Abstract
Daptomycin-nonsusceptible vancomycin-resistant Enterococcus faecium (VRE) strains are a formidable emerging threat to patients with comorbidities, leaving few therapeutic options in cases of severe invasive infections. Using a previously characterized isogenic pair of VRE strains from the same patient differing in their daptomycin susceptibilities (Etest MICs of 0.38 mg/liter and 10 mg/liter), we examined the effect of ceftaroline, ceftriaxone, and ampicillin on membrane fluidity and susceptibility of VRE to surface binding and killing by daptomycin and human cathelicidin antimicrobial peptide LL37. Synergy was noted in vitro between daptomycin, ampicillin, and ceftaroline for the daptomycin-susceptible VRE strain, but only ceftaroline showed synergy against the daptomycin-nonsusceptible VRE strain (∼2 log10 CFU reduction at 24 h). Ceftaroline cotreatment increased daptomycin surface binding with an associated increase in membrane fluidity and an increase in the net negative surface charge of the bacteria as evidenced by increased poly-l-lysine binding. Consistent with the observed biophysical changes, ceftaroline resulted in increased binding and killing of daptomycin-nonsusceptible VRE by human cathelicidin LL37. Using a pair of daptomycin-susceptible/nonsusceptible VRE strains, we noted that VRE is ceftaroline resistant, yet ceftaroline confers significant effects on growth rate as well as biophysical changes on the cell surface of VRE that can potentiate the activity of daptomycin and innate cationic host defense peptides, such as cathelicidin. Although limited to just 2 strains, these finding suggest that additional in vivo and in vitro studies need to be done to explore the possibility of using ceftaroline as adjunctive anti-VRE therapy.
INTRODUCTION
Loss of susceptibility to daptomycin is an increasing concern among vancomycin-resistant Enterococcus faecium (VRE) (1). When faced with invasive infections by daptomycin-nonsusceptible VRE, clinicians have limited therapeutic options. Of great concern are the lack of a bactericidal agent, antibiotic-associated side effects such as linezolid-induced thrombocytopenia and quinupristin-dalfopristin (QD)-associated myalgias, and drug-drug interactions such as microsomal P450 effects of QD and serotonin syndrome concerns with linezolid and concomitant serotonin reuptake inhibitors. Therefore, a great need exists for infectious-disease physicians practicing in tertiary medical centers with patients at high risk for VRE infections, e.g., bone marrow and liver transplant recipients, to develop innovative pharmacotherapies to treat such patients (2, 3). The clinical dilemma faced by physicians treating these patients is further compounded by the facts that novel therapeutics targeting VRE are lacking and that single or combination antibiotics with in vitro activity against VRE have not been clinically validated by appropriate trials (4).
Our group has previously shown a perhaps counterintuitive effect of ampicillin in converting daptomycin from a bacteriostatic to a bactericidal antibiotic against an ampicillin-resistant bloodstream VRE, with successful clearance of refractory bacteremia by this organism using daptomycin plus ampicillin combination therapy (5). In this study, ampicillin also conferred increased susceptibility of VRE to killing by innate cationic host defense peptides (HDPs) such as cathelicidin LL37. To further explore the possible benefit of beta-lactams against VRE, we examined ampicillin and ceftaroline each in combination with daptomycin against a previously characterized daptomycin-nonsusceptible VRE strain and its isogenic parent strain (6).
MATERIALS AND METHODS
Bacterial strains and antimicrobial susceptibility testing.
Daptomycin-susceptible VRE strain 8019 and the isogenic daptomycin-nonsusceptible strain 5938 later isolated from the same patient were previously characterized phenotypically and genotypically (6). VRE strain 5938 was previously reported as having a daptomycin MIC of 192 mg/liter by Etest. However, upon retrieval of this isolate from the freezer for these studies, the daptomycin MIC by Etest was decreased to 10 mg/liter, consistent with the instability of daptomycin nonsusceptibility previously reported (7). Antimicrobial susceptibility testing was performed by using CLSI methods or the Epsilometer test according to the manufacturer's instructions (bioMérieux, Durham, NC) (8). Time-kill assays were performed in duplicate using an initial bacterial inoculum of 106 CFU/ml in Luria-Bertani broth (LB) supplemented with 50 mg/liter calcium, with antibiotic concentrations chosen to encompass readily achievable serum levels of each agent during clinical treatment regimens (9–13). Quantitative bacterial counts were determined at 0, 6, and 24 h of incubation at 37°C. LB was chosen because it yielded more consistent results than when using Mueller-Hinton broth.
Cell surface characterization studies.
The cell envelopes of the 8019 and 5938 strains were evaluated morphologically and structurally following overnight cultures with study strains in the presence and absence of antibiotics, ampicillin at 50 mg/liter or ceftaroline at 1 mg/liter. Transmission electron microscopy (TEM) was used to determine cell wall thickness and septation, and membrane fluidity was analyzed by fluorescence polarization using 6-diphenyl-1,3,5-hexatriene as previously described (6). Fluorescein isothiocyanate (FITC)-labeled poly-l-lysine (PLL) binding assays were performed using flow cytometry methods previously described (5). PLL is a polycationic molecule used to study the interactions of cationic peptides with charged bacterial envelopes. In this analysis, the extent of bacterial-bound FITC-labeled PLL reflects the relative net negative surface charge. A total of 10,000 events were counted and analyzed using a BD FACSCalibur system (Becton, Dickinson Labware, San Jose, CA). Data are expressed as mean relative fluorescent units ± standard deviation (SD). At least two independent experiments of triplicate samples were performed.
Daptomycin binding assays.
To determine if various beta-lactam antibiotics were able to impact the ability of daptomycin to bind to the VRE membrane, the organism was grown in LB at 37°C to an optical density at 600 nm (OD600) of 0.6 in the presence or absence of ampicillin at 40 mg/liter, ceftaroline at 5 mg/liter, or ceftriaxone at 20 mg/liter and then incubated for 20 min with Bodipy-fluorescein-labeled daptomycin (Bo-DAP; supplied by Cubist Pharmaceuticals, Lexington, MA). Concentrations of Bo-DAP used were 8 mg/liter for staining of the daptomycin-susceptible VRE and 16 mg/liter for staining of the daptomycin-nonsusceptible VRE. These concentrations of labeled daptomycin were established by pilot studies as optimal for fluorescence microscopy (data not shown). The activity of Bo-DAP has consistently shown an approximately 2-fold increased MIC compared to that of the unlabeled daptomycin molecule (MIC of 1 mg/liter for strain 8019 and 16 to 32 mg/liter for strain 5938). Excess unincorporated label was removed by washing the cells three times in LB. The cells were counterstained with 2 mg/liter 4′,6-diamidino-2-phenylindole (DAPI) in the final LB wash to visualize the nucleoid and then imaged using a Delta Vision deconvolution microscope (Applied Precision, Inc., Issaquah, WA) as previously described (5).
Human cathelicidin LL37 killing assays.
Human cathelicidin LL37 (net charge + 6 at pH 7.5) was purchased from AnaSpec, Inc. (Fremont, CA). The VRE strain was grown to stationary phase in LB either in the absence or presence of the different antibiotics, pelleted and washed in phosphate-buffered saline (PBS), and resuspended to an OD600 of 0.5 in PBS. Bacteria were diluted to 103 CFU/ml in RPMI and 5% LB containing 1× MICs of LL37 (2 μM for strain 8019 and 4 μM for strain 5938) and incubated at 37°C. Aliquots (10 μl) were plated on blood agar after 2 h of incubation, and colonies were enumerated after 24 h to quantify the percentage of surviving bacteria (±SD). Results represent three separate experiments performed in duplicate.
LL37 binding assays.
The VRE strains were grown in the presence or absence of antibiotics as in the Bo-DAP preparation steps described above, diluted 1:1 in phenol red-free RPMI 1640 medium (Invitrogen, Grand Island, NY) to a final volume of 200 μl with 6-carboxytetramethylrhodamine (TAMRA)-labeled LL37 (American Peptide, Sunnyvale, CA) to a final concentration of 4 μM, and incubated for 45 min at 37°C with shaking. Bacteria were pelleted, washed 3 times with RPMI, counterstained with 2 mg/liter DAPI in the final wash, and visualized using a DeltaVision deconvolution microscope (Applied Precision, Inc., Issaquah, WA).
Statistics.
Statistical evaluations of the differences in survival in the presence of various cationic peptides and differences in PLL binding were performed via Mann-Whitney U test (GraphPad Prism 5.0; GraphPad Software, Inc., San Diego, CA). Differences in cell wall thickness, membrane fluidity, and Bo-DAP or LL37 binding studies were evaluated by nonpaired t test. P values of <0.05 were considered statistically significant.
RESULTS
Daptomycin, vancomycin, and LL37 MIC activity against VRE, in combination with ceftaroline.
Daptomycin and vancomycin MICs were determined for VRE strains 8019 (daptomycin susceptible) and 5938 (daptomycin nonsusceptible) in the presence of increasing concentrations of ampicillin or ceftaroline, as well as selected concentrations of other beta-lactams. Both strains had MICs (mg/liter) in Mueller-Hinton broth as follows: ceftaroline, >32; ampicillin, >64; piperacillin, >64; ceftriaxone, >64; cefazolin, >64; linezolid, 2. Of interest, however, was that ceftaroline had appreciable effects on growth on both VRE strains, most notable of which was a dose-dependent decrease in maximal bacterial density (see Fig. S1 in the supplemental material). Morphologically, bacteria grown in the presence of ceftaroline also showed increased chain length (data not shown). Results in Table 1 show a decreased daptomycin MIC of VRE strain 5938 in the presence of ceftaroline across the concentration range expected in vivo after dosing 600 mg every 12 h (q12h). Minimal effects were seen for other beta-lactam agents at much higher concentrations.
TABLE 1.
Daptomycin and vancomycin MICs of strains 8019 (daptomycin susceptible) and 5938 (daptomycin resistant) in Mueller-Hinton agar plates containing various beta-lactams at selected concentrations by Etestc
| Beta-lactam (mg/liter)b | MIC (mg/liter) |
|||
|---|---|---|---|---|
| Strain 8019 |
Strain 5938 |
|||
| DAP | VAN | DAPa | VAN | |
| None | 0.38 | >256 | 10 | >256 |
| CPT (1) | 0.38 | >256 | 6 | >256 |
| CPT (2) | 0.38 | >256 | 2 | >256 |
| CPT (4) | 0.25 | >256 | 2 | >256 |
| CPT (8) | 0.19 | >256 | 0.75 | 24 |
| AMP (25) | 0.25 | >256 | 6 | >256 |
| AMP (50) | 0.25 | >256 | 6 | >256 |
| PIP (20) | 0.38 | >256 | 6 | >256 |
| CRO (20) | 0.38 | >256 | 6 | >256 |
| CZL (20) | 0.38 | >256 | 6 | >256 |
VRE strain 5938 has been previously reported as initially having a DAP MIC of 192 mg/liter by Etest. However, upon retrieval of this isolate from the freezer, the DAP MIC by Etest was decreased to 10 mg/liter, consistent with the instability of DAP nonsusceptibility previously reported.
CPT, ceftaroline; AMP, ampicillin; PIP, piperacillin; CRO, ceftriaxone; CZL, cefazolin; LIN, linezolid.
Mutation differences between isogenic strains 8019 and 5938 have been previously characterized by whole genome sequencing (6). MICs (mg/liter) of strains 8019 and 5938 to various antibiotics are as follows: CPT, >32; AMP, >64; PIP, >64; CRO, >64; CZL, >64; LIN, 2.
MICs to cathelicidin LL37 in RPMI-5% LB for strains 8019 and 5938 were 2 μM and 4 μM, respectively, consistent with tracking of the susceptibility of daptomycin to cationic antimicrobial peptides (14). No change in LL37 MIC was observed for strain 8019 in the presence of ceftaroline at 2 mg/liter or ampicillin at 50 mg/liter or for strain 5938 in ampicillin at 50 mg/liter. In ceftaroline at 2 mg/liter, the LL37 MIC decreased consistently (n = 4 experiments) to 2 μM for strain 5938.
Ceftaroline enhanced daptomycin killing of both daptomycin-susceptible and -nonsusceptible VRE.
In vitro kill curve studies were performed for VRE strains 8019 (Fig. 1, left) and 5938 (Fig. 1, right) by using daptomycin alone or in combination with ceftriaxone, ampicillin, or ceftaroline. The concentration of daptomycin utilized was below the MIC, whereas the concentration of the beta-lactam antibiotic was chosen to reflect concentrations achievable with standard dosing. Mean linezolid concentrations of 8 mg/liter expected with standard doses and 4× MIC were performed in parallel for comparison (13). At the concentrations chosen, growth was seen for daptomycin alone. Synergy was seen with ampicillin and ceftaroline against daptomycin-susceptible VRE 8019. However, for daptomycin-nonsusceptible strain 5938, synergy (e.g., 2 log10 reduction in CFU) was seen only with ceftaroline plus daptomycin. Ceftriaxone did not enhance daptomycin killing of either strain. Linezolid showed bacteriostatic activity at 8 mg/liter, with CFU counts at 6 and 24 h nearly identical to those in the starting inoculum.
FIG 1.
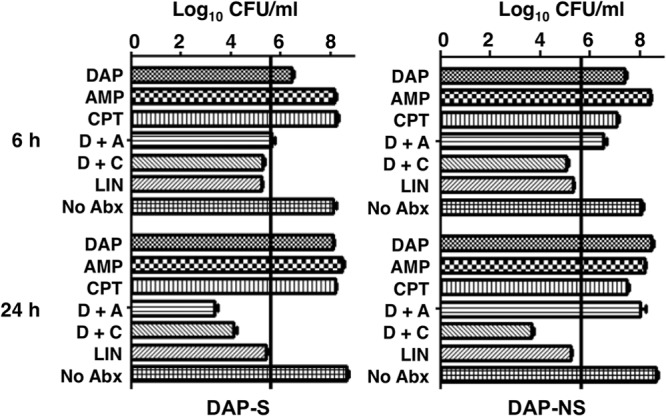
In vitro antibiotic killing assays performed in calcium-supplemented LB (50 mg/liter) for daptomycin-susceptible VRE strain 8019 (left) and daptomycin-nonsusceptible VRE strain 5938 (right) demonstrating log10 CFU/ml at 6 h (top) and 24 h (bottom). Vertical bar denotes the starting inoculum. Concentrations of antibiotics used were daptomycin at 1 mg/liter for the susceptible strain and 8 mg/liter for the nonsusceptible strain, ampicillin at 40 mg/liter, ceftaroline at 5 mg/liter, and linezolid at 8 mg/liter. Note only daptomycin plus ceftaroline resulted in a net killing of 2 log10 CFU/ml at 24 h against the daptomycin-nonsusceptible strain.
Ceftaroline alters cell wall thickness.
Growth of daptomycin-susceptible and -nonsusceptible VRE resulted in differences in cell wall thickness with subinhibitory antibiotic exposures, as shown in Fig. 2 (top left). There were no differences in cell wall thickness between the 2 strains grown in the absence of antibiotic. Against daptomycin-susceptible strain 8019, ampicillin and ceftaroline resulted in decreases in cell wall thickness, while the opposite was seen for daptomycin-resistant strain 5938. We had previously noted significant increases in cell division septum formation for strain 5938 compared to that for strain 8019 (6). Ceftaroline markedly reduced septum formation in strain 8019 (36% compared to 47%, P < 0.05, chi-square). Against strain 5938, no significant changes in septum formation were seen with ceftaroline (54%) compared to growth control (58%).
FIG 2.
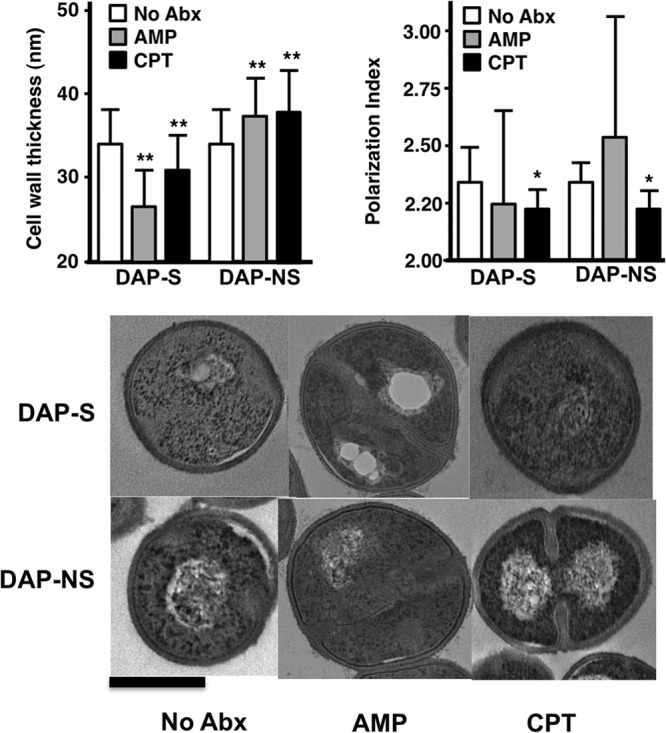
Cell wall thickness (top left; mean ± SD) and cell membrane polarization fluidity (top right; mean ± SD) in daptomycin-susceptible and -nonsusceptible VRE when exposed to subinhibitory concentrations of ampicillin at 50 mg/liter or ceftaroline at 1 mg/liter. Cell wall thickness measurements were performed on 100 to 140 cells for each group. The polarization index is expressed as degree of fluorescence polarization. Membrane fluidity is inversely corrected to polarization index. *, P < 0.05; **, P < 0.0001 compared to the control by unpaired t test. Bottom panels demonstrate representative electron micrographs of daptomycin-susceptible (top row) and daptomycin-nonsusceptible (bottom row) bacteria grown under different antibiotic conditions as shown. The dark bar at the bottom left denotes 500 nm.
Ceftaroline increases cell membrane fluidity.
Previous work has shown that daptomycin resistance in Enterococcus faecium and Enterococcus faecalis may be accompanied by decreases in membrane fluidity (6, 15). Interestingly, our previous characterization of these 2 strains showed no changes in this property between the strains under standard growth conditions (6). Growth of strain 5938 in ceftaroline at 1 mg/liter showed significant increases in membrane fluidity, as evidenced by mean polarization index changes by ceftaroline. For strain 8019, membrane fluidity was not significantly different (Fig. 2, top right).
Ceftaroline decreases net surface charge.
Strains 8019 and 5938 were grown in antibiotic-free or ceftaroline- or ampicillin-supplemented medium and subjected to PLL binding studies. As seen in Fig. 3, strain 5938 exhibited significantly less binding to PLL compared to that of strain 8019, as can be expected upon loss of daptomycin susceptibility. Against strain 8019, only ceftaroline but not ampicillin resulted in an increase in net negative surface charge. Against strain 5938, both ampicillin and ceftaroline resulted in increased PLL binding, reflective of increased negative surface charge on the bacterial surface.
FIG 3.
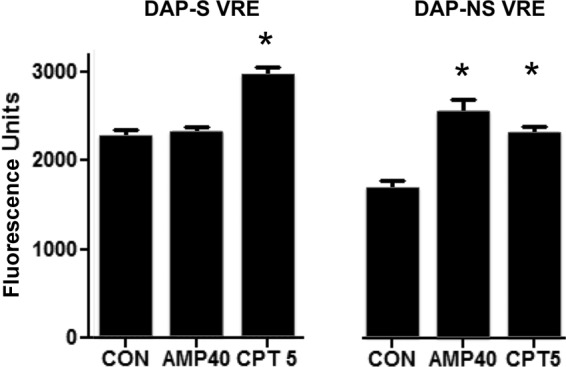
PLL binding of daptomycin-nonsusceptible and isogenic-susceptible VRE when grown in the presence of ampicillin (AMP) at 40 mg/liter or ceftaroline (CPT) at 5 mg/liter compared to the control (CON) without antibiotic. Increase binding is indicative of increase in negative charge. *, P < 0.05 compared to the control.
Ceftaroline increases cell surface daptomycin binding.
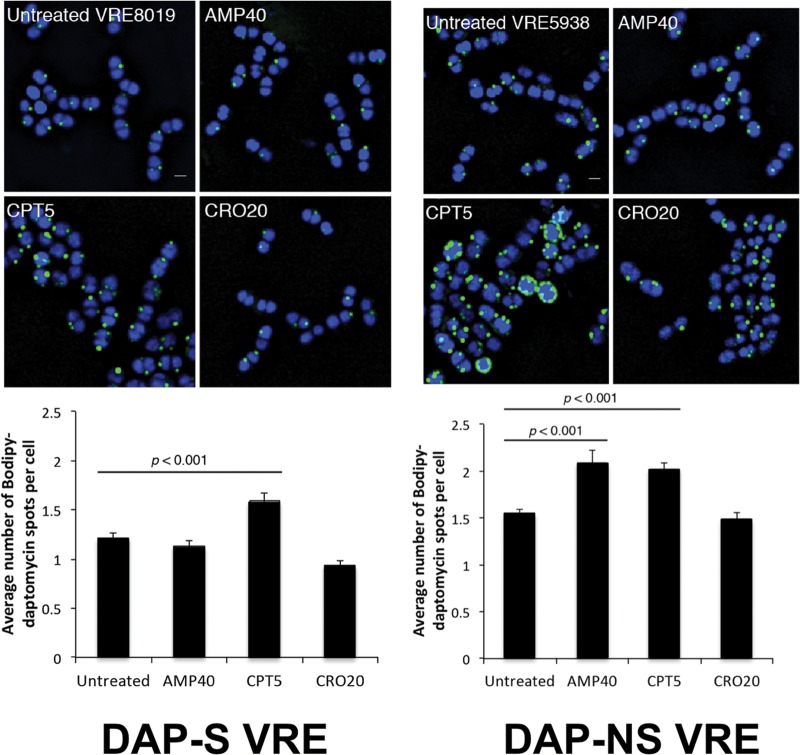
VRE strains 8019 and 5938 were labeled with 8 mg/liter Bo-DAP, demonstrating reduced relative binding of DAP for nonsusceptible strain 5938 compared to susceptible strain 8019 (see Fig. S2 in the supplemental material). To measure the effect of various antibiotics on Bo-DAP binding, VRE strains 8019 and 5938 were labeled with 8 mg/liter and 16 mg/liter Bo-DAP, respectively, after growth in LB containing no antibiotic, ampicillin at 40 mg/liter, ceftriaxone at 20 mg/liter, or ceftaroline at 5 mg/liter (Fig. 4). Daptomycin binding on the cell surface was significantly increased by ceftaroline for both strains, as evidenced by the number of binding foci/cell, without differences in intensity of the foci. Ampicillin increased the number of daptomycin binding foci only for strain 5938 and not for strain 8019. We tested ceftriaxone, as many clinicians might consider the convenience of once daily dosing, but found no effect to increase binding of daptomycin.
FIG 4.
Effects of ampicillin (AMP) at 40 mg/liter, ceftaroline (CPT) at 5 mg/liter, or ceftriaxone (CRO) at 20 mg/liter on daptomycin (DAP) binding using a bodipy-labeled daptomycin (Bo-DAP) molecule on daptomycin-susceptible (left) or daptomycin-nonsusceptible (right) VRE strains using 8 mg/liter or 16 mg/liter Bo-DAP, respectively. DAPI nucleoid staining was used as a counterstain. The labeled cells are seen in the top panels, and the quantitative statistics of number of DAP-binding foci per cell are shown below.
Ceftaroline increases killing of and binding to human cathelicidin LL37.
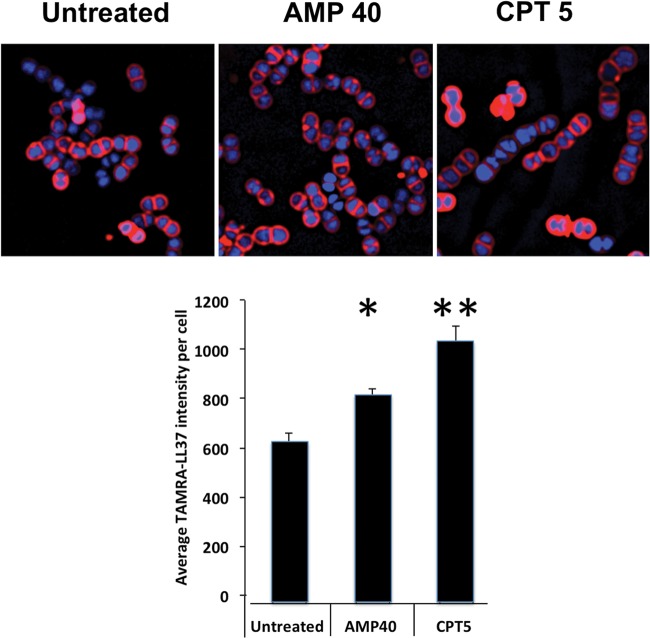
Given that daptomycin bound to calcium is a de facto antimicrobial peptide (16), examination of similarities or differences on the effects of ceftaroline or ampicillin on human cathelicidin LL37 killing of the two strains was of interest. Strains 8019 and 5938 were grown to stationary phase in LB media without antibiotic or with ampicillin at 40 mg/liter, ceftriaxone at 20 mg/liter, or ceftaroline at 5 mg/liter and exposed to LL37 killing assays at MICs for 2 h. Ceftaroline-treated VRE strains 8019 (Fig. 5, left) and 5938 (Fig. 5, right) were significantly more susceptible to LL37 killing than untreated controls. The concentration of ampicillin required to achieve comparable enhancement of LL37 killing was approximately 10-fold higher, at 50 mg/liter. Given that linezolid is the only approved therapy for VRE bloodstream infections, we also examined its ability at 8 mg/liter to sensitize VRE to LL37 and found that it also provided sensitization of strains 8019 and 5938 to LL37 killing (Fig. 5). Figure 6 shows that growth of VRE 5938 in ampicillin at 40 mg/liter or ceftaroline resulted in increased LL37 (4 μM) binding compared to that of cells grown in antibiotic-free medium. Quantitation shows significantly increasing intensity of binding/cell.
FIG 5.
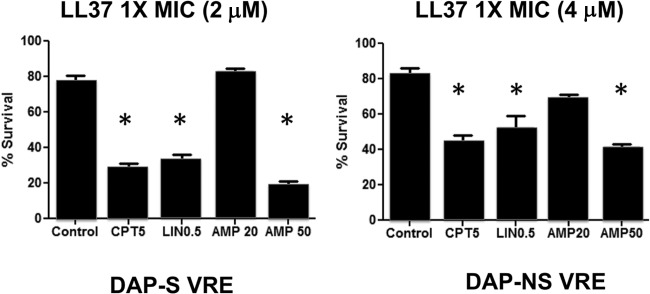
Human cathelicidin LL37 killing assays (2 h) at 1× MIC for daptomycin-susceptible (left) and -nonsusceptible (right) VRE strains after growth in media containing ampicillin (AMP), ceftaroline (CPT), or linezolid (LIN) at the indicated concentrations (mg/liter). *, P < 0.05 compared to the control.
FIG 6.
Effect of growth in media containing ampicillin at 40 mg/liter (AMP 40) or ceftaroline at 5 mg/liter (CPT 5) on binding of TAMRA-LL37 (4 µM) compared to the antibiotic-untreated cells for daptomycin-nonsusceptible VRE. The labeled cells are seen on the top panels, and the quantitative statistics of mean binding intensity/cell are shown below. *, P < 0.05; **, P < 0.01 compared to the control.
DISCUSSION
The lack of available therapies against daptomycin-nonsusceptible VRE requires innovative strategies to fulfill this need. We used a pair of previously characterized VRE strains that differ by their daptomycin susceptibility. We noted a very unstable phenotype in the daptomycin-nonsusceptible strain, consistent with what has been previously reported. Daptomycin susceptibility is driven in large part by differential expressions of various two-component regulatory systems influencing surface charge and other properties of the bacterial surface that are modulated based on environmental conditions (17). Consistent with this property, the daptomycin MIC can be readily increased in strain 5938 upon serial passage in daptomycin-containing media (unpublished observations).
The cephalosporin class of antibiotics has traditionally been considered inactive against E. faecium, which represents the majority of VRE species in which daptomycin nonsusceptibility has emerged. Of interest, however, we noted that ceftaroline was not inert against these 2 strains, as shown by considerable effects on growth, especially on the maximal bacterial density achieved after 24 h (see Fig. S1 in the supplemental material). We had also noted microscopically that ceftaroline results in significant chaining of the bacteria, with resultant lower CFU/OD600 than bacteria grown in antibiotic-free medium (data not shown). These phenotypes are part of other studies in our lab on the effects of beta-lactams on bacterial autolysins. We are currently investigating the differential effect of class A penicillin binding protein (PBP) inactivation on susceptibility to daptomycin and antimicrobial peptides and whether ceftaroline and/or ampicillin exert their effects on VRE through these targets (15). Additional evidence by others suggests that low-molecular-weight PBPs, such as ddcP, which influences d-alanine-d-alanine carboxypeptidase activity (16), warrant study as well, particularly with the possible tangential effects on vancomycin MIC by ceftaroline.
For daptomycin-nonsusceptible VRE strain 5938, daptomycin at 8 mg/liter, which would be close to the maximum free drug concentration in vivo (10), allows bacterial growth that is not different from growth control. Ceftriaxone showed no appreciable synergy with daptomycin against both daptomycin-susceptible and -nonsusceptible strains 8019 and 5938, respectively. Consistent with our prior data (5), ampicillin resulted in synergy with daptomycin against strain 8019, but this synergy was lost upon emergence of daptomycin resistance in strain 5938. Of great interest, however, is that ceftaroline showed synergy with daptomycin against both strains, achieving >2 log10 CFU killing at 24 h. This combination regimen was considerably more potent than linezolid, the only drug currently approved for VRE bacteremia (13), which was entirely bacteriostatic against both strains. Nevertheless, we did find that linezolid did sensitize VRE to killing by cathelicidin LL37, suggesting that in vivo the drug may yield greater activity than kill curves might suggest. Although not ideal, linezolid has been used to treat VRE bacteremia successfully and is approved for this indication (13).
In support of the in vitro findings of killing of daptomycin plus ceftaroline against VRE, we have shown that ceftaroline (i) increases daptomycin surface binding against daptomycin-susceptible and -nonsusceptible VRE, with parallel increases in poly-l-lysine binding suggesting an increase in the net negative charge, (ii) increases membrane fluidity for daptomycin-nonsusceptible VRE, a property that would be expected to increase susceptibility to daptomycin given that daptomycin-nonsusceptible strains show a decreased membrane fluidity phenotype, and (iii) sensitizes VRE to killing by the cationic antimicrobial peptide human cathelicidin LL37, via increased LL37 binding to the surface of VRE. A summary of the changes is presented in Table 2. Note that ampicillin and ceftaroline resulted in decreases in cell wall thickness in the daptomycin-susceptible strain and increases in the nonsusceptible strain (Table 2). Collectively, these data provide an interesting pattern on analysis. First, it appears that enhancement of daptomycin binding by ampicillin may not be consistent across VRE strains, as the daptomycin-susceptible strain in this study did not behave like the strain in our prior study (5). Nevertheless, it appears that marked decreases in cell wall thickness induced by ampicillin on this strain may somehow render the organism more daptomycin susceptible independent of daptomycin binding. Second, in the setting of increased cell wall thickness that occurs with both ceftaroline and ampicillin, a concomitant increase in membrane fluidity may be necessary to result in daptomycin synergy. Ampicillin, which failed to increase membrane fluidity, failed to show synergy with daptomycin against the daptomycin-nonsusceptible strain. Finally, increased daptomycin binding may not be necessary or sufficient for enhanced daptomycin activity. Daptomycin-susceptible VRE showed synergy with ampicillin plus daptomycin without an improvement in Bo-DAP or poly-l-lysine binding. On the other hand, increased Bo-DAP and poly-l-lysine by ampicillin in the daptomycin-nonsusceptible strain did not result in synergy.
TABLE 2.
Summary of changes induced on daptomycin-susceptible and -nonsusceptible VRE by ampicillin or ceftaroline
| Characteristic | Resulta |
|
|---|---|---|
| DAP-susceptible VRE | DAP-nonsusceptible VRE | |
| Synergy with DAP | ||
| AMP | + | − |
| CPT | + | + |
| Effect on cell wall thickness | ||
| AMP | ↓↓ | ↑ |
| CPT | ↓ | ↑ |
| Effect on membrane fluidity | ||
| AMP | − | − |
| CPT | − | ↑ |
| Poly-l-lysine binding | ||
| AMP | − | ↑ |
| CPT | ↑ | ↑ |
| Bodipy-DAP binding | ||
| AMP | − | ↑ |
| CPT | ↑ | ↑ |
| LL37 binding and activity | ||
| AMP | ↑ | ↑ |
| CPT | ↑↑ | ↑↑ |
+, yes; −, no; ↓↓, more-marked decrease; ↓, decrease; ↑↑, more-marked increase; ↑, increase.
These data are built upon an increasing line of evidence demonstrating that the in vitro effects of antibiotics are not fully appreciated by current methods of susceptibility testing. Specifically, beta-lactam antibiotics appear to have profound effects on beta-lactam-resistant Gram-positive organisms. Similar to the effects on VRE by ampicillin that we previously discussed (5), we have shown that antistaphylococcal beta-lactams increase susceptibility of methicillin-resistant Staphylococcus aureus (MRSA) to daptomycin and innate immunity (14, 18). Not all beta-lactams are equal in this effect, as PBP-1 binding beta-lactams are better at potentiating daptomycin activity than non-PBP-1-specific beta-lactams (19).
The development of daptomycin nonsusceptibility appears to be multifactorial and complex and, therefore, effects of drugs that restore its susceptibility would be expected to be equally complex (5, 20–22). Daptomycin-nonsusceptible enterococci are different from their susceptible counterparts in sensing cell wall stress (via liaFSR) (15, 16, 18), phospholipid composition (via cls) (20–22), and increased proclivity to septation (via ezrA) (6). While we did not perform analysis of phospholipid content with ceftaroline therapy, we noticed changes in septation and cell wall thickness induced by ceftaroline. Perhaps ceftaroline, similar to what has been observed with ceftobiprole (23), binds PBP5 and alters the surface physiology to allow enhanced daptomycin and HDP activity.
It is important to point out that these data are limited to a single daptomycin-susceptible/nonsusceptible pair of VRE strains. As it is well appreciated that clinical strains are quite heterogeneous, additional in vitro and in vivo studies are needed to explore the effects of ceftaroline on VRE as potential adjunctive therapy and to determine whether all the changes we observed in these strains are generalizable to other clinical VRE strains. Several of us are currently exploring in vitro PK/PD modeling of ceftaroline combination therapy against other VRE strains, while others are completing checkerboard studies of daptomycin plus various beta-lactams, including ceftaroline, against a larger collection of VRE strains, some of which are daptomycin nonsusceptible. While we did not have the opportunity to employ this combination clinically, we did recently publish a report showing in vitro synergy using daptomycin and ceftaroline in the successful treatment of E. faecalis aortic valve endocarditis (24). Optimal dosing of daptomycin or ceftaroline, should a clinician choose this combination, remains to be determined, although we might suggest 8 to 10 mg/kg of body weight/day of daptomycin given prior studies showing increased efficacy of higher doses compared to that of ≤6 mg/kg/day (25).
Supplementary Material
ACKNOWLEDGMENTS
Ceftaroline powder was obtained from Forest Pharmaceuticals. The research described in the manuscript was conducted at the sole discretion of the authors without the knowledge or support of Forest Laboratories, Inc., Forest Research Institute, Inc., or Cerexa, Inc.
G.S. has received speaking honoraria from Cubist, Forest, and Pfizer Pharmaceuticals, consulting fees from Cubist and Forest Pharmaceuticals, and research grant support from Forest Pharmaceuticals. W.R. has received grant funding and speaking honoraria from Cubist and consults for The Medicines Company. R.H. has received research funding from Cubist. V.N. is on the Scientific Advisory Board of Trius Therapeutics.
Funding for this research was provided by U54 HD071600-01 09/26/2011-06/30/2016 NICHD on Developmental and Translational Pharmacology of Pediatric Antimicrobial Therapy (G.S. and V.N.), the Great Lakes Regional Center for Excellence in Biodefense and Emerging Infectious Disease Research (AI057153; V.N.), and R01GM073898 (J.P.). No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published ahead of print 23 December 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02274-13.
REFERENCES
- 1.Kelesidis T, Humphries Uslan DZ, Pegues DA. 2011. Daptomycin nonsusceptible enterococci: an emerging challenge for clinicians. Clin. Infect. Dis. 52:228–234. 10.1093/cid/ciq113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orloff SL, Busch AM, Olyaei AJ, Corless CL, Benner KG, Flora KD, Rosen HR, Rabkin JM. 1999. Vancomycin-resistant Enterococcus in liver transplant patients. Am. J. Surg. 177:418–422. 10.1016/S0002-9610(99)00083-5 [DOI] [PubMed] [Google Scholar]
- 3.Kamboj M, Chung D, Seo SK, Pamer EG, Sepkowitz KA, Jakubowski AA, Papanicolaou G. 2010. The changing epidemiology of vancomycin-resistant Enterococcus (VRE) bacteremia in allogeneic hematopoietic stem cell transplant (HSCT) recipients. Biol. Blood Marrow Transplant. 16:1576–1581. 10.1016/j.bbmt.2010.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arias CA, Contreras GA, Murray BE. 2010. Management of multidrug-resistant enterococcal infections. Clin. Microbiol. Infect. 16:555–562. 10.1111/j.1469-0691.2010.03214.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakoulas G, Bayer AS, Pogliano J, Tsuji BT, Yang SJ, Mishra NN, Nizet V, Yeaman MR, Moise PA. 2012. Ampicillin enhances daptomycin- and cationic host defense peptide-mediate killing of ampicillin and vancomycin-resistant Enterococcus faecium. Antimicrob. Agents Chemother. 56:838–844. 10.1128/AAC.05551-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Humphries RM, Kelesidis T, Tewhey R, Rose WE, Schork N, Nizet N, Sakoulas G. 2012. Genotypic and phenotypic evaluation of the evolution of high-level daptomycin non-susceptibility in vancomycin-resistant Enterococcus faecium. Antimicrob. Agents Chemother. 56:6051–6053. 10.1128/AAC.01318-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rose WE, Leonard SN, Sakoulas G, Kaatz GW, Zervos MM, Sheth A, Carpenter CF, Rybak MJ. 2008. Daptomycin activity against Staphylococcus aureus following vancomycin exposure in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 52:831–836. 10.1128/AAC.00869-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CLSI 2013. Performance standards for antimicrobial susceptibility testing; 23rd informational supplement. CLSI document M100-S23. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 9.Forest Laboratories Inc 10 October 2012, accession date Ceftaroline fosamil package insert. Forest Laboratories Inc., New York, NY: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/200327s000lbl.pdf [Google Scholar]
- 10.Dvorchik BH, Brazier D, DeBruin MF, Arbeit RD. 2003. Daptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjects. Antimicrob. Agents Chemother. 47:1318–1323. 10.1128/AAC.47.4.1318-1323.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foulds G. 1983. Pharmacokinetics of sulbactam/ampicillin in humans: a review. Rev. Infect. Dis. 8(Suppl 5):S503–S511 [DOI] [PubMed] [Google Scholar]
- 12.Patel IH, Chen S, Parsonnet Hackman MR, Brooks MA, Konikoff J, Kaplan SA. 1981. Pharmacokinetics of ceftriaxone in humans. Antimicrob. Agents Chemother. 20:634–641. 10.1128/AAC.20.5.634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dryden MS. 2011. Linezold pharmacokinetics and pharmacodynamics in clinical treatment. J. Antimicrob. Chemother. 66(Suppl 4):iv7–iv15 [DOI] [PubMed] [Google Scholar]
- 14.Dhand A, Bayer AS, Pogliano J, Yang SJ, Bolaris M, Nizet V, Wang G, Sakoulas G. 2011. Use of antistaphylococcal beta-lactams to increase daptomycin activity in eradicating persistent bacteremia due to methicillin-resistant Staphylococcus aureus: role of daptomycin binding. Clin. Infect. Dis. 53:158–163. 10.1093/cid/cir340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rice LB, Carias LL, Rudin S, Hutton R, Marshall S, Hassan M, Josseaume N, Dubost L, Marie A, Arthur M. 2009. Role of class A penicillin-binding proteins in the expression of beta-lactam resistance in Enterococcus faecium. J. Bacteriol. 191:3649. 10.1128/JB.01834-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Paganelli FL, Bierschenk D, Kuiper A, Bonten MJM, Willems RJL, van Schaik W. 2012. Genome-wide identification of ampicillin resistance determinants in Enterococcus faecium. PLoS Genet. 8(6):e1002804. 10.1371/journal.pgen.1002804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawada-Matsuo M, Yoshida Y, Nakamura N, Konatsuzawa H. 2011. Role of two-component systems in the resistance of Staphylococcus aureus to antibacterial agents. Virulence 2:427–430. 10.4161/viru.2.5.17711 [DOI] [PubMed] [Google Scholar]
- 18.Sakoulas G, Okumura CY, Thienphrapa, Olson J, Nonejuie P, Dam Q, Dhand A, Pogliano J, Yeaman MR, Hensler ME, Bayer AS, Nizet V. 3 December 2013. Nafcillin enhances innate immune-mediated killing of methicillin-resistant Staphylococcus aureus. J. Mol. Med. 10.1007/s00109-013-1100-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berti A, Sakoulas G, Nizet V, Tewhey R, Rose WE. 2013. β-Lactam antibiotics targeting PBP1 selectively enhance daptomycin activity against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 57:5005–5012. 10.1128/AAC.00594-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mishra NN, Bayer AS, Tran TT, Shamoo Y, Mileykovskaya E, Dowhan W, Guan Z, Arias CA. 2012. Daptomycin resistance in enterococci is associated with distinct alterations of cell membrane phospholipid content. PLoS One 7(8):e43958. 10.1371/journal.pone.0043958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munita JM, Panesso D, Diaz L, Tran TT, Reyes J, Wanger A, Murray BE, Arias CA. 2012. Correlation between mutations in liaFSR of Enterococcus faecium and MIC of daptomycin: revisiting daptomycin breakpoints. Antimicrob. Agents Chemother. 56:4354–4359. 10.1128/AAC.00509-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arias CA, Panesso D, McGrath DM, Qin X, Mojica MF, Miller C, Diaz L, Tran TT, Rincon S, Barbu EM, Reyes J, Roh JH, Lobos E, Sodergren E, Pasqualini R, Arap W, Quinn JP, Shamoo Y, Murray BE, Weinstock GM. 2011. Genetic basis for in vivo daptomycin resistance in enterococci. N. Engl. J. Med. 365:892–900. 10.1056/NEJMoa1011138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xavier H, Amoroso A, Coyette J, Joris B. 2010. Interaction of ceftobiprole with the low-affinity PBP 5 of Enterococcus faecium. Antimicrob. Agents Chemother. 54:953–955. 10.1128/AAC.00983-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakoulas G, Noneluie P, Nizet V, Pogliano J, Crum-Cianflone N, Haddad F. 2013. Treatment of high-level gentamicin-resistant Enterococcus faecalis endocarditis with daptomycin plus ceftaroline. Antimicrob. Agents Chemother. 57:4042–4045. 10.1128/AAC.02481-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King EA, McCoy D, Desai S, Nyirenda T, Bicking K. 2011. Vancomycin-resistant Enterococcal bacteremia and daptomycin: are higher doses necessary? J. Antimicrob. Chemother. 66:2112–2118. 10.1093/jac/dkr255 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.