrations, generating the following first-order polynomial formula: SD = γ(0.0022 + 0. 0629 × C), where C is the ceftazidime concentration and γ was identified to be 1.98. Individual pharmacokinetic parameters were identified using the population of one utility in BigNPAG.
(ii) Cefepime.
Pharmacokinetic parameter estimates for cefepime-treated patients were derived from a validated population pharmacokinetic model of VAP patients at our institution who received high-dose, prolonged-infusion cefepime, as previously described (22), using CLCR and total body weight (TBW) as covariates of K10 (elimination rate constant) and V1 (volume of distribution in the central compartment), respectively.
Pharmacodynamic indices.
Following the determination of pharmacokinetic parameters for each individual, simulations of each patient's steady-state serum concentration-time profiles were conducted (WinNonlin software; Pharsight Corp., Mountain View, CA) based on their treatment drug dosing regimen. In the simulations, protein binding of 15% was assumed for ceftazidime and cefepime to derive free (ƒ) drug concentrations. The treatment drug MIC for the isolated Gram-negative pathogen and the free drug exposure value were used to calculate the following pharmacodynamic indices: % ƒT > MIC, the ratio of maximum free drug concentration to MIC (ƒCmax/MIC), the ratio of minimum free drug concentration to MIC (ƒCmin/MIC), and the ratio of the free drug area under the concentration curve to MIC (ƒAUC/MIC). For patients with polymicrobial infections, the Gram-negative bacillus with the highest treatment drug MIC was used to calculate the pharmacodynamic indices.
Statistical analysis.
Classification and regression tree (CART) analysis (Salford Systems, San Diego, CA) was employed to distinguish microbiological success from failure on the basis of pharmacodynamic exposure. Pearson's chi-square test or Fisher's exact test, where appropriate, was used for categorical data, including CART-derived breakpoints of microbiological response. Continuous variables were analyzed by Student's t test or the Mann-Whitney U test. Logistic regression analysis was performed to identify which pharmacodynamic parameter(s), when controlling for confounding variables, was independently associated with microbiological success. Statistical analyses were performed with SigmaStat software (SPSS Inc., San Rafael, CA). A P value of <0.05 was considered statistically significant.
RESULTS
Patient characteristics and microbiological response.
Seventy-three patients with VAP were evaluable for microbiological outcome; 18 patients received ceftazidime and 55 patients were treated with cefepime. Patient characteristics and the frequency of each dosing regimen are presented in Table 1. Combination therapy was common but did not appear to have any impact on microbiological response. Pseudomonas aeruginosa was the most common infecting pathogen (63.0%; n = 46) followed by Klebsiella pneumoniae (8.2%; n = 6), Enterobacter cloacae (8.2%; n = 6), Escherichia coli (4.1%; n = 3), Haemophilus influenzae (4.1%; n = 3), Serratia marcescens (4.1%; n = 3), and Acinetobacter baumannii (2.7%; n = 2).
TABLE 1.
Patient characteristics and dosing regimens by drug
| Patient characteristic | Value |
|---|---|
| No. (%) of males | 50 (68.5) |
| Mean (SD) age (yr) | 52.8 (21.7) |
| Mean (SD) wt (kg) | 78.4 (19.2) |
| Mean (SD) APACHE II score | 16.6 (6.5) |
| Mean (SD) ClCR (ml/min) | 106.6 (50.8) |
| No. (%) receiving the following ceftazidime regimen: | 18 (24.7) |
| 2 g every 24 h (0.5-h infusion) | 1 (5.5) |
| 2 g every 12 h (0.5-h infusion) | 2 (11.1) |
| 2 g every 8 h (0.5-h infusion) | 7 (38.9) |
| 3 g every 24 h (continuous infusion) | 7 (38.9) |
| 4.5 g every 24 h (continuous infusion) | 1 (5.5) |
| No. (%) receiving the following cefepime regimen: | 55 (75.3) |
| 1 g every 12 h (0.5-h infusion) | 15 (27.2) |
| 1 g every 8 h (0.5-h infusion) | 10 (18.2) |
| 2 g every 12 h (0.5-h infusion) | 2 (3.6) |
| 2 g every 8 h (0.5-h infusion) | 20 (36.4) |
| 2 g every 8 h (3-h infusion) | 8 (14.5) |
Microbiological response was classified as successful in 58.9% of patients. When stratified by microbiological response, the demographics of the populations were similar (Table 2). Additionally, there was no difference in microbiological response rates between ceftazidime-treated patients and cefepime-treated patients. However, the MIC (median [range]) was significantly higher in patients with microbiological failure (3 [0.094 to 96] versus 1 [0.047 to 32] μg/ml; P = 0.006).
TABLE 2.
Univariate analysis for association with microbiological responsea
| Variable | Microbiological failure (n = 30) | Microbiological success (n = 43) | P value |
|---|---|---|---|
| Patient age (yr) | 54.4 (22.7) | 51.6 (21.2) | 0.600 |
| No. (%) of male patients | 19 (63.3) | 31 (72.1) | 0.592 |
| Patient wt (kg) | 81.3 (25.0) | 76.4 (13.8) | 0.287 |
| ClCR (ml/min) | 115.5 (51.4) | 100.4 (50.0) | 0.214 |
| APACHE II score | 16.1 (6.47) | 17.0 (6.5) | 0.553 |
| MIC, median (range) | 3 (1.5–8.0) | 1 (0.25–3.75) | 0.006 |
| No. (%) of patients with combination therapy | 23 (76.7) | 29 (67.4) | 0.553 |
| No. (%) of patients with ceftazidime therapy | 5 (27.8) | 13 (72.2) | 0.295 |
| No. (%) of patients with cefepime therapy | 25 (45.5) | 30 (54.5) | 0.295 |
All data are listed as mean (SD) unless otherwise specified.
Population pharmacokinetic model.
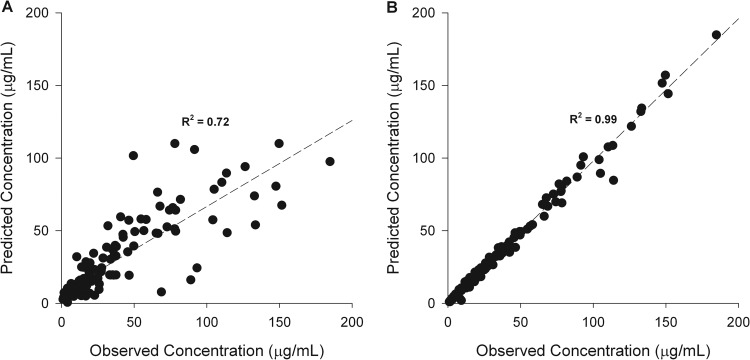
Thirty-two patients with serum ceftazidime concentrations were used to develop the population pharmacokinetic model for this agent. An average of five serum samples (range, 2 to 7) were collected for each patient. Seventeen patients received CI and 15 received II ceftazidime, with the dosage adjusted based on renal function. The final population pharmacokinetic parameters derived from ceftazidime-treated patients are shown in Table 3. The predicted versus observed (pre-Bayesian) population plot for the model fit the population well (Fig. 1A), with r2, bias, and precision values of 0.72, 0.36, and 20.31 μg/ml, respectively. The individual predicted maximum a posteriori (MAP) Bayesian versus observed concentration plot (Fig. 1B) had r2, bias, and precision values of 0.99, −0.011, and 0.96 μg/ml, respectively.
TABLE 3.
Population pharmacokinetic estimates for ceftazidime in patients with VAP
| Parametera | Mean | Median | SD |
|---|---|---|---|
| Cli (liters/h) | 3.84 | 4.22 | 2.71 |
| Cls | 0.05 | 0.04 | 0.036 |
| V1 (liters) | 10.86 | 11.65 | 6.08 |
| K12 (h−1) | 3.35 | 1.57 | 4.29 |
| K21 (h−1) | 2.4 | 1.34 | 2.6 |
CLi, nonrenal clearance; CLs, proportion of creatinine clearance estimate contributing to renal clearance; V1, volume of distribution of the central compartment; K12, microtransfer rate constant from the central to the peripheral compartment; K21, microtransfer rate constant from the peripheral compartment to the central compartment.
FIG 1.
(A) Population predicted versus observed ceftazidime concentrations using the mean parameter estimates from the population pharmacokinetic model. (B) Individual predicted versus observed ceftazidime concentrations with mean population parameters as the maximum a posteriori Bayesian estimates.
Pharmacodynamic indices.
Of the 18 ceftazidime patients, 15 (83.3%) achieved serum ƒT > MIC exposures of 100%. The median (range) % ƒT > MIC, ƒCmax/MIC, ƒCmin/MIC, and ƒAUC/MIC values were 100 (31.3 to 100), 42.5 (1.8 to 351.4), 6.26 (0.1 to 62.8), and 483.6 (19.8 to 3,030). Forty-two (76.3%) of the 55 cefepime patients had 100% ƒT > MIC. The median (range) % ƒT > MIC, ƒCmax/MIC, ƒCmin/MIC, and ƒAUC/MIC values were 100 (0.8 to 100), 29.5 (1.1 to 2,318), 6.14 (0.03 to 318.1), and 293.3 (4.2 to 17,399).
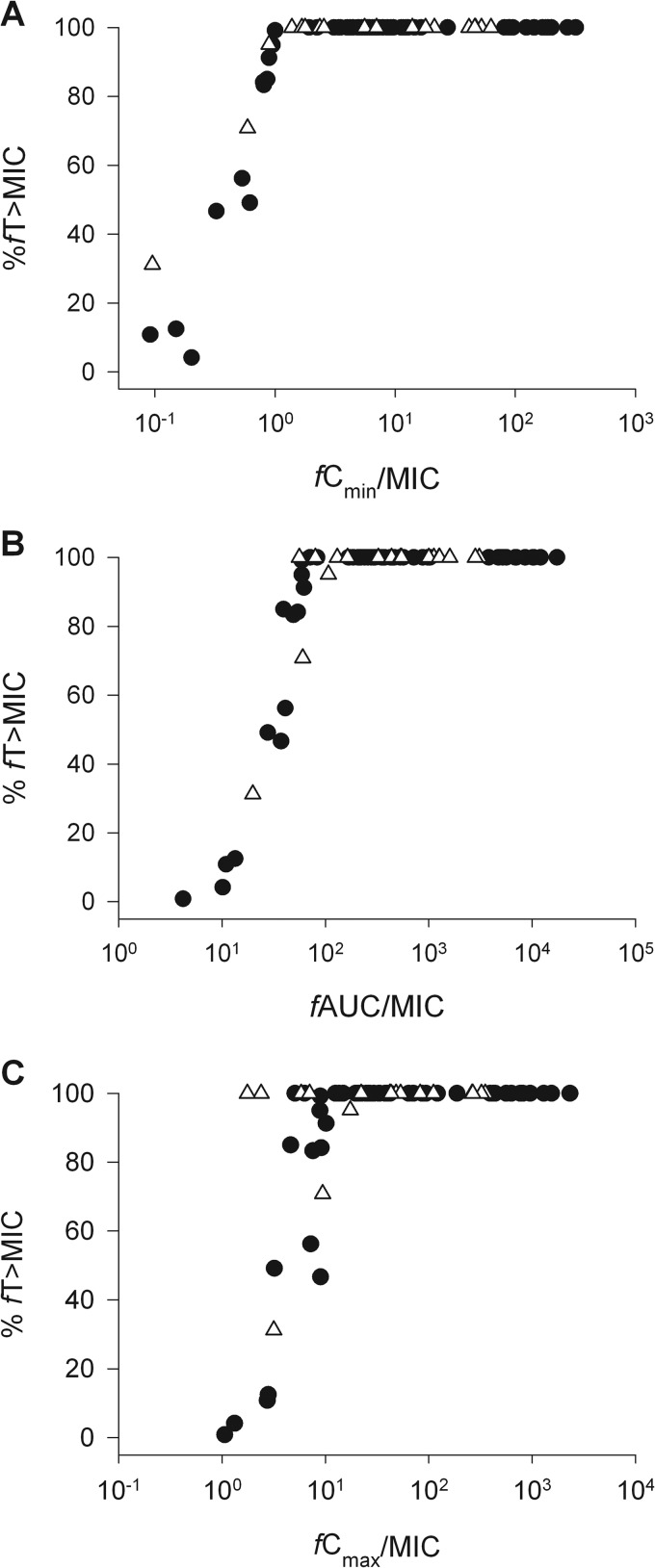
When the populations were combined, most patients (78.1%) achieved serum ƒT > MIC exposures of 100%, presumably because of the high-dose regimens recommended for patients on the VAP clinical pathway at our institution and low MICs in many situations. As a result, the median (range) % ƒT > MIC for the total study population was 100 (0.8 to 100). Modest collinearity was observed between % ƒT > MIC and the other pharmacodynamic parameters despite significant plateaus at 100% ƒT > MIC (Fig. 2). However, due to the range of MICs (ceftazidime MICs, 0.25 to 32 μg/ml; cefepime MICs, 0.047 to 96 μg/ml), ƒCmax/MIC, ƒCmin/MIC, and ƒAUC/MIC exposures differed widely, resulting in median (range) values of 32.7 (1.1 to 2,318.3), 6.14 (0.03 to 318.1), and 299.6 (4.2 to 17,398.6).
FIG 2.
Colinearity between %ƒT > MIC and ƒCmax/MIC (A), ƒCmin/MIC (B), and ƒAUC/MIC (C) for the 73 patients with VAP treated with ceftazidime (open triangles) and cefepime (closed circles).
Pharmacodynamic breakpoint.
The CART-derived pharmacodynamic parameters values partitioning microbiological success versus failure are included in Table 4. These identified breakpoints were tested for statistical significance in univariate analysis, and all pharmacodynamic indices remained significant predictors of response. Multivariate logistic regression analysis was conducted to assess confounding variables and is presented in Table 5. The following variables were entered into the model: % ƒT > MIC, APACHE II, ClCR, and combination therapy. MIC was not entered in the model as this variable is accounted for in determination of the pharmacodynamic parameters. When controlling for APACHE II (odds ratio [OR], 1.01 [95% confidence interval, 0.93 to 1.09]; P = 0.85) and combination therapy (OR, 0.74 [0.25 to 2.19]; P = 0.74), achieving greater than 53% ƒT > MIC remained a significant predictor of microbiological response. No patient characteristics (e.g., age, weight, ClCR) were statistically predictive of response.
TABLE 4.
Results from CART and univariate logistic regression analyses to determine which pharmacodynamic indices significantly predict microbiological response and the associated breakpoints for success
| Parameter | Classification and regression |
Logistic regression |
|||
|---|---|---|---|---|---|
| % breakpoint | % response rate (above, below) | P | Odds ratio (95% confidence interval) | P | |
| % ƒT > MIC | 53 | 63.6, 14.3 | 0.017 | 10.50 (1.19–92.48) | 0.034 |
| ƒAUC/MIC | 529 | 92.3, 40.4 | <0.001 | 17.68 (3.73–83.79) | <0.001 |
| ƒCmax/MIC | 6 | 67.7, 9.1 | <0.001 | 21.00 (2.51–175.56) | 0.005 |
| ƒCmin/MIC | 12 | 92.0, 41.7 | <0.001 | 16.10 (3.40–76.21) | <0.001 |
TABLE 5.
Results of the multivariate logistic regression analysis for predicting microbiological success
| Model parameter | Odds ratio (95% confidence interval) | P |
|---|---|---|
| % ƒT > MIC > 53 | 10.3 (1.15–92.28) | 0.04 |
| APACHE II score | 1.01 (0.93–1.09) | 0.85 |
| Combination therapy | 0.74 (0.25–2.19) | 0.59 |
DISCUSSION
There have been a number of investigations evaluating cephalosporin pharmacodynamics in humans (12, 13, 27, 28). Little of the attention has focused on a single site of infection among the members of an exclusively adult critically ill population. The potential advantages of employing such a focus are 2-fold. First, doing so eliminates the uncertainty that comes with extrapolating composite data from various populations (i.e., data from healthy volunteers and other sites of infection), as significant variability in pharmacokinetic parameters can be seen between healthy and infected individuals (29). This is an important distinction, which in turn improves the likelihood of emulating the true population of interest. Second, antibiotic concentrations at the site of infection can differ from those seen in serum, depending on the physiochemical properties of the compound and the infected tissue (30). Furthermore, physiological changes and interpatient variations in critically ill patients can drastically affect the pharmacokinetics of cephalosporins (31–34). The considerations mentioned above make the use of a standard dose for cephalosporins (in this case, ceftazidime and cefepime) unlikely to provide appropriate exposures for all patients and could lead to higher rates of failure, resistance, and, in some cases, toxicity (35, 36). In the present study, population pharmacokinetic models were used to derive the pharmacokinetic parameters in serum of patients with VAP treated with either ceftazidime or cefepime in order to predict individual exposures and their correlation with microbiological response.
With the increased prevalence of MDR Gram-negative organisms in VAP, ceftazidime's and cefepime's spectrum of activity has sparked rejuvenated interest in understanding their pharmacodynamics. Furthermore, such data are paramount in decision support for the development of dosing regimens for new antimicrobials. Target pharmacodynamic exposures are traditionally derived from in vitro and in vivo animal infections models, with limited human data to validate these findings. Human studies with cephalosporins have produced variable % ƒT > MIC exposure data for microbiological success (12, 13, 37). Others have suggested enhanced β-lactam activity by maintaining a ƒCmin/MIC > 4 to 6 (37, 38). In the current study of VAP patients, attainment of specific pharmacodynamic exposures was associated with favorable microbiological response for antipseudomonal cephalosporins, including a serum ƒT > MIC value compatible with animal infection models (9–11). While all pharmacodynamic indices displayed predictive relationships, our observations are consistent with findings from other studies in that microbiological success was significantly associated with achieving free drug exposures of greater than 53% ƒT > MIC.
Muller and colleagues saw favorable clinical and microbiological outcomes with ceftazidime in nosocomial pneumonia when patients had a greater than 45% ƒT > MIC (13). Their population pharmacokinetic model was derived from 75 patients with nosocomial pneumonia, 8 healthy volunteers, and 6 additional ICU patients. We developed a ceftazidime model specifically in the VAP population to accurately estimate drug exposures for all 18 included ceftazidime patients. The cefepime model used here is the same utilized in a pharmacodynamic analysis of cefepime in patients infected with P. aeruginosa performed at our institution by Crandon and colleagues (12). All sites of infection were included except the urinary tract, and the researchers found an association with microbiological failure in patients with less than 60% ƒT > MIC for cefepime. A strength of the current study is that the models used were derived strictly from a population of patients with VAP and identified similar ƒT > MICs.
Given the high proportion of relatively low MICs (median, 2 μg/ml) against the causative pathogens, % ƒT > MIC was not uniformly distributed across the population, resulting in a majority of patients with 100% ƒT > MIC. We observed collinearity between ƒT > MIC and the other pharmacodynamic indices (Fig. 2), which we believe is responsible for the significant correlation with response identified with all parameters. This observation is not uncommon; Li and colleagues saw a similar occurrence in a pharmacodynamic analysis of meropenem in lower respiratory tract infection, where ƒT > MIC and ƒCmin/MIC were highly colinear and predictive of microbiological success (39). Like the current study results, the median % ƒT > MIC was 100%, with approximately 80% of patients achieving this value. In the current study, ƒCmax/MIC and ƒAUC/MIC breakpoints were also significantly predictive of microbiological success, emphasizing the relationship between exposure and response. Due to our knowledge surrounding the pharmacodynamics of cephalosporins, only the % ƒT > MIC value was used when conducting multivariate analyses.
This study is not without limitations. Although this is a retrospective study of data from two different time periods, the mainstay of treatment for VAP is antibiotic chemotherapy. Therefore, we employed microbiological response as an endpoint, instead of clinical response or mortality, to alleviate any concerns surrounding changes in the standard of care for VAP patients. Second, while ceftazidime concentration data were available for each patient, cefepime exposures were estimated using a validated population pharmacokinetic model based on patient covariates. This model was derived from cefepime-treated patients with VAP at the same institution and with the drugs used in dosages similar to those used in the current analysis, and many of these patients contributed concentrations to the original model. Last, assessing patients who were treated with two different agents allowed for a larger sample size but did introduce a potential confounder, which should be considered when interpreting these findings. However, given the similarity between the two agents, it is not unreasonable to predict they would have similar pharmacodynamic targets.
In patients with VAP, a significant relationship between antibiotic exposure and microbiological outcome was observed with antipseudomonal cephalosporin therapy. Notably, a serum ƒT > MIC greater than 53% was associated with microbiological success. In addition to these cephalosporins, these data may have application in the design of optimal dosing regimens for future cephalosporins under development for the treatment of VAP.
Footnotes
Published ahead of print 16 December 2013
REFERENCES
- 1.Chastre J, Fagon JY. 2002. Ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 165:867–903. 10.1164/ajrccm.165.7.2105078 [DOI] [PubMed] [Google Scholar]
- 2.Bassetti M, Taramasso L, Giacobbe DR, Pelosi P. 2012. Management of ventilator-associated pneumonia: epidemiology, diagnosis and antimicrobial therapy. Expert Rev. Anti Infect. Ther. 10:585–596. 10.1586/eri.12.36 [DOI] [PubMed] [Google Scholar]
- 3.Richards DM, Brogden RN. 1985. Ceftazidime: a review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs 29:105–161. 10.2165/00003495-198529020-00002 [DOI] [PubMed] [Google Scholar]
- 4.Masuyoshi S, Hiraoka M, Inoue M, Tomatsu K, Hirano M, Mitsuhashi S. 1989. Comparison of the in vitro and in vivo antibacterial activities of cefepime (BMY-28142) with ceftazidime, cefuzonam, cefotaxime and cefmenoxime. Drugs Exp. Clin. Res. 15:1–10 [PubMed] [Google Scholar]
- 5.Clynes N, Scully BE, Neu HC. 1989. The use of cefepime (BMY 28142) to treat respiratory infections. Diagn. Microbiol. Infect. Dis. 12:257–260. 10.1016/0732-8893(89)90023-0 [DOI] [PubMed] [Google Scholar]
- 6.Grassi GG, Grassi C. 1993. Cefepime: overview of activity in vitro and in vivo. J. Antimicrob. Chemother. 32(Suppl B):87–94. 10.1093/jac/32.suppl_B.87 [DOI] [PubMed] [Google Scholar]
- 7.Okamoto MP, Nakahiro RK, Chin A, Bedikian A, Gill MA. 1994. Cefepime: a new fourth-generation cephalosporin. Am. J. Hosp. Pharm. 51:463–467 [PubMed] [Google Scholar]
- 8.American Thoracic Society and Infectious Diseases Society of America 2005. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 171:388–416. 10.1164/rccm.200405-644ST [DOI] [PubMed] [Google Scholar]
- 9.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1–10. 10.1086/516284 [DOI] [PubMed] [Google Scholar]
- 10.Drusano GL. 2004. Antimicrobial pharmacodynamics: critical interactions of ‘bug and drug'. Nat. Rev. Microbiol. 2:289–300. 10.1038/nrmicro862 [DOI] [PubMed] [Google Scholar]
- 11.Craig WA. 1995. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn. Microbiol. Infect. Dis. 22:89–96. 10.1016/0732-8893(95)00053-D [DOI] [PubMed] [Google Scholar]
- 12.Crandon JL, Bulik CC, Kuti JL, Nicolau DP. 2010. Clinical pharmacodynamics of cefepime in patients infected with Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 54:1111–1116. 10.1128/AAC.01183-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muller AE, Punt N, Mouton JW. 2013. Optimal exposures of ceftazidime predict the probability of microbiological and clinical outcome in the treatment of nosocomial pneumonia. J. Antimicrob. Chemother. 68:900–906. 10.1093/jac/dks468 [DOI] [PubMed] [Google Scholar]
- 14.Infectious Diseases Society of America 2004. Bad bugs, no drugs: as antibiotic discovery stagnates, a public health crisis brews. Infectious Diseases Society of America, Alexandria, VA [Google Scholar]
- 15.Interagency Task Force on Antimicrobial Resistance 2001. Action plan to combat antimicrobial resistance; part I: domestic. http://www.cdc.gov/drugresistance/actionplan/aractionplan.pdf Accessed 21 May 2013
- 16.Boucher HW, Talbot GH, Benjamin, Bradley DKJ, Guidos RJ, Jones RN, Murray BE, Bonomo RA, Gilbert D; Infectious Diseases Society of America 2013. 10 x '20 progress–development of new drugs active against gram-negative bacilli: an update from the infectious diseases society of America. Clin. Infect. Dis. 56:1685–1694. 10.1093/cid/cit152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. 1985. APACHE II: a severity of disease classification system. Crit. Care Med. 13:818–829. 10.1097/00003246-198510000-00009 [DOI] [PubMed] [Google Scholar]
- 18.Nicolau DP, McNabb J, Lacy MK, Quintiliani R, Nightingale CH. 2001. Continuous versus intermittent administration of ceftazidime in intensive care unit patients with nosocomial pneumonia. Int. J. Antimicrob. Agents 17:497–504. 10.1016/S0924-8579(01)00329-6 [DOI] [PubMed] [Google Scholar]
- 19.Nicasio AM, Eagye KJ, Nicolau DP, Shore E, Palter M, Pepe J, Kuti JL. 2010. Pharmacodynamic-based clinical pathway for empiric antibiotic choice in patients with ventilator-associated pneumonia. J. Crit. Care 25:69–77. 10.1016/j.jcrc.2009.02.014 [DOI] [PubMed] [Google Scholar]
- 20.Benko AS, Cappelletty DM, Kruse JA, Rybak MJ. 1996. Continuous infusion versus intermittent administration of ceftazidime in critically ill patients with suspected Gram-negative infections. Antimicrob. Agents Chemother. 40:691–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicolau DP, Nightingale CH, Banevicius MA, Fu Q, Quintiliani R. 1996. Ceftazidime serum bactericidal activity: continuous infusion versus intermittent injections. Antimicrob. Agents Chemother. 40:61–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicasio AM, Ariano RE, Zelenitsky SA, Kim A, Crandon JL, Kuti JL, Nicolau DP. 2009. Population pharmacokinetics of high-dose, prolonged-infusion cefepime in adult critically ill patients with ventilator-associated pneumonia. Antimicrob. Agents Chemother. 53:1476–1481. 10.1128/AAC.01141-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leary R, Jelliffe R, Schumitzky A, van Guilder M. 2002. A unified parametric/nonparametric approach to population PK/PD modeling, abstr 302, p 11 Abstr. 11th Annu. Meet. Popul. Appr. Group Eur Paris, France [Google Scholar]
- 24.Bustad A, Terziivanov D, Leary R, Port R, Schumitzky A, Jelliffe R. 2006. Parametric and nonparametric population methods: their comparative performance in analysing a clinical dataset and two Monte Carlo simulation studies. Clin. Pharmacokinet. 45:365–383 [DOI] [PubMed] [Google Scholar]
- 25.Yamaoka K, Nakagawa T, Uno T. 1978. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J. Pharmacokinet. Biopharm. 6:165–175. 10.1007/BF01117450 [DOI] [PubMed] [Google Scholar]
- 26.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. 10.1159/000180580 [DOI] [PubMed] [Google Scholar]
- 27.McKinnon PS, Paladino JA, Schentag JJ. 2008. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int. J. Antimicrob. Agents 31:345–351. 10.1016/j.ijantimicag.2007.12.009 [DOI] [PubMed] [Google Scholar]
- 28.Lee SY, Kuti JL, Nicolau DP. 2007. Cefepime pharmacodynamics in patients with extended spectrum beta-lactamase (ESBL) and non-ESBL infections. J. Infect. 54:463–468. 10.1016/j.jinf.2006.09.004 [DOI] [PubMed] [Google Scholar]
- 29.Mouton JW, Punt N, Vinks AA. 2005. A retrospective analysis using Monte Carlo simulations to evaluate recommended ceftazidime dosing regimens in healthy volunteers, patients with cystic fibrosis, and patients in the intensive care unit. Clin. Ther. 27:762–772. 10.1016/j.clinthera.2005.06.013 [DOI] [PubMed] [Google Scholar]
- 30.Boselli E, Breih D, Rimmele T, Poupelin JC, Saux MC, Chassard D, Allaouchiche B. 2004. Plasma and lung concentrations of ceftazidime administered in continuous infusion to critically ill patients with severe nosocomial pneumonia. Intensive Care Med. 30:989–991. 10.1007/s00134-004-2171-2 [DOI] [PubMed] [Google Scholar]
- 31.Roberts JA, Lipman J. 2009. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit. Care Med. 37:840–851. 10.1097/CCM.0b013e3181961bff [DOI] [PubMed] [Google Scholar]
- 32.Lipman J, Wallis SC, Rickard C. 1999. Low plasma cefepime levels in critically ill septic patients: pharmacokinetic modeling indicates improved troughs with revised dosing. Antimicrob. Agents Chemother. 43:2559–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gómez CM, Cordingly JJ, Palazzo MG. 1999. Altered pharmacokinetics of ceftazidime in critically ill patients. Antimicrob. Agents Chemother. 43:1798–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Georges B, Conil JM, Seguin T, Ruiz S, Minville V, Cougot P, Decun JF, Gonzalez H, Houin G, Fourcade O, Saivin S. 2009. Population pharmacokinetics of ceftazidime in intensive care unit patients: influence of glomerular filtration rate, mechanical ventilation, and reason for admission. Antimicrob. Agents Chemother. 53:4483–4489. 10.1128/AAC.00430-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taccone FS, Laterre PF, Dugernier T, Spapen H, Delattre I, Wittebole X, De Backer D, Layeux B, Wallemacq P, Vincent JL, Jacobs F. 2010. Insufficient beta-lactam concentrations in the early phase of severe sepsis and septic shock. Crit. Care 14:R126. 10.1186/cc9091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andes D, Anon J, Jacobs MR, Craig WA. 2004. Application of pharmacokinetics and pharmacodynamics to antimicrobial therapy of respiratory tract infections. Clin. Lab. Med. 24:477–502. 10.1016/j.cll.2004.03.009 [DOI] [PubMed] [Google Scholar]
- 37.Tam VH, McKinnon PS, Akins RL, Drusano GL, Rybak MJ. 2003. Pharmacokinetics and pharmacodynamics of cefepime in patients with various degrees of renal function. Antimicrob. Agents Chemother. 47:1853–1861. 10.1128/AAC.47.6.1853-1861.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manduru M, Mihm LB, White RL, Friedrich LV, Flume PA, Bosso JA. 1997. In vitro pharmacodynamics of ceftazidime against Pseudomonas aeruginosa isolates from cystic fibrosis patients. Antimicrob. Agents Chemother. 41:2053–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li C, Du X, Kuti JL, Nicolau DP. 2007. Clinical pharmacodynamics of meropenem in patients with lower respiratory tract infections. Antimicrob. Agents Chemother. 51:1725–1730. 10.1128/AAC.00294-06 [DOI] [PMC free article] [PubMed] [Google Scholar]