Abstract
Community-acquired pneumonia (CAP) is an important childhood health problem. Penicillin remains appropriate for treating children with CAP. Clinical data are lacking on disease evolution in children treated with different posologic schemes of aqueous penicillin G. To assess if there were differences in disease evolution between children with CAP treated with 6 or 4 daily doses of aqueous penicillin G, we reviewed the medical charts of hospitalized patients 2 months to 11.5 years of age. Pneumonia was radiologically confirmed based on the detection of pulmonary infiltrate or pleural effusion on the chest radiograph taken on admission and read by a pediatric radiologist blinded to the clinical data. The total daily dose of aqueous penicillin G was 200,000 IU/kg of body weight. Data were recorded on admission, during disease evolution up to the 7th day of treatment, and at the final outcome. The results of hospitalization and the daily frequency of physical signs suggestive of pneumonia were assessed. The subgroups comprised 120 and 144 children who received aqueous penicillin G in 6 or 4 daily doses, respectively. Children ≥5 years of age were more frequent in the 4-daily-doses subgroup (16.0% versus 4.2%; respectively, P = 0.02). There were no differences between the compared subgroups in terms of final outcomes, lengths of hospitalization, durations of aqueous penicillin G use, frequencies of aqueous penicillin G substitution, or daily frequencies of tachypnea, fever, chest retraction, lower chest recession, nasal flaring, and cyanosis up to the 7th day of treatment. The studied posologic regimens were similarly effective in treating children hospitalized with a radiologically confirmed CAP diagnosis. Aqueous penicillin G (200,000 IU/kg/day) may be given in 4 daily doses to children with CAP.
INTRODUCTION
Community-acquired pneumonia (CAP) is an important childhood health problem that accounts for approximately 1.396 million child deaths (1) and 154 million cases (2, 3) annually among children under 5 years old around the world. In clinical practice, it is not routinely possible to identify the etiological agent of the disease (4). Based on a wide range of evidence, Streptococcus pneumoniae is recognized as the most common bacterial agent of CAP (5). This is the rationale behind choosing antibiotics that target this pathogen (4). That is why penicillin and its derivatives remain the appropriate antimicrobial agents to treat children with CAP (6). When hospitalization is required, the first-line drug is intravenous aqueous penicillin G (7).
In the 1990s, pneumococcal resistance to penicillin had been widely recognized as a potential problem but the increased dose of penicillin was shown to be effective in overcoming this problem as long as the infection did not affect the central nervous system (8). This finding may be explained by the time-dependent killing characteristic of penicillin (9). That is, the period of time during which the level of penicillin in serum is higher than the MIC is predictive of the therapeutic efficacy (10). Regarding pneumococcal CAP, the penicillin dosing regimen should provide a high enough serum concentration to exceed the pneumococcal MIC for 40 to 50% of the dosing interval (9). Classically, aqueous penicillin G was recommended to be given in 6 daily doses (11). However, its use in 4 daily doses is easier, cheaper, and more comfortable (12). To the best of our knowledge, there is no clinical evidence of comparisons of the equivalency of aqueous penicillin G divided into 6 or 4 daily doses among children with CAP. We aimed to study this research question.
MATERIALS AND METHODS
A retrospective study of children hospitalized with CAP between January 1998 and October 2005 was conducted at University Hospital in Salvador (northeast Brazil). The hospital admittance log book was reviewed, and the medical charts of all CAP cases were reviewed. Children ≥2 months old who were treated with aqueous penicillin G (daily dose of 200,000 IU/kg of body weight) for at least 48 h and who had a chest radiograph taken on admission were included. Children with chronic debilitating illnesses, immunodeficiency, severe malnutrition, other concomitant infections, or nosocomial pneumonia from other hospitals were excluded.
Every chest radiograph was read by a pediatric radiologist blinded to the clinical information for the purpose of this study. CAP was confirmed whenever a pulmonary infiltrate or pleural effusion was described. This radiographic reading was performed in accordance with the standardized interpretation recommended by the World Health Organization (13). The data retrieved from the medical charts included age, gender, and complaints and physical signs suggestive of pneumonia on admission and during daily evolution up to the 7th day of treatment, in addition to final outcome, discharge date, and other therapeutic items which were used during the hospital stay. All data were registered in a predefined form. Data regarding the highest grade of axillary temperature and respiratory rate (RR) found in the medical chart were collected.
For the purpose of analysis, an axillary temperature of ≥37.5°C was defined as fever (14); RRs of ≥50 breaths/min among children <12 months old or ≥40 breaths/min among children ≥12 months old (15) or of ≥30 in children ≥60 months old (16) were defined as tachypnea. The software Anthro, versions 1.02 and 3.22 (CDC and WHO), was used to perform the nutritional evaluation in accordance with the National Centre for Health Statistics, United States standard (17). A z score under −3.00 for the weight-for-age index defined severe malnutrition. As part of the analysis, the British Thoracic Society (BTS) guidelines were used to assess severity retrospectively, and they included RR >70 breaths/min for infants, RR >50 breaths/min for older children, difficulty in breathing, nasal flaring, cyanosis, grunting when calm, and lower chest recession (18). The assignment of severity and the measures of clinical course and final outcome were based on available records.
The study group was divided according to the use of a posologic regimen of aqueous penicillin G of 6 or 4 daily doses. This division was feasible because the recommended routine therapeutic management of hospitalized children with CAP in the Federal University of Bahia Hospital had changed over time. Up to 2001, the usual treatment was aqueous penicillin G in 6 daily doses, and from 2002 on, it has been aqueous penicillin G in 4 daily doses. No other changes in the hospital policy regarding antimicrobial stewardship, supportive therapy, or discharge occurred at the hospital during the study period. The subgroups were compared on admission and during disease evolution while aqueous penicillin G was given. Primary outcomes comprised death, transference to the intensive care unit, and length of hospital stay. Categorical variables were compared by using chi-square or Fisher's exact tests as appropriate; continuous variables were assessed by using the Student t test or Mann-Whitney U test, taking into account the variable distribution. The statistical tests were two tailed and the software SPSS (version 9.0) was used for analysis. The study was approved by the ethics committee of the Federal University of Bahia Hospital (082/9).
RESULTS
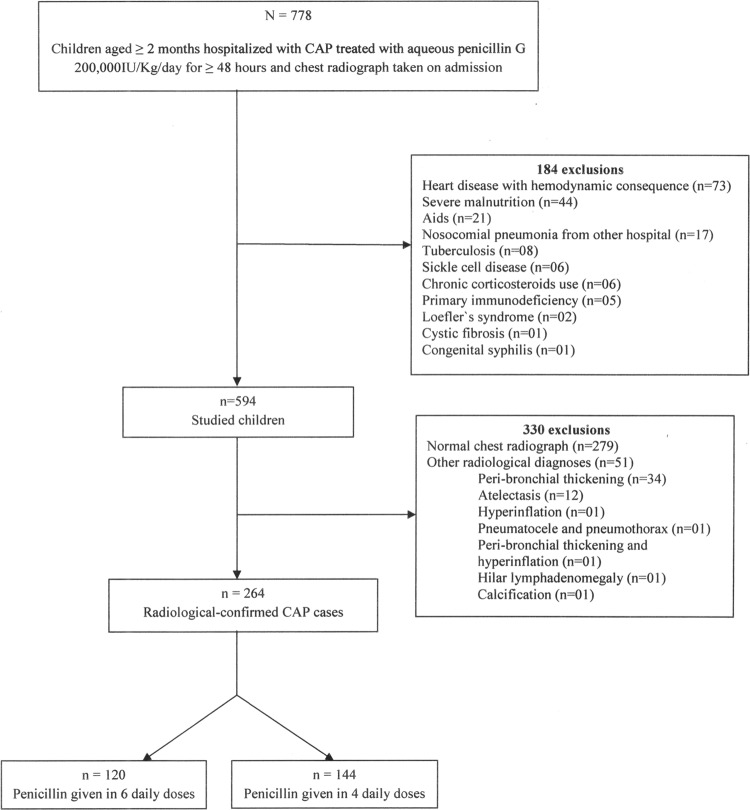
Overall, 778 children fulfilled the inclusion criteria, of which 264 (34.8%) were eligible. Figure 1 shows the flow chart of the study, with the excluded cases. Overall, 157 (59.5%) patients were males and the median age was 20 months (25th to 75th percentiles, 11 to 40; range, 2 months to 11.5 years). When aqueous penicillin G was started, the most common complaints were fever (94.7%), cough (87.1%), and respiratory discomfort (44.7%), and the most frequent findings were tachypnea (79.0%), fever (59.6%), crackles (44.3%), wheezing (31.4%), chest retraction (30.3%), and lower chest recession (27.7%). The study group comprised 120 (45.5%) and 144 (54.5%) children who received aqueous penicillin G in 6 or 4 daily doses, respectively (Fig. 1). The baseline characteristics of the children, the physical examination results, and the severity assessment of disease are shown in Table 1. No children presented with grunting. The radiological findings are presented in Table 2.
FIG 1.
Flow chart of the step-by-step selection of children hospitalized with community-acquired pneumonia (CAP) treated with aqueous penicillin G in 6 or 4 daily doses.
TABLE 1.
Baseline characteristics of children hospitalized with community-acquired pneumonia and treated with different posologic regimens of aqueous penicillin G in a university hospital in Salvador, Brazil
| Child characteristics | No. (%) of children receiving daily posologic penicillin regimen of: |
P | |
|---|---|---|---|
| 6 doses (n = 120) | 4 doses (n = 144) | ||
| Male gender | 70 (58.3) | 87 (60.4) | 0.7 |
| Age | |||
| 2–11 mo | 41 (34.1) | 36 (25.0) | 0.1b |
| 1–4 yr | 74 (61.7) | 85 (59.0) | 0.7b |
| ≥5 yr | 5 (4.2) | 23 (16.0) | 0.002b |
| Assessment on admission | |||
| Tachypneaa | 70/90 (77.8) | 92/115 (80.0) | 0.7 |
| Fevera | 69/105 (65.7) | 74/135 (54.8) | 0.09 |
| Chest retraction | 34 (28.3) | 46 (31.9) | 0.5 |
| Severity (BTS)c | |||
| Severe CAP | 78 (65.0) | 92 (63.9) | 0.9 |
| Difficulty breathing | 47 (39.2) | 60 (41.7) | 0.7 |
| RR, >70 breaths/mina | 9/33 (27.3) | 7/30 (23.3) | 0.7 |
| RR, >50 breaths/mina | 31/57 (54.4) | 37/85 (43.5) | 0.2 |
| Lower chest recession | 34 (28.3) | 39 (27.1) | 0.8 |
| Nasal flaring | 4 (3.3) | 10 (6.9) | 0.2 |
| Cyanosis | 3 (2.5) | 1 (0.7) | 0.3 |
Numerators indicate the number of children with the finding and denominators indicate the total number of children examined for the finding. The information was missing for some children, which is why the numerators differ.
The frequencies in each age group were compared in the posologic regimens subgroups in a bivariate analysis.
BTS, British Thoracic Society; RR, respiratory rate.
TABLE 2.
Radiological findings of children hospitalized with community-acquired pneumonia and treated with different posologic regimens of aqueous penicillin G in a university hospital in Salvador, Brazil
| Radiological findings | No. (%) of children receiving daily posologic penicillin regimen of: |
P | |
|---|---|---|---|
| 6 doses (n = 120) | 4 doses (n = 144) | ||
| Presence of pulmonary infiltrate | 112 (93.3) | 113 (78.5) | 0.001 |
| Classification of pulmonary infiltratea | |||
| Alveolar | 106/112 (94.6) | 109/113 (96.5) | 0.5 |
| Interstitial | 5/112 (4.5) | 1/113 (0.9) | 0.1 |
| Interstitial-alveolar | 1/112 (0.9) | 3/113 (2.6) | 0.6 |
| Atelectasis | 9 (7.5) | 6 (4.2) | 0.2 |
| Peribronchial thickening | 1 (0.8) | 5 (3.5) | 0.2 |
| Alveolar consolidation | 2 (1.7) | 2 (1.4) | 1.0 |
| Pleural effusion | 15 (12.5) | 39 (27.1) | 0.003 |
Numerators indicate the number of children with the finding and denominators indicate the total number of children examined for the finding. The information was missing for some children, which is why the numerators differ.
Overall, the median length of hospital stay was 7 days (25th to 75th percentiles, 5 to 11; range, 2 to 59 days), and no patient died or was transferred to the intensive care unit. Moreover, every patient was discharged after improvement. The median length of aqueous penicillin G use was 4 days (25th to 75th percentiles, 3 to 6; range, 2 to 17 days). When children treated with aqueous penicillin G given in 6 or 4 daily doses were compared, the medians of hospitalization lengths in days were 7 days (25th to 75th percentiles, 5 to 12; range, 2 to 59 days) and 7.5 days (25th to 75th percentiles, 5 to 11; range, 2 to 31 days) (P = 0.6), respectively. There were no differences between the subgroups in the duration of aqueous penicillin G use (data not shown).
Among 204 (77.3%) cases, the initial treatment with aqueous penicillin G was changed to oral amoxicillin as a step-down therapy. Aqueous penicillin G was substituted for other antibiotics in 60 (22.7%) patients, of whom 2 (3.3%) patients received aqueous penicillin G initially in 6 daily doses, and this posologic regimen was changed to 4 daily doses after 2 days of treatment, at the discretion of the assistant pediatrician. The subsequent antibiotics were ceftriaxone (n = 15; 25%), ceftriaxone plus oxacillin (n = 12; 20%), oxacillin (n = 9; 15%), oxacillin plus amikacin and erythromycin (n = 5; 8.3% each), chloramphenicol (n = 4; 6.7%), ceftriaxone plus oxacillin plus amikacin (n = 3; 5%), cephalothin (n = 2; 3.3%), and azithromycin, cefotaxime, and trimethoprim-sulfamethoxazole (n = 1; 1.7% each). No differences were detected in the frequencies of aqueous penicillin G substitutions in children in whom aqueous penicillin G was divided into 6 or 4 daily doses (27.5% versus 18.8%; P = 0.09). This finding was not modified by stratified analysis by age (data not shown). Children in whom aqueous penicillin G was substituted for another antibiotic besides amoxicillin were excluded from the comparative analysis regarding daily clinical findings during evolution. No differences were found in the daily frequencies of tachypnea, fever, chest retraction, lower chest recession, nasal flaring, and cyanosis up to the 7th day of treatment (Table 3), when no children presented grunting.
TABLE 3.
Frequency of clinical findings among children hospitalized with community-acquired pneumonia and treated with aqueous penicillin G in 6 or 4 daily doses
| Clinical finding | Daily frequency (%) of indicated finding ona: |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0b |
Day 1 |
Day 2 |
Day 3 |
Day 4 |
Day 5 |
Day 6 |
Day 7 |
|||||||||
| 6 daily doses | 4 daily doses | 6 daily doses | 4 daily doses | 6 daily doses | 4 daily doses | 6 daily doses | 4 daily doses | 6 daily doses | 4 daily doses | 6 daily doses | 4 daily doses | 6 daily doses | 4 daily doses | 6 daily doses | 4 daily doses | |
| Tachypnea | 77.8 (70/90) | 80.0 (92/115) | 69.2 (63/91) | 61.7 (71/115) | 56.5 (61/108) | 51.2 (64/125) | 54.5 (54/99) | 52.4 (55/105) | 50.0 (36/72) | 46.7 (35/75) | 40.4 (19/47) | 35.7 (20/56) | 33.3 (12/36) | 37.5 (15/40) | 33.3 (8/24) | 14.8 (4/27) |
| Fever | 65.7 (69/105) | 54.8 (74/135) | 36.3 (41/113) | 37.7 (43/114) | 30.2 (35/116) | 38.5 (50/130) | 29.0 (31/107) | 23.9 (28/117) | 24.7 (20/81) | 27.1 (23/85) | 19.2 (10/52) | 17.7 (11/62) | 9.8 (4/41) | 12.2 (6/49) | 8.3 (2/24) | 6.3 (2/32) |
| Chest retraction | 28.3 (34/120) | 31.9 (46/144) | 20.0 (24/120) | 15.3 (22/144) | 13.3 (16/120) | 11.1 (16/144) | 13.8 (15/109) | 7.4 (9/121) | 6.1 (5/82) | 5.6 (5/89) | 7.5 (4/53) | 1.5 (1/65) | 2.3 (1/43) | 0 | 4.2 (1/24) | 0 |
| Lower chest recession | 28.3 (34/120) | 27.1 (39/144) | 15.8 (19/120) | 11.1 (16/144) | 10.8 (13/120) | 10.4 (15/144) | 10.1 (11/109) | 6.6 (8/121) | 14.6 (12/82) | 10.1 (9/89) | 9.4 (5/53) | 1.5 (1/65) | 4.7 (2/43) | 0 | 4.2 (1/24) | 3.0 (1/33) |
| Nasal flaring | 3.3 (4/120) | 6.9 (10/144) | 3.3 (4/120) | 2.1 (3/144) | 2.5 (3/120) | 0.7 (1/144) | 1.8 (2/109) | 0.8 (1/121) | 1.2 (1/82) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cyanosis | 2.5 (3/120) | 0.7 (1/144) | 0.8 (1/120) | 0.7 (1/144) | 0 | 0.7 (1/144) | 0.9 (1/109) | 0 | 1.2 (1/82) | 1.1 (1/89) | 1.9 (1/53) | 0 | 0 | 0 | 4.2 (1/24) | 0 |
Values in parentheses indicate the number of children with the finding (numerator) and the total number of children examined for the finding (denominator). The denominator changed day by day as children were discharged.
Day 0 was the day when aqueous penicillin G treatment was initiated.
Supportive therapy provided on admission included rapid-acting inhaled bronchodilator (60.2%), intravenous hydration (saline solution plus 5% dextrose in water [1:4]) (58.7%), antipyretic drugs (58.3%), systemic corticosteroids (23.5%), oxygen (5.3%), and electrolyte therapy (4.2%). The only detected difference was higher frequency of intravenous hydration in the 4-daily-doses subgroup (66% versus 50%; P = 0.009).
DISCUSSION
This investigation demonstrates that children with radiologically confirmed CAP treated with 200,000 IU/kg/day of aqueous penicillin G in 6 or 4 daily doses presented similar outcomes. Therefore, our data depict similarity of the effectiveness of the studied posologic regimens through several indicators, such as similar durations of aqueous penicillin G use or of hospitalization and similar frequencies of antibiotic substitution, in addition to no death or intensive care unit transference in any of the studied subgroups. In a multicenter, prospective observational study in children with severe CAP due to S. pneumoniae, the use of 200,000 IU/kg/day of aqueous penicillin G in 4 daily doses was proposed and good effectiveness of this posologic regimen was demonstrated (19). The rationale for the use of 200,000 IU/kg/day of aqueous penicillin G was to overcome penicillin resistance in pneumococcal CAP by increasing the dose and, therefore, increasing the duration of the serum concentration of penicillin above the MIC (10, 20). The administration in 4 daily doses was based on a more comfortable posologic regimen, with lower administration costs, better use of human resources, and reduction of the daily use of equipment because of fewer daily procedures performed on the patients.
Nonetheless, the compared subgroups were slightly different on admission. Of note, children ≥5 years old were more frequently placed in the 4-daily-doses subgroup (16.0% versus 4.2%; P = 0.02) (Table 1). Significant differences were also found in the frequencies of pulmonary infiltrate and pleural effusion (Table 2), which points to higher frequencies of pleural effusion in the 4-daily-doses subgroup. That is, the frequency of pleural effusion among children hospitalized with CAP increased over time. A sharp increase from 18 to 43 episodes of CAP with pleural effusion per 100,000 children under 5 years old has been registered in Spain (21). This finding has also been described in other countries, and it has been linked with the spread of some serotypes of S. pneumoniae (22). Therefore, one can infer that the increase in the number of pleural effusion episodes reported herein was due to increases in pneumococcal infection in children with CAP, which was appropriately treated with both posologic regimens. That is why, despite the increase in pleural effusion frequency in one subgroup, no difference in outcome between subgroups was observed. It is possible also to infer that despite this fact, no difference in empyema development was noticed since children with empyema usually present with persistent fever (23). On the contrary, in this study, the evolution of fever during treatment was similar in both subgroups (Table 3). Actually, the evolutions of several typical physical signs among children with CAP were similar in both subgroups (Table 3). So, it is necessary to highlight that even though the subgroups were somehow different on admission, their outcomes were alike. A discrepancy can be noted regarding the percentages of intravenous hydration prescriptions. It is important to emphasize that such prescriptions were the initial ones before the administration of any therapeutic item and physicians' subjectivity may had been the basis for these.
Certain methodological limitations in this study should be highlighted. The assignments were convenience based as the hospital standard practice changed over time, with the standard being 6 daily doses up through 2001, and then changing to 4 doses in 2002. As data were collected retrospectively, there was no control for measuring variables. Also, data were collected from patients hospitalized during a long time period. However, this investigation was performed in a teaching hospital where standardized procedures are used and the data were collected using strict criteria. Because they were not available, no MICs or pharmacokinetic data were collected. Nonetheless, no previous study has addressed the research question investigated herein.
To the best of our knowledge, this is the first study to show the clinical equivalency between the use of 200,000 IU/kg/day of aqueous penicillin G given in 6 or 4 daily doses for children with CAP. Despite some methodological limitations, we demonstrated that children using the aforementioned posologic regimens had no differences in markers of clinical outcomes such as hospitalization length, duration of aqueous penicillin G use, antibiotic substitution, intensive care unit transference, or death. The use of aqueous penicillin G in 4 daily doses also offers additional advantages related to lower costs, which include reduction in the use of equipment and a rational use of human resources involved in assistance, and is a practical and effective posologic regimen to be employed in clinical practice.
ACKNOWLEDGMENTS
We are grateful to members of the Medical Chart Unit of the Professor Hosannah de Oliveira Pediatric Center, Federal University of Bahia, Salvador, Bahia, Brazil, for their cooperation in getting the medical charts that were reviewed.
C.M.N.-C. is an investigator from the Brazilian Council for Scientific and Technological Development (CNPq). The CNPq had no role in the design, collection, analysis, or interpretation of the data or in the writing of or the decision to submit the manuscript for publication.
We received no funding.
We declare that we have no conflicts of interest.
Footnotes
Published ahead of print 16 December 2013
REFERENCES
- 1.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, Mathers C, Black RE, Child Health Epidemiology Reference Group of WHO and UNICEF 2012. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 379:2151–2161. 10.1016/S0140-6736(12)60560-1 [DOI] [PubMed] [Google Scholar]
- 2.Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. 2008. Epidemiology and etiology of childhood pneumonia. Bull. World Health Organ. 86:408–416. 10.2471/BLT.07.048769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madhi SA, De Wals P, Grijalva CG, Grimwood K, Grossman R, Ishiwada N, Lee PI, Nascimento-Carvalho C, Nohynek H, O′Brien KL, Vergison A, Wolter J. 2013. The burden of childhood pneumonia in the developed world: a review of the literature. Pediatr. Infect. Dis. J. 32:e119–e127. 10.1097/INF.0b013e3182784b26 [DOI] [PubMed] [Google Scholar]
- 4.Kabra SK, Lodha R, Pandey RM. 2010. Antibiotics for community acquired pneumonia in children. Cochrane Database Syst. Rev. 6:CD004874. 10.1002/14651858.CD004874.pub3 [DOI] [PubMed] [Google Scholar]
- 5.Nascimento-Carvalho CM. 2001. Etiology of childhood community acquired pneumonia and its implication of vaccination. Braz. J. Infect. Dis. 5:87–97. 10.1590/S1413-86702001000200007 [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Lovatel R, Nicolete D, Sinzkel E, Matiello J, Staszko K, Lincho C. 2008. Empiric antibiotic therapy in children with community-acquired pneumonia. Indian Pediatr. 45:554–558 [PubMed] [Google Scholar]
- 7.Bradley JS, Byington CL, Shah SS, Alyerson B, Carter ER, Harrison C, Kaplan SL, Mace SE, McCracken GH, Jr, Moore MR, St Peter SD, Stockwell JA, Swanson JT, Pediatric Infectious Diseases Society and the Infectious Diseases Society of America 2011. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by Pediatric Infectious Diseases Society and Infectious Diseases Society of America. Clin. Infect. Dis. 53:25–76. 10.1093/cid/cir531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs MR. 1999. Drug-resistant Streptococcus pneumoniae: rational antibiotic choices. Am. J. Med. 106:19S–25S [DOI] [PubMed] [Google Scholar]
- 9.Jacobs MR. 2001. Optimization of antimicrobial therapy using pharmacokinetic and pharmacodynamic parameters. Clin. Microbiol. Infect. 7:589–596. 10.1046/j.1198-743x.2001.00295.x [DOI] [PubMed] [Google Scholar]
- 10.Ponte C, Parra A, Nieto E, Soriano F. 1996. Development of experimental pneumonia by infection with penicillin-insensitive Streptococcus pneumoniae in guinea pigs and their treatment with amoxicillin, cefotaxime, and meropenem. Antimicrob. Agents Chemother. 40:2698–2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giachetto G, Pirez MC, Nanni L, Martinez A, Montano A, Algorta G, Kaplan SL, Ferrari AM. 2004. Ampicillin and penicillin concentration in serum and pleural fluid of hospitalized children with community-acquired pneumonia. Pediatr. Infect. Dis. J. 23:625–629. 10.1097/01.inf.0000128783.11218.c9 [DOI] [PubMed] [Google Scholar]
- 12.Nascimento-Carvalho CM, Cardoso MR, Brandileone MC, Ferrero F, Camargos P, Berezin E, Ruvinsky R, Sant′anna C, March MF, Feris-Iglesias J, Maggi R, Benguigui Y, CARIBE Group 2009. Penicillin/ampicillin efficacy among children with severe pneumonia due to penicillin-resistant pneumococcus (MIC = 4 μg/ml). J. Med. Microbiol. 58:1390–1392. 10.1099/jmm.0.007765-0 [DOI] [PubMed] [Google Scholar]
- 13.Cherian T, Mulholland EK, Carlin JB, Ostensen H, Amin R, de Campo M, Greenberg D, Lagos R, Lucero M, Madhi SA, O′Brien KL, Obaro S, Steinhoff MC, the WHO Radiology Working Group 2005. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull. World Health Organ. 83:353–359 [PMC free article] [PubMed] [Google Scholar]
- 14.El-Radhi AS, Barry W. 2006. Thermometry in paediatric practice. Arch. Dis. Child. 91:351–356. 10.1136/adc.2005.088831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization 2008. Integrated management of childhood illness. World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/publications/2008/9789241597289_eng.pdf [Google Scholar]
- 16.Nascimento-Carvalho CM. 2001. Physical signs in children with pneumonia. Indian Pediatr. 38:307–308 [PubMed] [Google Scholar]
- 17.World Health Organization 2008. Training course on child growth assessment. World Health Organization, Geneva, Switzerland: http://www.who.int/childgrowth/training/module_b_measuring_growth.pdf [Google Scholar]
- 18.Harris M, Clark J, Coote N, Fletcher P, Harnden A, McKean M, Thomson A, British Thoracic Society Standards of Care Committee 2011. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax 66:ii1–ii23. 10.1136/thoraxjnl-2011-200598 [DOI] [PubMed] [Google Scholar]
- 19.Cardoso MR, Nascimento-Carvalho CM, Ferrero F, Berezin E, Ruvinsky R, Camargos P, Sant′anna C, Brandileone MC, March MF, Feris-Iglesias J, Maggi R, Benguigui Y, CARIBE Group 2008. Penicillin resistant pneumococcus and risk of treatment failure in pneumonia. Arch. Dis. Child. 93:221–225. 10.1136/adc.2006.111625 [DOI] [PubMed] [Google Scholar]
- 20.Aguado-García JM, Martín-Herrero JE, Lumbreras-Bermejo C. 2004. Bacterial resistance and pharmacodynamics as the basis for prescribing antibiotics in respiratory infections. Enferm. Infecc. Microbiol. Clin. 22:230–237 (In Spanish.) 10.1157/13059054 [DOI] [PubMed] [Google Scholar]
- 21.Deiros Bronte L, Baquero-Artigao F, García-Miguel MJ, Hernández González N, Peña García P, del Castillo Martín F. 2006. Parapneumonic pleural effusion: an 11-year review. An. Pediatr. (Barc.) 64:40–45 (In Spanish.) 10.1016/S1695-4033(06)70007-8 [DOI] [PubMed] [Google Scholar]
- 22.Goldbart AD, Leibovitz E, Porat N, Givon-Lavi N, Drukmann I, Tal A, Greenberg D. 2009. Complicated community acquired pneumonia in children prior to the introduction of the pneumococcal conjugated vaccine. Scand. J. Infect. Dis. 41:182–187. 10.1080/00365540802688378 [DOI] [PubMed] [Google Scholar]
- 23.Niemi E, Korppi M. 2011. Parapneumonic empyema in children before the era of pneumococcal vaccination. Acta Paediatr. 100:1230–1233. 10.1111/j.1651-2227.2011.02290.x [DOI] [PubMed] [Google Scholar]