Abstract
Current monotherapy against visceral leishmaniasis has serious side effects, and resistant Leishmania strains have been identified. Amphotericin B (AmB) has shown an extraordinary antileishmanial efficacy without emergence of resistance; however, toxicity has limited its general use. Results obtained showed, using a fixed-ratio analysis, that the combination of diallyl thiosulfinate (allicin) and AmB ranged from moderately synergic to synergic at low concentrations (0.07 μM AmB plus 35.45 μM allicin induced 95% growth inhibition). None of the treatments, alone or in combination, had noticeable adverse effects on macrophages (Mϕ) in the concentration range examined (allicin, 0.5, 1, 5 and 10 μM; AmB, 0.05, 0.075, and 0.1 μM). Allicin, AmB, or the combination did not affect the infection rate (percentage of infected Mϕ) of Leishmania. Allicin enhanced the activity of AmB on intracellular amastigotes of Leishmania donovani and L. infantum (ca. 45% reduction of amastigote burden with 0.05 μM AmB plus 10 μM allicin); this represented nearly a 2-fold reduction in the 50% inhibitory concentration (IC50) of the antibiotic added alone. Results point toward the possible utility of testing this combination in vivo to reduce the toxicity associated with monotherapy with AmB.
INTRODUCTION
Human Leishmania infections are present in all inhabited continents, with around 12 million people infected, 2 million new cases every year, and estimates of around 350 million people living in areas at risk (http://who.int/health-topics/leishmaniasis.htm). Leishmaniasis is a spectral infection with a range of clinical presentations, from self-healing dermal infections to deadly processes. Cutaneous leishmaniasis is by large the most common disease, but visceral leishmaniasis (caused by Leishmania donovani and L. infantum [=L. chagasi]) is also present in all continents, and the infections produce severe disease. The distribution of these infections has greatly expanded, and coinfection of Leishmania and HIV is very frequent (1). In addition to human cases, canine leishmaniasis caused by L. infantum (Mediterranean Basin) and L. chagasi (South America) constitutes a first-order pathology in veterinary clinics besides the zoonotical importance of dogs as reservoirs. The main control system of leishmaniasis in both humans and dogs is chemotherapy. However, antileishmanial drugs have some important shortcomings, including high toxicity and teratogenicity in some cases, absence of parasitological cure in most cases, and unaffordable prices for some of the compounds and presentations. Moreover, in some areas, Leishmania strains resistant to commonly used drugs, particularly antimonials, have emerged (2). Liposomal amphotericin (AmB), paromomycin, and miltefosine were considered the most promising drugs for chemotherapy of leishmaniasis (http://www.who.int/tdr/diseases-topics/leishmaniasis/en/). However, for the large part, these drugs were introduced over 40 years ago, and new active compounds or combinations against Leishmania are needed (3, 4).
The pharmaceutical industry has experienced a contraction during recent years, resulting in very few companies being present in the market. It is anticipated that investment and intercompany competition and consequently the launch of new antiparasitic agents will be reduced (5). One of the alternative chemotherapeutic approaches is the use of combinations of effective existing drugs whose toxicity precluded them from being widely used with chemically unrelated compounds of reduced toxicity. The antibiotic AmB, besides being the standard treatment for systemic fungal infections, has shown an extraordinary antileishmanial efficacy. Its main mechanism of action, binding to ergosterol-containing membranes of Leishmania (6), probably explains the lack of significant emergence of resistance to the compound. However, toxicity has limited its general use, and different low-toxicity preparations have been developed (i.e., liposomes), but their high price limits their standard use (7). Some low-cost vehicles for the antibiotic have been tested in vitro and in vivo (i.e., albumin microspheres [8] and polylactic-co-glycolic acid [PLGA] [9, 10]), although without further development. For its part, allicin (diallyl thiosulfinate = 2-propene-1-sulfinothioic acid S-2-propenyl ester), a natural product present in plants of the family Alliaceae, with antibacterial (11), antifungal (Candida) (12), and antiprotozoal (Plasmodium and Leishmania promastigotes) (13, 14) activities, has been found to exhibit a notable antileishmanial effect on the intracellular stages of L. donovani and L. infantum without substantial cytotoxicity for mammalian cells (15).
On these grounds, our approach was the exploration of the potential synergistic or additive antileishmanial effect of the combination of AmB and allicin (low micromolar concentrations of AmB plus micromolar allicin), thus avoiding the toxic concentrations needed with AmB in monotherapy. Results obtained in vitro against both promastigotes and intracellular amastigotes of L. donovani and L. infantum showed that allicin significantly enhanced the leishmanicidal activity of AmB and therefore reduced the required amount of AmB to eliminate intracellular infection of Mϕ by Leishmania.
MATERIALS AND METHODS
Parasites.
L. infantum (MCAN/ES/2001/UCM-9) is an autochthonous isolate obtained at the Clinical Service of the Department of Animal Health, Faculty of Veterinary Medicine (UCM), from a naturally infected dog (Madrid, Spain). L. donovani isolate Khartoum 1246 (MHOM/SD/43/124) was provided by A. Toraño (Department of Immunology, Instituto de Salud Carlos III, Madrid, Spain). Both species were routinely maintained as promastigotes in 25-ml culture flasks at 26°C in RPMI 1640 medium (Lonza Group, Switzerland) supplemented with 10% heat-inactivated (30 min at 56°C) fetal bovine serum (FBS; Sera Laboratories International, Horsted Keynes, United Kingdom) and 100 U/ml of penicillin plus 100 μg/ml of streptomycin (BioWhittaker, Verviers, Belgium).
Drugs.
Fungizone (deoxycholate-dispersed amphotericin B) was a gift from Bristol-Myers Squibb (France). Stabilized allicin was obtained as liquid Allisure (5,000 ppm) from Allicin International Ltd. (Rye, East Sussex, United Kingdom). For the in vitro experiments, 10 mM stock solutions of amphotericin B deoxycholate (AmB) in dimethyl sulfoxide (DMSO; Sigma, St. Louis, MO) were prepared. Further dilutions were freshly made in RPMI 1640 medium. The final DMSO concentration never exceeded 0.5% in the culture medium and had no effect on cell growth. Dilutions of allicin were directly performed in culture medium.
Promastigote assay. (i) Cell counts.
Preliminary tests to determine the 50% inhibitory concentrations (IC50s) of the individual drugs were performed. For the combination assay, the IC50s of the single drugs, previously determined by cell counts, were combined. Leishmania log-phase promastigotes were cultured in 10-ml sterile polystyrene tubes (initial concentration, 106 parasites/ml; 2 ml/tube) and treated with 0.07 μM AmB or 30 μM allicin or the combination of both (0.07 μM AmB plus 30 μM allicin). Cultures were counted 24, 48, and 72 h posttreatment in a Neubauer chamber, and the viability was determined with trypan blue exclusion dye (0.4% in phosphate-buffered saline [PBS]). Experiments were performed in triplicate.
(ii) alamarBlue assay for promastigote proliferation and synergism of the drug combination.
Mid-log-phase promastigotes were seeded in 96-well culture plates (Corning, New York, NY) at an initial plating density of 2.5 × 105 cells/well (200 μl/well). Cultures were exposed for 24 h to various concentrations of the drugs and their combinations. Promastigote proliferation and inhibition were determined with alamarBlue reagent (AbDSerotec Ltd., United Kingdom) (10%, vol/vol) by following the manufacturer's recommendations by reading fluorescence intensity (550-nm excitation wavelength and 590-nm emission wavelength) in a FLUOstar Omega (BMG Labtech) fluorimeter after 72 h of incubation with the dye. Untreated cultures, wells without cells and the maximal concentration from each compound and their combinations, and wells with culture medium and alamarBlue (10%, vol/vol) were included as controls. Results for growth inhibition were expressed as percentage of growth in untreated control cultures. Cultures were performed at least in triplicate.
alamarBlue assays with increasing concentrations of the single drugs were performed first, and appropriate drug combination ratios were tested according to the results obtained. Drugs were combined at constant ratios (0.01:5, 0.01:10, and 0.01:20 [μM AmB/μM allicin]) by following a modified fixed-ratio method (16), and their dose-effect relationships were assessed by the Chou-Talalay combination index (CI) method using CalcuSyn software (17, 18). This method is based on the multiple-drug effect equation derived from the median-effect principle and the mass action law. Results provide a quantitative determination for synergism (CI < 1), additivity (CI = 1), and antagonism (CI > 1). The software allows the calculation of the dose reduction index (DRI), a parameter which indicates the fold dose reduction allowed in a drug combination to reach a given degree of inhibition compared to the drug as a single agent. It provides other dose-effect-related parameters such as the potency (Dm) of the drugs alone or in combination, this representing the concentration which inhibits cell growth by 50%, and the shape of the dose-effect curve (m), where m of 1, >1, and <1 indicate hyperbolic, sigmoidal, and flat sigmoidal curves, respectively. The linear correlation coefficient (r) of the median-effect plot represents the conformity of the data to the mass action law.
Cytotoxicity assay for mammalian cells.
Toxicity for mammalian cells was determined ex vivo in murine (female BALB/c mice; Harlan Barcelona, Spain) peritoneal Mϕ isolated by peritoneal lavage, resuspended in RPMI 1640 medium supplemented with 10% FBS, and seeded in 96-well culture plates (4 × 106 cells/ml; 200 μl/well). Plates were incubated overnight to allow cell adherence at 37°C in a 5% CO2–95% air mixture. Different concentrations of allicin (10, 30, and 40 μM) plus AmB (0.1 and 0.5 μM) were added to the cultures. At the end of the drug exposure period (24 h), the medium was removed; wells were replenished with fresh medium and incubated for another 24 h. The viability of cells was determined by the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) reduction assay as described previously (19). Briefly, 50 μl of MTT (Sigma) solution (5 mg/ml in sterile PBS) was added to the wells, and the plates were kept for 4 h at 37°C in a humidified atmosphere protected from light. Formazan crystals were dissolved with 200 μl of DMSO and 25 μl/well of Sorensen's glycine buffer (0.1 M glycine and 0.1 M NaCl, adjusted to pH 10.5 with 1 M NaOH) and added to stop the reaction. Absorbance was read at 570 nm in a microplate reader. Untreated cells and wells without cells and the maximal concentration from each compound were included. Three independent experiments were carried out.
Amastigote assay and determination of the synergism of the drug combination.
Infection of Mϕ was carried out with the modification developed by us (15, 20). In brief, 2.5 × 104 J774 cells/well (200 μl/well) were cultured in 8-well Lab-Tek chambers (Nunc, Roskilde, Denmark) for 16 h to ensure adherence. Stationary-phase Leishmania promastigotes (day 7 to 10) were centrifuged (370 × g, 10 min, without brake) in a 5810R centrifuge (Eppendorf, Germany) through a Ficoll gradient (0, 10% in medium 199, and 30% in sterile PBS). Metacyclic parasites recovered were washed and opsonized with 15% normal mouse serum (Jackson ImmunoResearch, United Kingdom) in a solution (1/1, vol/vol) of RPMI medium and Hanks' balanced salt solution (HBSS; Sigma), 0.15 mM CaCl2, and 1 mM MgCl2 for 30 min at 37°C and 5% CO2. Infection was carried out overnight at 33°C at a parasite/Mϕ ratio of 10:1 in 5% CO2. Noninternalized promastigotes were removed by thorough washing, and fresh media containing the appropriate compounds dilutions were added to the wells. Cultures were treated with AmB (0.05, 0.075, and 0.1 μM) and allicin (0.05, 1, 5, and 10 μM) administered alone and in combination for 48 h. Unless otherwise stated, mitomycin C (Calbiochem, Merck, USA) at 1 μg/ml was added to avoid cell line proliferation. Untreated cultures, cultures treated with mitomycin C, and cultures treated with 0.5% DMSO were included as negative controls. Slides were fixed and stained (May-Grünwald Giemsa; Merck Darmstadt, Germany), and the number of amastigotes/100 cells and percent infected cells were determined. Drug activity was expressed as percentage of growth inhibition compared to that in untreated cultures. Three independent experiments were performed at least in triplicate.
Statistical analysis.
Graphs were generated and statistical analysis was performed using GraphPad Prism 5. Statistical significance of differences between mean values obtained in the different groups was determined by repeated-measures generalized linear model analysis, one-way analysis of variance (ANOVA), and Bonferroni test. Differences were considered significant at a P value of <0.05.
RESULTS
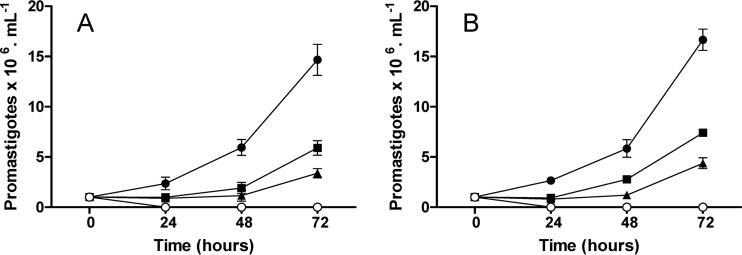
Figure 1 shows the results obtained by cell counting in the preliminary test on the effect of the combination of AmB and allicin. Similar results were obtained with both species, L. donovani (Fig. 1A) and L. infantum (Fig. 1B). Both compounds, AmB (0.07 μM) and allicin (30 μM), reduced by more than 50% the Leishmania growth after 24 h of exposure, although a certain recovery of cell multiplication was observed on the 48-h and 72-h samplings. Combination of AmB (0.07 μM) and allicin (30 μM), however, provoked the cell death of the majority of the leishmanial population, and actually, after 72 h, L. infantum counts reached a mere 3 × 103 ml−1, this representing a reduction of more than 99% compared to the untreated cultures. These results were suggestive of a positive interaction (additive or synergic) between the compounds. To determine the effect of the combination, drugs were tested at constant micromolar ratios (0.01:5, 0.01:10, and 0.01:20) (AmB/allicin); growth inhibition was estimated with the alamarBlue fluorimetric method. The two Leishmania species behaved similarly; Table 1 shows the results obtained for L. infantum promastigotes. In all cases, data obtained agreed with mass law action (r) and inhibition followed a sigmoid curve (m > 1). Dm values, corresponding to IC50s, obtained for the individual drugs tested were in the range of the previously estimated concentrations (22.1 μM for allicin and 0.09 μM for AmB). Growth inhibition results showed the significantly higher effect of the combination with allicin even with 0.01 μM AmB. Thus, whereas AmB administered alone did not induce at this concentration any inhibition of promastigotes' multiplication, the combination with 20 μM allicin provoked a 37.5% growth reduction. This effect of allicin was more evident with 0.02 μM AmB, since when the antibiotic was combined with 20 μM allicin, 50% inhibition was reached (IC50), and with 40 μM allicin, 89% (IC90) of the multiplication was stopped. Similarly, an AmB concentration of 0.08 μM, close to the IC50 of this compound, with 40 μM allicin reduced almost completely the multiplication of the promastigotes. It is noteworthy that microscopic examination of the promastigotes subjected to the combinations showed that they were not viable and that no recovery of the cultures was found (data not shown). The combined effect of the two compounds in terms of synergism-antagonism was complex, as shown at Table 2. Generally speaking, all combinations allowed reduction of the concentrations required for AmB (DRI), and synergism (CI < 1) was evident only with the fixed ratio (0.01 μM AmB plus 5 μM allicin) (Fig. 2). Combination allowed a 7- to 10-fold reduction of the AmB dose required to provoke a 90 to 95% reduction in the promastigotes' multiplication.
FIG 1.
Effects of 30 μM allicin (■), 0.07 μM AmB (▲), and the combination (30 μM allicin plus 0.07 μM AmB) (○) on the multiplication of L. donovani (A) and L. infantum (B) promastigotes after 24, 48, and 72 h. ●, untreated control cultures. Data are means ± standard deviations of an experiment in triplicate.
TABLE 1.
Effects of AmB and allicin and their combination on promastigotes of Leishmania infantum determined by alamarBlue assaya
| D1-D2 combination ratio | AmB concn (μM) | Allicin concn (μM) | Growth Inhibition (%)b | m | Dm (μM) | r |
|---|---|---|---|---|---|---|
| 0.01:5 | 0.0025 | 1.25 | 3.5 ± 0.59 | |||
| 0.005 | 2.5 | 3.4 ± 0.98 | ||||
| 0.01 | 5 | 3.5 ± 0.45 | ||||
| 0.02 | 10 | 3.6 ± 2.84 | ||||
| 0.04 | 20 | 15.0 ± 1.32 | ||||
| 0.08 | 40 | 99.0 ± 0.03 | ||||
| 0.16 | 80 | 99.0 ± 0.02 | 2.655 | 11.71 | 0.90 | |
| 0.01:10 | 0.0025 | 2.5 | 3.1 ± 1.54 | |||
| 0.005 | 5 | 3.3 ± 1.49 | ||||
| 0.01 | 10 | 4.0 ± 2.02 | ||||
| 0.02 | 20 | 52.0 ± 2.22 | ||||
| 0.04 | 40 | 97.0 ± 1.65 | ||||
| 0.08 | 80 | 99.0 ± 0.11 | ||||
| 0.16 | 160 | 99.0 ± 0.12 | 1.998 | 21.96 | 0.91 | |
| 0.01:20 | 0.0025 | 5 | 1.42 ± 1.05 | |||
| 0.005 | 10 | 2.09 ± 1.13 | ||||
| 0.01 | 20 | 37.5 ± 1.52 | ||||
| 0.02 | 40 | 89.0 ± 2.31 | ||||
| 0.04 | 80 | 99.0 ± 0.07 | ||||
| 0.08 | 160 | 99.0 ± 0.04 | ||||
| 0.16 | 320 | 99.0 ± 0.02 | 2.478 | 26.30 | 0.94 |
Drugs were combined at constant ratios (0.01:5, 0.01:10, and 0.01:20) and their dose-effect parameters were assessed by the Chou-Talalay method (17) using CalcuSyn software. Dm (median-effect dose) signifies the potency and it is the concentration which inhibits cell growth by 50%; m is the shape of the dose-effect curve, where m values of 1, >1, and <1 indicate hyperbolic, sigmoidal, and flat sigmoidal curves, respectively; r represents the linear correlation coefficient of the median-effect plot (conformity of the data to the mass action law principle). The m, Dm (μM), and r values for AmB (drug 1 [D1]) were 1.451, 0.09, and 0.86, respectively, and those for allicin (D2) were 2.066, 22.13, and 0.90, respectively.
Results are expressed as the means ± standard deviations of three independent experiments.
TABLE 2.
Dose-effect relationships of the combination of AmB and allicin on Leishmania infantum promastigotesa
| D1-D2 combination ratio | % growth inhibition (EDn) | CI | DRI |
Dose required (μM) |
||
|---|---|---|---|---|---|---|
| AmB | Allicin | AmB | Allicin | |||
| 0.01:5 | 50 | 0.79 (moderate synergism) | 3.89 | 1.89 | 0.023 | 11.69 |
| 75 | 0.65 (synergism) | 5.48 | 2.13 | 0.035 | 17.69 | |
| 90 | 0.54 (synergism) | 7.73 | 2.39 | 0.053 | 26.75 | |
| 95 | 0.49 (synergism) | 11.52 | 2.74 | 0.071 | 35.45 | |
| 0.01:10 | 50 | 1.23 (moderate antagonism) | 4.14 | 1.01 | 0.022 | 21.94 |
| 75 | 1.16 (slight antagonism) | 5.31 | 1.03 | 0.037 | 36.49 | |
| 90 | 1.09 (nearly additive) | 6.81 | 1.05 | 0.061 | 60.71 | |
| 95 | 1.05 (nearly additive) | 8.06 | 1.07 | 0.085 | 85.82 | |
| 0.01:20 | 50 | 1.33 (moderate antagonism) | 6.92 | 0.84 | 0.015 | 31.22 |
| 75 | 1.19 (slight antagonism) | 9.47 | 0.91 | 0.023 | 47.48 | |
| 90 | 1.07 (nearly additive) | 12.96 | 1.01 | 0.036 | 72.23 | |
| 95 | 0.99 (nearly additive) | 16.05 | 1.06 | 0.048 | 96.07 | |
Drugs were combined at constant ratios (0.01:5, 0.01:10, and 0.01:20), and their dose-effect relationships were assessed by the Chou-Talalay method (17) using CalcuSyn software. CI was calculated by the combination index equation. CI of <1, 1, and >1 indicate synergism, additive effect, and antagonism, respectively, at different effective doses (ED50, ED75, ED90, and ED95). Synergistic effects (CI < 1) are in bold. DRI indicates the fold dose reduction allowed in a drug combination to reach a given degree of inhibition compared to the drug as a single agent. Computer-simulated dose-required values of each drug in combination to reach a given effect level are included.
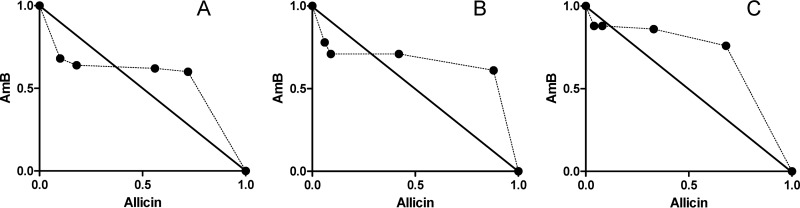
FIG 2.
Representative normalized isobolograms of the interaction of AmB with allicin at a fixed ratio (0.01µM AmB to 5 µM allicin). Lines intersect at the x and y axes at the normalized concentrations corresponding to the EC50 (●) (A), EC75 (■) (B), and EC90 (▲) (C) when the compounds were administered alone. The same symbols are used for the concentrations found with the AmB-plus-allicin combination to elicit the same effect as the drugs added alone (EC50, EC75, and EC90). Dr, dose required (µM AmB + µM allicin); EC, effective concentration.
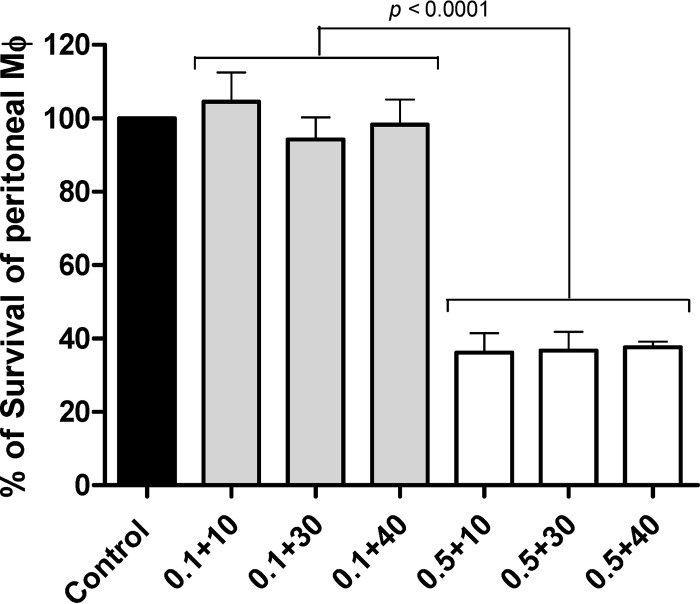
Given the results obtained with the extracellular stage of Leishmania, interaction was tested on intracellular amastigotes. Toxicity of the combination of AmB plus allicin was assayed on peritoneal Mϕ from BALB/c mice. None of the concentrations of allicin employed (10 through 40 μM) in the combination induced any significant toxicity as assessed by MTT assay (Fig. 3). However, 0.5 μM AmB, irrespective of the allicin concentration used, significantly (P < 0.001) inhibited the viability of Mϕ, more than 60%. As expected, no toxicity for mammalian cells was found with 0.1 μM AmB. This value is in the range of reported IC50s for Leishmania and supported the exploration of the combined treatment with allicin on the multiplication of intracellular amastigotes of Leishmania given the apparent absence of toxicity of the dialylsulfide up to 40 μM. Only concentrations below 0.1 μM AmB and 10 μM allicin were tested (Table 3). For comparative purposes, the effect of both compounds administered alone was also included. Since antileishmanial effect was determined by cell counting, drugs were combined at a nonconstant ratio in a checkerboard manner. As expected, AmB alone showed an approximate IC50 of 0.1 μM, and the maximal concentration of allicin (10 μM) reduced by ca. 30% the multiplication of amastigotes. Tested concentrations of the drugs, added alone or in combination, did not significantly affect the viability and infection rate of Mϕ under our experimental conditions; infection rates ranged from 47.3% ± 3.8% to 67.0% ± 3.5%, and the results obtained with the two Leishmania species (L. infantum and L. donovani) were similar (data not shown). An interaction between the compounds was found from moderately synergistic with the lowest concentrations to antagonistic with the highest concentration used (0.1 μM AmB plus 10 μM allicin) (Fig. 4A). In spite of this deduced interaction (CI), the most significant finding of the experiment was that 10 μM allicin could induce ca. 50% reduction of amastigotes' multiplication when administered with 0.05 μM AmB. It should be pointed out that for the particular L. infantum isolate employed, this AmB concentration administered alone had almost no effect (7.3% ± 5.5% growth inhibition) on the multiplication of intracellular amastigotes. This represents a 2-fold reduction in the dose required for the antibiotic.
FIG 3.
Effect of the combination of AmB and allicin on the ex vivo survival of mouse peritoneal macrophages (Mϕ). Numbers on the x axis represent the concentration (μM) of AmB plus concentration (μM) of allicin. Solid bar, untreated control Mϕ; gray bars, Mϕ cultures treated with 0.1 μM AmB; white bars, Mϕ cultures treated with 0.5 μM AmB. Results are the means ± standard deviations of three independent experiments.
TABLE 3.
Effects of AmB, allicin, and their combination on the multiplication of intracellular amastigotes of Leishmania infantum in J774 cellsa
| Drug combination nonconstant ratio (μM) |
% infectionb | % growth inhibitionb | CI | DRI |
||
|---|---|---|---|---|---|---|
| AmB | Allicin | AmB | Allicin | |||
| 0 | 0 | 64.7 ± 2.5 | 0 | |||
| 0.05 | 67.0 ± 3.5 | 7.3 ± 5.5 | ||||
| 0.075 | 64.7 ± 4.5 | 25.8 ± 5.1 | ||||
| 0.1 | 58.3 ± 2.1 | 50.1 ± 5.5 | ||||
| 0.05 | 60.3 ± 2.1 | 1.3 ± 4.2 | ||||
| 1 | 61.7 ± 5.7 | 15.7 ± 4.6 | ||||
| 5 | 56.7 ± 3.1 | 24.9 ± 1.8 | ||||
| 10 | 63.0 ± 4.4 | 32.8 ± 3.9 | ||||
| 0.05 | 0.05 | 62.7 ± 3.1 | 26.1 ± 3.1** | 0.75 (moderate synergism) | 1.51 | 11.28 |
| 1 | 60.0 ± 4.0 | 28.5 ± 4.4** | 0.80 (moderate synergism) | 1.55 | 6.04 | |
| 5 | 56.7 ± 1.5 | 34.2 ± 6.3*** | 1.30 (moderate antagonism) | 1.67 | 1.42 | |
| 10 | 56.3 ± 1.5 | 45.6 ± 8.5*** | 1.58 (antagonism) | 1.91 | 0.94 | |
| 0.075 | 0.05 | 56.0 ± 9.5 | 47.4 ± 4.5*** | 0.82 (moderate synergism) | 1.29 | 19.68 |
| 1 | 54.0 ± 8.2 | 55.8 ± 10.9*** | 0.79 (moderate synergism) | 1.42 | 11.96 | |
| 5 | 59.0 ± 4.4 | 54.8 ± 2.0*** | 1.13 (nearly additive) | 1.41 | 2.34 | |
| 10 | 59.0 ± 2.6 | 54.0 ± 3.6*** | 1.58 (antagonism) | 1.39 | 1.15 | |
| 0.1 | 0.05 | 47.3 ± 3.8 | 61.4 ± 3.8* | 0.90 (slight synergism) | 1.13 | 27.58 |
| 1 | 53.3 ± 3.8 | 61.5 ± 3.3* | 0.95 (nearly additive) | 1.13 | 13.86 | |
| 5 | 53.0 ± 3.6 | 64.5 ± 5.0** | 1.18 (slight antagonism) | 1.17 | 3.00 | |
| 10 | 47.7 ± 1.5 | 64.9 ± 0.9** | 1.50 (antagonism) | 1.18 | 1.51 | |
Drugs were combined at a nonconstant ratio and their dose-effect relationships assessed by the Chou-Talalay method (17) using CalcuSyn software. CI was calculated by the combination index equation. CI of <1, 1, and >1 indicate synergism, additive effect, and antagonism, respectively, at the different drug combination doses. Synergistic effects (CI < 1) are in bold. DRI indicates the fold dose reduction allowed in a drug combination at the different data points compared with each drug alone. Statistical differences between the effects of AmB alone and the combination of AmB plus allicin are indicated as follows: *, P < 0.05; **, P < 0.001; and ***, P < 0.0001.
Values are means ± standard deviations.
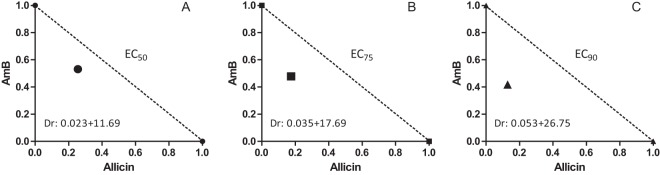
FIG 4.
Representative isobolograms of the in vitro interaction between AmB and allicin on intracellular amastigotes of L. donovani. Results correspond to the nonconstant-ratio experiments using 0.05 μM (A), 0.075 μM (B), and 0.1 μM (C) AmB and 0.05, 1, 5, and 10 μM allicin. Black circles indicate the IC50 of the combination at the concentrations on the x and y axes.
DISCUSSION
The aim of this work was the evaluation of the in vitro antileishmanial effect of the combination of AmB and allicin with the final purpose of reducing the required doses of the antibiotic and therefore the toxicity associated with its use. Results showed, using a fixed-ratio analysis with the extracellular stage (17, 18) and a checkerboard analysis with amastigotes, that the combination of the two compounds was from moderately synergic to synergic at low concentrations against both promastigotes (0.07 μM AmB plus 35.45 μM allicin induced 95% growth inhibition) and amastigotes (ca. 45% reduction with 0.05 μM AmB plus 10 μM allicin); this represents nearly a 2-fold reduction in the IC50 of the antibiotic added alone (15, 21). The differences observed between promastigotes, with a 7- to 11-fold reduction of AmB, and amastigotes, with a ca. 2-fold reduction, support an interest in using intracellular amastigotes in drug screening (9, 21, 22). It is clear that in vitro and ex vivo tests measure only the antiparasitic effect and that no direct prediction of the effect in vivo can be drawn from the results (23). Analysis showed that some drug combinations were classified as antagonist in spite of reaching a highly inhibitory effect in amastigotes and particularly promastigotes. This finding, previously observed in anti-Plasmodium screening (24, 25), suggests that even drugs associated with an indifferent effect in vitro could be useful therapeutic partners in vivo. In addition, differential interaction between AmB and allicin found in promastigotes and amastigotes of Leishmania could give us some clues on the mechanism of action of the drugs used. The main mechanism of action of AmB against Leishmania is related to the preferential binding to ergosterol (6), which impairs the permeability of membranes, resulting in loss of small cations and causing cell death (26, 27). This mechanism is also responsible for the fungicidal activity of the antibiotic. The part mechanistic basis of the antiproliferative, and in particular the leishmanial, activity of allicin (15, 19) is not clearly understood, although several cellular targets have been incriminated (28). Apparently allicin produces an enhancement of fungicidal activity of AmB by inhibiting ergosterol trafficking from the plasma membrane to the vacuole membrane in Candida (a member of the Fungi) (29). Leishmania also has considerable amounts of ergosterol on the plasma membrane. Resistance to AmB in clinical isolates of L. donovani has been associated, besides the presence of cholesta-5,7, 24-trien-3β-ol instead of ergosterol, with the upregulation of the thiol metabolic pathway (30). No thiol contents have been yet determined by us in allicin-treated Leishmania, but interference with thiols has been considered the main mechanism of action for allicin (28, 31). The synergy observed could be related to the impairment of the thiol metabolic pathway provoked by allicin, thus enhancing the effect of AmB. Whether or not this enhancement of AmB effect in the presence of allicin is responsible for the synergism found by us in amastigotes and, particularly, promastigotes of Leishmania needs further experimentation, which is under way.
Chemotherapy of leishmaniasis, both human and animal, is not yet solved in spite of the substantial efforts made on basic aspects of Leishmania biology. An alternative approach to monotherapy is the combination of drugs using well-established chemotherapeutic agents (i.e., miltefosine plus amphotericin B or antimonials plus amphotericin B) (32); in addition, these drug combinations have been common in veterinary clinical practice. Our results showed the apparently synergistic effect of allicin and AmB in the micromolar range. Data obtained with drug combinations in vitro have many limitations in spite of the dose-effect relationship analysis carried out (i.e., unforeseen interactions and pharmacokinetics) (18). However, the 2-fold reduction in the required dose of AmB against intracellular amastigotes and the possibility of using higher nontoxic doses of allicin (>10 μM) suggest exploration of this combination in vivo.
ACKNOWLEDGMENTS
M.J.C. has a predoctoral fellowship from the Ministry of Economy and Competitivity (MINECO). Research was supported by CICYT grant AGL2009-13009.
Amphotericin B was kindly donated by J. J. Torrado (Faculty of Pharmacy, Universidad Complutense). Excellent technical help by Beatriz Rojas is acknowledged.
Footnotes
Published ahead of print 23 December 2013
REFERENCES
- 1.Desjeux P. 2004. Leishmaniasis: current situation and new perspectives. Comp. Immunol. Microbiol. Infect. Dis. 27:305–318. 10.1016/j.cimid.2004.03.004 [DOI] [PubMed] [Google Scholar]
- 2.Croft SL, Sundar S, Fairlamb AH. 2006. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 19:11–126. 10.1128/CMR.19.1.111-126.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvar J, Croft SL, Olliaro P. 2006. Chemotherapy in the treatment and control of leishmaniasis. Adv. Parasitol. 61:224–274 http://dx.doi.org/10.1016/S0065-308X(05)61006-8 [DOI] [PubMed] [Google Scholar]
- 4.Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, Peeling RW, Alvar J, Boelaert M. 2007. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat. Rev. Microbiol. 5:873–882. 10.1038/nrmicro1748 [DOI] [PubMed] [Google Scholar]
- 5.Woods DJ, Knauer CS. 2010. Discovery of veterinary antiparasitic agents in the 21st century: a view from industry. Int. J. Parasitol. 40:1177–1185. 10.1016/j.ijpara.2010.04.005 [DOI] [PubMed] [Google Scholar]
- 6.Seifert K. 2011. Structures, targets and recent approaches in anti-leishmanial drug discovery and development. Open Med. Chem. J. 5:31–39. 10.2174/1874104501105010031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sundar S, Chatterjee M. 2006. Visceral leishmaniasis—current therapeutic modalities. Indian J. Med. Res. 123:345–352 [PubMed] [Google Scholar]
- 8.Sánchez-Brunete JA, Dea MA, Rama S, Bolás F, Alunda JM, Torrado-Santiago S, Torrado JJ. 2005. Influence of the vehicle on the properties and efficacy of microparticles containing amphotericin B. J. Drug Target. 13:225–233. 10.1080/10611860500097107 [DOI] [PubMed] [Google Scholar]
- 9.Ordóñez-Gutiérrez L, Espada-Fernández R, Dea-Ayuela MA, Torrado JJ, Bolás-Fernández F, Alunda JM. 2007. In vitro effect of new formulations of amphotericin B on amastigote and promastigote forms of Leishmania infantum. Int. J. Antimicrob. Agents 30:325–329. 10.1016/j.ijantimicag.2007.05.013 [DOI] [PubMed] [Google Scholar]
- 10.Van de Ven H, Paulussen C, Feijens PB, Matheeeussen A, Rombaut P, Kayaert P, Van den Mooter G, Weyenberger W, Cos P, Maes L, Ludwig A. 2012. PLGA nanoparticles and nanosuspensions with amphotericin B: potent in vitro and in vivo alternatives to Fungizone and AmBisome. J. Control. Release 161:795–803. 10.1016/j.jconrel.2012.05.037 [DOI] [PubMed] [Google Scholar]
- 11.Cutler RR, Wilson P. 2004. Antibacterial activity of a new, stable, aqueous extract of allicin against methicillin-resistant Staphylococcus aureus. Br. J. Biomed. Sci. 61:71–74 [DOI] [PubMed] [Google Scholar]
- 12.Khodavandi A, Alizadeh F, Ramal NS, Sidik SM, Toman F, Sekawi Z, Jahromi MA, Ng KP, Chong PP. 2011. Comparison between efficacy of allicin and fluconazole against Candida albicans in vitro and in a systemic candidiasis mouse model. FEMS Microbiol. Lett. 315:87–93. 10.1111/j.1574-6968.2010.02170.x [DOI] [PubMed] [Google Scholar]
- 13.McClure DC, Noland LL, Zatyrka SA. 1996. Antileishmanial properties of Allium sativum extracts and derivatives. Acta Hortic. 426:183–191 [Google Scholar]
- 14.Waag T, Gelhaus C, Rath J, Stich A, Leippe M, Schirmeister T. 2010. Allicin and derivates are cysteine protease inhibitors with antiparasitic activity. Bioorg. Med. Chem. Lett. 20:5541–5543. 10.1016/j.bmcl.2010.07.062 [DOI] [PubMed] [Google Scholar]
- 15.Corral-Caridad MJ, Moreno I, Toraño A, Domínguez M, Alunda JM. 2012. Effect of allicin on promastigotes and intracellular amastigotes of Leishmania donovani and L. infantum. Exp. Parasitol. 132:475–482. 10.1016/j.exppara.2012.08.016 [DOI] [PubMed] [Google Scholar]
- 16.Seifert K, Croft SL. 2006. In vitro and in vivo interactions between miltefosine and other antileishmanial drugs. Antimicrob. Agents Chemother. 50:73–79. 10.1128/AAC.50.1.73-79.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chou TC, Hayball M. 1996. CalcuSyn for Windows, Multiple-Drug Dose-Effect analyzer and manual. Biosoft, Cambridge, United Kingdom [Google Scholar]
- 18.Chou TC. 2008. Preclinical versus clinical drug combination studies. Leuk. Lymphoma 49:2059–2080. 10.1080/10428190802353591 [DOI] [PubMed] [Google Scholar]
- 19.Plumb JA. 2004. Cell sensitivity assays: the MTT assay. Methods Mol. Med. 88:165–169. 10.1385/1-59259-406-9:165 [DOI] [PubMed] [Google Scholar]
- 20.Méndez S, Nell M, Alunda JM. 1996. Leishmania infantum: infection of macrophages in vitro with promastigotes. Int. J. Parasitol. 26:619–622. 10.1016/0020-7519(96)00037-9 [DOI] [PubMed] [Google Scholar]
- 21.Vermeersch M, da Luz RI, Toté K, Timmermans JP, Cos P, Maes L. 2009. In vitro susceptibilities of Leishmania donovani promastigote and amastigote stages to antileishmanial reference drugs: practical relevance of stage-specific differences. Antimicrob. Agents Chemother. 53:3855–3859. 10.1128/AAC.00548-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sereno D, Cordeiro da Silva A, Mathieu-Daude F, Ouaissi A. 2007. Advances and perspectives in Leishmania cell based drug-screening procedures. Parasitol. Int. 56:3–7. 10.1016/j.parint.2006.09.001 [DOI] [PubMed] [Google Scholar]
- 23.Seifert K, Munday J, Syeda T, Croft S. 2011. In vitro interactions between sitamaquine and amphotericin B, sodium stibogluconate, miltefosine, paromomycin and pentamidine against Leishmania donovani. J. Antimicrob. Chemother. 66:850–854. 10.1093/jac/dkq542 [DOI] [PubMed] [Google Scholar]
- 24.Wiesner J, Henschker D, Hurchinson DB, Beck E, Jomaa H. 2002. In vitro and in vivo synergy of fosmidomycin, a novel antimalarial drug, with clindamycin. Antimicrob. Agents Chemother. 46:2889–2894. 10.1128/AAC.46.9.2889-2894.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fivelman QL, Adagu IS, Warhurst DC. 2004. Modified fixed-ratio isobologram method for studying in vitro interactions between atovaquone and proguanil or dihydroartemisin against drug-resistant strains of Plasmodium falciparum. Antimicrob. Agents Chemother. 48:4097–4102. 10.1128/AAC.48.11.4097-4102.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berman JD, Badaro R, Thakur CP, Wasunna KM, Behbehani K, Davidson R, Kuzoe F, Pang L, Weerasuriya K, Bryceson ADM. 1998. Efficacy and safety of liposomal amphotericin B (AmBisome) for visceral leishmaniasis in endemic developing countries. Bull. World Health Organ. 76:25–32 [PMC free article] [PubMed] [Google Scholar]
- 27.Larabi M, Yardley V, Loiseau PM, Appel M, Legrand P, Gulik A, Bories C, Croft SL, Barratt G. 2003. Toxicity and antileishmanial activity of a new stable lipid suspension of amphotericin B. Antimicrob. Agents Chemother. 47:3774–3779. 10.1128/AAC.47.12.3774-3779.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabinkov A, Miron T, Konstantinovski L, Wilchek M, Mirelman D, Weiner I. 1998. The mode of action of allicin: trapping of radicals and interaction with thiol containing proteins. Biochim. Biophys. Acta 1379:233–244. 10.1016/S0304-4165(97)00104-9 [DOI] [PubMed] [Google Scholar]
- 29.Ogita A, Fujita K-I, Tanaka T. 2009. Enhancement of the fungicidal activity of amphotericin B by allicin: effects on intracellular ergosterol trafficking. Planta Med. 75:222–226. 10.1055/s-0028-1088376 [DOI] [PubMed] [Google Scholar]
- 30.Purkait B, Kumar A, Nandi N, Sardar AH, Das S, Kumar S, Pandey K, Ravidas V, Kumar M, De T, Singh D, Das P. 2012. Mechanism of amphotericin B resistance in clinical isolates of Leishmania donovani. Antimicrob. Agents Chemother. 56:1031–1041. 10.1128/AAC.00030-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miron T, Listowsky I, Wilchek M. 2010. Reaction mechanisms of allicin and allyl-mixed disulfides with proteins and small thiol molecules. Eur. J. Med. Chem. 45:1912–1918. 10.1016/j.ejmech.2010.01.031 [DOI] [PubMed] [Google Scholar]
- 32.Olliaro P. 2010. Drug combinations for visceral leishmaniasis. Curr. Opin. Infect. Dis. 23:595–602. 10.1097/QCO.0b013e32833fca9d [DOI] [PubMed] [Google Scholar]