Abstract
Doripenem and ertapenem have demonstrated efficacy against several NDM-1-producing isolates in vivo, despite having high MICs. In this study, we sought to further characterize the efficacy profiles of humanized regimens of standard (500 mg given every 8 h) and high-dose, prolonged infusion of doripenem (2 g given every 8 h, 4-h infusion) and 1 g of ertapenem given intravenously every 24 h and the comparator regimens of ceftazidime at 2 g given every 8 h (2-h infusion), levofloxacin at 500 mg every 24 h, and aztreonam at 2 g every 6 h (1-h infusion) against a wider range of isolates in a murine thigh infection model. An isogenic wild-type strain and NDM-1-producing Klebsiella pneumoniae and eight clinical NDM-1-producing members of the family Enterobacteriaceae were tested in immunocompetent- and neutropenic-mouse models. The wild-type strain was susceptible to all of the agents, while the isogenic NDM-1-producing strain was resistant to ceftazidime, doripenem, and ertapenem. Clinical NDM-1-producing strains were resistant to nearly all five of the agents (two were susceptible to levofloxacin). In immunocompetent mice, all of the agents produced ≥1-log10 CFU reductions of the isogenic wild-type and NDM-1-producing strains after 24 h. Minimal efficacy of ceftazidime, aztreonam, and levofloxacin against the clinical NDM-1-producing strains was observed. However, despite in vitro resistance, ≥1-log10 CFU reductions of six of eight clinical strains were achieved with high-dose, prolonged infusion of doripenem and ertapenem. Slight enhancements of doripenem activity over the standard doses were obtained with high-dose, prolonged infusion for three of the four isolates tested. Similar efficacy observations were noted in neutropenic mice. These data suggest that carbapenems are a viable treatment option for infections caused by NDM-1-producing Enterobacteriaceae.
INTRODUCTION
New Delhi metallo-β-lactamase (NDM-1) is a novel carbapenemase that was first identified in a Klebsiella pneumoniae isolate in India in 2008 (1). Since then, it has disseminated at a high rate among Enterobacteriaceae isolates both within the Indian subcontinent and worldwide (2–4). The treatment options for infections caused by NDM-1-producing isolates are extremely limited, as these enzymes are known to hydrolyze penicillins, cephalosporins, and carbapenems while typically retaining susceptibility to only colistin and tigecycline (2), neither of which has been shown to be a dependable option on the basis of in vitro time-kill data and case reports (5–8). Aztreonam is not readily inactivated by metallo-β-lactamases, including NDM-1, and may represent another potential option for therapy (1, 9). However, NDM-1-producing strains often coproduce other β-lactamases, such as CTX-M- and CMY-type enzymes, that possess the ability to hydrolyze aztreonam, further limiting the utility of all β-lactam antibiotics (1). Furthermore, in vivo efficacy data evaluating potential treatment options for NDM-1-producing isolates are sparse and clinical experience in treating infections caused by these isolates is limited to case reports.
Unlike in vivo observations with isolates that produce the serine-carbapenemase KPC, carbapenem monotherapy has demonstrated reductions of NDM-1-producing bacterial isolates in a murine thigh infection model, even though the time that the concentration of an unbound antibiotic remains above the MIC during the dosing interval (ƒT>MIC) needed for efficacy was not achieved for all of the isolates (10, 11). Previous work by our group has demonstrated a reduction in bacterial density with human simulated monotherapy regimens of ertapenem and high-dose, prolonged infusion of doripenem against an isogenically constructed NDM-1-producing strain and four clinical NDM-1-producing strains in a murine thigh infection model (10). Since these results were both surprising and promising, it is essential to validate them with additional in vivo studies before determining the potential clinical utility of carbapenems for treating infections caused by NDM-1-producing organisms. A high-dose, prolonged doripenem infusion regimen (2 g given every 8 h as a 4-h infusion) was initially evaluated in the previous study for its potential to achieve the requisite pharmacodynamic target needed for efficacy against these resistant pathogens. Since considerable efficacy was observed with this regimen despite in vitro resistance, a standard dose of doripenem (500 mg given every 8 h) also warrants further study. Furthermore, only four clinical isolates were included in the initial study. Efficacy data from a larger range of clinical NDM-1-producing Enterobacteriaceae isolates, with additional comparator agents, are needed to best characterize the potential clinical utility of these regimens. In the present study, we sought to describe the in vivo efficacy of human simulated doses of both high-dose, prolonged-infusion and standard regimens of doripenem, ertapenem, ceftazidime, aztreonam, and levofloxacin against an isogenic pair consisting of a wild-type and an NDM-1-producing strain and eight clinical NDM-1-producing isolates in a murine thigh infection model.
MATERIALS AND METHODS
Antimicrobial test agents.
Commercially available ertapenem (Invanz, Merck & Co., Inc., Whitehouse Station, NJ), doripenem (Doribax; Janssen Pharmaceuticals, Inc., Raritan, NJ), ceftazidime (Fortaz; GlaxoSmithKline, Philadelphia, PA), aztreonam (Azactam; Bristol-Myers Squibb, Princeton, NJ), and levofloxacin (Sagent Pharmaceuticals, Schaumburg, IL) were obtained from the pharmacy department at Hartford Hospital for use in all in vivo experiments. Each drug was reconstituted according to the manufacturer's prescribing information and then further diluted in normal saline to the concentrations required for dosing. Dosing solutions were prepared just prior to each experiment, stored under refrigeration, and discarded after 24 h.
Bacterial isolates.
An isogenic pair consisting of a wild-type K. pneumoniae strain (KP 454) and an NDM-1-producing strain derived by conjugation and four clinical NDM-1-producing Enterobacteriaceae isolates (EC 389, EC 393, KP 425, and KP 450) were provided by Patrice Nordmann, Hospital Bicêtre (Le Kremlin-Bicêtre, France) (10). The ertapenem and doripenem MICs for these isolates were determined via Etest (AB bioMérieux, Solna, Sweden) according to the manufacturer's instructions. The MICs of ceftazidime, aztreonam, and levofloxacin for these isolates were determined by broth microdilution consistent with the Clinical and Laboratory Standards Institute (CLSI) methodology (12). Pseudomonas aeruginosa ATCC 27853 was used as the quality control strain for all three agents evaluated by broth microdilution. MIC determinations were conducted in triplicate, and MICs are reported as modal values unless otherwise noted. Four additional clinical NDM-1-producing Enterobacteriaceae isolates (KP 476, KP 477, KP 478, and ECL 70) were kindly provided by Christine Lascols, International Health Management Associates, Inc. (Schaumburg, IL) (13). The MICs of all five of the agents evaluated were determined by broth microdilution as outlined above. All of the isolates were screened for KPC, OXA-48-like, VIM, IMP, and NDM carbapenemases, as well for extended-spectrum β-lactamases (TEM, SHV, and CTX-M) and AmpC β-lactamases (CMY, DHA, FOX, MOX, ACC, and ACT), as previously described (13). Isolates were stored at −80°C in double-strength skim milk (Remel, Lenexa, KS). Prior to each in vivo experiment, isolates were subcultured twice onto Trypticase soy agar with 5% sheep blood (BD Biosciences, Sparks, MD) and incubated at 35°C for 18 to 24 h.
Immunocompetent-mouse thigh infection model.
This study was reviewed and approved by the Institutional Animal Care and Use Committee at Hartford Hospital, Hartford, CT. Pathogen-free ICR mice weighing 20 to 22 g were acquired from Harlan Laboratories (Indianapolis, IN). All animals were maintained in accordance with the National Research Council's recommendations and were provided food and water ad libitum. Three days prior to inoculation for in vivo experiments, an intraperitoneal injection of uranyl nitrate (5 mg/kg) was given to produce a predictable level of renal impairment in order to slow antibiotic clearance (14). Two hours prior to the initiation of antimicrobial therapy, both thighs of each animal were inoculated intramuscularly with 0.1 ml of an inoculum solution containing the test isolate at 108 CFU/ml of normal saline.
Neutropenic thigh infection model.
The isogenic wild-type and NDM-1-producing strains and two of the clinical NDM-1-producing isolates were also evaluated in a neutropenic infection model for efficacy comparison. The mice used in the neutropenic-mouse model underwent the same procedures as described for the immunocompetent-mouse model and were also given intraperitoneal injections of cyclophosphamide (Baxter, Deerfield, IL) at 100 and 150 mg/kg 1 and 4 days prior to inoculation, respectively, to induce neutropenia (14). Furthermore, an inoculum solution of 107 CFU/ml was used to produce thigh infection in neutropenic mice.
In vivo efficacy.
For each isolate, groups of three mice were administered a human simulated regimen of doripenem, ertapenem, ceftazidime, aztreonam, or levofloxacin beginning 2 h after inoculation. Humanized regimens previously developed and validated by our group to simulate the human pharmacodynamic profile of 2 g of doripenem given intravenously (i.v.) every 8 h as a 4-h infusion, 1 g of ertapenem given i.v. every 24 h, 2 g of ceftazidime given i.v. every 8 h as a 2-h infusion, 2 g of aztreonam given i.v. every 6 h as a 1-h infusion, and 500 mg of levofloxacin given every 24 h were used throughout (15–19). Since in vivo efficacy has been demonstrated previously with high-dose, prolonged infusion of doripenem, a standard doripenem dose of 500 mg given i.v. every 8 h was also evaluated against the four isolates with the highest doripenem MICs in the immunocompetent-mouse model and all of the isolates in the neutropenic-mouse model (20). Each dose within these regimens was administered as a 0.2-ml subcutaneous injection, with dosing regimens repeated when necessary to complete 24 h of therapy. Three control animals for each isolate were given normal saline in the same volume, by the same route, and at the same frequency as the most frequently administered treatment regimen over 24 h. Groups of untreated control mice were harvested at 0 h, while the control mice that received normal saline and all of the treated mice were harvested at 24 h. Mice that failed to survive to 24 h were harvested at the time of expiration and were included in the final data analysis. Harvesting consisted of euthanization by CO2 exposure, followed by cervical dislocation. Thighs were then removed from sacrificed animals and homogenized individually in normal saline. Serial dilutions of thigh homogenate were plated onto Trypticase soy agar with 5% sheep blood for determination of bacterial density. For experiments conducted in the neutropenic thigh infection model, efficacy was calculated as the change in bacterial density (in log10 CFU) in treated mice after 24 h from the starting bacterial densities in 0-h control animals. In order to control for the effect of the host and associated variability of 0-h control bacterial density between isolates, efficacy in experiments conducted in the immunocompetent-mouse model was calculated as the change in bacterial density in treated mice after 24 h compared to that in the 24-h immunocompetent control mice (10, 21). The efficacy of human simulated high-dose, prolonged infusion of doripenem and the standard regimen of ertapenem was evaluated previously for the isogenic pair, EC 389, EC393, and KP 425 and has been included to provide a complete comparison (10). The observed efficacies of the two doripenem regimens and the ertapenem regimen were compared for each isolate by one-way repeated-measures analysis of variance. A P value of <0.05 was defined a priori as statistically significant.
RESULTS
Bacterial isolates.
An isogenic pair consisting of wild-type K. pneumoniae 454, the NDM-1-producing strain derived from it, and a group of eight clinical NDM-1-producing Enterobacteriaceae isolates, were used. Phenotypic profiles for the five antimicrobials evaluated and the known genotypic profiles for all of the isolates included in the efficacy studies are described in Table 1 (10, 13). Briefly, the wild-type strain (KP 454) was susceptible to all five antibiotics tested, while the addition of NDM-1 to this strain resulted in in vitro resistance to ceftazidime, ertapenem, and doripenem according to current CLSI breakpoints. Most of the clinical strains used coproduced a variety of other β-lactamases and were resistant to nearly all of the β-lactam agents used in this study. Furthermore, six of the eight clinical strains were also resistant to levofloxacin.
TABLE 1.
Phenotypic and genotypic profiles of Enterobacteriaceae isolates used in the in vivo efficacy studies described here
| Isolate | Known β-lactamase content (10, 13) | MIC (μg/ml)a |
||||
|---|---|---|---|---|---|---|
| DOR | ETP | CAZ | ATM | LEV | ||
| K. pneumoniae 454 | None | 0.03 | 0.012 | 0.25 | ≤0.25 | ≤0.06 |
| K. pneumoniae 454 + NDM-1 plasmid | NDM-1 | 4 | 16 | >128 | 2 | 0.125 |
| E. coli 389 | NDM-1, TEM-1, CTX-M-15 | 8 | >32 | >128 | >256 | 32 |
| E. coli 393 | NDM-1, CTX-M-15, TEM-1, OXA-1, OXA-2 | 4 | >32 | >128 | >256 | 32 |
| K. pneumoniae 425 | NDM-1, TEM-1, SHV-11, CTX-M-15, CMY-16, OXA-1, OXA-9, OXA-10 | 32 | >32 | >128 | 256 | 0.5 |
| K. pneumoniae 450 | NDM-1, SHV-11, SHV-28, TEM-1, CTX-M-15, OXA-1, OXA-9 | >32 | >32 | >128 | >256 | 16 |
| K. pneumoniae 476 | NDM-1 | 64 | 128 | >128 | 64 | >32 |
| K. pneumoniae 477 | NDM-1, SHV-2A, AmpC, DHA-1 | 64 | 128 | >128 | 128b | >32 |
| K. pneumoniae 478 | NDM-1, CTX-M-15 | >64 | 128 | >128 | >256 | >32 |
| E. cloacae 70 | NDM-1 | 64 | 64 | >128 | 128 | 0.5 |
DOR, doripenem; ETP, ertapenem; CAZ, ceftazidime; ATM, aztreonam; LEV, levofloxacin.
Median value reported in the absence of a mode.
In vivo efficacy.
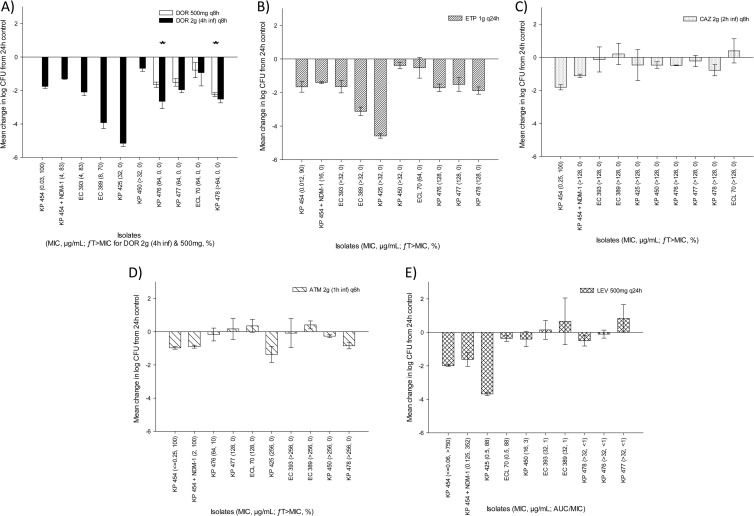
The isogenic pair and all eight clinical isolates were evaluated in the immunocompetent-mouse model. In these studies, the mean bacterial density (± the standard deviation) in 0-h control mice was 6.82 ± 0.32 log10 CFU per thigh and was maintained at 6.82 ± 1.23 log10 CFU per thigh after 24 h. The efficacy results of the immunocompetent-mouse studies are shown in Fig. 1. All five of the antimicrobials tested achieved a ≥1-log10 CFU reduction of the isogenic wild-type and NDM-1-producing strains. Consistent with ceftazidime MICs of >128 μg/ml (ƒT>MIC, 0%), levofloxacin MICs of ≥64 μg/ml (ƒT>MIC, ≤10%), and levofloxacin MICs of ≥0.5 μg/ml (AUC/MIC ratio, ≤88), none of these three agents produced consistent reductions in bacterial density across the eight clinical isolates evaluated. However, the high-dose, prolonged doripenem infusion regimen and the ertapenem regimen both resulted in substantial bacterial reductions for all eight clinical strains, even in the light of six of these isolates having doripenem MICs of ≥32 μg/ml (ƒT>MIC, 0%) and all eight isolates having ertapenem MICs of >32 μg/ml (ƒT>MIC, 0%). While doripenem efficacy against the four isolates was demonstrated with both regimens, with doripenem MICs of ≥64 μg/ml, numerically greater reductions of all four of the bacterial isolates evaluated were attained with the high-dose, prolonged-infusion regimen than with the standard 500-mg dose, and the difference was statistically significant for two of these isolates (Fig. 1A).
FIG 1.
Efficacies of human simulated regimens of 500 mg of doripenem (DOR) given every 8 h and 2 g given every 8 h as a 4-h infusion (A), 1 g of ertapenem (ETP) given every 24 h (B), 2 g of ceftazidime (CAZ) given i.v. every 8 h as a 2-h infusion (C), 2 g of aztreonam (ATM) given i.v. every 6 h as a 1-h infusion (D), and 500 mg of levofloxacin (LEV) given i.v. every 24 h (E) against a group of NDM-1-producing Enterobacteriaceae isolates (CLSI susceptibility breakpoints: doripenem, ≤1 μg/ml; ertapenem, ≤0.5 μg/ml; ceftazidime, ≤4 μg/ml; aztreonam, ≤4 μg/ml; levofloxacin, ≤2 μg/ml [45]) in an immunocompetent-mouse thigh infection model. Error bars represent standard deviations. For isolates where both doripenem regimens were evaluated, the asterisks denote a statistically significantly greater observed efficacy of 2 g of doripenem given every 8 h as a 4-h infusion than of 500 mg of doripenem given every 8 h and 1 g of ertapenem given every 24 h (P < 0.05).
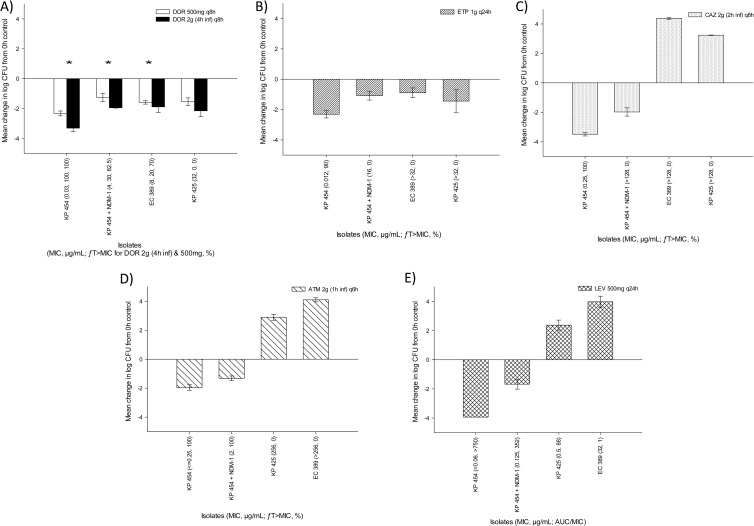
The isogenic wild-type and NDM-1-producing strains plus two of the clinical NDM-1-producing strains (EC 380 and KP 425) were also evaluated in a neutropenic-mouse model in order to best ascertain the efficacy of the regimens evaluated without the impact of the host. In these studies, the mean bacterial density in 0-h control mice (± the standard deviation) was 5.99 ± 0.49 log10 CFU per thigh and increased to a mean of 9.10 ± 0.51 log10 CFU per thigh after 24 h. The results of the neutropenic studies are depicted in Fig. 2. Similar trends in efficacy with respect to phenotypic and pharmacodynamic profiles were noted between the immunocompetent- and neutropenic-mouse studies.
FIG 2.
Efficacies of human simulated regimens of 500 mg of doripenem (DOR) given every 8 h and 2 g of doripenem given every 8 h as a 4-h infusion (A), 1 g of ertapenem (ETP) given every 24 h (B), 2 g of ceftazidime (CAZ) given i.v. every 8 h as a 2-h infusion (C), 2 g of aztreonam (ATM) given i.v. every 6 h as a 1-h infusion (D), and 500 mg of levofloxacin (LEV) given i.v. every 24 h (E) against a group of NDM-1-producing Enterobacteriaceae isolates (CLSI susceptibility breakpoints: doripenem, ≤1 μg/ml; ertapenem, ≤0.5 μg/ml; ceftazidime, ≤4 μg/ml; aztreonam, ≤4 μg/ml; levofloxacin, ≤2 μg/ml [45]) in a neutropenic-mouse thigh infection model. Error bars represent standard deviations. The asterisks denote a statistically significantly greater observed efficacy of 2 g of doripenem given every 8 h as a 4-h infusion than of 500 mg of doripenem given every 8 h and 1 g of ertapenem given every 24 h (P < 0.05).
DISCUSSION
The rate at which the metallo-β-lactamase NDM-1 has disseminated globally among members of the family Enterobacteriaceae is alarming (2). Without antimicrobials with novel mechanisms of action readily available, evaluation of the potential clinical utility of existing agents for the treatment of infections caused by these resistant pathogens is imperative. Since preliminary data on the in vivo efficacy of carbapenem regimens for NDM-1-producing organisms looked promising, we sought to corroborate these findings by using an expanded group of NDM-1-producing isolates and including other comparator agents (ceftazidime, aztreonam, and levofloxacin) whose pharmacodynamic profiles have previously been well characterized in the same in vivo infection model.
In the present study, in vivo efficacy was observed with all of the regimens tested against the wild-type K. pneumoniae strain, as expected. Comparable efficacies of levofloxacin, aztreonam, and high-dose, prolonged infusion of doripenem against the isogenically constructed NDM-1-producing strain were also observed, as predicted on the basis of the pharmacodynamic targets required for efficacy of an AUC/MIC ratio of ≥125, an ƒT>MIC of ≥50%, and an ƒT>MIC of ≥40%, respectively (22–24). However, ertapenem and ceftazidime also demonstrated efficacy against the isogenic strain, even though the ƒT>MIC achieved with these agents for this strain was 0%.
Neither ceftazidime nor aztreonam nor levofloxacin produced reliable efficacy across the eight clinical NDM-1-producing isolates evaluated in the immunocompetent-mouse model or the two isolates that were also evaluated in the neutropenic-mouse model, as anticipated on the basis of pharmacodynamic exposures of these isolates that were achieved. Even though efficacy of ceftazidime against the isogenic NDM-1-producing strain was noted when the ƒT>MIC was 0%, minimal efficacy against the eight clinical strains with similar phenotypic profiles was seen, as anticipated, given NDM-1's efficient hydrolysis of most cephalosporins, coupled with the coproduction of additional β-lactamases by the clinical isolates (1). While variable efficacy of levofloxacin against the two clinical strains was noted, with a MIC of 0.5 μg/ml, essentially no activity against the other six strains was observed, with MICs of ≥16 μg/ml. These data were consistent with the previously published pharmacodynamic target for fluoroquinolones against Gram-negative pathogens of an AUC/MIC ratio of ≥125, as the AUC/MIC ratio was ≤88 for these eight clinical strains (24). A lack of consistent efficacy was also noted with aztreonam across the eight resistant clinical strains, consistent with a ƒT>MIC of ≤10% for these isolates (18, 23). While aztreonam is not a substrate for metallo-β-lactamase enzymes such as NDM-1, monotherapy is not a viable option for treating infections with NDM-1-producing Enterobacteriaceae because of the high likelihood of the coproduction of CTX-M- and CMY-type β-lactamases, as evidenced by our results with the clinical strains (1, 9).
Even though the requisite 40% ƒT>MIC needed for efficacy was achieved in only two of the eight clinical isolates with doripenem and in no isolates with ertapenem, bacterial reductions were achieved with both agents against all of the clinical strains evaluated in the immunocompetent- and neutropenic-mouse models (22). Furthermore, ≥1-log10 CFU reductions of six of the eight clinical strains were achieved with both the doripenem and ertapenem regimens in the immunocompetent-mouse model. Similarly, reductions of the two isolates used in the neutropenic-mouse model were also observed with these regimens. When the doripenem regimens were compared directly, the high-dose, prolonged-infusion regimen of 2 g given every 8 h as a 4-h infusion showed a slight enhancement in efficacy over the standard regimen of 500 mg given every 8 h. The unanticipated activity of the carbapenems against these resistant NDM-1-producing isolates may be supported by the enzyme's reduced hydrolytic efficiency for carbapenems. In an enzyme kinetic study, NDM-1 exhibited less efficient hydrolysis of imipenem and meropenem than IMP-1 but was similar to that of VIM-2, which is known to hydrolyze carbapenems more slowly than other metallo-β-lactamases (1, 25). Because of this variation in the hydrolytic efficiency of carbapenems among the various metallo-β-lactamases, the efficacy observed with carbapenems in the present study cannot be readily extrapolated to strains that produce other metallo-β-lactamases without additional in vivo evaluation against VIM- and IMP-producing strains.
While a great majority of the published case reports focus solely on the emergence and detection of NDM-1-producing strains in patients from around the world, some also describe the clinical course and treatment outcomes for these patients. It appears that a majority of the cases that include clinical outcomes have predominantly been treated successfully, most often with colistin- and/or amikacin-based regimens (26–39). It is likely that this collection of largely positive case reports is a result of publication bias and should be interpreted cautiously, since a small number of clinical failures with these same regimens have also been reported (6–8, 40). Nonetheless, consistent with the in vivo efficacy of carbapenems demonstrated in this study, four case reports have been published to date describing the successful treatment of infections caused by NDM-1 producers with meropenem- or ertapenem-based regimens (41–44). The first case was a patient with urosepsis caused by an NDM-1-producing Escherichia coli strain susceptible to meropenem, imipenem, tigecycline, colistin, and trimethoprim-sulfamethoxazole who successfully cleared the infection after an initial course of meropenem, followed by trimethoprim-sulfamethoxazole (41). A second patient with prostatitis, pyelonephritis, and an NDM-1-producing E. coli strain isolated from a clean-catch urine culture also demonstrated clinical improvement and microbiologic clearance of urine cultures with an ertapenem course of 2 g daily, followed by a single dose of fosfomycin, despite the isolate's resistance to ertapenem (42). Another patient who developed ventilator-associated pneumonia, likely caused by NDM-1-producing Acinetobacter baumannii, was treated with 7 days of meropenem and metronidazole. While the initial isolate recovered from a bronchoalveolar lavage sample and an oral cavity swab was resistant to meropenem, the patient recovered; however, it was unclear if this organism represented infection or colonization (43). A fourth patient treated for an abscess of the right iliac fossa also showed clinical improvement after 2 weeks of meropenem. However, adequate abscess drainage may have played a critical role in his recovery (44). While these clinical data are scarce and confounded by combination regimens and other clinical variables, they do appear to support the potential utility of carbapenems for treating NDM-1-producing Enterobacteriaceae infections and the discordance observed in this study between the phenotypic profile and in vivo efficacy.
Consistent with phenotypic profiles and pharmacodynamic targets, ceftazidime, aztreonam, and levofloxacin did not show consistent efficacy against the NDM-1-producing strains evaluated in this study, particularly in the absence of a functioning immune system. Only the simulated carbapenem regimens including standard and high-dose, prolonged infusion of doripenem and ertapenem demonstrated efficacy against these pathogens in both immunocompetent- and neutropenic-mouse thigh infection models, even in the light of elevated MICs. While there are currently new β-lactamase inhibitors and other novel drug targets in various phases of development, these data suggest that carbapenem-based regimens may represent a viable treatment option for infections caused by NDM-1-producing isolates in the interim.
ACKNOWLEDGMENTS
This study was supported with internal funds from the Center for Anti-Infective Research and Development, Hartford Hospital.
We acknowledge Amira Bhalodi, Shawn MacVane, Kevin Connors, Debora Santini, Lindsay Tuttle, Jennifer Hull, Henry Christensen, Mary Anne Banevicius, Lucinda Lamb, and Pam Tessier (Center for Anti-Infective Research & Development, Hartford Hospital) for their assistance with the animal experimentation and Christina Sutherland and Pam Tessier for their assistance with MIC determinations. We thank Delphine Girlich (Department of Bacteriology-Virology, Hospital Bicêtre, Le Kremlin-Bicêtre, France) for determining MICs and constructing the isogenic strains. We also thank Christine Lascols and Meredith Hackel (International Health Management Associates, Inc., Schaumburg, IL) for providing clinical NDM-1-producing strains.
Footnotes
Published ahead of print 30 December 2013
REFERENCES
- 1.Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, Lee K, Walsh TR. 2009. Characterization of a new metallo-beta-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 53:5046–5054. 10.1128/AAC.00774-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shakil S, Azhar EI, Tabrez S, Kamal MA, Jabir NR, Abuzenadah AM, Damanhouri GA, Alam Q. 2011. New Delhi metallo-β-lactamase (NDM-1): an update. J. Chemother. 23:263–265 http://www.researchgate.net/publication/51724850_New_Delhi_metallo-lactamase_(NDM-1)_an_update [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) 2010. Detection of Enterobacteriaceae isolates carrying metallo-beta-lactamase—United States, 2010. MMWR Morb. Mortal. Wkly. Rep. 59:750 http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5924a5.htm [PubMed] [Google Scholar]
- 4.Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, Uma C, Doumith M, Giske CG, Irfan S, Krishnan P, Kumar AV, Maharjan S, Mushtaq S, Noorie T, Paterson DL, Pearson A, Perry C, Pike R, Rao B, Ray U, Sarma JB, Sharma M, Sheridan E, Thirunarayan MA, Turton J, Upadhyay S, Warner M, Welfare W, Livermore DM, Woodford N. 2010. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect. Dis. 10:597–602. 10.1016/S1473-3099(10)70143-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albur M, Noel A, Bowker K, MacGowan A. 2012. Bactericidal activity of multiple combinations of tigecycline and colistin against NDM-1-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 56:3441–3443. 10.1128/AAC.05682-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poirel L, Ozdamar M, Ocampo-Sosa AA, Turkoglu S, Ozer UG, Nordmann P. 2012. NDM-1-producing Klebsiella pneumoniae now in Turkey. Antimicrob. Agents Chemother. 56:2784–2785. 10.1128/AAC.00150-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan HLW, Poon LM, Chan SG, Teo JWP. 2011. The perils of medical tourism: NDM-1 positive Escherichia coli causing febrile neutropenia in a medical tourist. Singapore Med. J. 52:299–302 http://smj.sma.org.sg/5204/5204cr1.pdf [PubMed] [Google Scholar]
- 8.Mulvey MR, Grant JM, Plewes K, Roscoe D, Boyd DA. 2011. New Delhi metallo-β-lactamase in Klebsiella pneumoniae and Escherichia coli, Canada. Emerg. Infect. Dis. 17:103–106. 10.3201/eid1701.101358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bush K. 2013. The ABCD's of β-lactamase nomenclature. J. Infect. Chemother. 19:549–559. 10.1007/s10156-013-0640-7 [DOI] [PubMed] [Google Scholar]
- 10.Wiskirchen DE, Nordmann P, Crandon JL, Nicolau DP. 2013. Efficacy of humanized carbapenem exposures against New Delhi metallo-beta-lactamase (NDM-1) producing Enterobacteriaceae in a murine infection model. Antimicrob. Agents Chemother. 57:3936–3940. 10.1128/AAC.00708-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiskirchen DE, Crandon JL, Nicolau DP. 2013. Impact of various conditions on the efficacy of dual carbapenem therapy against KPC-producing Klebsiella pneumoniae. Int. J. Antimicrob. Agents 41:582–585. 10.1016/j.ijantimicag.2013.02.015 [DOI] [PubMed] [Google Scholar]
- 12.Clinical Laboratory Standards Institute 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 9th ed CLSI publication M07–A9 Clinical Laboratory Standard Institute, Wayne, PA [Google Scholar]
- 13.Lascols C, Peirano G, Hackel M, Laupland KB, Pitout JD. 2013. Surveillance and molecular epidemiology of Klebsiella pneumoniae isolates that produce carbapenemases: first report of OXA-48-like enzymes in North America. Antimicrob. Agents Chemother. 57:130–136. 10.1128/AAC.01686-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andes D, Craig WA. 1998. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob. Agents Chemother. 42:2375–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeRyke CA, Banevicius MA, Fan HW, Nicolau DP. 2007. Bactericidal activities of meropenem and ertapenem against extended-spectrum-β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a neutropenic mouse thigh model. Antimicrob. Agents Chemother. 51:1481–1486. 10.1128/AAC.00752-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crandon JL, Bulik CC, Nicolau DP. 2009. In vivo efficacy of 1- and 2-gram human simulated prolonged infusions of doripenem against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 53:4352–4356. 10.1128/AAC.00282-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crandon JL, Schuck VJ, Banevicius MA, Beaudoin ME, Nichols WW, Tanudra MA, Nicolau DP. 2012. Comparative in vitro and in vivo efficacies of human simulated doses of ceftazidime and ceftazidime-avibactam against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 56:6137–6146. 10.1128/AAC.00851-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crandon JL, Nicolau DP. 2013. Human simulated studies of aztreonam and aztreonam-avibactam to evaluate activity against challenging gram-negative organisms, including metallo-β-lactamase producers. Antimicrob. Agents Chemother. 57:3299–3306. 10.1128/AAC.01989-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onyeji CO, Bui KQ, Owens RC, Nicolau SP, Quintiliani R, Nightingale CH. 1999. Comparative efficacies of levofloxacin and ciprofloxacin against Streptococcus pneumoniae in a mouse model of experimental septicaemia. Int. J. Antimicrob. Agents 12:107–114. 10.1016/S0924-8579(98)00087-9 [DOI] [PubMed] [Google Scholar]
- 20.Kim A, Banevicius MA, Nicolau DP. 2008. In vivo pharmacodynamic profiling of doripenem against Pseudomonas aeruginosa by simulating human exposures. Antimicrob. Agents Chemother. 52:2497–2502. 10.1128/AAC.01252-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keel RA, Crandon JL, Nicolau DP. 2011. Efficacy of human simulated exposures of ceftaroline administered at 600 milligrams every 12 hours against phenotypically diverse Staphylococcus aureus isolates. Antimicrob. Agents Chemother. 55:4028–4032. 10.1128/AAC.00372-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turnidge JD. 1998. The pharmacodynamics of beta-lactams. Clin. Infect. Dis. 27:10–22. 10.1086/514622 [DOI] [PubMed] [Google Scholar]
- 23.Craig WA. 2003. Basic pharmacodynamics of antibacterials with clinical applications to the use of β-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. North Am. 17:479–501. 10.1016/S0891-5520(03)00065-5 [DOI] [PubMed] [Google Scholar]
- 24.Forrest A, Nix DE, Ballow CH, Goss TF, Birmingham MC, Schentag JJ. 1993. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob. Agents Chemother. 37:1073–1081. 10.1128/AAC.37.5.1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Queenan AM, Shang W, Flamm R, Bush K. 2010. Hydrolysis and inhibition profiles of β-lactamases from molecular classes A to D with doripenem, imipenem, and meropenem. Antimicrob. Agents Chemother. 54:565–569. 10.1128/AAC.01004-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darley E, Weeks J, Jones L, Daniel V, Wootton M, MacGowan A, Walsh T. 2012. NDM-1 polymicrobial infections including Vibrio cholera. Lancet 380:1358. 10.1016/S0140-6736(12)60911-8 [DOI] [PubMed] [Google Scholar]
- 27.Tsang KY, Luk S, Lo JY, Tsang TY, Lai ST, Hg TK. 2012. Hong Kong experiences the ‘Ultimate superbug': NDM-1 Enterobacteriaceae. Hong Kong Med. J. 18:439–441 http://www.hkmj.org/abstracts/v18n5/439.htm [PubMed] [Google Scholar]
- 28.Flateau C, Janvier F, Delacour H, Males S, Ficko C, Andriamanantena D, Jeannot K, Merens A, Rapp C. 2012. Recurrent pyelonephritis due to NDM-1 metallo-beta-lactamase producing Pseudomonas aeruginosa in a patient returning from Serbia, France, 2012 Euro Surveill. 17:pii=20311 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20311 [PubMed] [Google Scholar]
- 29.Mochon AB, Garner OB, Hindler JA, Krogstad P, Ward KW, Lewinski MA, Rasheed JK, Anderson KF, Limbago B, Humphries RM. 2011. New Delhi metallo-β-lactamase (NDM-1)-producing Klebsiella pneumoniae: case report and laboratory detection strategies. J. Clin. Microbiol. 49:1667–1670. 10.1128/JCM.00183-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roy S, Viswanathan R, Singh AK, Das P, Basu S. 2011. Sepsis in neonates due to imipenem-resistant Klebsiella pneumoniae producing NDM-1 in India. J. Antimicrob. Chemother. 66:1411–1413. 10.1093/jac/dkr068 [DOI] [PubMed] [Google Scholar]
- 31.Stone NR, Woodford N, Livermore DM, Howard J, Pike R, Mushtaq S, Perry C, Hopkins S. 2011. Breakthrough bacteraemia due to tigecycline-resistant Escherichia coli with New Delhi metallo-β-lactamase (NDM)-1 successfully treated with colistin in a patient with calciphylaxis. J. Antimicrob. Chemother. 66:2677–2678. 10.1093/jac/dkr337 [DOI] [PubMed] [Google Scholar]
- 32.Chien JM, Koh TH, Chan KS, Chuah TH, Tan TT. 2012. Successful treatment of NDM-1 Klebsiella pneumoniae bacteraemia in a neutropenic patient. Scand. J. Infect. Dis. 44:312–314. 10.3109/00365548.2011.633549 [DOI] [PubMed] [Google Scholar]
- 33.Pasteran F, Albornoz E, Faccone D, Gomez S, Valenzuela C, Morales M, Estrada P, Valenzuela L, Matheu J, Guerriero L, Arbizu E, Calderon Y, Ramon-Pardo P, Corso A. 2012. Emergence of NDM-1-producing Klebsiella pneumoniae in Guatemala. J. Antimicrob. Chemother. 67:1795–1797. 10.1093/jac/dks101 [DOI] [PubMed] [Google Scholar]
- 34.Hu H, Hu Y, Pan Y, Liang H, Wang H, Wang X, Hao Q., Yang X, Yang X, Xiao X, Luan C, Yang Y, Cui Y, Yang Rk, Gao G, Song Y, Zhu B. 2012. Novel plasmid and its variant harboring both a bla(NDM-1) gene and type IV secretion system in clinical isolates of Acinetobacter lwoffii. Antimicrob. Agents Chemother. 56:1698–1702. 10.1128/AAC.06199-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lowman W, Sriruttan C, Nana T, Bosman N, Duse A, Venturas J, Clay C, Coetzee J. 2011. NDM-1 has arrived: first report of a carbapenem resistance mechanism in South Africa. S. Afr. Med. J. 101:873–875 http://www.scielo.org.za/scielo.php?pid=S0256-95742011001200015&script=sci_arttext [PubMed] [Google Scholar]
- 36.Chihara S, Okuzumi K, Yamamoto Y, Oikawa S, Hishinuma A. 2011. First case of New Delhi metallo-β-lactamase 1-producing Escherichia coli infection in Japan. Clin. Infect. Dis. 52:153–154. 10.1093/cid/ciq054 [DOI] [PubMed] [Google Scholar]
- 37.Khajuria A, Praharaj AK, Grover N, Kumar M. 2013. First report of blaNDM-1 in Raoultella ornithinolytica. Antimicrob. Agents Chemother. 57:1092–1093. 10.1128/AAC.02147-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sidjabat H, Nimmo GR, Walsh TR, Binotto E, Htin A, Hayashi Y, Li J, Nation RL, George N, Paterson DL. 2011. Carbapenem resistance in Klebsiella pneumoniae due to the New Delhi metallo-β-lactamase. Clin. Infect. Dis. 52:481–484. 10.1093/cid/ciq178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoang TH, Wertheim H, Minh NB, Duong TN, Anh DD, Phuong TT, Son TH, Izumiya H, Ohnishi M, Shibayama K, Hien NT. 2013. Carbapenem-resistant Escherichia coli and Klebsiella pneumoniae strains containing New Delhi metallo-beta-lactamase isolated from two patients in Vietnam. J. Clin. Microbiol. 51:373–374. 10.1128/JCM.02322-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poirel L, Benouda A, Hays C, Nordmann P. 2011. Emergence of NDM-1-producing Klebsiella pneumoniae in Morocco. J. Antimicrob. Chemother. 66:2781–2783. 10.1093/jac/dkr384 [DOI] [PubMed] [Google Scholar]
- 41.Samuelsen O, Thilesen CM, Heggelund L, Vada AN, Kummel A, Sundsfjord A. 2011. Identification of NDM-1-producing Enterobacteriaceae in Norway. J. Antimicrob. Chemother. 66:670–683. 10.1093/jac/dkq483 [DOI] [PubMed] [Google Scholar]
- 42.Peirano G, Ahmed-Bentley J, Woodford N, Pitout J. 2011. New Delhi metallo-β-lactamase from traveler returning to Canada. Emerg. Infect. Dis. 17:242–244. 10.3201/eid1702.101313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hrabak J, Stolbova M, Studentova V, Fridrichova M, Chudackova E, Zemlickova H. 2012. NDM-1 producing Acinetobacter baumannii isolated from a patient repatriated to the Czech Republic from Egypt, July 2011 Euro Surveill. 17:pii=20085 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20085 [PubMed] [Google Scholar]
- 44.Oteo J, Domingo-Garcia D, Fernandez-Romero S, Saez D, Guiu A, Cuevas O, Lopez-Brea M, Campos J. 2012. Abdominal abscess due to NDM-1-producing Klebsiella pneumoniae in Spain. J. Med. Microbiol. 61:864–867. 10.1099/jmm.0.043190-0 [DOI] [PubMed] [Google Scholar]
- 45.Clinical and Laboratory Standards Institute 2013. Performance standards for antimicrobial susceptibility testing; 23rd informational supplement. CLSI M100-S23. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]