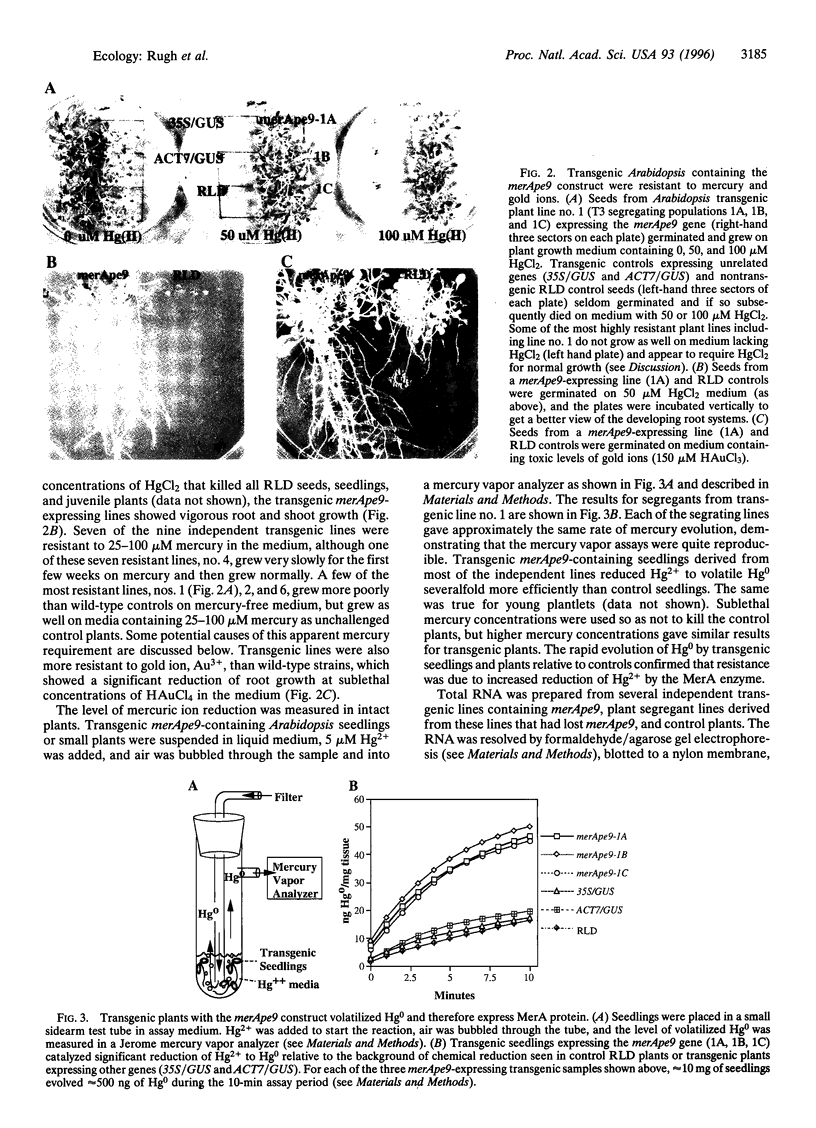
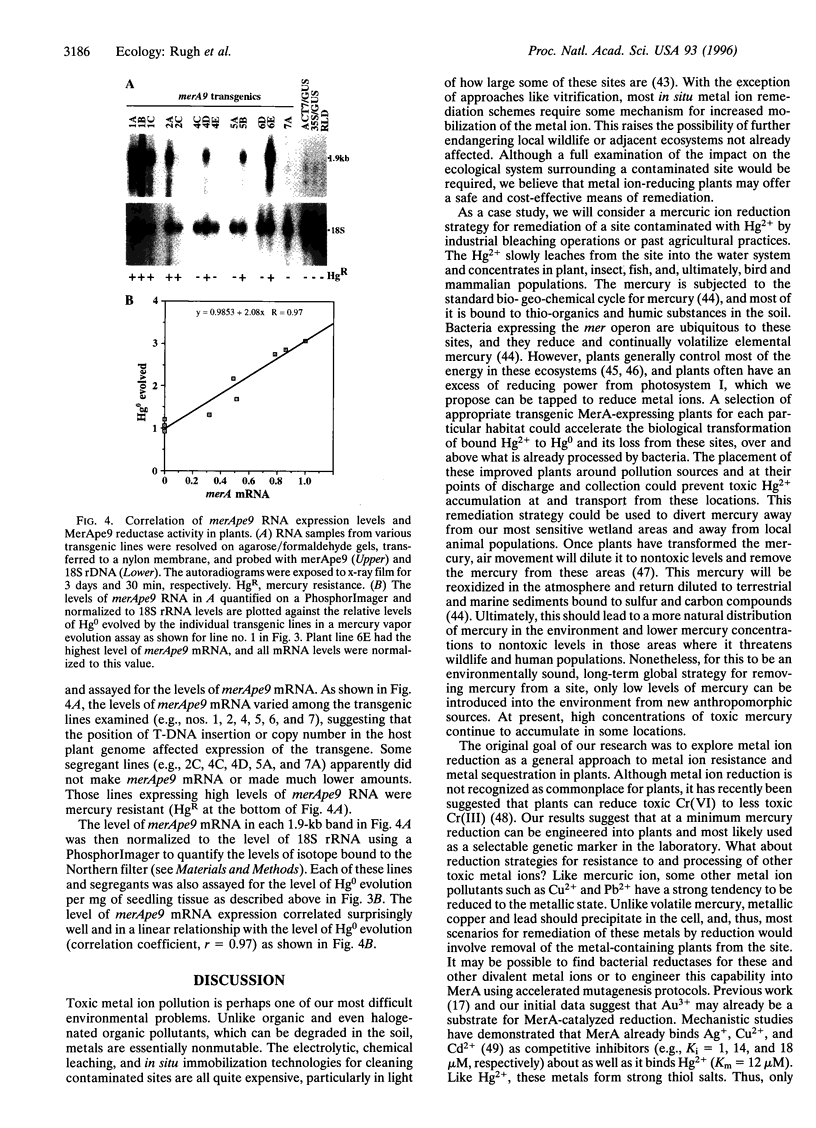
Abstract
With global heavy metal contamination increasing, plants that can process heavy metals might provide efficient and ecologically sound approaches to sequestration and removal. Mercuric ion reductase, MerA, converts toxic Hg2+ to the less toxic, relatively inert metallic mercury (Hg0) The bacterial merA sequence is rich in CpG dinucleotides and has a highly skewed codon usage, both of which are particularly unfavorable to efficient expression in plants. We constructed a mutagenized merA sequence, merApe9, modifying the flanking region and 9% of the coding region and placing this sequence under control of plant regulatory elements. Transgenic Arabidopsis thaliana seeds expressing merApe9 germinated, and these seedlings grew, flowered, and set seed on medium containing HgCl2 concentrations of 25-100 microM (5-20 ppm), levels toxic to several controls. Transgenic merApe9 seedlings evolved considerable amounts of Hg0 relative to control plants. The rate of mercury evolution and the level of resistance were proportional to the steady-state mRNA level, confirming that resistance was due to expression of the MerApe9 enzyme. Plants and bacteria expressing merApe9 were also resistant to toxic levels of Au3+. These and other data suggest that there are potentially viable molecular genetic approaches to the phytoremediation of metal ion pollution.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrineau P., Gilbert P., Jackson W. J., Jones C. S., Summers A. O., Wisdom S. The DNA sequence of the mercury resistance operon of the IncFII plasmid NR1. J Mol Appl Genet. 1984;2(6):601–619. [PubMed] [Google Scholar]
- Barrineau P., Summers A. O. A second positive regulatory function in the mer (mercury resistance) operon. Gene. 1983 Nov;25(2-3):209–221. doi: 10.1016/0378-1119(83)90225-1. [DOI] [PubMed] [Google Scholar]
- Eckenrode V. K., Arnold J., Meagher R. B. Comparison of the nucleotide sequence of soybean 18S rRNA with the sequences of other small-subunit rRNAs. J Mol Evol. 1984;21(3):259–269. doi: 10.1007/BF02102358. [DOI] [PubMed] [Google Scholar]
- Fox B. S., Walsh C. T. Mercuric reductase: homology to glutathione reductase and lipoamide dehydrogenase. Iodoacetamide alkylation and sequence of the active site peptide. Biochemistry. 1983 Aug 16;22(17):4082–4088. doi: 10.1021/bi00286a014. [DOI] [PubMed] [Google Scholar]
- Fox B., Walsh C. T. Mercuric reductase. Purification and characterization of a transposon-encoded flavoprotein containing an oxidation-reduction-active disulfide. J Biol Chem. 1982 Mar 10;257(5):2498–2503. [PubMed] [Google Scholar]
- Hamlett N. V., Landale E. C., Davis B. H., Summers A. O. Roles of the Tn21 merT, merP, and merC gene products in mercury resistance and mercury binding. J Bacteriol. 1992 Oct;174(20):6377–6385. doi: 10.1128/jb.174.20.6377-6385.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower R. C., Meagher R. B. Divergence and differential expression of soybean actin genes. EMBO J. 1985 Jan;4(1):1–8. doi: 10.1002/j.1460-2075.1985.tb02309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Lee K. A., Guertin D., Sonenberg N. mRNA secondary structure as a determinant in cap recognition and initiation complex formation. ATP-Mg2+ independent cross-linking of cap binding proteins to m7I-capped inosine-substituted reovirus mRNA. J Biol Chem. 1983 Jan 25;258(2):707–710. [PubMed] [Google Scholar]
- McKinney E. C., Ali N., Traut A., Feldmann K. A., Belostotsky D. A., McDowell J. M., Meagher R. B. Sequence-based identification of T-DNA insertion mutations in Arabidopsis: actin mutants act2-1 and act4-1. Plant J. 1995 Oct;8(4):613–622. doi: 10.1046/j.1365-313x.1995.8040613.x. [DOI] [PubMed] [Google Scholar]
- Moore M. D., Kaplan S. Identification of intrinsic high-level resistance to rare-earth oxides and oxyanions in members of the class Proteobacteria: characterization of tellurite, selenite, and rhodium sesquioxide reduction in Rhodobacter sphaeroides. J Bacteriol. 1992 Mar;174(5):1505–1514. doi: 10.1128/jb.174.5.1505-1514.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray E. E., Lotzer J., Eberle M. Codon usage in plant genes. Nucleic Acids Res. 1989 Jan 25;17(2):477–498. doi: 10.1093/nar/17.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nriagu J. O. A silent epidemic of environmental metal poisoning? Environ Pollut. 1988;50(1-2):139–161. doi: 10.1016/0269-7491(88)90189-3. [DOI] [PubMed] [Google Scholar]
- Nriagu J. O., Pacyna J. M. Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature. 1988 May 12;333(6169):134–139. doi: 10.1038/333134a0. [DOI] [PubMed] [Google Scholar]
- Ogata M., Aikoh H. Mechanism of metallic mercury oxidation in vitro by catalase and peroxidase. Biochem Pharmacol. 1984 Feb 1;33(3):490–493. doi: 10.1016/0006-2952(84)90246-6. [DOI] [PubMed] [Google Scholar]
- Phillips G. J., Kushner S. R. Determination of the nucleotide sequence for the exonuclease I structural gene (sbcB) of Escherichia coli K12. J Biol Chem. 1987 Jan 5;262(1):455–459. [PubMed] [Google Scholar]
- Platt D., Abshagen U., Mühlberg W., Horn H. J., Schmitt-Rüth R., Vollmar J. The influence of age and multimorbidity on the pharmacokinetics and metabolism of spironolactone. Arch Gerontol Geriatr. 1984 Jul;3(2):147–159. doi: 10.1016/0167-4943(84)90006-2. [DOI] [PubMed] [Google Scholar]
- Rensing C., Kües U., Stahl U., Nies D. H., Friedrich B. Expression of bacterial mercuric ion reductase in Saccharomyces cerevisiae. J Bacteriol. 1992 Feb;174(4):1288–1292. doi: 10.1128/jb.174.4.1288-1292.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinderle S. J., Booth J. E., Williams J. W. Mercuric reductase from R-plasmid NR1: characterization and mechanistic study. Biochemistry. 1983 Feb 15;22(4):869–876. doi: 10.1021/bi00273a025. [DOI] [PubMed] [Google Scholar]
- Scheller H. V., Huang B., Hatch E., Goldsbrough P. B. Phytochelatin synthesis and glutathione levels in response to heavy metals in tomato cells. Plant Physiol. 1987 Dec;85(4):1031–1035. doi: 10.1104/pp.85.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L. M. Characterization of translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2971–2996. doi: 10.1093/nar/10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers A. O. Organization, expression, and evolution of genes for mercury resistance. Annu Rev Microbiol. 1986;40:607–634. doi: 10.1146/annurev.mi.40.100186.003135. [DOI] [PubMed] [Google Scholar]
- Summers A. O., Sugarman L. I. Cell-free mercury(II)-reducing activity in a plasmid-bearing strain of Escherichia coli. J Bacteriol. 1974 Jul;119(1):242–249. doi: 10.1128/jb.119.1.242-249.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Goldsbrough P. B. Functional homologs of fungal metallothionein genes from Arabidopsis. Plant Cell. 1994 Jun;6(6):875–884. doi: 10.1105/tpc.6.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]