Abstract
The novel lincosamide resistance gene lnu(E), truncated by insertion of an ISEnfa5-cfr-ISEnfa5 segment, was identified in Streptococcus suis. The gene lnu(E) encodes a 173-amino-acid protein with ≤69.4% identity to other lincosamide nucleotidyltransferases. The lnu(E) gene and its promoter region were de novo synthesized, and Staphylococcus aureus RN4220 carrying a shuttle vector with the cloned lnu(E) gene showed a 16-fold increase in the lincomycin MIC. Mass spectrometry experiments demonstrated that Lnu(E) catalyzed the nucleotidylation of lincomycin.
TEXT
To date, the three lincosamides, lincomycin, clindamycin, and pirlimycin, are widely used to treat staphylococcal and streptococcal infections in veterinary medicine. Lincomycin alone or in the combination lincomycin-spectinomycin is licensed in the European Union for the control of a number of bacterial pathogens involved in infections of the respiratory, gastrointestinal, and urinary/genital tracts as well as wound and skin infections in cattle, swine, horses, dogs, cats, and poultry (1). In contrast, pirlimycin is approved only for the control of staphylococci and streptococci from cases of bovine mastitis (2). Clindamycin is widely used in human medicine (3) but is also approved for use in cats and dogs, mainly for the treatment of skin and soft tissue infections, periodontitis, and osteomyelitis (4).
Resistance to lincosamides can be due to a number of different genes which specify different resistance mechanisms (http://faculty.washington.edu/marilynr/). The highest MICs of lincosamides are commonly seen in the presence of rRNA methylases which modify the ribosomal binding site for macrolides, lincosamides, and streptogramin B antibiotics (MLSB phenotype) and thereby confer cross-resistance to these antimicrobial agents (5). Resistance to lincosamides only (L phenotype) is exclusively based on the inactivation of lincosamides by nucleotidylation via O-nucleotidyltransferases (6). Since the first report of a lincosamide nucleotidyltransferase gene, lnu(A) (formerly linA) (7), a number of lnu genes have been described in different bacteria from animal, human, and environmental sources, including lnu(B) (8), lnu(C) (9), lnu(D) (10), and lnu(F) (11). Most of these lnu genes confer resistance to lincomycin and pirlimycin, but their MICs for clindamycin are often below the breakpoints for resistance (2, 9, 10). Of all Lnu proteins, Lnu(A) is the best-studied enzyme (6). The corresponding gene, lnu(A), has been detected in many staphylococcal species (2), and plasmids seem to play an important role in the interspecies transfer of lnu(A) (3, 12).
In a previous study, we identified the cfr-carrying plasmid pStrcfr, isolated from porcine Streptococcus suis S10 in Beijing, China (13). In this plasmid, cfr was bracketed by two copies of insertion sequence ISEnfa5, located in the same orientation, and direct target site duplications of 3 bp (GAT) were found immediately upstream and downstream of this segment (13). Further analysis identified a 522-bp open reading frame into which this ISEnfa5-cfr-ISEnfa5 segment was inserted (Fig. 1). The intact reading frame possibly coded for a 173-amino-acid (aa) protein that showed 69.4% identity to the lincosamide nucleotidyltransferase Lnu(A) from Staphylococcus haemolyticus (GenBank accession no. YP_758631.1) (12). Since an amino acid identity of <80% is considered indicative of a novel type of Lnu protein, the designations lnu(E) and Lnu(E) were tentatively provided by the nomenclature center for MLS resistance genes (http://faculty.washington.edu/marilynr/) for the new gene and the corresponding protein, respectively. Figure 2 shows a homology tree of the Lnu(E) protein with other lincosamide nucleotidyltransferases deposited in the GenBank database. As assumed from the initial amino acid comparison, Lnu(E) was most closely related to Lnu(A). In a multisequence alignment, Lnu(E) exhibited distinctly lower identities of 24.3% and 25.9% to Lnu(C) from Streptococcus agalactiae (GenBank accession no. AY928180) and Lnu(D) from Streptococcus uberis (GenBank accession no. EF452177), respectively. No significant homology was found between Lnu(E) and Lnu(B) from Enterococcus faecium (GenBank accession no. AJ238249) or Lnu(F) from Salmonella enterica serovar Stanley (GenBank accession no. EU118119).
FIG 1.
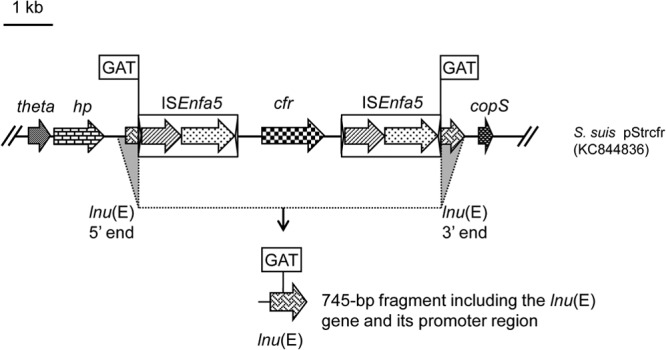
Schematic presentation of the lun(E) gene truncated by insertion of an ISEnfa5-cfr-ISEnfa5 segment in plasmid pStrcfr from S. suis S10 and the de novo-synthesized 745-bp segment which included the lnu(E) gene (522 bp) and its promoter region (223 bp) used for functional confirmation of lnu(E) as a lincosamide resistance gene.
FIG 2.
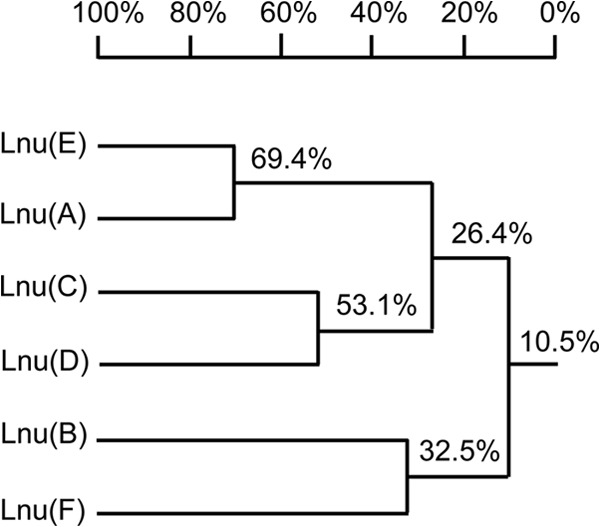
Homology tree of Lnu proteins based on multiple sequence alignment, produced by using DNAMAN software (Lynnon-BioSoft, Ontario, Canada).
Since the original lnu(E) gene in S. suis was truncated by ISEnfa5-cfr-ISEnfa5 in S. suis, a PCR-directed search (primers lnuE-F [5′-AGACCTCCAAATAACTTA-3′] and lnuE-R [5′-AAATGACCTTCTTTCTTA-3′]; annealing temperature, 48°C; amplicon size, 450 bp) for an intact and functionally active lnu(E) gene was conducted among unrelated streptococcal and enterococcal isolates. Unfortunately, negative results were obtained for all 94 enterococci and 97 streptococci (including 58 S. suis isolates) of food animal origin isolated during 2008 to 2012, suggesting a low prevalence of lnu(E) in these bacteria (data not shown). To investigate whether an intact lnu(E) gene confers lincosamide resistance, a 745-bp fragment (Fig. 1) which included the lnu(E) coding and promoter regions was synthesized by Genewiz Biotechnology Company (Jiangsu, China). The sequence of this synthetic fragment corresponded exactly to that found in plasmid pStrcfr from S. suis S10 but, however, (i) without the ISEnfa5-cfr-ISEnfa5 segment and (ii) without one of the two 3-bp direct repeats generated during insertion of the ISEnfa5-cfr-ISEnfa5 segment into the lnu(E) reading frame, but (iii) with XbaI and BamHI restriction sites in the noncoding regions at the 5′ and 3′ termini. Sequence analysis was conducted to confirm that the sequence of this synthetic segment corresponds exactly to the original S. suis sequence. This synthetic fragment was ligated into the XbaI- and BamHI-digested Escherichia coli-Enterococcus faecalis shuttle plasmid vector pAM401, which also replicates in Staphylococcus aureus. The recombinant plasmid pAM401-lnu(E) was then transformed into S. aureus RN4220. Susceptibility testing of RN4220+pAM401-lnu(E) exhibited 16-fold increases in the MICs for lincomycin (8 mg/liter) but only 2-fold increases in the MICs for clindamycin (0.12 mg/liter) and pirlimycin (1 mg/liter), compared to the recipient strain S. aureus RN4220 and S. aureus RN4220 carrying the “empty” shuttle vector (Table 1). In addition, no MIC changes were observed for erythromycin, streptomycin, gentamicin, kanamycin, vancomycin, cefazolin, oxacillin, ciprofloxacin, enrofloxacin, sulfamethoxazole-trimethoprim, spiramycin, tilmicosin, and chloramphenicol (data not shown).
TABLE 1.
MICs of S. aureus RN4220 and strains containing the lnu(E)-carrying recombinant plasmid or the corresponding vector-only plasmids
| Bacterial strain | MIC (mg/liter) of drug: |
||
|---|---|---|---|
| Lincomycin | Pirlimycin | Clindamycin | |
| S. aureus RN4220 | 0.5 | 0.5 | 0.06 |
| S. aureus RN4220+pAM401 | 0.5 | 0.5 | 0.06 |
| S. aureus RN4220+pAM401-lnu(E)a | 8 | 1 | 0.12 |
This intact lun(E) gene and its promoter region were de novo synthesized by Genewiz Biotechnology Company.
In a comparison between Lnu(E) and Lnu(A), two domains involved in the binding of the cofactor for the nucleotidylation reaction, ATP or GTP, were found to be well conserved (6). The first domain in Lnu(E), consisting of Val-Asp-Val-Leu-Thr-Gly-Lys, was identical to that in Lnu(A), while the second domain, Lys-Leu-Val-Lys-Lys, was very similar to the Lys-Val-Ile-Gln/Lys-Lys sequence in Lnu(A), with Leu, Val, and Ile representing nonpolar and hydrophilic amino acids with similar biological functions. Whether these amino acid exchanges in the second functional domain have an impact on the relatively low lincosamide MICs conferred by Lnu(E) in S. aureus RN4220 remains to be determined. However, the L phenotype, with resistance to lincomycin but susceptibility to clindamycin and borderline susceptibility to pirlimycin, conferred by this novel nucleotidyltransferase was similar to the phenotypes observed with the lincosamide nucleotidyltransferases Lnu(A), Lnu(C), and Lnu(D) (2, 9, 10, 12). These results suggested that the intact lnu(E) reading frame—even if synthesized de novo—might represent a novel, functionally active lnu gene.
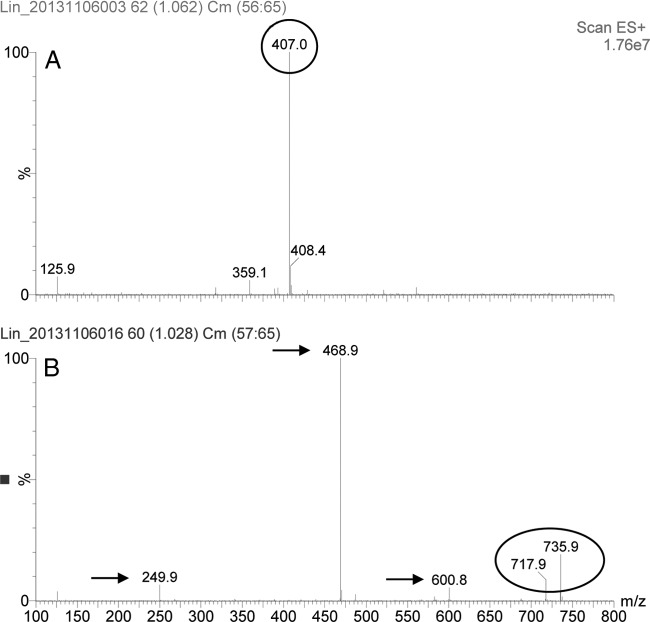
To analyze the mechanism of resistance conferred by Lnu(E), S. aureus RN4220+pAM401-lnu(E) cells in comparison to S. aureus RN4220+pAM401 cells (control) were tested for their ability to inactivate lincomycin. The assay was conducted in liquid medium by ultra-high-performance liquid chromatography coupled with Quattro LC triple quadrupole tandem mass spectrometry (UPLC-MS/MS; Waters, Milford, MA, USA) (Fig. 3). Spectra were acquired in a full MS scan (mass range from m/z 200 to 800; maximum ion time, 100 ms), followed by a collision-induced dissociation (CID) with argon as the collision gas. The most abundant ions were detected at the collision energy of 25 eV. The spectra showed the presence of the nonmodified lincomycin (407.0 Da) in Fig. 3A (control) whereas the 735.9-Da and the 717.9-Da peaks in Fig. 3B represent the nucleotidylated lincomycin with and without one H2O molecule, respectively. Other fragments of 600.8, 468.9, and 249.9 Da are believed to result from leakage of the adenine moiety or the adenosine moiety of the compound, respectively, as previously described in the inactivation of lincomycin by Lnu(C) (9) or Lnu(D) (10). These results suggest that the inactivation products represent nucleotidylated lincomycin as previously described (9, 10) and confirm that the Lnu(E) protein represents in fact a novel type of lincosamide nucleotidyltransferase.
FIG 3.
UPLC-MS/MS analysis of S. aureus RN4220+pAM401 cells (control sample) (A) and S. aureus RN4220+pAM401-lnu(E) cells (B). The 407.0-Da peak in panel A represents the nonmodified lincomycin, whereas the 735.9-Da and the 717.9-Da peaks in panel B represent the nucleotidylated lincomycin and the nucleotidylated lincomycin which has lost one H2O molecule, respectively. Results are shown for fragments observed after CID. Fragments indicated by arrows are believed to result from leakage of the adenine or the adenosine moiety of the compound.
In conclusion, a novel lincomycin resistance gene, lnu(E), conferring low-level lincomycin resistance in staphylococci, was detected. Since this gene was nonfunctional in the original porcine S. suis isolate due to the insertion of an ISEnfa5-cfr-ISEnfa5 segment, de novo synthesis was employed to confirm its functionality. Although we could not detect a naturally occurring, functionally active lnu(E) gene in our strain collections, it is nevertheless most likely that such a gene exists since its inactivated version was present on a naturally occurring S. suis plasmid (13). Routine surveillance for lnu(E) in bacteria of human and animal origin is warranted and might help to clarify whether S. suis is the original host, in which bacteria this gene is present, and how widespread it is.
Nucleotide sequence accession number.
The sequence of the lnu(E) gene was deposited in GenBank under the accession no. KF287643.
ACKNOWLEDGMENTS
The work was funded by grants from the National Basic Research Program of China (2013CB127200), and by grant number 01KI1014D (MedVet-Staph) of the German Federal Ministry of Education and Research (BMBF) provided through the German Aerospace Center (DLR).
Footnotes
Published ahead of print 23 December 2013
REFERENCES
- 1.Schwarz S, Werckenthin C, Alesík E, Wieler LH, Wallmann J. 2007. Susceptibility of bacterial pathogens against lincomycin/spectinomycin (1/2), penicillin G/neomycin (1/1), and penicillin G/dihydrostreptomycin (1/1) as determined in the BfT-GermVet monitoring program 2004–2006. Berl. Münch. Tierärztl. Wochenschr. 120:363–371 [PubMed] [Google Scholar]
- 2.Lüthje P, Schwarz S. 2006. Antimicrobial resistance of coagulase-negative staphylococci from bovine subclinical mastitis with particular reference to macrolide-lincosamide resistance phenotypes and genotypes. J. Antimicrob. Chemother. 57:966–969. 10.1093/jac/dkl061 [DOI] [PubMed] [Google Scholar]
- 3.Lozano C, Aspiroz C, Saenz Y, Ruiz-Garcia M, Royo-Garcia G, Gomez-Sanz E, Ruiz-Larrea F, Zarazaga M, Torres C. 2012. Genetic environment and location of the lnu(A) and lnu(B) genes in methicillin-resistant Staphylococcus aureus and other staphylococci of animal and human origin. J. Antimicrob. Chemother. 67:2804–2808. 10.1093/jac/dks320 [DOI] [PubMed] [Google Scholar]
- 4.Noli C, Boothe D. 1999. Macrolides and lincosamides. Vet. Dermatol. 10:217–223. 10.1046/j.1365-3164.1999.00176.x [DOI] [PubMed] [Google Scholar]
- 5.Leclercq R. 2002. Mechanisms of resistance to macrolides and lincosamides: nature of the resistance elements and their clinical implications. Clin. Infect. Dis. 34:482–492. 10.1086/324626 [DOI] [PubMed] [Google Scholar]
- 6.Brisson-Noël A, Delrieu P, Samain D, Courvalin P. 1988. Inactivation of lincosaminide antibiotics in Staphylococcus. Identification of lincosaminide O-nucleotidyltransferases and comparison of the corresponding resistance genes. J. Biol. Chem. 263:15880–15887 [PubMed] [Google Scholar]
- 7.Brisson-Noël A, Courvalin P. 1986. Nucleotide sequence of gene linA encoding resistance to lincosamides in Staphylococcus haemolyticus. Gene 43:247–253. 10.1016/0378-1119(86)90213-1 [DOI] [PubMed] [Google Scholar]
- 8.Lüthje P, Schwarz S. 2007. Molecular basis of resistance to macrolides and lincosamides among staphylococci and streptococci from various animal sources collected in the resistance monitoring program BfT-GermVet. Int. J. Antimicrob. Agents 29:528–535. 10.1016/j.ijantimicag.2006.12.016 [DOI] [PubMed] [Google Scholar]
- 9.Achard A, Villers C, Pichereau V, Leclercq R. 2005. New lnu(C) gene conferring resistance to lincomycin by nucleotidylation in Streptococcus agalactiae UCN36. Antimicrob. Agents Chemother. 49:2716–2719. 10.1128/AAC.49.7.2716-2719.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petinaki E, Guerin-Faublee V, Pichereau V, Villers C, Achard A, Malbruny B, Leclercq R. 2008. Lincomycin resistance gene lnu(D) in Streptococcus uberis. Antimicrob. Agents Chemother. 52:626–630. 10.1128/AAC.01126-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heir E, Lindstedt BA, Leegaard TM, Gjernes E, Kapperud G. 2004. Prevalence and characterization of integrons in blood culture Enterobacteriaceae and gastrointestinal Escherichia coli in Norway and reporting of a novel class 1 integron-located lincosamide resistance gene. Ann. Clin. Microbiol. Antimicrob. 3:12. 10.1186/1476-0711-3-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lüthje P, von Kockritz-Blickwede M, Schwarz S. 2007. Identification and characterization of nine novel types of small staphylococcal plasmids carrying the lincosamide nucleotidyltransferase gene lnu(A). J. Antimicrob. Chemother. 59:600–606. 10.1093/jac/dkm008 [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Li D, Song L, Liu Y, He T, Liu H, Wu C, Schwarz S, Shen J. 2013. First report of the multi-resistance gene cfr in Streptococcus suis. Antimicrob. Agents Chemother. 57:4061–4063. 10.1128/AAC.00713-13 [DOI] [PMC free article] [PubMed] [Google Scholar]