Abstract
There are no well-matched, controlled studies comparing azithromycin with doxycycline for the treatment of complicated scrub typhus. A retrospective propensity score-matched case-control study was performed for patients who presented with complicated scrub typhus and were treated with doxycycline or azithromycin between 2001 and 2011. Data on comorbidities, clinical manifestations, laboratory studies, treatments, and outcomes were extracted for analysis. The clinical characteristics and outcomes of the azithromycin-treated group (n = 73) were compared to those of the doxycycline-treated group (n = 108). Of 181 patients, 73 from each group were matched by propensity scores. There were no significant differences in baseline characteristics between the matched groups. The treatment success and survival rates were not significantly different (89% [65/73 patients] versus 96% [70/73 patients] and 96% [70/73 patients] versus 96% [70/73 patients], respectively [P > 0.05]). No difference was observed in the time to defervescence or length of hospital stay between the two groups (P > 0.05). In complicated scrub typhus patients (n = 181), multivariate analysis showed that only APACHE II score was an independent risk factor for mortality (95% confidence interval, 1.11 to 1.56; P < 0.001). Our data suggest that outcomes of azithromycin therapy are comparable to those of doxycycline therapy in patients with complicated scrub typhus.
INTRODUCTION
Scrub typhus is a mite-borne rickettsiosis caused by Orientia tsutsugamushi, which is endemic or reemerging in eastern and southern Asia, northern Australia, and islands in the western Pacific and Indian Oceans (1). It is estimated that there are 1 million new cases each year and that 1 billion people are at risk of infection (2). Mortality rates can be as high as 30% if the disease is left untreated (3); however, administration of appropriate antibiotics reduces the mortality rate to less than 5%. Antibiotics that have shown efficacy toward scrub typhus include chloramphenicol, tetracyclines, macrolides, and rifampin (4).
A few clinical studies have shown that azithromycin is as effective as doxycycline for the treatment of mild to moderate scrub typhus (5–7). For this reason, azithromycin is considered an appropriate alternative for mild to moderate scrub typhus in areas where doxycycline-resistant scrub typhus is prevalent (8), and also for children under 8 years of age or in women during pregnancy (9), where doxycycline is contraindicated. However, there are few clinical data on the efficacy of different antibiotics for the treatment of severe, life-threatening scrub typhus (10), despite the fact that complicated cases of scrub typhus are not uncommon (11–13). Moreover, the efficacy of azithromycin for the treatment of severe complicated scrub typhus is largely unknown, because all randomized clinical trials using macrolides to date have excluded severe or complicated cases (5–7). Therefore, we performed this study to compare the outcomes of azithromycin therapy to those of doxycycline therapy in patients with complicated scrub typhus.
MATERIALS AND METHODS
Patients.
This study was approved by the Institutional Review Board of Chonnam National University Hospital, Gwang-ju, Republic of Korea. A waiver of consent was granted given the retrospective nature of the project.
This study included a retrospective review of adult patients (aged ≥16 years) with scrub typhus who were admitted to Chonnam National University Hospital (1,000 beds) from 2001 to 2011. A diagnosis of scrub typhus was made based on typical clinical manifestations and the results of a serologic test. Serologic testing was performed using a passive hemagglutination assay (PHA) against indirect O. tsutsugamushi antigen. A positive result was defined as a titer of ≥1:80 in a single serum sample or by a ≥4-fold increase in the follow-up titer (14). The PHA was performed using Genedia Tsutsu PHA II test kits (GreenCross SangA, Yongin, Republic of Korea).
Patients with complicated scrub typhus who were treated with doxycycline (100 mg orally every 12 h) or azithromycin (500 mg intravenously every 24 h) were included. Each patient's medical record was reviewed for information regarding age, sex, underlying disease, symptoms, signs, physical and laboratory findings at the time of admission, treatment, and outcome.
Definitions.
Complicated scrub typhus was defined by the following conditions (11, 12): (i) shock, defined by a systolic blood pressure of <90 mm Hg or a fall in systolic blood pressure of >40 mm Hg; (ii) acute kidney injury, defined by a serum creatinine increase of >2.0-fold or a glomerular filtration rate decrease of >50% from baseline; (iii) pneumonia with parenchymal lung lesions on a chest radiograph and cough or dyspnea; (iv) acute respiratory distress syndrome, defined by a ratio of arterial partial oxygen tension as a fraction of inspired oxygen of <200 mm Hg in the presence of bilateral infiltrates on a chest radiograph; (v) meningoencephalitis with neurologic symptoms and evidence of infection of the central nervous system, based on imaging studies or cerebrospinal fluid (CSF) counts of >5 leukocytes/mm3; (vi) gastrointestinal bleeding; and (vii) cholecystitis, defined by the presence of Murphy's sign and radiological evidence of gallbladder inflammation.
Mortality was defined as scrub typhus related if there was no other definite cause of death (15). Treatment was considered a failure when scrub typhus-related death occurred or if the initial antibiotic was changed to another agent(s) due to clinical deterioration, despite ≥5 days of antibiotic administration, without any other identifiable cause (7). Treatment was defined as successful if the agent used was not subsequently changed because of ineffectiveness and if scrub typhus-related death did not occur.
Statistical analysis.
Our major analysis compared the treatment outcomes between the doxycycline- and azithromycin-treated groups with complicated scrub typhus. We used propensity score adjustment to control for the following confounding variables: age (11, 13), white blood cell count (11, 13), creatinine level (16), albumin level (11, 17), and acute physiology and chronic health evaluation II (APACHE II) score (18). These variables are reported risk factors for complicated scrub typhus. This technique permitted pair-matched selection of 73 of the 108 patients in the doxycycline-treated group who were most similar to the patients in the azithromycin-treated group.
The Kolmogorov-Smirnov goodness-of-fit test was used to determine the distribution of each set of data for normality before subsequent analysis. Categorical variables were expressed as percentages of counts, and continuous variables were expressed as means ± standard deviations (SD) or as medians and interquartile ranges (IQR). Categorical variables were compared using the χ2 test or Fisher's exact test, as appropriate. Continuous variables were compared by the Student t test or the Mann-Whitney U test, as appropriate. Kaplan-Meier survival analysis in association with the log rank test was performed to compare the time to defervescence between groups. Logistic regression analysis was carried out, with inclusion of variables with P values of <0.10 in univariate analysis. All tests of significance were two-tailed, and P values of ≤0.05 were deemed to indicate statistical significance. Statistical analyses of the data were performed using SPSS v. 19.0 (SPSS, Chicago, IL).
RESULTS
Baseline characteristics of patients with complicated scrub typhus.
A total of 771 patients were diagnosed with scrub typhus at our institute during the study period. Of these, 615 (79.8%) patients were admitted, and 217 (28.1%) had complicated scrub typhus at presentation. A total of 181 patients with complicated scrub typhus who were initially treated with either doxycycline (doxycycline-treated group; n = 108) or azithromycin (azithromycin-treated group; n = 73) were analyzed.
The demographic and clinical characteristics of the 181 patients with complicated scrub typhus who were treated with doxycycline or azithromycin are shown in Table 1. No differences in demographic characteristics or underlying diseases were observed between the two groups. Rash was observed more commonly in the azithromycin-treated group than in the doxycycline-treated group (70% versus 82%, respectively; P = 0.05). Complications were as follows: pneumonia (84/181 patients [46%]), acute kidney injury (77/181 patients [43%]), shock (48/181 patients [27%]), meningitis (22/181 patients [13%]), acute respiratory distress syndrome (12/181 patients [6%]), gastrointestinal bleeding (12/181 patients [7%]), and cholecystitis (4/181 patients [2%]). Pneumonia was observed more commonly in the azithromycin-treated group than in the doxycycline-treated group (59% versus 38%, respectively; P < 0.01); frequencies of other complications were not significantly different between the two groups (P > 0.05). APACHE II scores at presentation and other initial laboratory findings were also not different between the two groups (P > 0.05) (Table 1).
TABLE 1.
Demographic and clinical characteristics of 181 patients with complicated scrub typhus
| Characteristic | Value for patients treated with: |
P value | |
|---|---|---|---|
| Doxycycline (n = 108) | Azithromycin (n = 73) | ||
| Demographic data | |||
| Age (yr)b | 68.7 ± 10.6 | 68.6 ± 11.3 | 0.99 |
| No. (%) of males | 35 (51) | 29 (40) | 0.14 |
| No. (%) of patients with underlying disease | 37 (34) | 28 (38) | 0.57 |
| Diabetes mellitus | 18 (17) | 15 (21) | 0.51 |
| Hypertension | 12 (11) | 8 (11) | 0.97 |
| Chronic liver disease | 9 (8) | 6 (8) | 0.98 |
| Chronic kidney disease | 2 (2) | 1 (1) | >0.99 |
| Cancer | 3 (3) | 2 (3) | >0.99 |
| No. (%) of patients with clinical symptoms and signs | |||
| Fever | 106 (98) | 68 (93) | 0.12 |
| Duration of fever before therapy (days)c | 7 (3, 8) | 6 (3, 7) | 0.16 |
| Rash | 75 (70) | 60 (82) | 0.05 |
| Eschar | 98 (91) | 67 (92) | 0.81 |
| No. (%) of patients with possible exposure history | |||
| Farming | 44 (41) | 41 (57) | 0.047 |
| Picnicking or climbing | 64 (59) | 32 (43) | 0.047 |
| No. (%) of patients with complications | |||
| Pneumonia | 41 (38) | 43 (59) | <0.01 |
| Acute kidney injury | 50 (46) | 27 (37) | 0.21 |
| Shock | 29 (27) | 19 (26) | 0.90 |
| Meningitis | 13 (12) | 9 (12) | 0.95 |
| Acute respiratory distress syndrome | 6 (6) | 6 (8) | 0.48 |
| Gastrointestinal bleeding | 8 (7) | 4 (6) | 0.76 |
| Cholecystitis | 3 (3) | 1 (1) | 0.65 |
| APACHE II scorea,b | 11.9 ± 5.3 | 11.5 ± 6.5 | 0.66 |
| Initial blood laboratory examination | |||
| White blood cell count (1,000/mm3)b | 9.5 ± 4.5 | 10.1 ± 5.3 | 0.89 |
| Hemoglobin concn (g/dl)b | 11.7 ± 1.6 | 12.0 ± 1.7 | 0.23 |
| Platelet count (1,000/mm3)b | 118 ± 69 | 125 ± 59 | 0.47 |
| C-reactive protein concn (mg/dl)b | 13.0 ± 7.1 | 14.0 ± 8.7 | 0.44 |
| Albumin concn (g/dl)b | 2.7 ± 0.6 | 2.8 ± 0.5 | 0.11 |
| Lactate dehydrogenase concn (IU/liter)c | 936 (719, 1,118) | 960 (711, 1,176) | 0.55 |
| Aspartate aminotransferase concn (IU/liter)c | 91 (67, 138) | 91 (58, 170) | 0.99 |
| Alanine aminotransferase concn (IU/liter)c | 56 (40, 56) | 67 (42, 111) | 0.23 |
| Creatinine concn (mg/dl)c | 1.2 (0.8, 1.9) | 1.0 (0.8, 2.0) | 0.82 |
| Length of hospital stay (days)c | 7 (6, 11) | 6 (5, 11) | 0.20 |
APACHE, acute physiology and chronic health evaluation.
Continuous variables are expressed as means ± SD and were compared by the Student t test.
Continuous variables are expressed as medians (IQR) and were compared by the Mann-Whitney U test.
Treatments and outcomes of patients with complicated scrub typhus.
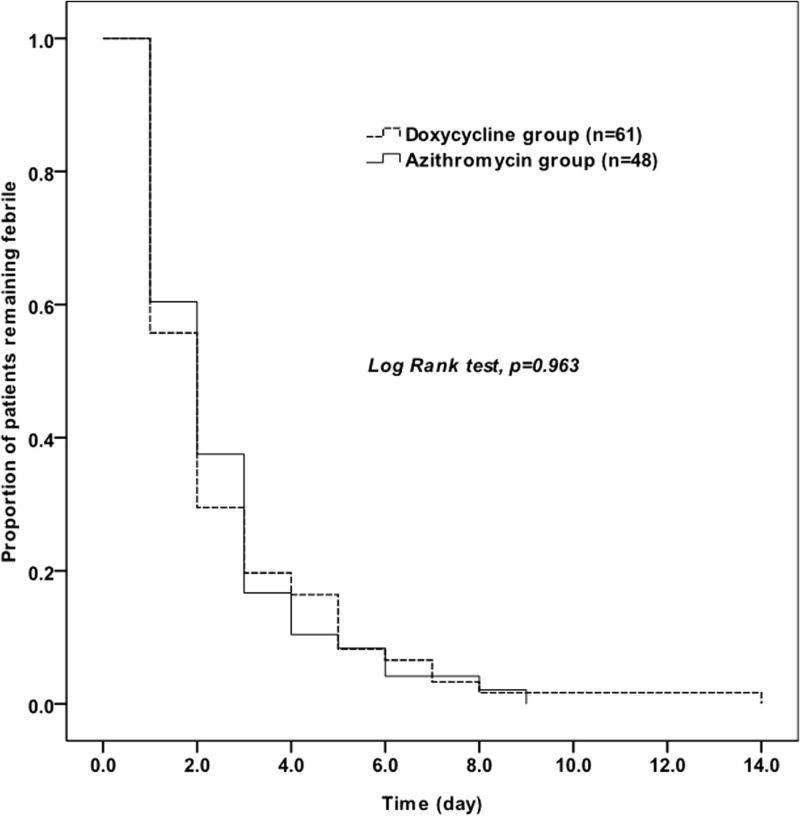
The 73 patients in the doxycycline-treated group were matched with the 73 patients in the azithromycin-treated group with the closest propensity scores. Following propensity score matching, both groups were well matched (Table 2); in particular, the incidences of pneumonia did not differ (56% versus 59%; P > 0.05). The treatments and outcomes of the 181 patients with complicated scrub typhus and the 146 propensity score-matched patients are shown in Table 3. The mean duration of antibiotic administration was shorter for the azithromycin-treated group (median, 5 days; IQR, 3 to 5 days) than for the doxycycline-treated group (median, 7 days; IQR, 7 to 7 days) (P < 0.01). In the unmatched analysis, no difference was observed in treatment success (92% and 96% for the doxycycline and azithromycin groups, respectively) or mortality (4% versus 4%) (P > 0.05) (Table 3). In the propensity score-matched case-control study, the treatment success (89% versus 96%) and mortality (4% versus 4%) rates were also not significantly different (P > 0.05). In addition, Kaplan-Meier curves in association with a log rank test of the time to defervescence also did not differ statistically between the two groups (Fig. 1).
TABLE 2.
Demographic and clinical characteristics of 146 patients with complicated scrub typhus after propensity score matching
| Characteristic | Value for patients treated with: |
P value | |
|---|---|---|---|
| Doxycycline (n = 73) | Azithromycin (n = 73) | ||
| Demographic data | |||
| Age (yr)b | 68.1 ± 11.3 | 68.6 ± 11.3 | 0.77 |
| No. (%) of males | 35 (48) | 29 (40) | 0.40 |
| No. (%) of patients with underlying disease | 23 (32) | 28 (38) | 0.49 |
| Diabetes mellitus | 10 (14) | 15 (21) | 0.38 |
| Hypertension | 7 (10) | 8 (11) | >0.99 |
| Chronic liver disease | 5 (7) | 6 (8) | >0.99 |
| Chronic kidney disease | 1 (1) | 1 (1) | >0.99 |
| Cancer | 3 (4) | 2 (3) | >0.99 |
| No. (%) of patients with clinical symptoms and signs | |||
| Fever | 72 (99) | 68 (93) | 0.06 |
| Duration of fever before therapy (days)c | 7 (4, 8) | 6 (3, 7) | 0.31 |
| Rash | 49 (67) | 60 (82) | 0.06 |
| Eschar | 67 (92) | 67 (92) | >0.99 |
| No. (%) of patients with possible exposure history | |||
| Farming | 33 (45) | 41 (57) | 0.246 |
| Picnicking or climbing | 40 (55) | 32 (43) | 0.246 |
| No. (%) of patients with complications | |||
| Pneumonia | 41 (56) | 43 (59) | 0.87 |
| Acute kidney injury | 29 (40) | 27 (37) | 0.87 |
| Shock | 19 (26) | 19 (26) | >0.99 |
| Meningitis | 12 (16) | 9 (12) | 0.64 |
| Acute respiratory distress syndrome | 1 (1) | 6 (8) | >0.99 |
| Gastrointestinal bleeding | 0 (0) | 4 (6) | 0.25 |
| Cholecystitis | 0 (0) | 1 (1) | >0.99 |
| APACHE II scorea,b | 11.5 ± 5.5 | 11.5 ± 6.5 | 0.99 |
| Initial blood laboratory examination | |||
| White blood cell count (1,000/mm3)b | 9.7 ± 4.9 | 10.1 ± 5.3 | 0.63 |
| Hemoglobin concn (g/dl)b | 11.7 ± 1.6 | 12.0 ± 1.7 | 0.35 |
| Platelet count (1,000/mm3)b | 124 ± 73 | 125 ± 59 | 0.88 |
| C-reactive protein concn (mg/dl)b | 12.2 ± 6.8 | 14.0 ± 8.7 | 0.20 |
| Albumin concn (g/dl)b | 2.7 ± 0.6 | 2.8 ± 0.5 | 0.23 |
| Lactate dehydrogenase concn (IU/liter)c | 958 (758, 1,130) | 960 (711, 1,176) | 0.98 |
| Aspartate aminotransferase concn (IU/liter)c | 91 (71, 138) | 91 (58, 170) | 0.76 |
| Alanine aminotransferase concn (IU/liter)c | 54 (43, 89) | 67 (42, 111) | 0.42 |
| Creatinine concn (mg/dl)c | 1.0 (0.8, 1.7) | 1.0 (0.8, 2.0) | 0.50 |
| Hospital stay (days)c | 8 (6, 12) | 6 (5, 11) | 0.030 |
APACHE, acute physiology and chronic health evaluation.
Continuous variables are expressed as means ± SD and were compared by the Student t test.
Continuous variables are expressed as medians (IQR) and were compared by the Mann-Whitney U test.
TABLE 3.
Comparison of treatment outcomes of 181 patients with complicated scrub typhus and 146 propensity score-matched patients
| Characteristic | Value for patients treated with: |
P value | Value for propensity score-matched patients treated with: |
P value | ||
|---|---|---|---|---|---|---|
| Doxycycline (n = 108) | Azithromycin (n = 73) | Doxycycline (n = 73) | Azithromycin (n = 73) | |||
| Duration of antibiotic administration (days)a | 7 (7, 7) | 5 (3, 5) | <0.01 | 7 (7, 7) | 5 (3, 5) | <0.01 |
| No. (%) of patients with treatment failure | 9 (8) | 3 (4) | 0.37 | 8 (11) | 3 (4) | 0.208 |
| No. (%) of deaths | 4 (4) | 3 (4) | >0.99 | 3 (4) | 3 (4) | >0.99 |
Continuous variables are expressed as medians (IQR) and were compared by the Mann-Whitney U test.
FIG 1.
Kaplan-Meier curves of the time to defervescence for 146 patients who initially had fever and received doxycycline or azithromycin for the treatment of complicated scrub typhus.
Risk factors for mortality in patients with complicated scrub typhus.
The risk factors for mortality in the 181 patients with complicated scrub typhus are shown in Table 4. In univariate analysis, shock at presentation, acute respiratory distress syndrome, and APACHE II score were risk factors for mortality in patients with severe scrub typhus. However, only APACHE II score was an independent risk factor for mortality in multivariate analysis (Table 4).
TABLE 4.
Risk factors for mortality in 181 patients with complicated scrub typhus
| Characteristic | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| Value |
P value | 95% CI |
P value | |||
| Surviving patients (n = 174) | Patients who died (n = 7) | Lower | Upper | |||
| No. (%) of patients with shock | 43 (25) | 5 (71) | 0.015 | |||
| No. (%) of patients with ARDSa | 10 (6) | 2 (29) | 0.070 | |||
| APACHE II scoreb,c | 11.2 ± 5.0 | 25.1 ± 7.7 | <0.001 | 1.11 | 1.56 | <0.001 |
ARDS, acute respiratory distress syndrome.
APACHE, acute physiology and chronic health evaluation.
Continuous variables are expressed as means ± SD and were compared by the Student t test.
Treatment failure in patients with complications.
The treatment success rates for patients with complications are shown in Table 5. Shock and acute respiratory distress syndrome were associated with treatment failure. Meningitis was significantly associated with treatment failure; the treatment failure rate was 23% (5/22 patients) for patients with meningoencephalitis, while the rate was 4.4% (7/159 patients) for patients without meningoencephalitis (P < 0.01). The treatment failure rates of doxycycline therapy and azithromycin therapy for patients with meningoencephalitis were 31% (4/13 patients) and 11% (1/9 patients), respectively (P = 0.36). Five patients (three with meningoencephalitis and two with pneumonia) were initially treated with doxycycline for ≥5 days, but treatment was later changed to azithromycin salvage therapy because of persistent fever and clinical deterioration, and these patients were successfully cured.
TABLE 5.
Treatment success rates for 181 patients with complications
| Complication | No. (%) of patients |
P value | |
|---|---|---|---|
| Treatment success (n = 169) | Treatment failure (n = 12) | ||
| Pneumonia | 77 (46) | 7 (58) | 0.39 |
| Acute kidney injury | 70 (41) | 7 (58) | 0.25 |
| Shock | 41 (24) | 7 (58) | 0.01 |
| Meningoencephalitis | 17 (10) | 5 (42) | <0.01 |
| Acute respiratory distress syndrome | 9 (5) | 3 (25) | 0.04 |
| Gastrointestinal bleeding | 12 (7) | 0 (0) | >0.99 |
| Cholecystitis | 4 (2) | 0 (0) | >0.99 |
DISCUSSION
We found that the treatment outcomes with azithromycin were not different from those with doxycycline in patients who presented with complicated scrub typhus.
The proportion and risk factors of severe or complicated cases of scrub typhus have been evaluated in several studies. Lee et al. (13) showed that 24% of patients admitted for rickettsiosis developed one or more organ dysfunctions, and organ dysfunction was associated with scrub typhus and delayed antibiotic administration. Kim et al. (11) showed that 43% of scrub typhus patients had severe scrub typhus, with complications such as pneumonia, renal failure, meningoencephalitis, shock, gastrointestinal bleeding, myocarditis, and death. These cases were associated with age (≥60 years), presentation without eschar, and laboratory findings such as white blood cell counts of >10,000/mm3 and serum albumin levels of ≤3.0 g/dl. In a multicenter study conducted in the Republic of Korea, Park et al. (12) reported that 10% of eschar-positive scrub typhus patients had central nervous system, respiratory, cardiac, or renal complications or septic shock. In the present study, complicated scrub typhus was observed in 28.1% of scrub typhus patients and 35% of hospitalized scrub typhus patients, consistent with the findings of previous studies (10 to 43%) (11, 12). Our results, as well as data from previous studies, show that complications in patients with scrub typhus are not uncommon.
Although complicated scrub typhus is commonly observed, the efficacies of different antibiotics for the treatment of severe, life-threatening scrub typhus are not established, because most clinical evidence on drug treatment is from cases of mild to moderate scrub typhus (4, 19). Moreover, there are no data on the efficacy of azithromycin for the treatment of complicated scrub typhus. A recent retrospective study showed that levofloxacin therapy is associated with mortality in severe scrub typhus, despite a favorable success rate in mild to moderate cases, suggesting that the treatment outcomes of antibiotics can be different for severe scrub typhus (10). In this study, we showed that the efficacy of azithromycin is comparable to that of doxycycline in complicated scrub typhus, suggesting that azithromycin can also be used as a primary agent for the treatment of severe scrub typhus infection. We confirmed this result in the propensity score-matched case-control study.
In our study, patients received short-duration antibiotic therapy while suffering from severe scrub typhus (medians of 7 and 5 days for the doxycycline and azithromycin groups, respectively). No systematic studies have reported the treatment duration of complicated scrub typhus, with the exception of anecdotal reports (20–22). Although 10 to 14 days of antibiotics were used to treat complicated scrub typhus in several case reports, our study suggests that short-duration antibiotics are sufficient for treatment of complicated scrub typhus. A well-designed study is needed to confirm this.
The anatomical site of infection should be taken into account when treating infectious diseases, because the appropriate treatment strategy and treatment outcome differ for different sites; bactericidal agents with good blood-brain penetration and an intravenous route of administration are usually preferable for central nervous system infection. In the present study, the mortality of patients with meningoencephalitis was not high, in accordance with previous studies (23, 24). However, central nervous system involvement was a factor associated with treatment failure, and treatment failure seemed to be more frequent in patients treated with doxycycline, based on some case reports (25, 26). In our study, doxycycline was administered orally because the intravenous form is not available in the Republic of Korea and many other countries. Suboptimal central nervous system penetration (27, 28) and bacteriostatic action (29) of oral doxycycline therapy and decreased absorption of doxycycline, caused by many other drugs used concomitantly, (25, 30) might contribute to the low success rate of treatment for central nervous system infection. The efficacy of azithromycin for central nervous system infection is controversial, because this drug penetrates the blood-brain barrier and lasts long in tissues, but its cerebrospinal fluid concentration is relatively low (31). However, azithromycin showed favorable treatment success rates in patients with meningoencephalitis and in some patients with meningoencephalitis who did not respond to doxycycline therapy in our study. In addition, intravenous administration, which can overcome low gastrointestinal absorption, as seen in critically ill patients and caused by concomitant medications, and its rapid bactericidal action compared to that of doxycycline (29) might have contributed to the success rate of azithromycin treatment.
It is controversial whether O. tsutsugamushi meningitis is caused by direct invasion or secondary vasculitis, since O. tsutsugamushi is an intracellular organism. However, Pai et al. showed that O. tsutsugamushi does invade the CSF; therefore, scrub typhus should be considered a cause of mononuclear meningitis (24). Consequently, we considered the CSF concentrations of therapeutic antibiotics and the endothelial cell cytosolic concentrations. The pharmacokinetics of azithromycin are characterized by high, persistent tissue concentrations and a longer half-life in tissue (32), which allow short-duration treatment. The therapeutic efficacy of intravenous azithromycin seen in our study suggests that the crucial factor is the endothelial cell cytosolic concentration of the antimicrobial agent rather than the cerebrospinal fluid concentration. In addition, the association of meningitis with treatment failure suggests the importance of the cerebrospinal fluid concentrations of drugs. Further clinical studies are needed to reach a conclusion on this matter.
The present study had several limitations. First, doxycycline resistance in scrub typhus was not measured, but doxycycline-resistant scrub typhus has not previously been reported in the Republic of Korea. Second, the route of antibiotic administration was different, as this was inevitable in the retrospective study design. Third, this study had methodological limitations because it was retrospective in design. Hence, some baseline characteristics, such as the proportion of farmers and frequencies of rash and pneumonia, were different between the two groups. We compensated for this by using propensity score matching. However, because the factors influencing the physician's choice of antibiotics were not determined, they might have influenced our results as unmeasured confounding factors in the analysis. Lastly, the number of complications, such as gastrointestinal bleeding and cholecystitis, was very low, so our results might not be applicable in such cases.
In conclusion, we are the first to report that the treatment outcomes of azithromycin therapy are comparable to those of doxycycline therapy in patients with complicated scrub typhus infection.
ACKNOWLEDGMENTS
There were no conflicts of interest, and no financial support was received for this study.
Footnotes
Published ahead of print 23 December 2013
REFERENCES
- 1.Raoult D, Roux V. 1997. Rickettsioses as paradigms of new or emerging infectious diseases. Clin. Microbiol. Rev. 10:694–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watt G, Parola P. 2003. Scrub typhus and tropical rickettsioses. Curr. Opin. Infect. Dis. 16:429–436. 10.1097/00001432-200310000-00009 [DOI] [PubMed] [Google Scholar]
- 3.Jensenius M, Fournier PE, Raoult D. 2004. Rickettsioses and the international traveler. Clin. Infect. Dis. 39:1493–1499. 10.1086/425365 [DOI] [PubMed] [Google Scholar]
- 4.Liu Q, Panpanich R. 2010. Antibiotics for treating scrub typhus. Cochrane Database Syst. Rev. 2010:CD002150. [DOI] [PubMed] [Google Scholar]
- 5.Phimda K, Hoontrakul S, Suttinont C, Chareonwat S, Losuwanaluk K, Chueasuwanchai S, Chierakul W, Suwancharoen D, Silpasakorn S, Saisongkorh W, Peacock SJ, Day NP, Suputtamongkol Y. 2007. Doxycycline versus azithromycin for treatment of leptospirosis and scrub typhus. Antimicrob. Agents Chemother. 51:3259–3263. 10.1128/AAC.00508-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim YS, Yun HJ, Shim SK, Koo SH, Kim SY, Kim S. 2004. A comparative trial of a single dose of azithromycin versus doxycycline for the treatment of mild scrub typhus. Clin. Infect. Dis. 39:1329–1335. 10.1086/425008 [DOI] [PubMed] [Google Scholar]
- 7.Kim DM, Yu KD, Lee JH, Kim HK, Lee SH. 2007. Controlled trial of a 5-day course of telithromycin versus doxycycline for treatment of mild to moderate scrub typhus. Antimicrob. Agents Chemother. 51:2011–2015. 10.1128/AAC.01460-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watt G, Chouriyagune C, Ruangweerayud R, Watcharapichat P, Phulsuksombati D, Jongsakul K, Teja-Isavadharm P, Bhodhidatta D, Corcoran KD, Dasch GA, Strickman D. 1996. Scrub typhus infections poorly responsive to antibiotics in northern Thailand. Lancet 348:86–89. 10.1016/S0140-6736(96)02501-9 [DOI] [PubMed] [Google Scholar]
- 9.Kim YS, Lee HJ, Chang M, Son SK, Rhee YE, Shim SK. 2006. Scrub typhus during pregnancy and its treatment: a case series and review of the literature. Am. J. Trop. Med. Hyg. 75:955–959 [PubMed] [Google Scholar]
- 10.Tsai CC, Lay CJ, Wang CL, Ho YH, Wang LS, Chen LK. 2010. Levofloxacin versus tetracycline antibiotics for the treatment of scrub typhus. Int. J. Infect. Dis. 14:e62–e67. 10.1016/j.ijid.2009.03.012 [DOI] [PubMed] [Google Scholar]
- 11.Kim DM, Kim SW, Choi SH, Yun NR. 2010. Clinical and laboratory findings associated with severe scrub typhus. BMC Infect. Dis. 10:108. 10.1186/1471-2334-10-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park SW, Lee CS, Lee CK, Kwak YG, Moon C, Kim BN, Kim ES, Kang JM, Oh MD. 2011. Severity predictors in eschar-positive scrub typhus and role of serum osteopontin. Am. J. Trop. Med. Hyg. 85:924–930. 10.4269/ajtmh.2011.11-0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee N, Ip M, Wong B, Lui G, Tsang OT, Lai JY, Choi KW, Lam R, Ng TK, Ho J, Chan YY, Cockram CS, Lai ST. 2008. Risk factors associated with life-threatening rickettsial infections. Am. J. Trop. Med. Hyg. 78:973–978 [PubMed] [Google Scholar]
- 14.Kim IS, Seong SY, Woo SG, Choi MS, Kang JS, Chang WH. 1993. Rapid diagnosis of scrub typhus by a passive hemagglutination assay using recombinant 56-kilodalton polypeptides. J. Clin. Microbiol. 31:2057–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang HC, Kim SH, Kim KH, Kim CJ, Lee S, Song KH, Jeon JH, Park WB, Kim HB, Park SW, Kim NJ, Kim EC, Oh MD, Choe KW. 2009. Salvage treatment for persistent methicillin-resistant Staphylococcus aureus bacteremia: efficacy of linezolid with or without carbapenem. Clin. Infect. Dis. 49:395–401. 10.1086/600295 [DOI] [PubMed] [Google Scholar]
- 16.Varghese GM, Abraham OC, Mathai D, Thomas K, Aaron R, Kavitha ML, Mathai E. 2006. Scrub typhus among hospitalised patients with febrile illness in South India: magnitude and clinical predictors. J. Infect. 52:56–60. 10.1016/j.jinf.2005.02.001 [DOI] [PubMed] [Google Scholar]
- 17.Lee CS, Min IS, Hwang JH, Kwon KS, Lee HB. 2010. Clinical significance of hypoalbuminemia in outcome of patients with scrub typhus. BMC Infect. Dis. 10:216. 10.1186/1471-2334-10-216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee CS, Hwang JH, Lee HB, Kwon KS. 2009. Risk factors leading to fatal outcome in scrub typhus patients. Am. J. Trop. Med. Hyg. 81:484–488 [PubMed] [Google Scholar]
- 19.Rajapakse S, Rodrigo C, Fernando SD. 2011. Drug treatment of scrub typhus. Trop. Doct. 41:1–4. 10.1258/td.2010.100311 [DOI] [PubMed] [Google Scholar]
- 20.Goswami D, Hing A, Das A, Lyngdoh M. 2013. Scrub typhus complicated by acute respiratory distress syndrome and acute liver failure: a case report from Northeast India. Int. J. Infect. Dis. 17:e644–e645. 10.1016/j.ijid.2012.12.023 [DOI] [PubMed] [Google Scholar]
- 21.Lee CH, Lee JH, Yoon KJ, Hwang JH, Lee CS. 2012. Peritonitis in patients with scrub typhus. Am. J. Trop. Med. Hyg. 86:1046–1048. 10.4269/ajtmh.2012.11-0586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SJ, Lee YH, Jeong DW, Choi EJ, Kim YJ, Lee JG, Lee YH. 2012. A case of scrub typhus complicated by acute calculous cholecystitis. Korean J. Fam. Med. 33:243–246. 10.4082/kjfm.2012.33.4.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silpapojakul K, Ukkachoke C, Krisanapan S. 1991. Rickettsial meningitis and encephalitis. Arch. Intern. Med. 151:1753–1757. 10.1001/archinte.1991.00400090051010 [DOI] [PubMed] [Google Scholar]
- 24.Pai H, Sohn S, Seong Y, Kee S, Chang WH, Choe KW. 1997. Central nervous system involvement in patients with scrub typhus. Clin. Infect. Dis. 24:436–440. 10.1093/clinids/24.3.436 [DOI] [PubMed] [Google Scholar]
- 25.Kim DM, Kim YS, Cho HY, Lee YB. 2011. Scrub typhus meningoencephalitis occurring during doxycycline therapy for Orientia tsutsugamushi. Diagn. Microbiol. Infect. Dis. 69:271–274. 10.1016/j.diagmicrobio.2010.10.005 [DOI] [PubMed] [Google Scholar]
- 26.Vallejo-Maroto I, Garcia-Morillo S, Wittel MB, Stiefel P, Miranda M, Pamies E, Aparicio R, Carneado J. 2002. Aseptic meningitis as a delayed neurologic complication of murine typhus. Clin. Microbiol. Infect. 8:826–827. 10.1046/j.1469-0691.2002.00502.x [DOI] [PubMed] [Google Scholar]
- 27.Dotevall L, Hagberg L. 1989. Penetration of doxycycline into cerebrospinal fluid in patients treated for suspected Lyme neuroborreliosis. Antimicrob. Agents Chemother. 33:1078–1080. 10.1128/AAC.33.7.1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yim CW, Flynn NM, Fitzgerald FT. 1985. Penetration of oral doxycycline into the cerebrospinal fluid of patients with latent or neurosyphilis. Antimicrob. Agents Chemother. 28:347–348. 10.1128/AAC.28.2.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strickman D, Sheer T, Salata K, Hershey J, Dasch G, Kelly D, Kuschner R. 1995. In vitro effectiveness of azithromycin against doxycycline-resistant and -susceptible strains of Rickettsia tsutsugamushi, etiologic agent of scrub typhus. Antimicrob. Agents Chemother. 39:2406–2410. 10.1128/AAC.39.11.2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deppermann KM, Lode H, Hoffken G, Tschink G, Kalz C, Koeppe P. 1989. Influence of ranitidine, pirenzepine, and aluminum magnesium hydroxide on the bioavailability of various antibiotics, including amoxicillin, cephalexin, doxycycline, and amoxicillin-clavulanic acid. Antimicrob. Agents Chemother. 11:901–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaruratanasirikul S, Hortiwakul R, Tantisarasart T, Phuenpathom N, Tussanasunthornwong S. 1996. Distribution of azithromycin into brain tissue, cerebrospinal fluid, and aqueous humor of the eye. Antimicrob. Agents Chemother. 40:825–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peters DH, Friedel HA, McTavish D. 1992. Azithromycin: a review of its antimicrobial activity, pharmacokinetic properties and clinical efficacy. Drugs 44:750–799. 10.2165/00003495-199244050-00007 [DOI] [PubMed] [Google Scholar]