Abstract
Biofilms play a role in the pathogenicity of pneumococcal infections. A pharmacodynamic in vitro model of biofilm was developed that allows characterization of the activity of antibiotics against viability and biomass by using in parallel capsulated (ATCC 49619) and noncapsulated (R6) reference strains. Naive biofilms were obtained by incubating fresh planktonic cultures for 2 to 11 days in 96-well polystyrene plates. Induced biofilms were obtained using planktonic bacteria collected from the supernatant of 6-day-old naive biofilms. Biomass production was more rapid and intense in the induced model, but the levels were similar for both strains. Full concentration responses fitting sigmoidal regressions allowed calculation of maximal efficacies and relative potencies of drugs. All antibiotics tested (amoxicillin, clarithromycin, solithromycin, levofloxacin, and moxifloxacin) were more effective against young naive biofilms than against old or induced biofilms, except macrolides/ketolides, which were as effective at reducing viability in 2-day-old naive biofilms and in 11-day-old induced biofilms of R6. Macrolides/ketolides, however, were less potent than fluoroquinolones against R6 (approximately 5- to 20-fold-higher concentrations needed to reduction viability of 20%). However, at concentrations obtainable in epithelial lining fluid, the viabilities of mature or induced biofilms were reduced 15 to 45% (amoxicillin), 17 to 44% (macrolides/ketolides), and 12 to 64% (fluoroquinolones), and biomasses were reduced 5 to 45% (amoxicillin), 5 to 60% (macrolides/ketolides), and 10 to 76% (fluoroquinolones), with solithromycin and moxifloxacin being the most effective and the most potent agents (due to lower MICs) in their respective classes. This study allowed the ranking of antibiotics with respect to their potential effectiveness in biofilm-related infections, underlining the need to search for still more effective options.
INTRODUCTION
Biofilm has been defined as a “microbially derived sessile community characterized by cells that are irreversibly attached to a substratum or interface or to each other, are embedded in a matrix of extracellular polymeric substance that they have produced, and exhibit an altered phenotype with respect to growth rate and gene transcription” (1). Biofilms are now considered to play a major role in pathogenesis, with more than 60% of all human bacterial infections possibly being associated with microbial growth within this type of structure (2). Persistence or recurrence of biofilm-associated infections may stem not only from their role as a reservoir for secondary bacterial dissemination (2, 3) or their interference with the host's responses (prevention of phagocytosis) (4, 5) but also from their capacity to impair antibiotic action. Possible factors decreasing antibiotic activity include diffusion barrier effects and phenotypic or metabolic variations accompanying the switch from a planktonic to a sessile mode of life that reduce their susceptibility to antibiotics (6–8).
Biofilms can develop on artificial surfaces, like medical devices, but also on tissues or mucus, as observed, for example, with Streptococcus pneumoniae in nasopharynx colonization (9), otitis media (9–11), or chronic rhinosinusitis (12). Therefore, in vitro (13–18) and in vivo (11, 19, 20) models of pneumococcal biofilms have been developed and used to study the pathophysiology of the infection as well as the activities of the antibiotics. None of these studies, however, developed a comprehensive and comparative pharmacodynamic model of the activity of antibiotics against biofilms of S. pneumoniae. Moreover, they focused on short maturity stages (14, 17, 21–25) that are probably poorly representative of the types of biofilms that develop in chronic infections or in infections occurring in deep airways (25, 26).
In the present work, we have set up in vitro models of pneumococcal biofilms at both young and old maturity stages in an attempt to mimic what takes place during short- and long-term infections by S. pneumoniae. The first model consists of naive biofilms, in which freshly grown bacteria are allowed to adhere on multiwell plates and to form a biofilm for up to 11 days. A second model consists of induced biofilms, in which bacteria collected from the supernatant of naive biofilms are used as a starting inoculum. This model may better take into account the adaptative process mediated by the quorum sensing molecules that takes place during biofilm maturation (27, 28) and which was already well demonstrated to take place with clinical isolates form other bacterial species (29, 30). The models have been tested with antibiotics representative of the 3 main classes of antibiotics active against S. pneumoniae, namely, amoxicillin (for β-lactams), clarithromycin (for macrolides), and levofloxacin and moxifloxacin (for fluoroquinolones). We also included solithromycin, a fluoroketolide active against macrolide-resistant strains (31) that has successfully completed phase II clinical trials in moderate to moderately severe community-acquired pneumonia (32).
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. pneumoniae (Klein) Chester reference strain ATCC 49619 (capsulated [serotype 19 F]; isolated from the sputum of a 75-year-old male) (33) and R6 ATCC BAA-255 (uncapsulated; derived from the capsulated clinical isolate D39) (34–36) were grown on Mueller-Hinton blood agar plates supplemented with 5% defibrinated horse blood at 37°C in a 5% CO2 atmosphere.
Antibiotics.
The tested antibiotics were obtained as microbiological standards from the following sources: clarithromycin (potency, 100%) from Teva Laboratories (Paris, France), solithromycin (potency, 100%) from Cempra Pharmaceuticals (Chapel Hill, NC), levofloxacin hemihydrate (potency, 97.5%) from Sanofi-Aventis Deutschland GmbH (Frankfurt, Germany), and moxifloxacin chlorhydrate (potency, 90.9%) from Bayer Schering Pharma AG (Berlin, Germany). Amoxicillin (potency, 100%) was procured as the corresponding branded product for human parenteral use distributed for clinical use in Belgium as Clamoxyl iv/im by GlaxoSmithKline s.a/n.v (Genval, Belgium). Sterile stock solutions of each antibiotic were prepared according to the manufacturer's instructions.
Susceptibility testing.
MICs were determined by microdilution following the guidelines of the Clinical and Laboratory Standards Institute (37) using cation-adjusted Mueller-Hinton broth (CA-MHB) (Becton, Dickinson and Company, Franklin Lakes, NJ) supplemented with 5% lysed horse blood, starting from overnight bacterial cultures diluted to an optical density at 620 nm (OD620) of 0.08 to 0.1 (corresponding to 0.5 McFarland standard). MICs were read after 18 to 24 h of incubation at 37°C.
Development of naive and induced biofilm models.
Ninety-six-well plates (European catalog no. 734-2327; VWR, Radnor, PA) were used as the support for the biofilm growth. In each well, 25 μl of bacterial culture (OD620 of 0.1) was added aseptically to 175 μl of cation-adjusted Mueller-Hinton broth (Becton, Dickinson Company, Franklin Lakes, NJ) supplemented with 5% lysed horse blood and 2% glucose (Sigma-Aldrich, St. Louis, MO). In preliminary experiments, we showed that biofilm formation was increased if CA-MHB supplemented with 5% lysed horse blood is used instead of Todd-Hewitt broth supplemented with 0.5% yeast extract and, for both of these media, by addition of 2% glucose. Under these conditions, the initial inoculum was approximately 5 × 107 CFU/ml (4.92 ± 1.22 107 CFU/ml for strain ATCC 49619 and 5.18 ± 0.64 107 CFU/ml for strain R6 in preliminary experiments [in triplicate from 2 independent pilot experiments]). The naive model of biofilm was obtained by incubating these plates for 2 to 11 days with medium replacement every 48 h. The induced model was produced by starting with an inoculum of 25 μl of the supernatant (free bacteria) from a 6-day-old biofilm, corresponding to an initial bacterial density of approximately 8.5 × 107 to 9 × 107 CFU/ml (8.5 ± 0.4 107 CFU/ml for strain ATCC 49619 and 8.9 ± 1.1 107 CFU/ml for strain R6, respectively, in preliminary experiments [in triplicate from 2 independent pilot experiments]). Biofilm culture was then performed as for the naive model. All cultures were incubated in a 5% CO2 atmosphere.
Determination of biofilm mass (crystal violet staining).
Biofilm mass was evaluated by measuring the absorbance of crystal violet, a cationic dye that quantitatively stains nonspecifically negatively charged biofilm constituents based on ionic interactions (38). After elimination of the medium, wells were washed once with phosphate-buffered saline (PBS) and dried for 1 h at 60°C, after which 150 μl of crystal violet (2.3% solution in 20% ethanol [Sigma-Aldrich, St. Louis, MO]) was added to each well and left at room temperature for 10 min. After the stain had been poured out, wells were washed under running water for 5 min, and the plates were dried. The dye bound to the plate was solubilized and homogenized by 1 h of incubation with 200 μl of 33% acetic acid. The absorbance of each well was measured at 570 nm using a microplate spectrophotometer (VersAmax Tunable microplate reader; Molecular Devices, Sunnyvale, CA).
Determination of bacterial viability within the biofilm by using resazurin.
Viability was determined using resazurin, a blue phenoxazin dye that is reduced by viable bacteria to the pink fluorescent compound resorufin (39, 40). After elimination of the medium and washing of the wells with PBS at room temperature, 200 μl of a 0.001% resazurin (Sigma-Aldrich) solution in CA-MHB was added to each well. Plates were then incubated at room temperature in the dark, and fluorescence was measured (λexcitation, 560 nm; λemission, 590 nm) thereafter using a microplate spectrofluorometer (SPECTRAmax Gemini XS; Molecular Devices). Preliminary experiments were done to determine the optimal time of incubation before plates were read (see Results and Table 1).
TABLE 1.
Time of incubation with resazurin needed to obtain a maximal fluorescence signal for biofilms of increasing maturity
| Biofilm maturity (days) | Incubation time before fluorescence reading (h) ina: |
|
|---|---|---|
| Naive model | Induced model | |
| 2 | 56 | 4.5 |
| 4 | 32 | 2 |
| 7 | 2 | 1 |
| 11 | 1 | 1 |
Shown are the incubation times necessary to reach the resorufin (RF) maximal fluorescence values measured by fluorimetry during kinetic studies (such as those illustrated for maturity stages of 2 and 11 days in Fig. 2). The studies were done using microplates containing naive and induced biofilms of strains ATCC 49619 and R6, which had maturity stages of 2, 4, 7, and 11 days. For each strain, the values are means of 4 to 8 independent determinations.
Antibiotic activity on bacterial viability within the matrix and on biofilm mass.
At specific stages of biofilm maturity, the culture medium (including unbound planktonic bacteria) was removed and replaced with fresh medium (control), medium supplemented with antibiotics at concentrations ranging from 10−4- to 103-fold their MIC (in order to obtain full concentration-effect relationships and calculate with accuracy the relevant pharmacodynamic parameters), or 1% sodium dodecyl sulfate (SDS), used as a positive control (full destruction of the biofilm and bacterial death) (41). After 24 h of incubation, the biofilm mass and the bacterial viability were measured using the crystal violet and resazurin assays, with data expressed as a percentage of the control value, using the formula [(valueAB − valueSDS)/(valueCT − valueSDS)] × 100, where valueAB, valueSDS, and valueCT are the absorbance or fluorescence signals recorded for biofilms incubated with antibiotic, SDS, or control medium, respectively.
Curve fitting and statistical analyses.
Curve fitting analyses were made using Graph-Pad Prism version 4.03 (GraphPad Software, San Diego, CA). Data were used to fit a sigmoid function (Hill equation, slope factor set to 1) by nonlinear regression. The fitted function was then used to determine two key pharmacodynamic descriptors of antibiotic activity, namely, (i) the relative maximal efficacy (Emax; maximal reduction in biofilm mass or viability as extrapolated for an infinitely large antibiotic concentration) and (ii) the relative potency (C20 or C50, i.e., the antibiotic concentration needed to achieve 20 or 50% reduction in bacterial viability within the biofilm or in biofilm mass). Confidence intervals at 95% (95% CI) and the standard errors of the mean (SEM) for the parameters of the Hill equation (Emin, Emax, and 50% effective concentration [EC50]) were obtained from GraphPad. SEM on log C20 were calculated as [log C20 (+5%) − log C20 (−5%)]/(2 × 1.96), where C20 (+5%) and C20 (−5%) are the concentrations yielding a 20% reduction in signal as calculated from the equations of the curves delimiting the 95% CI. Statistical analyses were performed with Graph Pad Instat version 3.06 (GraphPad Software).
RESULTS
Characterization of biofilm formation in the naive and induced models and validation of the methods of assay (biomass and viability).
In the first series of experiments, we compared the increases in biofilm mass over time for strains ATCC 49619 (capsulated) and R6 (uncapsulated). Figure 1 shows that with fresh planktonic cultures (naive model), crystal violet staining started to exponentially increase after about 5 days to reach an apparent plateau at day 8. This suggests that bacteria at days 6 to 8 are probably in a metabolic state that actively produces biofilm. We therefore developed a second model (referred to as the “induced” model), in which biofilm growth was initiated using planktonic bacteria collected from the supernatant of 6-day-old biofilms. With these bacteria, the biofilm mass started to increase after only 4 days to reach, after 8 to 9 days, a value that was 3 times higher than that of the naive model.
FIG 1.
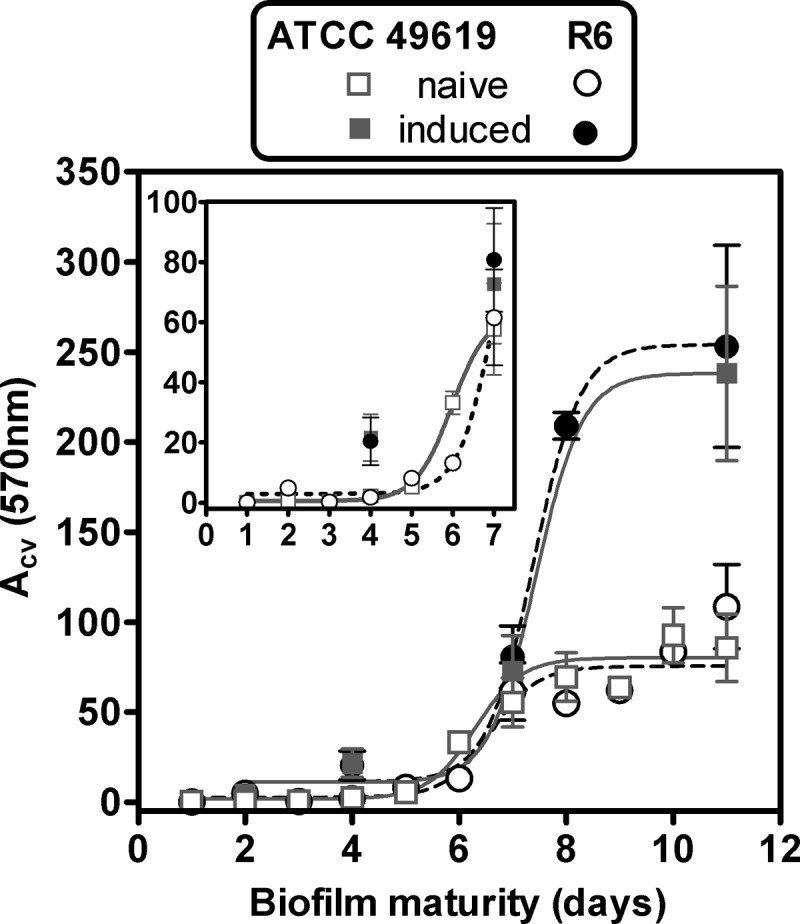
Evolution over time of matrix production (as evaluated by crystal violet [CV] absorbance) by the capsulated strain ATCC 49619 (gray squares) and the noncapsulated strain R6 (black circles) in the naive model (dotted lines, open symbols) and the induced model (solid lines, closed symbols). The inset shows the same data at higher scale for the first 7 days of incubation. All values are means ± standard deviations (SD) of 8 to 28 independent determinations. When not visible, the SD bars are smaller than the size of the symbols.
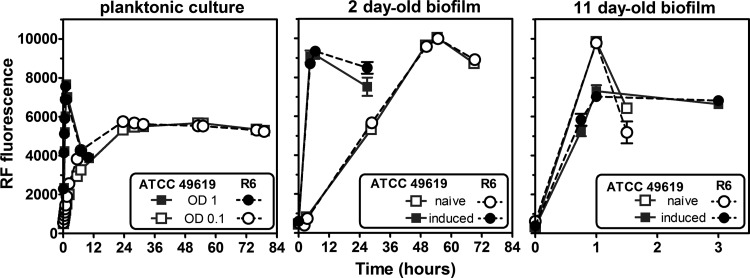
In the second series of experiments, we validated our viability assay based on the reduction of resazurin into resorufin. The rate at which this reduction occurs is indeed dependent on both the biofilm maturity and the number of metabolically active bacteria, but the reaction product may undergo additional enzymatic and nonenzymatic transformation(s) (42, 43), causing the fluorescent signal to increase and then decrease over time. Figure 2 shows the change in fluorescence recorded over time upon incubation at room temperature for (i) planktonic cells at different densities, and (ii) sessile cells in (a) young (2 days) and old (11 days) biofilms and (b) naive and induced biofilms. Under all conditions, the signal increased until it reached a maximal value, after which fluorescence remained stable (low-density planktonic culture or old induced biofilms) or decreased. The time needed to reach the maximal value was much longer for (i) planktonic cells at low density versus high density (left panel), (ii) young biofilms versus old biofilms (compare middle and right panels), and (iii) young naive versus young induced biofilms (middle panel). Accordingly, and for all subsequent experiments, fluorescence recordings were made at the fixed times shown in Table 1, based upon the type of sample examined. Using crystal violet staining, we checked that no biofilm growth occurred during incubation with resazurin, even when prolonged for more than 48 h, probably due to the fact that incubation with resazurin was performed in CA-MHB, which is not appropriate for growth as a biofilm.
FIG 2.
Evolution of resorufin (RF) fluorescence overtime with the capsulated strain ATCC 49619 (gray squares) and the noncapsulated strain R6 (black circles) in planktonic cultures (left panel) using starting inocula at an OD620 of 0.1 (open symbols) or 1 (closed symbols) or in biofilms (middle and right panels) at different maturity stages (2- and 11-day-old naive [open symbols] and induced [closed symbols] models). All values are means ± standard deviations (SD) of 3 independent determinations. When not visible, the SD bars are smaller than the size of the symbols.
Susceptibility testing.
The MICs of the antibiotics under study for the strains ATCC 49619 and R6 are shown in Table 2. Both strains were highly susceptible to all antibiotics, with solithromycin and levofloxacin demonstrating the highest and lowest activities, respectively.
TABLE 2.
MICs of antibiotics against the strains used in this study
| Antibiotic by class | MIC (mg/liter) for: |
|
|---|---|---|
| ATCC 49619 | R6 | |
| β-Lactams | ||
| Amoxicillin | 0.064 | 0.032 |
| Macrolides/ketolides | ||
| Clarithromycin | 0.032 | 0.064 |
| Solithromycin | 0.008 | 0.004 |
| Fluoroquinolones | ||
| Levofloxacin | 1 | 0.5 |
| Moxifloxacin | 0.125 | 0.064 |
Activities of antibiotics against biofilms (viability and biofilm mass).
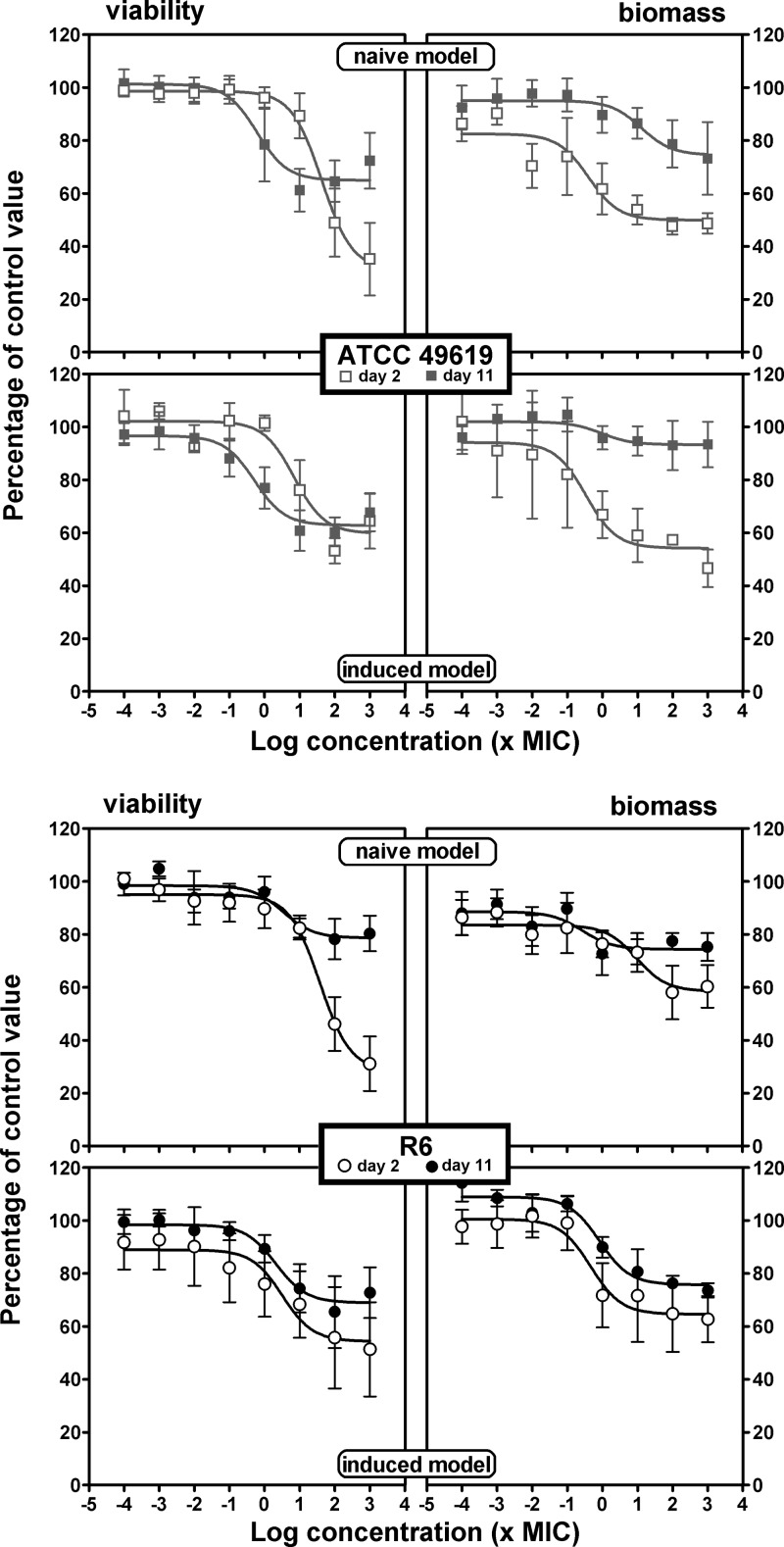
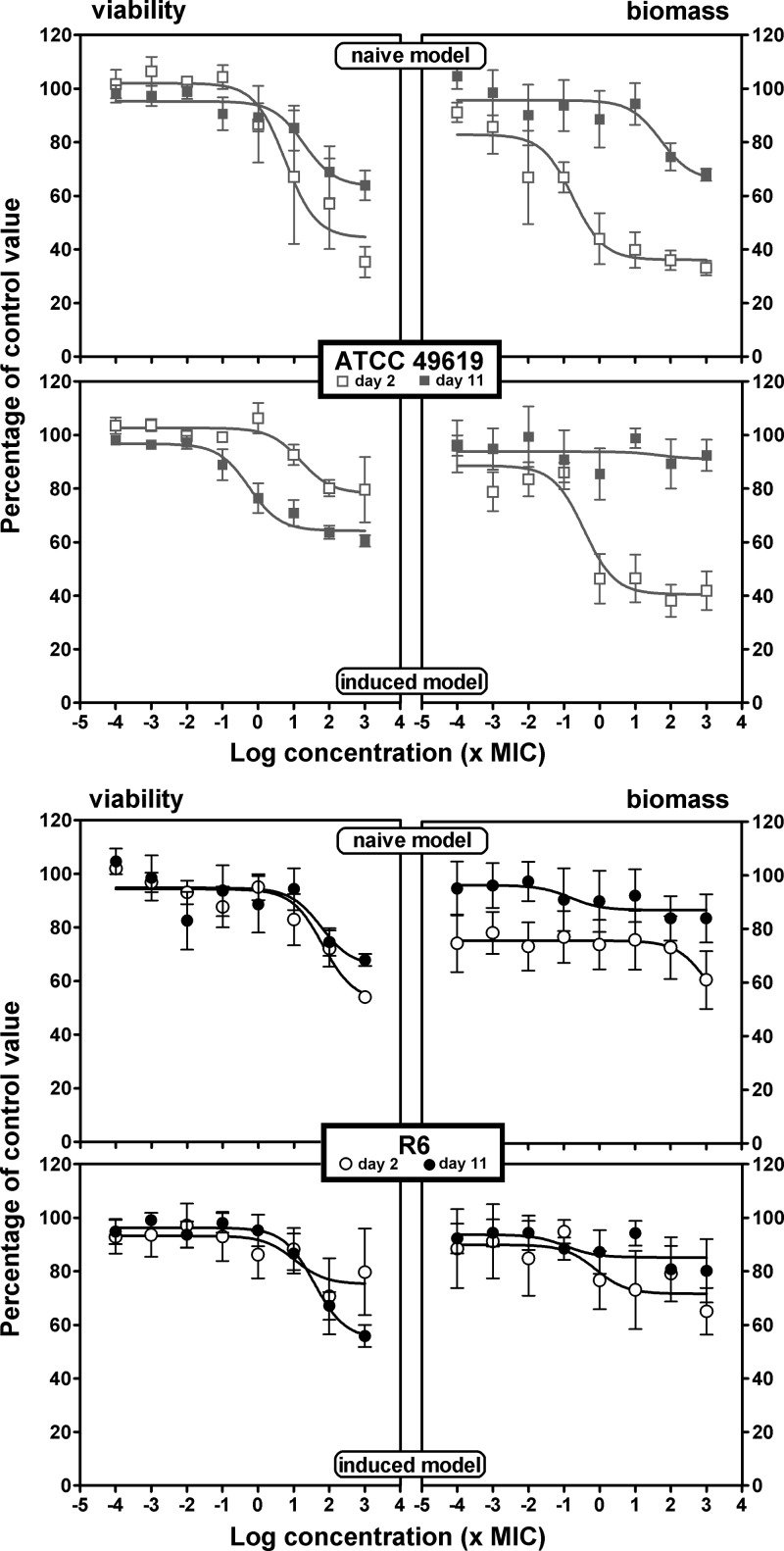
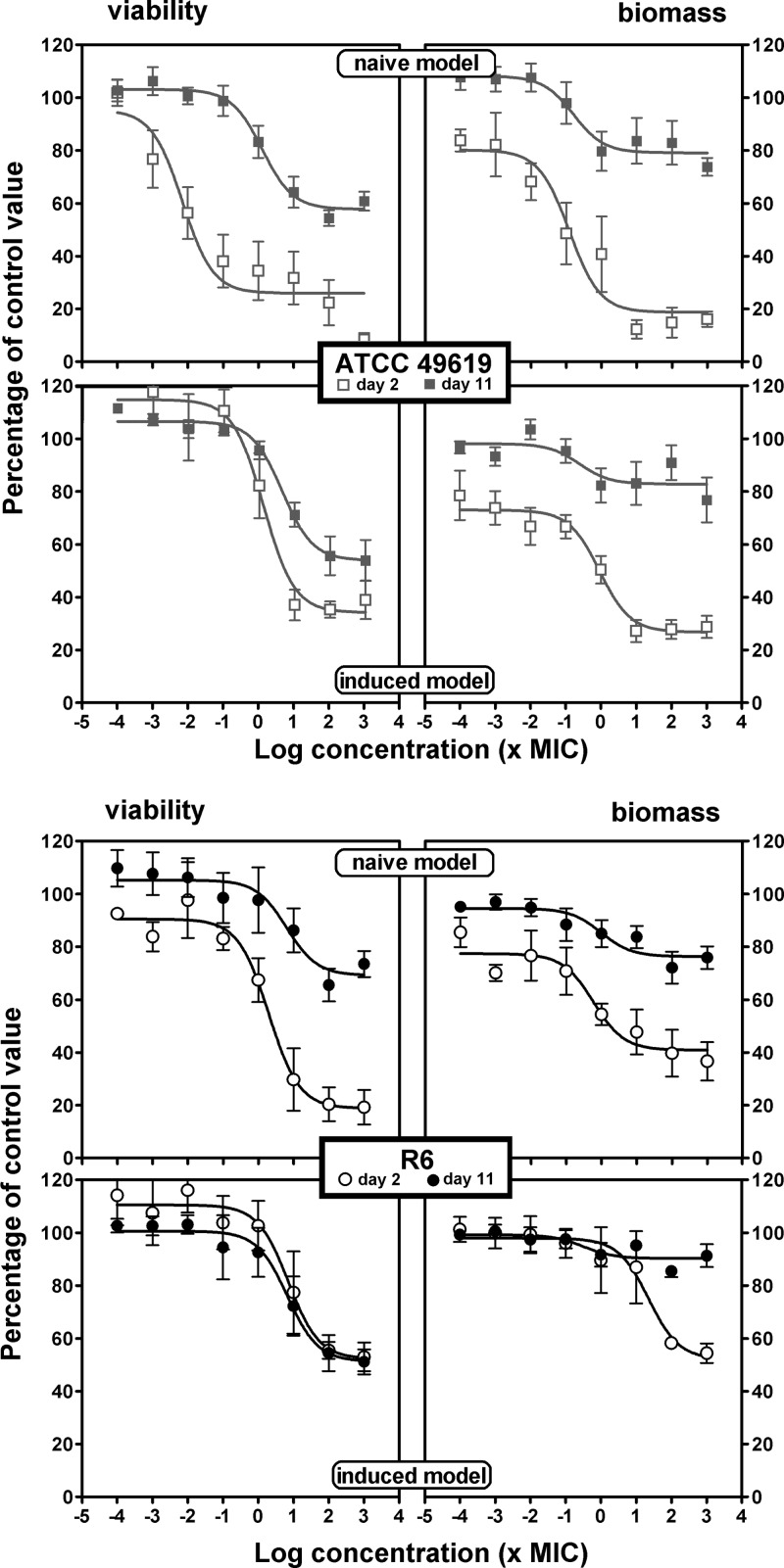
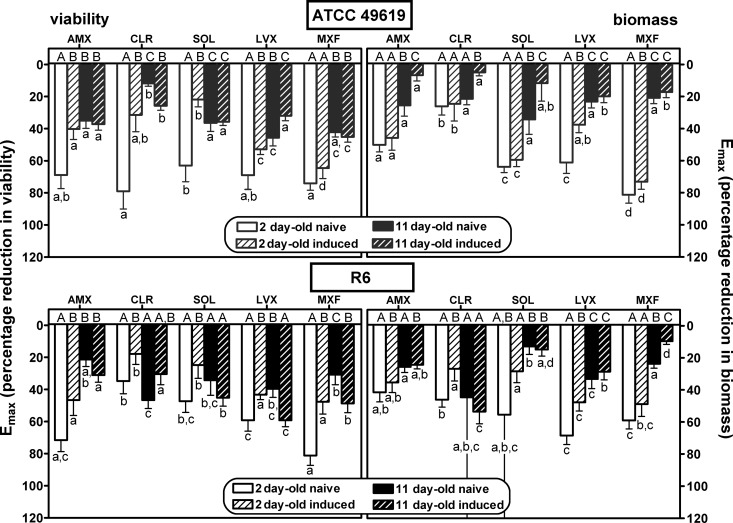
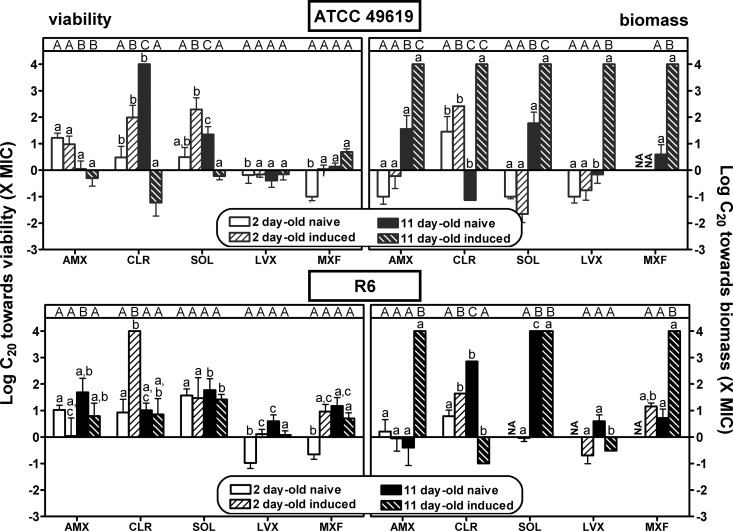
In the viability and biofilm mass experiments, we measured the effect of antibiotics on bacterial survival and biofilm mass after 24 h of incubation. We systematically compared naive and induced biofilms and, in each of these two models, the effects seen with young (2 days old) and old (11 days old) biofilms. Antibiotics were added to the medium over a wide range of concentrations to obtain full concentration-effect responses. Sigmoidal functions (Hill equations) with a slope factor of 1 could be fitted to all sets of data when plotted against the log10 value of the antibiotic concentration, which allowed direct comparison of the antibiotic maximal efficacies (Emax) and their relative potencies (C20 or C50). Graphical representations are shown in Fig. 3 (amoxicillin), 4 (solithromycin), and 5 (moxifloxacin), with additional antibiotics presented in the supplemental material (clarithromycin in Fig. S1 and levofloxacin in Fig. S2). Pharmacodynamic parameters evaluating relative efficacy and relative potency are compared in a pictorial fashion in Fig. 6 and 7, with numerical data provided as supplemental material (see Tables S1 and S2 for the ATCC 49619 and R6 strains, respectively).
FIG 3.
Concentration-response activity of amoxicillin against biofilms of ATCC 49619 (top) or R6 (bottom). Two-day-old (open symbols) or 11-day-old (closed symbols) biofilms from the naive model (upper panels for each strain) or the induced model (lower panels for each strain) were incubated with increasing concentrations of antibiotics for 24 h. The ordinate shows the change in viability (measured by the decrease in resorufin fluorescence [left panels]) or in biofilm mass (measured by the decrease in crystal violet absorbance [right panels]) as a percentage of the control value (no antibiotic present). All values are means ± SEM of 4 to 10 independent experiments performed in quadruplicate. When not visible, the error bars are smaller than the size of the symbols. The pertinent pharmacological descriptors of the curves are presented in Tables S1 and S2 in the supplemental material.
FIG 4.
Concentration-response activity of solithromycin against biofilms of ATCC 49619 (top) or R6 (bottom). Two-day-old (open symbols) or 11-day-old (closed symbols) biofilms from the naive model (upper panels for each strain) or the induced model (lower panels for each strain) were incubated with increasing concentrations of solithromycin for 24 h. The ordinate shows the change in viability (measured by the decrease in resorufin fluorescence [left panels]) or in biofilm mass (measured by the decrease in crystal violet absorbance [right panels]) as a percentage of the control value (no antibiotic present). All values are means ± SEM of 4 to 10 independent experiments performed in quadruplicate. When not visible, the error bars are smaller than the size of the symbols. The pertinent pharmacological descriptors of the curves are presented in Tables S1 and S2 in the supplemental material.
FIG 5.
Concentration-response activity of moxifloxacin against biofilms of ATCC 49619 (top) or R6 (bottom). Two-day-old (open symbols) or 11-day-old (closed symbols) biofilms from the naive model (upper panels for each strain) or the induced model (lower panels for each strain) were incubated with increasing concentrations of moxifloxacin for 24 h. The ordinate shows the change in viability (measured by the decrease in resorufin fluorescence [left panels]) or in biofilm mass (measured by the decrease in crystal violet absorbance [right panels]) as a percentage of the control value (no antibiotic present). All values are means ± SEM of 4 to 10 independent experiments performed in quadruplicate. When not visible, the error bars are smaller than the size of the symbols. The pertinent pharmacological descriptors of the curves are presented in Tables S1 and S2 in the supplemental material.
FIG 6.
Comparison of antibiotic maximal efficacies (Emax) expressed as percentages of reduction in viability (left panels) or biomass (right panels) compared to that in the control (no antibiotic) for 2- and 11-day-old naive and induced biofilms of strain ATCC 49619 (upper panels [gray bars]) or R6 (lower panels [black bars]). AMX, amoxicillin; CLR, clarithromycin; SOL, solithromycin; LVX, levofloxacin; MXF, moxifloxacin. Values were calculated as means ± SEM using the Hill equation of the concentration-response curves presented in Fig. 3 to 5 and Fig. S1 and S2 in the supplemental material. (Also see Tables S1 and S2 in the supplemental material for numerical values.) Statistical analyses were performed by one-way analysis of variance (ANOVA) with Tukey's posttest for multiple comparisons; values with different letters are significantly different from each other (P < 0.05). Lowercase letters indicate comparison between antibiotics for each type of biofilm, and capital letters indicate comparison between different types of biofilms for each antibiotic.
FIG 7.
Comparison of antibiotic relative potencies (C20) expressed in multiples of the MIC with respect to viability (left panels) or biomass (right panels) for 2- and 11-day-old naive and induced biofilms of strain ATCC 49619 (upper panels [gray bars]) or R6 (lower panels [black bars]). AMX, amoxicillin; CLR, clarithromycin; SOL, solithromycin; LVX, levofloxacin; MXF, moxifloxacin. Values were calculated as means ± SEM (calculated from the 95% confidence interval band around the curve) using the Hill equation of the concentration-response curves presented in Fig. 3 to 5 and Fig. S1 and S2 in the supplemental material. (Also see Tables S1 and S2 in the supplemental material for numerical values.) Statistical analyses were performed by one-way ANOVA with Tukey's posttest for multiple comparisons; values with different letters are significantly different from each other (P < 0.05). Lowercase letters indicate comparison between antibiotics for each type of biofilm, and capital letters indicate comparison between different types of biofilms for each antibiotic. NA, not applicable (a “Top” value of the Hill equation of <80%). When not reached at the maximal value tested, C20 values were set at 4 (log scale).
Considering first the effect of antibiotics on viability (left panels) and focusing on efficacy (maximal effect), we see that all antibiotics were globally most effective against 2-day-old naive biofilms, with a loss of viability ranging from 35% (clarithromycin for R6) to 81% (moxifloxacin) compared to control values (no antibiotic added). Eleven-day-old naive biofilms and 2- or 11-day-old induced biofilms showed much less reduction of viability that did not exceed approximately 40% for amoxicillin, clarithromycin, and solithromycin and reached 32 to 65% for levofloxacin and moxifloxacin, respectively. No systematic difference in efficacies was observed between biofilms formed with the ATCC 49619 and those with R6, except again for macrolides (clarithromycin and solithromycin), which were poorly active against biofilms formed with the R6 strain, even if young and naive. Examination then of relative potencies, showed that fluoroquinolones were more potent (lower C20 values, close to the MIC) than the other drugs against 2-day-old naive biofilms, while macrolides/ketolides were systematically less potent against 2-day-old induced or 11-day-old naive biofilms of strain ATCC 49619. C50 values were much higher or could not be determined under most conditions.
Considering now the activity of antibiotics on biomass (right panels), we globally see that the effects, although developing often in parallel to those described for viability, resulted in much lower maximal efficacy (no more than 50% reduction) for amoxicillin and clarithromycin, whatever the condition. Solithromycin showed a much larger maximal efficacy than clarithromycin against ATCC 49619, except against 11-day-old induced biofilms. Conversely, no systematic difference was seen for strain R6 between these 3 antibiotics. Globally, fluoroquinolones were the most active at reducing the biomass of young biofilms (especially moxifloxacin), but this difference from the other antibiotics was not maintained with 11-day-old biofilms in either naive or induced models. Against 2-day-old biofilms, C20 values were globally similar to those observed against viability, with only amoxicillin and solithromycin showing slightly higher potencies (lower C20 values) against biomass. Against 11-day-old biofilms, potencies were globally low; in many cases, a 20% reduction was not reached even at the highest antibiotic concentration tested.
DISCUSSION
In this study, we have developed an in vitro model that allows (i) a quantification of the biofilm mass and bacterial viability in naive and induced streptococcal biofilms at different stages of maturity and (ii) a pharmacodynamic evaluation of the activity of antibiotics. The model uses the widely accepted polystyrene support (14, 16, 23, 25, 44, 45), but with important changes from previous studies concerning (i) the medium used for biofilm growth, (ii) the maturity stages investigated and the impact of adaptation (naive versus induced biofilm), and (iii) the method used to quantify bacterial viability.
With respect to the culture medium, we optimized the conditions of culture not only by adding 2% glucose as recommended previously to increase biofilm formation (20, 45) but also by selecting the medium recommended by CLSI (37) for culture and susceptibility testing of S. pneumoniae.
With respect to maturity stages, we compared young (2 days) to mature (11 days) biofilms because the latter may represent a more relevant model to study antibiotic activity against persistent forms of infections in deep tissues, where biofilms are suspected to play a role (2, 25, 26). Most of the studies performed so far to evaluate antibiotic activity have indeed used young biofilms only (13, 23, 24), and for those that also considered mature biofilms, only antibiotic effects on the matrix were evaluated (15, 21). We furthermore show that an adaptation process of bacteria is important (viz. growth of induced versus naive biofilms). This is probably related to quorum sensing factors, such as the competence-stimulating peptide (CSP), which is produced during biofilm formation and increases bacterial adherence (46).
With respect to quantification of bacterial viability within the biofilm, the method used, namely, resazurin reduction into resorufin, has already been applied to quantify viability of Staphylococcus aureus in biofilms (39, 40, 47). We showed here that it can be applied to S. pneumoniae biofilms, provided the time after which readings are made is carefully selected to capture the maximal fluorescence signal, which critically depends on both the number of bacteria and the degree of biofilm maturity (since the decrease in the signal can also occur upon too prolonged incubation due to further metabolization in nonfluorescent dihydroresorufin) (42, 48). Because of its proportionality with the number of bacteria, this approach may help in avoiding pitfalls inherent in the other more commonly used method to assess bacterial viability in biofilms, namely, CFU counting after sonication (18, 23, 24, 49, 50). This approach, indeed, was shown to underestimate viability because of the difficulty of quantitatively recovering bacteria from the matrix while at the same time avoiding killing these bacteria (51, 52).
Combining crystal violet staining (for quantification of biomass) with resazurin reduction allows the obtaining of two complementary pieces of information concerning the development of the biofilm, as recently done with S. aureus biofilms (47). With those two tools, we show here that the kinetics of biofilm development of S. pneumoniae are quite different from those of S. aureus with respect to both the rate of attachment and the amount of matrix. Attachment of S. pneumonaie is much slower than for S. aureus biofilms (47). This may result from a lower expression of adhesins in S. pneumoniae than in S. aureus (which produces several adhesins, such as the so-called “microbial surface components recognizing adhesive matrix molecules” [MSCRAMMs] or the polysaccharide intercellular adhesin [PIA]) (53). In streptococci, adhesion capacity is described as being highly variable, depending on the phenotype of the colonies (opaque or transparent) and the presence of a capsule (19, 24), yet, phenotypic variation or downregulation of the capsule may occur during biofilm maturation (15, 54). In our hands, no major difference in biofilm formation and maturation was observed between a capsulated strain and an uncapsulated strain, but the demonstration is of limited value, since the strains are not isogenic and may therefore differ by other characteristics than the presence of a capsule. With respect to matrix, the higher production observed in streptococci compared to staphylococci could be ascribed to the fact that mature staphylococcal biofilms are characterized by a disassembly phenomenon (mediated by secreted proteases or surfactant-like peptides and regulated by depletion in nutrients in the external environment) (55), which may regulate and limit matrix production. Yet, to our knowledge, this process has never been observed for S. pneumoniae biofilms.
We also show here that bacteria released from a preformed biofilm are more prone not only to produce matrix but also to multiply within the biofilm, producing globally thicker structures filled with more bacteria. This suggests that a bacterial adaptation process has taken place during maturation of the naive biofilm. This hypothesis can be placed in correlation with the observation that the protein expression patterns differ between planktonic forms of S. pneumoniae and the same strains growing in biofilms with respect to proteins involved in virulence, adhesion, and resistance (49).
Moving now to the quantitative assessment of antibiotic activity, the first and most salient observation is that all responses (for both biomass and bacterial viability) could be analyzed by using the model (Hill equation) commonly used for the analysis of drug-concentration relationships (56) and already applied by us for the study of antibiotic activities against both extracellular and intracellular Gram-positive and Gram-negative bacteria (57, 58), as well as against S. aureus biofilms (47). This model offers the possibility to clearly distinguish between two distinct properties of antibiotics, namely, (i) their maximal relative efficacy (using the Emax parameter of the Hill equation), which measures the ability of the antibiotic to reduce the biomass or the number of viable bacteria (expressed here as the percentage of the value observed with untreated biofilms) and (ii) their relative potency (the C50 or C20 parameter), which tells us which drug concentration is needed to obtain a given fraction of its maximal effect, taking into account the type and level of maturation of the biofilm. We therefore suggest that the approach proposed here is more informative than the simple determination of MBIC (minimal biofilm inhibitory concentration) (59) performed in other studies (13, 59) and which gives only a static parameter to describe antibiotic activity against biofilms. As clearly shown here, there is a large divergence between the changes in these two key properties when moving from young to mature and from naive to induced biofilms, with the main consistent changes being related to maximal relative efficacies. Thus, and as for S. aureus biofilms (47), antibiotic efficacy for reducing both the bacterial viability and the amount of matrix markedly decreases upon biofilm maturation. However, we see here that antibiotic efficacy is also decreased when biofilms are formed from trained bacteria (induced biofilms) as opposed to untrained ones (naive biofilms). As a result, and quite interestingly, the effect of antibiotics on bacterial viability was, in most cases, weakened to a similar extent for 11-day-old naive biofilms and 2-day-old induced biofilms compared to 2-day-old naive biofilms. Globally also, antibiotics are least effective against 11-day-old induced biofilms. This may have major implications in terms of chemotherapy, since a reduction in maximal efficacy corresponds to a situation in which a sizeable proportion of bacteria become refractive to the bactericidal effects of antibiotics whatever their concentration in the medium.
The following key observations may also require attention. First, we see that the maximal relative efficacies of antibiotics are somewhat lower (less reduction) when examining the decrease of biomass compared to that of viability for 11-day-old naive biofilms or for 2-day-old induced biofilms and become very low against 11-day-old induced biofilm. This is consistent with the fact that antibiotics primarily act on bacteria and not on the matrix and that destructuration, subsequent to bacterial killing, may become more difficult as the matrix becomes thicker. Second, in contrast to the marked and consistent changes seen for maximal relative efficacies, changes in relative potencies were either minimal or nonsystematic when the effects of age or induction were considered. Detailed analysis here is, however, hampered by the fact that reduction in either viability or biomass was often weak as no C50 could be determined. Nevertheless, the data clearly suggest that the effects of maturation and induction on antibiotic activity are related to an apparent reduction in the proportion of reachable targets (bacterial refractory state) and not to a decrease in target apparent affinity (bacterial intrinsic susceptibility). Third, antibiotic maximal efficacies did not differ markedly between the two strains examined, except for clarithromycin and solithromycin, which were more efficient against the capsulated strain in the 2-day-old naive biofilm. This could be related to the capacity of macrolides to downregulate capsule formation (60), since capsule is associated with tolerance to antibiotics (61). Of note also, clarithromycin and solithromycin were less affected than other antibiotics when induced bacteria were used to build up the biofilm. This could be related to the known inhibitory effect of macrolides on quorum sensing (62). Accordingly, macrolides have been shown to increase antibiotic activity on biofilms for S. aureus (63).
Our study suffers from at least three limitations. First, we only used two nonisogenic reference strains rather than a collection of clinical isolates obtained from patients with evidence of in vivo formation of biofilms. The present study must therefore be viewed as a first pharmacological investigation establishing a model and delineating its main properties with respect to a panel of clinically used antibiotics. The model may now be further explored by using isogenic strains differing in their expression, their capsule, or virulence factors and by including clinical strains harboring relevant resistance patterns or different serotypes. Second, biofilms were obtained on an artificial support, which is far from the conditions prevailing in the infected body compartments. However, it has been demonstrated (i) that the gene expression profile of S. pneumoniae isolates collected from lung tissue resembles that of bacteria grown in biofilm in polystyrene plates (7) and (ii) that biofilm-derived pneumococci (represented here by induced biofilms) possess an enhanced ability to adhere to living support, such as polystyrene coated with epithelial cells (64), which is considered a better model to study biofilm development (65). Adhesins expressed in contact with artificial or viable surfaces may be different (64), however, and therefore may affect biofilm properties. Also, there are reports suggesting that the ability to form early biofilms in vitro does not reflect virulence potential in vivo (17) and does not necessarily correlate with the clinical presentation of pneumococcal disease (22). Finally, bacteria were exposed to constant concentrations of antibiotics, and records were made at only one fixed time point. These conditions do not mimic the pharmacokinetic profile of the drugs in the lung and do not inform us about differences in progression of the effects seen. The model, therefore, must be viewed as a first approach open for improvement.
With these limitations, however, our work can be examined in a more clinical perspective, considering the range of antibiotic concentrations reached in human bronchoalveolar lavage fluid (Table 3). Among the drugs investigated, fluoroquinolones and clarithromycin are more effective against 2-day-old naive biofilms, causing an approximately 70 to 80% reduction in viability. Clarithromycin activity, however, is severely hampered today by high resistance rates around the globe (66–68). Against mature or induced biofilms and with the range of clinically relevant concentrations, fluoroquinolones reduce viability more than amoxicillin and macrolides/ketolides, highlighting a potentially greater activity for the first class of drugs. This is consistent with a previous, noncomparative study showing that moxifloxacin, at concentrations that can be achieved in the bronchial mucosa during therapy, was able to inhibit biofilm synthesis and induce slime disruption (23). However, differences among classes are not major, and other considerations, like resistance rates or patients' susceptibility to undesirable effects or drug interactions, are important determinants to take into account in antibiotic selection. Moxifloxacin and solithromycin may thus offer an advantage over the other molecules within their pharmacological class since both molecules are less prone to select resistance than others in their respective class (31, 69). Moreover, moxifloxacin MICs have remained stable over the last 10 years, despite extensive usage (70, 71), and preclinical studies show that solithromycin is barely affected by mechanisms conferring resistance to other macrolides (31, 72).
TABLE 3.
Activities of antibiotics under study on biofilms exposed to concentrations found in epithelial lining fluid
| Antibiotic (daily dose, mg)a | ELFb concn (mg/liter) | Reference | Biofilm modelc | % viability/matrix loss in: |
|||
|---|---|---|---|---|---|---|---|
| ATCC 49619 |
R6 |
||||||
| Viability | Matrix | Viability | Matrix | ||||
| AMX (3,000) | 0.25–1.7 | 73 | 2-day-old naive | 7–27 | 47–50 | 16–44 | 28–38 |
| 11-day-old naive | 30–34 | 10–19 | 15–20 | 25–26 | |||
| 2-day-old induced | 13–32 | 42–45 | 36–44 | 33–35 | |||
| 11-day-old induced | 33–37 | 5–6 | 25–30 | 21–24 | |||
| Strong | 13–37 | 5–45 | 15–44 | 21–35 | |||
| CLR (1,000) | 4–34 | 74 | 2-day-old naive | 74–74 | 24–26 | 79–80 | 42–46 |
| 11-day-old naive | 42–42 | 22–22 | 28–30 | 6–17 | |||
| 2-day-old induced | 22–30 | 16–23 | 17–17 | 33–35 | |||
| 11-day-old induced | 26–26 | 5–5 | 29–30 | 21–24 | |||
| Strong | 22–42 | 5–23 | 17–30 | 6–35 | |||
| SOL (400) | 1–7.6 | 75 | 2-day-old naive | 53–55 | 64–64 | 39–46 | 30–44 |
| 11-day-old naive | 32–36 | 25–33 | 40–44 | 13–13 | |||
| 2-day-old induced | 19–22 | 59–60 | 24–25 | 28–28 | |||
| 11-day-old induced | 36–37 | 8–9 | 40–44 | 15–15 | |||
| Strong | 19–37 | 8–60 | 24–44 | 13–28 | |||
| LVX (1,000) | 2.8–23 | 76 | 2-day-old naive | 74–74 | 53–60 | 63–78 | 60–67 |
| 11-day-old naive | 29–40 | 22–23 | 12–26 | 22–31 | |||
| 2-day-old induced | 37–50 | 34–37 | 35–42 | 40–46 | |||
| 11-day-old induced | 28–31 | 19–20 | 42–56 | 28–29 | |||
| Strong | 28–50 | 19–37 | 12–56 | 22–46 | |||
| MXF (400) | 3.5–20 | 77 | 2-day-old naive | 74–74 | 81–81 | 79–81 | 59–59 |
| 11-day-old naive | 40–42 | 21–21 | 28–30 | 23–24 | |||
| 2-day-old induced | 61–64 | 72–73 | 41–47 | 36–76 | |||
| 11-day-old induced | 38–44 | 17–17 | 44–48 | 10–10 | |||
| Strong | 28–64 | 17–73 | 28–48 | 10–76 | |||
AMX, amoxicillin; CLR, clarithromycin; SOL, solithromycin; LVX, levofloxacin; MXF, moxifloxacin.
ELF, epithelial lining fluid.
“Strong” represents compilation of data obtained for 11-day-old naive biofilms and 2- or 11-day-old induced biofilms.
Supplementary Material
ACKNOWLEDGMENTS
We thank Julia Bauer for expert advice when developing the biofilm model used in this study and Jean-Paul Coutelier (De Duve Institute, Université catholique de Louvain) for access to specialized instrumentation. N.M.V. is Boursière of the Belgian Fonds pour la Recherche dans l'Industrie et l'Agriculture (FRIA), and F.V.B. is Maître de Recherches of the Belgian Fonds de la Recherche Scientifique (FRS-FNRS).
This work was supported by the Fonds de la Recherche Scientifique (grant 3.4530.12) and the Interuniversity Attraction Poles Programme initiated by the Belgian Science Policy Office (program IAP P7/28).
P.M.T. and F.V.B. received research grants from Cempra Pharmaceuticals.
Footnotes
Published ahead of print 16 December 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01858-13.
REFERENCES
- 1.Donlan RM, Costerton JW. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167–193. 10.1128/CMR.15.2.167-193.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moscoso M, Garcia E, Lopez R. 2009. Pneumococcal biofilms. Int. Microbiol. 12:77–85. 10.2436/20.1501.01.84 [DOI] [PubMed] [Google Scholar]
- 3.Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322. 10.1126/science.284.5418.1318 [DOI] [PubMed] [Google Scholar]
- 4.Hall-Stoodley L, Stoodley P. 2009. Evolving concepts in biofilm infections. Cell. Microbiol. 11:1034–1043. 10.1111/j.1462-5822.2009.01323.x [DOI] [PubMed] [Google Scholar]
- 5.Lopez D, Vlamakis H, Kolter R. 2010. Biofilms. Cold Spring Harb. Perspect. Biol. 2:a000398. 10.1101/cshperspect.a000398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart PS. 1996. Theoretical aspects of antibiotic diffusion into microbial biofilms. Antimicrob. Agents Chemother. 40:2517–2522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oggioni MR, Trappetti C, Kadioglu A, Cassone M, Iannelli F, Ricci S, Andrew PW, Pozzi G. 2006. Switch from planktonic to sessile life: a major event in pneumococcal pathogenesis. Mol. Microbiol. 61:1196–1210. 10.1111/j.1365-2958.2006.05310.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simoes M. 2011. Antimicrobial strategies effective against infectious bacterial biofilms. Curr. Med. Chem. 18:2129–2145. 10.2174/092986711795656216 [DOI] [PubMed] [Google Scholar]
- 9.Torretta S, Marchisio P, Drago L, Baggi E, De Vecchi E, Garavello W, Nazzari E, Pignataro L, Esposito S. 2012. Nasopharyngeal biofilm-producing otopathogens in children with nonsevere recurrent acute otitis media. Otolaryngol. Head Neck Surg. 146:991–996. 10.1177/0194599812438169 [DOI] [PubMed] [Google Scholar]
- 10.Hall-Stoodley L, Hu FZ, Gieseke A, Nistico L, Nguyen D, Hayes J, Forbes M, Greenberg DP, Dice B, Burrows A, Wackym PA, Stoodley P, Post JC, Ehrlich GD, Kerschner JE. 2006. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA 296:202–211. 10.1001/jama.296.2.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reid SD, Hong W, Dew KE, Winn DR, Pang B, Watt J, Glover DT, Hollingshead SK, Swords WE. 2009. Streptococcus pneumoniae forms surface-attached communities in the middle ear of experimentally infected chinchillas. J. Infect. Dis. 199:786–794. 10.1086/597042 [DOI] [PubMed] [Google Scholar]
- 12.Sanderson AR, Leid JG, Hunsaker D. 2006. Bacterial biofilms on the sinus mucosa of human subjects with chronic rhinosinusitis. Laryngoscope 116:1121–1126. 10.1097/01.mlg.0000221954.05467.54 [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Castillo M, Morosini MI, Valverde A, Almaraz F, Baquero F, Canton R, del Campo R. 2007. Differences in biofilm development and antibiotic susceptibility among Streptococcus pneumoniae isolates from cystic fibrosis samples and blood cultures. J. Antimicrob. Chemother. 59:301–304. 10.1093/jac/dkl482 [DOI] [PubMed] [Google Scholar]
- 14.Munoz-Elias EJ, Marcano J, Camilli A. 2008. Isolation of Streptococcus pneumoniae biofilm mutants and their characterization during nasopharyngeal colonization. Infect. Immun. 76:5049–5061. 10.1128/IAI.00425-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall-Stoodley L, Nistico L, Sambanthamoorthy K, Dice B, Nguyen D, Mershon WJ, Johnson C, Hu FZ, Stoodley P, Ehrlich GD, Post JC. 2008. Characterization of biofilm matrix, degradation by DNase treatment and evidence of capsule downregulation in Streptococcus pneumoniae clinical isolates. BMC Microbiol. 8:173. 10.1186/1471-2180-8-173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.del Prado G, Ruiz V, Naves P, Rodriguez-Cerrato V, Soriano F, del Carmen PM. 2010. Biofilm formation by Streptococcus pneumoniae strains and effects of human serum albumin, ibuprofen, N-acetyl-l-cysteine, amoxicillin, erythromycin, and levofloxacin. Diagn. Microbiol. Infect. Dis. 67:311–318. 10.1016/j.diagmicrobio.2010.03.016 [DOI] [PubMed] [Google Scholar]
- 17.Lizcano A, Chin T, Sauer K, Tuomanen EI, Orihuela CJ. 2010. Early biofilm formation on microtiter plates is not correlated with the invasive disease potential of Streptococcus pneumoniae. Microb. Pathog. 48:124–130. 10.1016/j.micpath.2010.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marks LR, Reddinger RM, Hakansson AP. 2012. High levels of genetic recombination during nasopharyngeal carriage and biofilm formation in Streptococcus pneumoniae. mBio 3(5):e00200–12. 10.1128/mBio.00200-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trappetti C, Ogunniyi AD, Oggioni MR, Paton JC. 2011. Extracellular matrix formation enhances the ability of Streptococcus pneumoniae to cause invasive disease. PLoS One 6:e19844. 10.1371/journal.pone.0019844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yadav MK, Chae SW, Song JJ. 2012. In vitro Streptococcus pneumoniae biofilm formation and in vivo middle ear mucosal biofilm in a rat model of acute otitis induced by S. pneumoniae. Clin. Exp. Otorhinolaryngol. 5:139–144. 10.3342/ceo.2012.5.3.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drago L, Mattina R, Legnani D, Romano CL, Vianello E, Ricci C, De Vecchi E. 2011. Modulation of biofilm of strains isolated from patients with chronic obstructive pulmonary disease by levofloxacin, moxifloxacin, ciprofloxacin, amoxicillin/clavulanic acid and ceftriaxone. Int. J. Immunopathol. Pharmacol. 24:1027–1035 [DOI] [PubMed] [Google Scholar]
- 22.Tapiainen T, Kujala T, Kaijalainen T, Ikaheimo I, Saukkoriipi A, Renko M, Salo J, Leinonen M, Uhari M. 2010. Biofilm formation by Streptococcus pneumoniae isolates from paediatric patients. APMIS 118:255–260. 10.1111/j.1600-0463.2010.02587.x [DOI] [PubMed] [Google Scholar]
- 23.Roveta S, Schito AM, Marchese A, Schito GC. 2007. Activity of moxifloxacin on biofilms produced in vitro by bacterial pathogens involved in acute exacerbations of chronic bronchitis. Int. J. Antimicrob. Agents 30:415–421. 10.1016/j.ijantimicag.2007.06.029 [DOI] [PubMed] [Google Scholar]
- 24.Sanchez CJ, Kumar N, Lizcano A, Shivshankar P, Dunning Hotopp JC, Jorgensen JH, Tettelin H, Orihuela CJ. 2011. Streptococcus pneumoniae in biofilms are unable to cause invasive disease due to altered virulence determinant production. PLoS One 6:e28738. 10.1371/journal.pone.0028738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trappetti C, Kadioglu A, Carter M, Hayre J, Iannelli F, Pozzi G, Andrew PW, Oggioni MR. 2009. Sialic acid: a preventable signal for pneumococcal biofilm formation, colonization, and invasion of the host. J. Infect. Dis. 199:1497–1505. 10.1086/598483 [DOI] [PubMed] [Google Scholar]
- 26.Chaney EJ, Nguyen CT, Boppart SA. 2011. Novel method for non-invasive induction of a middle-ear biofilm in the rat. Vaccine 29:1628–1633. 10.1016/j.vaccine.2010.12.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cogan NG, Keener JP. 2004. The role of the biofilm matrix in structural development. Math. Med. Biol. 21:147–166. 10.1093/imammb/21.2.147 [DOI] [PubMed] [Google Scholar]
- 28.Hall-Stoodley L, Costerton JW, Stoodley P. 2004. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2:95–108. 10.1038/nrmicro821 [DOI] [PubMed] [Google Scholar]
- 29.Dai L, Yang L, Parsons C, Findlay VJ, Molin S, Qin Z. 2012. Staphylococcus epidermidis recovered from indwelling catheters exhibit enhanced biofilm dispersal and “self-renewal” through downregulation of agr. BMC Microbiol. 12:102. 10.1186/1471-2180-12-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woo JK, Webb JS, Kirov SM, Kjelleberg S, Rice SA. 2012. Biofilm dispersal cells of a cystic fibrosis Pseudomonas aeruginosa isolate exhibit variability in functional traits likely to contribute to persistent infection. FEMS Immunol. Med. Microbiol. 66:251–264. 10.1111/j.1574-695X.2012.01006.x [DOI] [PubMed] [Google Scholar]
- 31.McGhee P, Clark C, Kosowska-Shick KM, Nagai K, Dewasse B, Beachel L, Appelbaum PC. 2010. In vitro activity of CEM-101 against Streptococcus pneumoniae and Streptococcus pyogenes with defined macrolide resistance mechanisms. Antimicrob. Agents Chemother. 54:230–238. 10.1128/AAC.01123-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oldach D, Clark K, Schranz J, Das A, Craft JC, Scott D, Jamieson BD, Fernandes P. 2013. Randomized, double-blind, multicenter phase 2 study comparing the efficacy and safety of oral solithromycin (CEM-101) to those of oral levofloxacin in the treatment of patients with community-acquired bacterial pneumonia. Antimicrob. Agents Chemother. 57:2526–2534. 10.1128/AAC.00197-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jorgensen JH, Doern GV, Ferraro MJ, Knapp CC, Swenson JM, Washington JA. 1992. Multicenter evaluation of the use of Haemophilus test medium for broth microdilution antimicrobial susceptibility testing of Streptococcus pneumoniae and development of quality control limits. J. Clin. Microbiol. 30:961–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Avery OT, Macleod CM, McCarty M. 1944. Studies on the chemical nature of the substance inducing transformation of pneumococcal types: induction of transformation by a desoxyribonucleic acid fraction isolated from Pneumococcus type III. J. Exp. Med. 79:137–158. 10.1084/jem.79.2.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoskins J, Alborn WE, Jr, Arnold J, Blaszczak LC, Burgett S, DeHoff BS, Estrem ST, Fritz L, Fu DJ, Fuller W, Geringer C, Gilmour R, Glass JS, Khoja H, Kraft AR, Lagace RE, LeBlanc DJ, Lee LN, Lefkowitz EJ, Lu J, Matsushima P, McAhren SM, McHenney M, McLeaster K, Mundy CW, Nicas TI, Norris FH, O'Gara M, Peery RB, Robertson GT, Rockey P, Sun PM, Winkler ME, Yang Y, Young-Bellido M, Zhao G, Zook CA, Baltz RH, Jaskunas SR, Rosteck PR, Jr, Skatrud PL, Glass JI. 2001. Genome of the bacterium Streptococcus pneumoniae strain R6. J. Bacteriol. 183:5709–5717. 10.1128/JB.183.19.5709-5717.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iannelli F, Pearce BJ, Pozzi G. 1999. The type 2 capsule locus of Streptococcus pneumoniae. J. Bacteriol. 181:2652–2654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clinical and Laboratory Standards Institute 2012. Performance standards for antimicrobial susceptibility testing. 22nd informational supplement (MS100-S22). Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 38.Christensen GD, Simpson WA, Bisno AL, Beachey EH. 1982. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect. Immun. 37:318–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tote K, Vanden Berghe D, Maes L, Cos P. 2008. A new colorimetric microtitre model for the detection of Staphylococcus aureus biofilms. Lett. Appl. Microbiol. 46:249–254. 10.1111/j.1472-765X.2007.02298.x [DOI] [PubMed] [Google Scholar]
- 40.Tote K, Horemans T, Vanden Berghe D, Maes L, Cos P. 2010. Inhibitory effect of biocides on the viable masses and matrices of Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 76:3135–3142. 10.1128/AEM.02095-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gawande PV, LoVetri K, Yakandawala N, Romeo T, Zhanel GG, Cvitkovitch DG, Madhyastha S. 2008. Antibiofilm activity of sodium bicarbonate, sodium metaperiodate and SDS combination against dental unit waterline-associated bacteria and yeast. J. Appl. Microbiol. 105:986–992. 10.1111/j.1365-2672.2008.03823.x [DOI] [PubMed] [Google Scholar]
- 42.Natto MJ, Savioli F, Quashie NB, Dardonville C, Rodenko B, de Koning HP. 2012. Validation of novel fluorescence assays for the routine screening of drug susceptibilities of Trichomonas vaginalis. J. Antimicrob. Chemother. 67:933–943. 10.1093/jac/dkr572 [DOI] [PubMed] [Google Scholar]
- 43.Piwonski HM, Goomanovsky M, Bensimon D, Horovitz A, Haran G. 2012. Allosteric inhibition of individual enzyme molecules trapped in lipid vesicles. Proc. Natl. Acad. Sci. U. S. A. 109:E1437–E1443. 10.1073/pnas.1116670109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moscoso M, Garcia E, Lopez R. 2006. Biofilm formation by Streptococcus pneumoniae: role of choline, extracellular DNA, and capsular polysaccharide in microbial accretion. J. Bacteriol. 188:7785–7795. 10.1128/JB.00673-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurola P, Tapiainen T, Sevander J, Kaijalainen T, Leinonen M, Uhari M, Saukkoriipi A. 2011. Effect of xylitol and other carbon sources on Streptococcus pneumoniae biofilm formation and gene expression in vitro. APMIS 119:135–142. 10.1111/j.1600-0463.2010.02703.x [DOI] [PubMed] [Google Scholar]
- 46.Trappetti C, Gualdi L, Di Meola L, Jain P, Korir CC, Edmonds P, Iannelli F, Ricci S, Pozzi G, Oggioni MR. 2011. The impact of the competence quorum sensing system on Streptococcus pneumoniae biofilms varies depending on the experimental model. BMC Microbiol. 11:75. 10.1186/1471-2180-11-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bauer J, Siala W, Tulkens PM, Van Bambeke F. 2013. A combined pharmacodynamic quantitative and qualitative model reveals the potent activity of daptomycin and delafloxacin against Staphylococcus aureus biofilms. Antimicrob. Agents Chemother. 57:2726–2737. 10.1128/AAC.00181-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Brien J, Wilson I, Orton T, Pognan F. 2000. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 267:5421–5426. 10.1046/j.1432-1327.2000.01606.x [DOI] [PubMed] [Google Scholar]
- 49.Allegrucci M, Hu FZ, Shen K, Hayes J, Ehrlich GD, Post JC, Sauer K. 2006. Phenotypic characterization of Streptococcus pneumoniae biofilm development. J. Bacteriol. 188:2325–2335. 10.1128/JB.188.7.2325-2335.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marks LR, Parameswaran GI, Hakansson AP. 2012. Pneumococcal interactions with epithelial cells are crucial for optimal biofilm formation and colonization in vitro and in vivo. Infect. Immun. 80:2744–2760. 10.1128/IAI.00488-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scherba G, Weigel RM, O'Brien WD., Jr 1991. Quantitative assessment of the germicidal efficacy of ultrasonic energy. Appl. Environ. Microbiol. 57:2079–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ceri H, Olson ME, Morck DW. 2004. Methods for biofilm study and assays for biofilm susceptibility and target recognition: approaches to deal with the biofilm mode of life, p 53–85 In Jordan R, Willams D, Charaf U. (ed), Advances in biofilm science and engineering. Cytergy Publishing, Bozeman, MT [Google Scholar]
- 53.Otto M. 2008. Staphylococcal biofilms. Curr. Top. Microbiol. Immunol. 322:207–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hammerschmidt S, Wolff S, Hocke A, Rosseau S, Muller E, Rohde M. 2005. Illustration of pneumococcal polysaccharide capsule during adherence and invasion of epithelial cells. Infect. Immun. 73:4653–4667. 10.1128/IAI.73.8.4653-4667.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boles BR, Horswill AR. 2011. Staphylococcal biofilm disassembly. Trends Microbiol. 19:449–455. 10.1016/j.tim.2011.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ross EM, Kenakin T. 2001. Pharmacodynamics: mechanism of drug action and the relationship between drug concentration and effect, p 31–43 In Hardman J, Limbird LE. (ed), Goodman & Gilman's the pharmacological basis of therapeutics. McGraw-Hill Medical Publishing Division, New York, NY [Google Scholar]
- 57.Barcia-Macay M, Seral C, Mingeot-Leclercq MP, Tulkens PM, Van Bambeke F. 2006. Pharmacodynamic evaluation of the intracellular activities of antibiotics against Staphylococcus aureus in a model of THP-1 macrophages. Antimicrob. Agents Chemother. 50:841–851. 10.1128/AAC.50.3.841-851.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buyck JM, Tulkens PM, Van Bambeke F. 2013. Pharmacodynamic evaluation of the intracellular activity of antibiotics towards Pseudomonas aeruginosa PAO1 in a model of THP-1 human monocytes. Antimicrob. Agents Chemother. 57:2310–2318. 10.1128/AAC.02609-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moskowitz SM, Foster JM, Emerson J, Burns JL. 2004. Clinically feasible biofilm susceptibility assay for isolates of Pseudomonas aeruginosa from patients with cystic fibrosis. J. Clin. Microbiol. 42:1915–1922. 10.1128/JCM.42.5.1915-1922.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brook I, Hausfeld JN. 2006. In vitro effects of penicillin and telithromycin on the expression of Streptococcus pneumoniae capsule. J. Antimicrob. Chemother. 58:678–679. 10.1093/jac/dkl261 [DOI] [PubMed] [Google Scholar]
- 61.Fernebro J, Andersson I, Sublett J, Morfeldt E, Novak R, Tuomanen E, Normark S, Normark BH. 2004. Capsular expression in Streptococcus pneumoniae negatively affects spontaneous and antibiotic-induced lysis and contributes to antibiotic tolerance. J. Infect. Dis. 189:328–338. 10.1086/380564 [DOI] [PubMed] [Google Scholar]
- 62.Gillis RJ, Iglewski BH. 2004. Azithromycin retards Pseudomonas aeruginosa biofilm formation. J. Clin. Microbiol. 42:5842–5845. 10.1128/JCM.42.12.5842-5845.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fujimura S, Sato T, Mikami T, Kikuchi T, Gomi K, Watanabe A. 2008. Combined efficacy of clarithromycin plus cefazolin or vancomycin against Staphylococcus aureus biofilms formed on titanium medical devices. Int. J. Antimicrob. Agents 32:481–484. 10.1016/j.ijantimicag.2008.06.030 [DOI] [PubMed] [Google Scholar]
- 64.Sanchez CJ, Shivshankar P, Stol K, Trakhtenbroit S, Sullam PM, Sauer K, Hermans PW, Orihuela CJ. 2010. The pneumococcal serine-rich repeat protein is an intra-species bacterial adhesin that promotes bacterial aggregation in vivo and in biofilms. PLoS Pathog. 6:e1001044. 10.1371/journal.ppat.1001044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vidal JE, Ludewick HP, Kunkel RM, Zahner D, Klugman KP. 2011. The LuxS-dependent quorum-sensing system regulates early biofilm formation by Streptococcus pneumoniae strain D39. Infect. Immun. 79:4050–4060. 10.1128/IAI.05186-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jenkins SG, Farrell DJ. 2009. Increase in pneumococcus macrolide resistance, United States. Emerg. Infect. Dis. 15:1260–1264. 10.3201/eid1508.081187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van Bambeke F, Reinert RR, Appelbaum PC, Tulkens PM, Peetermans WE. 2007. Multidrug-resistant Streptococcus pneumoniae infections: current and future therapeutic options. Drugs 67:2355–2382. 10.2165/00003495-200767160-00005 [DOI] [PubMed] [Google Scholar]
- 68.Low DE. 2013. What is the relevance of antimicrobial resistance on the outcome of community-acquired pneumonia caused by Streptococcus pneumoniae? (Should macrolide monotherapy be used for mild pneumonia?) Infect. Dis. Clin. North Am. 27:87–97. 10.1016/j.idc.2012.11.013 [DOI] [PubMed] [Google Scholar]
- 69.Credito K, Kosowska-Shick K, McGhee P, Pankuch GA, Appelbaum PC. 2010. Comparative study of the mutant prevention concentrations of moxifloxacin, levofloxacin, and gemifloxacin against pneumococci. Antimicrob. Agents Chemother. 54:673–677. 10.1128/AAC.01353-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simoens S, Verhaegen J, van Bleyenbergh P, Peetermans WE, Decramer M. 2011. Consumption patterns and in vitro resistance of Streptococcus pneumoniae to fluoroquinolones. Antimicrob. Agents Chemother. 55:3051–3053. 10.1128/AAC.00019-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Patel SN, McGeer A, Melano R, Tyrrell GJ, Green K, Pillai DR, Low DE. 2011. Susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. Antimicrob. Agents Chemother. 55:3703–3708. 10.1128/AAC.00237-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Farrell DJ, Sader HS, Castanheira M, Biedenbach DJ, Rhomberg PR, Jones RN. 2010. Antimicrobial characterisation of CEM-101 activity against respiratory tract pathogens, including multidrug-resistant pneumococcal serogroup 19A isolates. Int. J. Antimicrob. Agents 35:537–543. 10.1016/j.ijantimicag.2010.01.026 [DOI] [PubMed] [Google Scholar]
- 73.Cole PJ, Roberts DE, Davies SF, Knight RK. 1983. A simple oral antimicrobial regimen effective in severe chronic bronchial suppuration associated with culturable Haemophilus influenzae. J. Antimicrob. Chemother. 11:109–113. 10.1093/jac/11.2.109 [DOI] [PubMed] [Google Scholar]
- 74.Patel KB, Xuan D, Tessier PR, Russomanno JH, Quintiliani R, Nightingale CH. 1996. Comparison of bronchopulmonary pharmacokinetics of clarithromycin and azithromycin. Antimicrob. Agents Chemother. 40:2375–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rodvold KA, Gotfried MH, Still JG, Clark K, Fernandes P. 2012. Comparison of plasma, epithelial lining fluid, and alveolar macrophage concentrations of solithromycin (CEM-101) in healthy adult subjects. Antimicrob. Agents Chemother. 56:5076–5081. 10.1128/AAC.00766-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Conte JE, Jr, Golden JA, McIver M, Little E, Zurlinden E. 2007. Intrapulmonary pharmacodynamics of high-dose levofloxacin in subjects with chronic bronchitis or chronic obstructive pulmonary disease. Int. J. Antimicrob. Agents 30:422–427. 10.1016/j.ijantimicag.2007.05.023 [DOI] [PubMed] [Google Scholar]
- 77.Soman A, Honeybourne D, Andrews J, Jevons G, Wise R. 1999. Concentrations of moxifloxacin in serum and pulmonary compartments following a single 400 mg oral dose in patients undergoing fibre-optic bronchoscopy. J. Antimicrob. Chemother. 44:835–838. 10.1093/jac/44.6.835 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.