Abstract
Painful blinding keratitis and fatal granulomatous amebic encephalitis are caused by the free-living amebae Acanthamoeba spp. Several prescription eye medications are used to treat Acanthamoeba keratitis, but the infection can be difficult to control because of recurrence of infection. For the treatment of encephalitis, no single drug was found useful, and in spite of the use of a combination of multiple drugs, the mortality rate remains high. Therefore, efficient, novel drugs are urgently needed for the treatment of amebic keratitis and granulomatous amebic encephalitis. In this study, we identified corifungin, a water-soluble polyene macrolide, as amebicidal. In vitro, it was effective against both the trophozoites and the cysts. Transmission electron microscopy of Acanthamoeba castellanii incubated with corifungin showed the presence of swollen mitochondria, electron-dense granules, degeneration of cytoplasm architecture, and loss of nuclear chromatin structure. These changes were followed by lysis of amebae. Corifungin also induced the encystment process of A. castellanii. There were alterations in the cyst cell wall followed by lysis of the cysts. Corifungin is a promising therapeutic option for keratitis and granulomatous amebic encephalitis.
INTRODUCTION
Free-living Acanthamoeba spp. cause keratitis, a serious eye infection that can occur in healthy individuals wearing contact lenses, as well as chronic granulomatous amebic encephalitis (GAE) leading to death in immunocompromised persons. Acanthamoeba has a worldwide distribution and is the most common ameba found in the environment. Coincident with the number of Acanthamoeba keratitis cases in the United States has been an increase in developing countries. Wearing of contact lenses is now recognized as the leading risk factor for keratitis (1–3). In the United States, the estimated number of keratitis cases is 1.36 per million contact lens wearers (4, 5). GAE is a relatively rare disease. Approximately 150 cases have been reported worldwide (5). GAE results from inhalation of amebae through the nasal cavities and lungs, or introduction through skin lesions followed by access to the central nervous system by hematogenous spread or through the olfactory neuroepithelium (6). Clinical manifestations include headache, fever, nausea, vomiting, behavioral changes, stiff neck, lethargy, increased intracranial pressure, and, in the later stage, loss of consciousness, seizures, coma, and death (7–9).
The drugs recommended for the treatment of Acanthamoeba keratitis include polyhexamethylene biguanide (0.02%) or chlorhexidine (0.02%) along with a diamidine (either 0.1% propamidine or 0.1% hexamidine) (10). Corticosteroids are applied topically to control corneal inflammation, pain, and scleritis, particularly following keratoplasty (11). While this antimicrobial treatment can kill the trophozoites, the resistance of Acanthamoeba cysts to antimicrobials can lead to the recurrence of keratitis. For GAE, combination therapies were found more successful than single-drug therapies, and therefore, current therapeutic agents include a combination of ketoconazole, fluconazole, itraconazole, pentamidine isethionate, sulfadiazine, amphotericin B, azithromycin, rifampin, voriconazole, and miltefosine (12–15). Because of the lack of optimal antimicrobial therapy, GAE is often fatal, and fewer than 10 GAE patients have been successfully treated with multidrug regimens (16). Therefore, more effective drugs are urgently needed.
In this study, we first showed that the water-soluble macrolide corifungin produced in vitro trophozoite growth inhibition. Because of its efficacy against Acanthamoeba trophozoites, we also confirmed its cysticidal effect.
MATERIALS AND METHODS
Maintenance of Acanthamoeba castellanii.
A. castellanii trophozoites isolated from human cases of amebic keratitis were kindly provided by Simon Kilvington (Public Health Laboratory, Bath, England). They were cultured at 30°C in Chang's liquid medium supplemented with 10% (vol/vol) fetal bovine serum (Equitech-Bio, Kerrville, TX) according to a modified technique (17). Trophozoites were harvested in logarithmic growth phase, usually 48 h after subculture, by chilling at 4°C and concentrated by centrifugation for 5 min at 300 × g.
In vitro studies of corifungin against A. castellanii trophozoites.
To determine the effect of corifungin on the growth of A. castellanii trophozoites, 5 × 105 amebae were incubated with 25 μM, 100 μM, and 200 μM corifungin for 24, 48, 72, 96, and 120 h. Control trophozoites were incubated with fresh medium only. Cell numbers were calculated by hemocytometer at the end of incubation. The percentage of viable trophozoites following exposure to different concentrations of corifungin was determined by the trypan blue exclusion method. Cells stained blue were considered nonviable while live cells were unstained. Data were presented as the mean and standard deviation of at least three independent experiments, each performed in duplicate.
To determine if corifungin induced cyst formation and to determine the effect of corifungin on cysts, 5 × 105 trophozoites were incubated with 200 μM corifungin for 24 to 120 h and the cell viability was evaluated using trypan blue staining.
Light microscopy.
Trophozoites (5 × 105) were incubated with 200 μM corifungin for 24, 48, 72, 96, and 120 h. At the end of each incubation period, samples were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer and postfixed with 1% (wt/vol) osmium tetroxide for 1 h. After being dehydrated with ethanol, samples were embedded in epoxy resin. Semithin sections stained with toluidine blue were examined with a light microscope (Optiphot; Nikon, Japan).
TEM.
For ultrastructural analysis, A. castellanii trophozoites were incubated with 200 μM corifungin for 24, 48, 72, 96, and 120 h and then fixed with a solution of 2.5% glutaraldehyde in 0.1 M cacodylate buffer. Samples were postfixed with 1% (wt/vol) osmium tetroxide, dehydrated with ethanol and propylene oxide, and embedded in epoxy resin. Thin sections (60 to 90 nm) were contrast stained with uranyl acetate and lead citrate and observed under a Zeiss EM-910 transmission electron microscope (TEM) (Carl Zeiss, Germany). To understand the effect of the induction of encystment of A. castellanii by corifungin, we also analyzed by TEM the ultrastructure of the cysts, after incubation of amebae with 200 μM corifungin until 120 h. The ultrastructural analysis of cysts was done by TEM according to the same protocol as that detailed above.
RESULTS
Effect of corifungin against A. castellanii in vitro.
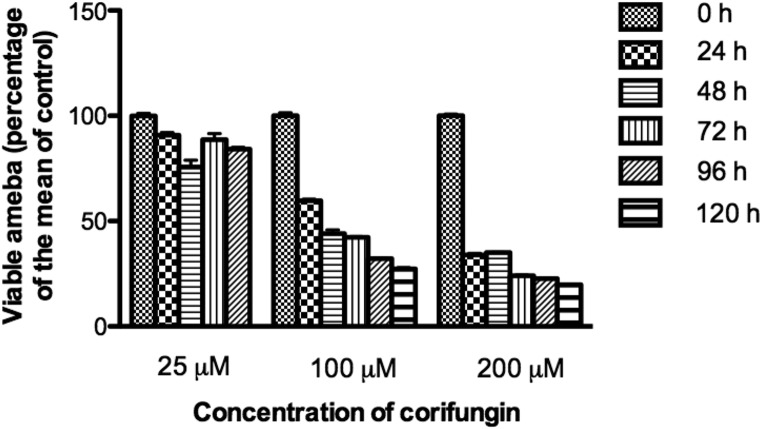
Corifungin did not inhibit A. castellanii trophozoite growth at 25 μM (Fig. 1). At 100 μM, there was 73% growth inhibition after 120 h of incubation (Fig. 1). At 200 μM corifungin, there was 80% growth inhibition after 120 h of incubation (Fig. 1). Based on this growth inhibition study, the 50% effective concentration (EC50) of corifungin, defined as that concentration of compound necessary to reduce the culture density to 50% of that of a vehicle-treated culture, was approximately 63 μM. A. castellanii trophozoites were next incubated with 200 μM corifungin for 24, 48, 72, 96, and 120 h, and the viability of A. castellanii was determined by the trypan blue exclusion method (Fig. 2).
FIG 1.
Effect of corifungin on A. castellanii growth. Trophozoites were incubated with 25, 100, and 200 μM corifungin for 24, 48, 72, 96, and 120 h, and viable trophozoites were calculated as the percentage of trophozoites compared with the means of controls (as 100%). Values plotted are means and standard deviations for three independent experiments.
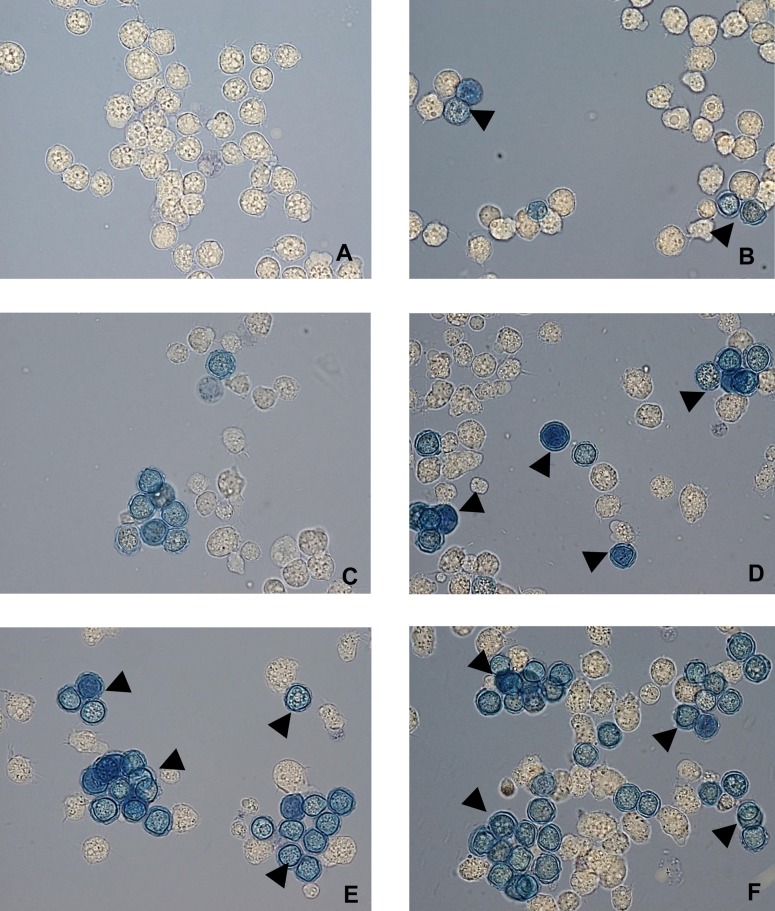
FIG 2.
A. castellanii viability determination by trypan blue exclusion method. Trophozoites were incubated with 200 μM corifungin for different time periods. (A) Amebae with fresh medium only. (B) Amebae incubated with corifungin for 24 h. (C) Amebae incubated with corifungin for 48 h. (D) Amebae incubated with corifungin for 72 h. (E) Amebae incubated with corifungin for 96 h. (F) Amebae incubated with corifungin for 120 h. Cells stained blue were considered nonviable (arrowheads). Magnification, ×60.
Corifungin induced encystation of A. castellanii.
Since corifungin showed 80% growth inhibition of trophozoites at 200 μM, this concentration was used for induction of the encystation process. Although corifungin induced encystment of trophozoites (Table 1), it also damaged and killed the cysts (Fig. 3).
TABLE 1.
Encystment of A. castellanii at different time points in the presence of 200 μM corifungin
| Time point (h) | Cyst formation (%) |
|---|---|
| 24 | 7 |
| 48 | 20 |
| 72 | 35 |
| 96 | 48 |
| 120 | 54 |
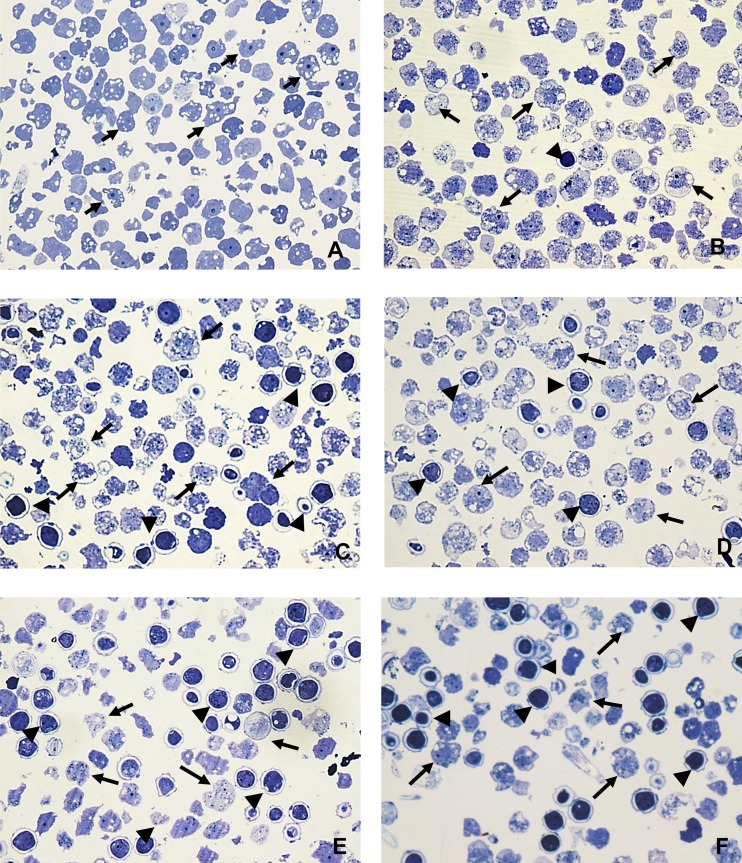
FIG 3.
Light microscopy of epoxy-embedded semithin sections, stained with toluidine blue, of A. castellanii trophozoites incubated in the presence and absence of corifungin. Trophozoites were incubated with 200 μM corifungin for different time periods. (A) Amebae (arrows) with fresh medium only. (B) Amebae incubated with 200 μM corifungin for 24 h. Arrows indicate trophozoites, and the arrowhead indicates a cyst. (C) Amebae incubated with 200 μM corifungin for 48 h. The damage in the trophozoites (arrows) is evident, and there was an increase in the number of the cysts (arrowheads). (D) Amebae incubated with 200 μM corifungin for 72 h. More damage is seen in the trophozoites (arrows), and damage is also found in the cysts (arrowheads). (E and F) Amebae incubated with 200 μM corifungin for 96 to 120 h. Cell remains of trophozoites (arrows) and more damage in the cysts (arrowheads) are seen. Magnification, ×60.
Light microscopy.
Examination of semithin sections of trophozoites incubated in the absence of corifungin showed the expected morphology (Fig. 3A). There was significant cellular damage to the trophozoites following incubation with corifungin. This was particularly evident at 48, 96, and 120 h (Fig. 3B to F).
Transmission electron microscopy.
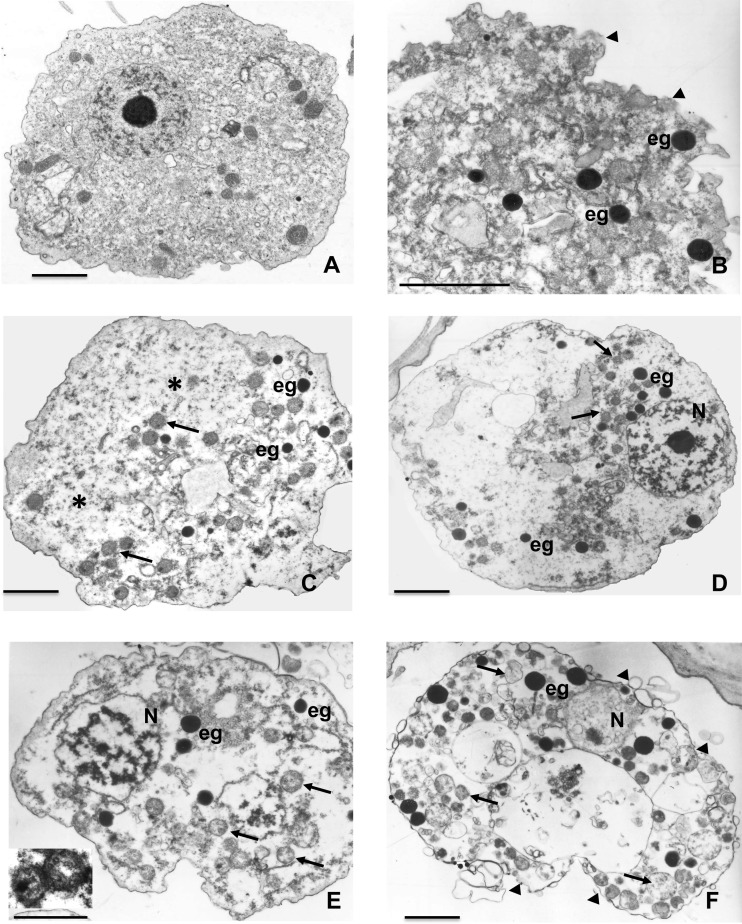
Transmission electron microscopy was also used to assess subcellular damage to A. castellanii induced by 200 μM corifungin at 24, 48, 72, 96, and 120 h (Fig. 4A to F). Ultrastructural analysis showed damage to the plasma membranes of trophozoites at 24 h. At 24 to 48 h, electron-dense granules appeared in the cytoplasm and overall cytoplasmic integrity and organelle structure were lost (Fig. 4B and C). At 72 and 96 h, electron-dense granules increased in number, cytoplasmic integrity was even more compromised, and the nuclear chromatin pattern was also altered (Fig. 4D and E). At 120 h, trophozoites began to lyse (Fig. 4F).
FIG 4.
Transmission electron microscopy of A. castellanii trophozoites incubated with 200 μM corifungin for different time periods. (A) Trophozoites treated with only fresh medium. (B) Trophozoites treated with corifungin for 24 h. The alteration of the plasma membrane is evident (arrowheads), and electron-dense granules (eg) appeared in the degenerated cytoplasm. (C) Trophozoites treated with corifungin for 48 h. Mitochondria (arrows) showed damage, and glycogen (*) was present. (D) Trophozoites treated with corifungin for 72 h. Mitochondria (arrows) are dilated, and the nucleus (N) shows alterations. (E) Trophozoites treated with corifungin for 96 h. The edematous mitochondria (arrows) and damaged nucleus (N) are evident. The inset image is the higher magnification of mitochondria. (F) Trophozoites treated with corifungin for 120 h. The ameba shows vacuolization, the electron-dense granules (eg) increase in number, and cell membrane damage is evident (arrowheads). Bars, 2 μm.
Exposure of cysts to corifungin produced many of the same effects on cytoplasmic integrity as those seen in trophozoites as well as alterations and swelling of the cyst wall (Fig. 5).
FIG 5.
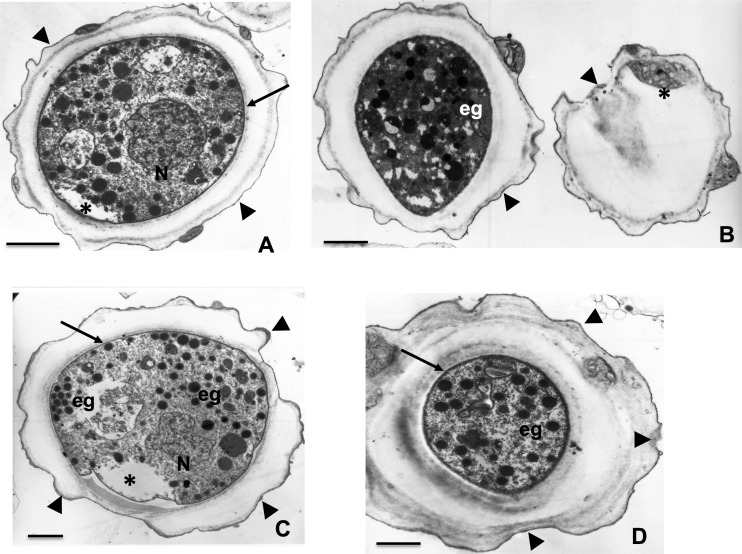
Transmission electron microscopy of A. castellanii cyst formation after incubation of trophozoites with 200 μM corifungin for 120 h. Encystment of the ameba is evident. Cell wall (arrowheads) is present. Inside the cyst, the trophozoite (arrow) presents signs of damage (*). Electron-dense granules also appear (eg). In many cysts, loss of the inner wall and cell wall disruption are observed (arrowheads). Bars, 2 μm.
DISCUSSION
While a wide range of antiparasitic, antimicrobial, and other pharmacologic agents has been tested against Acanthamoeba, these agents have shown limited activity and reports of successful treatment have been few (18–22). The current drugs of choice in treating Acanthamoeba keratitis are two biguanides and two diamidines. This treatment can be toxic and painful, and the topical application of corticosteroids to relieve pain may exacerbate disease (10, 23). Moreover, antimicrobial resistance and recurrent infections have prompted the search for new therapeutic agents for the treatment of Acanthamoeba keratitis.
Treatment of systemic infections, especially GAE, with multidrug regimens has also given mixed results because many of the drugs do not cross the blood-brain barrier. No single drug has been found effective against both the trophozoite and the cyst stages of Acanthamoeba. Therefore, a drug that would be amebicidal and cysticidal and that could cross the blood-brain barrier would be optimal for the treatment of Acanthamoeba infection.
We evaluated the polyene macrolide corifungin versus Acanthamoeba because an earlier study showed that it effectively killed another free-living ameba, Naegleria fowleri, with better efficacy than amphotericin B (24). Previous studies reported that a combination of amphotericin B and a second drug was effective in eradicating a skin infection and GAE caused by Acanthamoeba (25, 26). Amphotericin B has significant toxicity and is a hydrophobic molecule with negligible solubility in aqueous solution (27). Use of amphotericin B often resulted in renal toxicity, manifested as azotemia and hypokalemia (10). Amphotericin B also may cause anemia, and many patients experience chills, fever, nausea, vomiting, and headache (28, 29). Given the mortality of GAE patients infected by A. castellanii, an efficient, water-soluble, nontoxic drug remains a highly desirable alternative to the conventional treatment. Corifungin, as a sodium salt of amphotericin B, is water soluble (>100 mg/ml) and is well tolerated in animals with minimal toxicity. In rats, it is safe up to a level of 250 mg/kg of body weight/day when administered by oral gavage for 28 days (pre-investigational new drug [pre-IND] document submitted to the U.S. Food and Drug Administration).
We have now shown that, in vitro, corifungin effectively kills the trophozoites of A. castellanii at 100 and 200 μM. The growth inhibition persisted throughout 120 h of incubation. Corifungin also induced the encystation process of A. castellanii. Current treatment failures are usually attributed to the ability of amebae to convert to cyst forms that are resistant to the currently employed drugs. Ultrastructural analysis showed that corifungin damaged the cyst wall and ultimately produced cyst lysis (Fig. 5).
Amphotericin B is a membrane-active drug that likely forms channel-like structures (pores) spanning the lipid bilayer (30–32). The transmission electron microscopy study results are consistent with a similar mechanism of action for corifungin. Corifungin damaged the plasma membrane of trophozoites as well as cytoplasmic organelle membranes. There was loss of the inner wall of cysts (Fig. 4 and 5). Corifungin targets mitochondria more effectively than amphotericin B in Aspergillus terreus (J. Tunac, unpublished observations) (24).
In GAE, Acanthamoeba enters into the central nervous system via the circulatory system, and many of the currently used drugs cannot cross the blood-brain barrier, leading to fatal consequences within days or weeks. In a previous study, we also confirmed the absence of detectable amebae in the brain of a corifungin-treated mouse model of primary amebic meningoencephalitis (24). This suggests that corifungin may have the ability to cross the blood-brain barrier because of enhanced solubility.
In conclusion, we identified corifungin as amebicidal and cysticidal for A. castellanii. Considering its earlier efficacy against another free-living ameba, N. fowleri, and its orphan-drug designation in the treatment of primary amebic meningoencephalitis, corifungin now represents a promising therapeutic option for Acanthamoeba infection.
ACKNOWLEDGMENTS
Anjan Debnath was supported by a Carlos Slim/BMGF partnership grant. This work was also partially supported by CONACyT grant (number 128317).
Footnotes
Published ahead of print 23 December 2013
REFERENCES
- 1.Donzis PB, Mondino BJ, Weissman BA, Bruckner DA. 1987. Microbial contamination of contact lens care systems. Am. J. Ophthalmol. 104:325–333 [DOI] [PubMed] [Google Scholar]
- 2.Illingworth CD, Cook SD, Karabatsas CH, Easty DL. 1995. Acanthamoeba keratitis: risk factors and outcome. Br. J. Ophthalmol. 79:1078–1082. 10.1136/bjo.79.12.1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marciano-Cabral F, Cabral G. 2003. Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev. 16:273–307. 10.1128/CMR.16.2.273-307.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seal DV. 2003. Acanthamoeba keratitis update-incidence, molecular epidemiology and new drugs for treatment. Eye (Lond.) 17:893–905. 10.1038/sj.eye.6700563 [DOI] [PubMed] [Google Scholar]
- 5.Trabelsi H, Dendana F, Sellami A, Sellami H, Cheikhrouhou F, Neji S, Makni F, Ayadi A. 2012. Pathogenic free-living amoebae: epidemiology and clinical review. Pathol. Biol. (Paris) 60:399–405. 10.1016/j.patbio.2012.03.002 [DOI] [PubMed] [Google Scholar]
- 6.Martinez AJ, Janitschke K. 1985. Acanthamoeba, an opportunistic microorganism: a review. Infection 13:251–256. 10.1007/BF01645432 [DOI] [PubMed] [Google Scholar]
- 7.Visvesvara GS, Moura H, Schuster FL. 2007. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol. Med. Microbiol. 50:1–26. 10.1111/j.1574-695X.2007.00232.x [DOI] [PubMed] [Google Scholar]
- 8.Khan NA. 2006. Acanthamoeba: biology and increasing importance in human health. FEMS Microbiol. Rev. 30:564–595. 10.1111/j.1574-6976.2006.00023.x [DOI] [PubMed] [Google Scholar]
- 9.Martinez AJ, Visvesvara GS. 1997. Free-living, amphizoic and opportunistic amebas. Brain Pathol. 7:583–598. 10.1111/j.1750-3639.1997.tb01076.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Visvesvara GS. 2010. Amebic meningoencephalitides and keratitis: challenges in diagnosis and treatment. Curr. Opin. Infect. Dis. 23:590–594. 10.1097/QCO.0b013e32833ed78b [DOI] [PubMed] [Google Scholar]
- 11.Schuster FL, Visvesvara GS. 2004. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int. J. Parasitol. 34:1001–1027. 10.1016/j.ijpara.2004.06.004 [DOI] [PubMed] [Google Scholar]
- 12.Aichelburg AC, Walochnik J, Assadian O, Prosch H, Steuer A, Perneczky G, Visvesvara GS, Aspock H, Vetter N. 2008. Successful treatment of disseminated Acanthamoeba sp. infection with miltefosine. Emerg. Infect. Dis. 14:1743–1746. 10.3201/eid1411.070854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maritschnegg P, Sovinz P, Lackner H, Benesch M, Nebl A, Schwinger W, Walochnik J, Urban C. 2011. Granulomatous amebic encephalitis in a child with acute lymphoblastic leukemia successfully treated with multimodal antimicrobial therapy and hyperbaric oxygen. J. Clin. Microbiol. 49:446–448. 10.1128/JCM.01456-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Webster D, Umar I, Kolyvas G, Bilbao J, Guiot MC, Duplisea K, Qvarnstrom Y, Visvesvara GS. 2012. Treatment of granulomatous amoebic encephalitis with voriconazole and miltefosine in an immunocompetent soldier. Am. J. Trop. Med. Hyg. 87:715–718. 10.4269/ajtmh.2012.12-0100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baig AM, Iqbal J, Khan NA. 2013. In vitro efficacies of clinically available drugs against growth and viability of an Acanthamoeba castellanii keratitis isolate belonging to the T4 genotype. Antimicrob. Agents Chemother. 57:3561–3567. 10.1128/AAC.00299-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walochnik J, Aichelburg A, Assadian O, Steuer A, Visvesvara G, Vetter N, Aspock H. 2008. Granulomatous amoebic encephalitis caused by Acanthamoeba amoebae of genotype T2 in a human immunodeficiency virus-negative patient. J. Clin. Microbiol. 46:338–340. 10.1128/JCM.01177-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivera F, Medina F, Ramirez P, Alcocer J, Vilaclara G, Robles E. 1984. Pathogenic and free-living protozoa cultured from the nasopharyngeal and oral regions of dental patients. Environ. Res. 33:428–440. 10.1016/0013-9351(84)90040-9 [DOI] [PubMed] [Google Scholar]
- 18.Walochnik J, Duchene M, Seifert K, Obwaller A, Hottkowitz T, Wiedermann G, Eibl H, Aspock H. 2002. Cytotoxic activities of alkylphosphocholines against clinical isolates of Acanthamoeba spp. Antimicrob. Agents Chemother. 46:695–701. 10.1128/AAC.46.3.695-701.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuster FL, Guglielmo BJ, Visvesvara GS. 2006. In-vitro activity of miltefosine and voriconazole on clinical isolates of free-living amebas: Balamuthia mandrillaris, Acanthamoeba spp., and Naegleria fowleri. J. Eukaryot. Microbiol. 53:121–126. 10.1111/j.1550-7408.2005.00082.x [DOI] [PubMed] [Google Scholar]
- 20.McBride J, Mullen AB, Carter KC, Roberts CW. 2007. Differential cytotoxicity of phospholipid analogues to pathogenic Acanthamoeba species and mammalian cells. J. Antimicrob. Chemother. 60:521–525. 10.1093/jac/dkm245 [DOI] [PubMed] [Google Scholar]
- 21.Walochnik J, Obwaller A, Gruber F, Mildner M, Tschachler E, Suchomel M, Duchene M, Auer H. 2009. Anti-acanthamoeba efficacy and toxicity of miltefosine in an organotypic skin equivalent. J. Antimicrob. Chemother. 64:539–545. 10.1093/jac/dkp215 [DOI] [PubMed] [Google Scholar]
- 22.Roberts CW, Henriquez FL. 2010. Drug target identification, validation, characterisation and exploitation for treatment of Acanthamoeba (species) infections. Exp. Parasitol. 126:91–96. 10.1016/j.exppara.2009.11.016 [DOI] [PubMed] [Google Scholar]
- 23.McClellan K, Howard K, Niederkorn JY, Alizadeh H. 2001. Effect of steroids on Acanthamoeba cysts and trophozoites. Invest. Ophthalmol. Vis. Sci. 42:2885–2893 [PubMed] [Google Scholar]
- 24.Debnath A, Tunac JB, Galindo-Gomez S, Silva-Olivares A, Shibayama M, McKerrow JH. 2012. Corifungin, a new drug lead against Naegleria, identified from a high-throughput screen. Antimicrob. Agents Chemother. 56:5450–5457. 10.1128/AAC.00643-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walia R, Montoya JG, Visvesvera GS, Booton GC, Doyle RL. 2007. A case of successful treatment of cutaneous Acanthamoeba infection in a lung transplant recipient. Transpl. Infect. Dis. 9:51–54. 10.1111/j.1399-3062.2006.00159.x [DOI] [PubMed] [Google Scholar]
- 26.Sheng WH, Hung CC, Huang HH, Liang SY, Cheng YJ, Ji DD, Chang SC. 2009. First case of granulomatous amebic encephalitis caused by Acanthamoeba castellanii in Taiwan. Am. J. Trop. Med. Hyg. 81:277–279 [PubMed] [Google Scholar]
- 27.Falk R, Domb AJ, Polacheck I. 1999. A novel injectable water-soluble amphotericin B-arabinogalactan conjugate. Antimicrob. Agents Chemother. 43:1975–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCurdy DK, Frederic M, Elkinton JR. 1968. Renal tubular acidosis due to amphotericin B. N. Engl. J. Med. 278:124–130. 10.1056/NEJM196801182780302 [DOI] [PubMed] [Google Scholar]
- 29.Proffitt RT, Satorius A, Chiang SM, Sullivan L, Adler-Moore JP. 1991. Pharmacology and toxicology of a liposomal formulation of amphotericin B (AmBisome) in rodents. J. Antimicrob. Chemother. 28(Suppl B):49–61. 10.1093/jac/28.suppl_B.49 [DOI] [PubMed] [Google Scholar]
- 30.Schuster FL, Visvesvara GS. 2004. Opportunistic amoebae: challenges in prophylaxis and treatment. Drug Resist. Updat. 7:41–51. 10.1016/j.drup.2004.01.002 [DOI] [PubMed] [Google Scholar]
- 31.Baginski M, Sternal K, Czub J, Borowski E. 2005. Molecular modelling of membrane activity of amphotericin B, a polyene macrolide antifungal antibiotic. Acta Biochim. Pol. 52:655–658 [PubMed] [Google Scholar]
- 32.Chattopadhyay A, Jafurulla M. 2011. A novel mechanism for an old drug: amphotericin B in the treatment of visceral leishmaniasis. Biochem. Biophys. Res. Commun. 416:7–12. 10.1016/j.bbrc.2011.11.023 [DOI] [PubMed] [Google Scholar]