Abstract
Indwelling medical devices have become a major source of nosocomial infections, especially Pseudomonas aeruginosa infections, which remain the most common cause of ventilator-associated pneumonia (VAP) in neonates and children. Using medical grade polyvinyl chloride endotracheal tubes (ETTs), the activity of tobramycin and polymyxin E was quantified in a simulated prevention and treatment static time-kill model using biofilm-forming P. aeruginosa. The model simulated three clinical conditions: (i) planktonic bacteria grown in the presence of antibiotics (tobramycin and polymyxin E) without ETTs, (ii) planktonic bacteria grown in the presence of P. aeruginosa, antibiotic, and ETTs (simulating prevention), and (iii) a 24-h-formed P. aeruginosa biofilm grown on ETTs prior to antibiotic exposure (simulating treatment). In the model simulating “prevention” (conditions 1 and 2 above), tobramycin alone or in combination with polymyxin E was more bactericidal than polymyxin E alone at 24 h using a concentration of greater than 2 times the MIC. However, after a 24-h-old biofilm was allowed to form on the ETTs, neither monotherapy nor combination therapy over 24 h exhibited bactericidal or bacteriostatic effects. Against the same pathogens, tobramycin and polymyxin E, alone or in combination, exhibited bactericidal activity prior to biofilm attachment to the ETTs; however, no activity was observed once biofilm formed on ETTs. These findings support surveillance culturing to identify pathogens for a rapid and targeted approach to therapy, especially when P. aeruginosa is a potential pathogen.
INTRODUCTION
Indwelling medical devices are a major source of nosocomial infections. In particular, patients requiring mechanical ventilation (intubation with an endotracheal tube [ETT]) face a high probability of contracting one of the most prevalent nosocomial infections, ventilator-associated pneumonia (VAP) (1–3). Neonatal and pediatric populations are at especially high risk for VAP because the current standard of care involves prolonged intubation without ETT exchange or tracheostomy, both common practice in adult patients. In neonates and infants, the inner diameter of the ETT is often 2.5 to 3.5 mm (the size of a thin straw), which complicates suctioning of secretions and confounds attempts to maintain patency. Despite aggressive bedside hygiene, Pseudomonas aeruginosa remains one of the most common causes of VAP in intubated children (2, 4, 5).
P. aeruginosa, often found on indwelling devices such as ETTs, forms a biofilm which serves as an ideal environment for antibiotic resistance, making VAP difficult to treat (6, 7). Biofilm on ETTs is considered to be a reservoir for infecting pathogens derived from oropharyngeal flora and gastric microaspiration and is highly correlated with lower airway infection and subsequent VAP (8–11). To date, few side-by-side studies have compared killing activity (defined as 99.9% kill) of tobramycin to that of polymyxin E against P. aeruginosa, especially in the context of ETT biofilm and VAP (12–15). The effect of monotherapy and/or combination therapy (synergistic versus antagonistic activity) must be assessed when evaluating antimicrobial drug therapy, especially in the presence of medical grade polyvinyl chloride (PVC) or conventional ETTs. For convenience, most studies investigating antibiotic susceptibility in formed biofilms have used PVC coupons rather than clinically available medical devices (16–18). However, most of the coupons made of PVC are not medical grade and, in many cases, do not contain equivalent plasticizer content. These differences result in different texture and flexibility characteristics of medical grade PVC products and PVC coupons used in biofilm experiments. Using clinically available ETTs, this study aimed both to assess the efficacy of antibiotics against planktonic versus biofilm-formed P. aeruginosa and to identify which antibiotic, alone or combination, demonstrates the best in vitro activity against P. aeruginosa in the context of VAP.
(This study was presented as a poster at the 52nd Interscience Conference of Antimicrobial Agents and Chemotherapy, San Francisco, CA, 7 to 12 September 2012 [19].)
MATERIALS AND METHODS
Bacterial isolates.
American Type Culture Collection (ATCC, Manassas, VA) strain 25668 was obtained. Reference strain PAO1 was obtained from Thomas Murray, Frank H. Netter MD School of Medicine, Quinnipiac University, North Haven, CT (20, 21). Prior to use, all bacteria were stored in tryptic soy broth (TSB; Difco Laboratories, Sparks, MD) with 15% glycerol and frozen at −80°C. Both strains are prolific biofilm producers (22, 23).
Antimicrobial agents.
Commercially available, chemical grade polymyxin E (lot 081M1525V) powder and chemical grade tobramycin (lot 090M1196V) powder were purchased from Sigma-Aldrich (St. Louis, MO). Tobramycin powder and polymyxin E powder were stored at 4°C. Both tobramycin and polymyxin E were diluted in sterile water, and a fresh stock was made each day and prior to every experiment. Tobramycin and polymyxin E were tested at one, two, four, and eight times their respective MICs at 0, 4, and 24 h after inoculation (24). Cation-adjusted Mueller-Hinton broth (CA-MHB; Difco Laboratories, Sparks, MD) supplemented with 25 mg/liter calcium, 12.5 mg/liter magnesium, and 0.25% dextrose (Fisher Scientific, Pittsburgh, PA) was used to obtain a suspension corresponding to a 0.7 to 0.8 McFarland standard to produce an initial inoculum of 5.5 to 6.0 × 106 CFU/ml. Colony counts were determined using tryptic soy agar (TSA; Difco, Becton, Dickinson Co., Sparks, MD) plates.
Susceptibility testing.
MIC tests were performed in triplicate using broth microdilution in accordance with Clinical and Laboratory Standards Institute (CLSI) and National Committee for Clinical Laboratory Standards (NCCLS) guidelines (25, 26). The MIC was defined as the minimum concentration of antibiotic that would inhibit the visual growth of the isolated organism. Minimum bactericidal concentrations (MBC) were also determined in triplicate for each antimicrobial agent using NCCLS guidelines (26). Bacteria were quantified using CFU/ml, and 5-μl aliquots were used for determination of MBC after 24 h incubation at 37°C using TSA (27).
Endotracheal tubes (ETTs).
Commercially available Sheridan uncuffed ETTs (Hudson RIC, Temecula, CA) (6.0-mm inner diameter [ID]) were obtained. Each ETT was cut into 0.6-cm-by-0.3-cm rectangular pieces (ETT chips) using a one-quarter-rectangle hand puncher (Fiskars Corporation, Helsinki, Finland) and sterilized with ethylene oxide gas prior to use in preformed- and formed-biofilm time-kill experiments (23). For comparisons, we also tested commercially available PVC coupons (part number RD 128-PVC; Biosurface Technologies Corp., Bozeman, MT) for preformed-biofilm P. aeruginosa PAO1 (16–18).
Biofilm formation.
Sterile ETT chips were placed in each well of a 24-well plate (BD Biosciences, San Jose, CA). The ETT chip was submerged with 2 ml of a final bacterial inoculum, either PAO1 or ATCC 25668, obtained as described above using TSB supplemented with 1% dextrose, 2% NaCl, and 25 mg/liter calcium (STSB) and a modified version of a previously described method (28). The well plate was incubated at 37°C under static conditions for 24 h to promote biofilm formation on ETT chips. After 24 h, each ETT chip was gently rinsed three times in sterile phosphate-buffered saline (PBS) (Fisher Scientific, Pittsburgh, PA).
Time-kill study.
Using a 24-h time-kill study, three clinical conditions were modeled using P. aeruginosa strains PAO1 and 25668: (i) planktonic bacteria in the presence of the antibiotics tobramycin and polymyxin E, without ETTs, (ii) planktonic bacteria grown in the presence of P. aeruginosa, antibiotics, and ETTs (simulating prevention), and (iii) a 24-h-formed P. aeruginosa biofilm on ETTs prior to antibiotic exposure (simulating treatment). Each time-kill experiment was carried out in a minimum of triplicate iterations. All antimicrobial agents were tested at one, two, four, and eight times their respective MICs with starting inocula of 5.5 × 106 to 6.0 × 106 CFU/ml adjusted to McFarland standards using a Vitek colorimeter (bioMérieux, Inc., Durham, NC) (18, 29).
Sample aliquots (0.1 ml) were removed from cultures at 0, 4, and 24 h after each tube was shaken using a vortexing device for 1 min to remove biofilm growth from the ETT chip (23). Antimicrobial carryover was accounted for by serial dilution (10- to 10,000-fold) of plated samples with normal saline or vacuum filtration. This methodology has a lower limit of detection of 2.0 log10 CFU/ml (29). Growth control tubes for each organism were prepared without antibiotic and run in parallel to the antibiotic test tubes.
For single antimicrobial agents, bactericidal activity (99.9% kill) was defined as a ≥3 log10 CFU/ml reduction at 24 h in colony count from the initial inoculum. Bacteriostatic activity was defined as a <3 log10 CFU/ml reduction at 24 h in colony count from the initial inoculum, while inactivity was defined as no observed reduction from the initial inoculum (24). For antibiotics evaluated in combination, synergy was defined as a ≥2 log10 CFU/ml decrease, indifference was defined as a 1 to 2 log10 CFU/ml change (increase or decrease), and antagonism was defined as a >2 log10 CFU/ml increase in growth compared to the most active single agent.
Data analysis.
All statistical analyses were performed using SPSS statistical software (IBM SPSS statistics version 20, IBM Corporation, Armonk, NY). After 24 h of exposure to an antimicrobial agent(s), the biofilm formation was quantified and bacteria were counted at 4 h and 24 h (with a lower limit of detection 2.0 log10 CFU/ml) to compare antimicrobial groups, concentrations, and strains using analysis of variance (ANOVA) followed by Tukey's post hoc analysis. Multiple regressions for the association between substrates and CFU/ml were analyzed. A P value of ≤0.05 indicated statistical significance.
RESULTS
The MIC for tobramycin was 0.5 μg/ml and for polymyxin E was 2 μg/ml for both the PAO1 and 25668 strains. The MBCs for tobramycin were 4 and 32 μg/ml and for polymyxin E were 16 and 64 μg/ml, respectively, for the Pseudomonas PAO1 and ATCC 25668 strains.
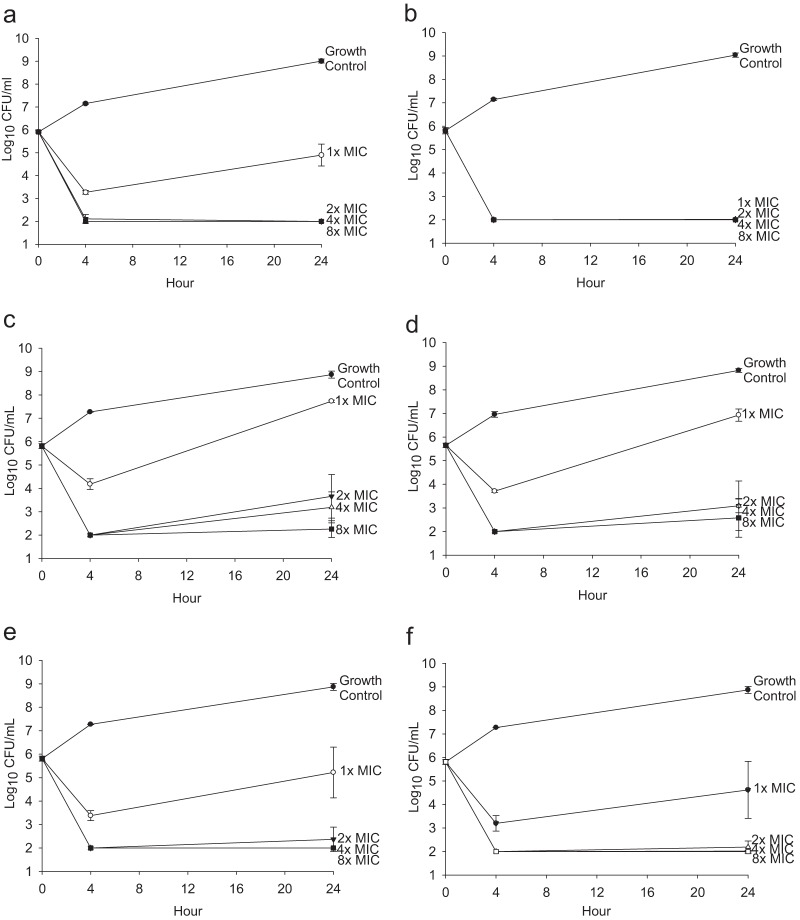
In the planktonic time-kill study, tobramycin demonstrated bactericidal activity against both Pseudomonas isolates at 24 h, with average decrease of 3.81 ± 0.16 log10 CFU/ml for all concentrations except 1 times the MIC for PAO1 (Fig. 1a and b). Polymyxin E demonstrated bacteriostatic activity at 2 times and 4 times the MIC (average decrease of 2.16 to 2.63 log10 CFU/ml) and bactericidal activity at 8 times the MIC (average decrease of 3.07 to 3.56 log10 CFU/ml) but inactivity at 1 times the MIC for both isolates at 24 h (Fig. 1c and d). The combination therapy at 2 times, 4 times, and 8 times the MIC demonstrated indifference, with >3.44 log10 CFU/ml kill for PAO1 and >3.46 log10 CFU/ml kill for 25668 at 24 h (Fig. 1e and f).
FIG 1.
Time kill against planktonic P. aeruginosa. Data shown represent the results for tobramycin against planktonic P. aeruginosa PAO1 (a) and 25668 (b), polymyxin E against planktonic P. aeruginosa PAO1 (c) and 25668 (d), and the combination of tobramycin and polymyxin E against planktonic P. aeruginosa PAO1 (e) and 25668 (f). Results are presented as means ± standard deviations.
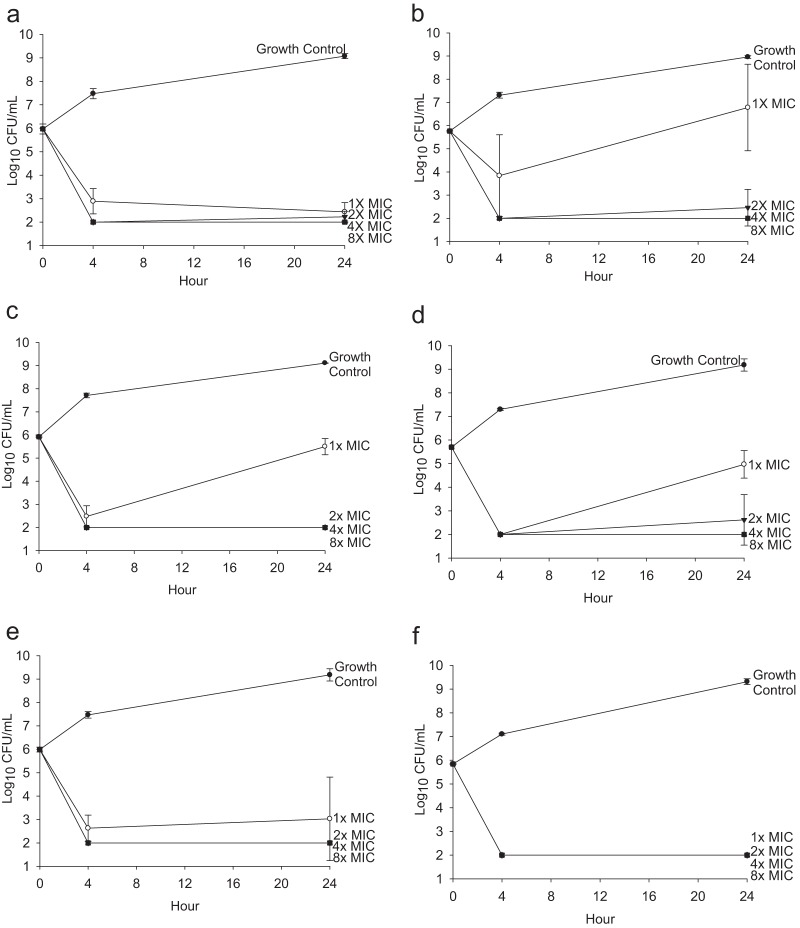
In the preformed-biofilm time-kill studies (simulating prevention) at 24 h, tobramycin demonstrated bactericidal activity against both Pseudomonas isolates (average decrease of >3.3 log10 CFU/ml), except 1 times the MIC for 25668, which showed inactivity (1.02 ± 1.86 log10 CFU/ml increase; Fig. 2a and b). Similarly, polymyxin E demonstrated bactericidal activity (average decrease of >3.08 log10 CFU/ml) at greater than 2 times the MIC but bacteriostatic activity at 1 times the MIC for both isolates at 24 h (Fig. 2c and d). The combination of tobramycin and polymyxin E demonstrated indifferent activity at all concentrations for both isolates (Fig. 2e and f).
FIG 2.
Time kill against pre-biofilm-formed P. aeruginosa. Data shown represent the results for tobramycin against P. aeruginosa PAO1 (a) and 25668 (b), polymyxin E against planktonic P. aeruginosa PAO1 (c) and 25668 (d), and the combination of tobramycin and polymyxin E against P. aeruginosa PAO1 (e) and 25668 (f) in the presence of ETT chips. Results are presented as means ± standard deviations.
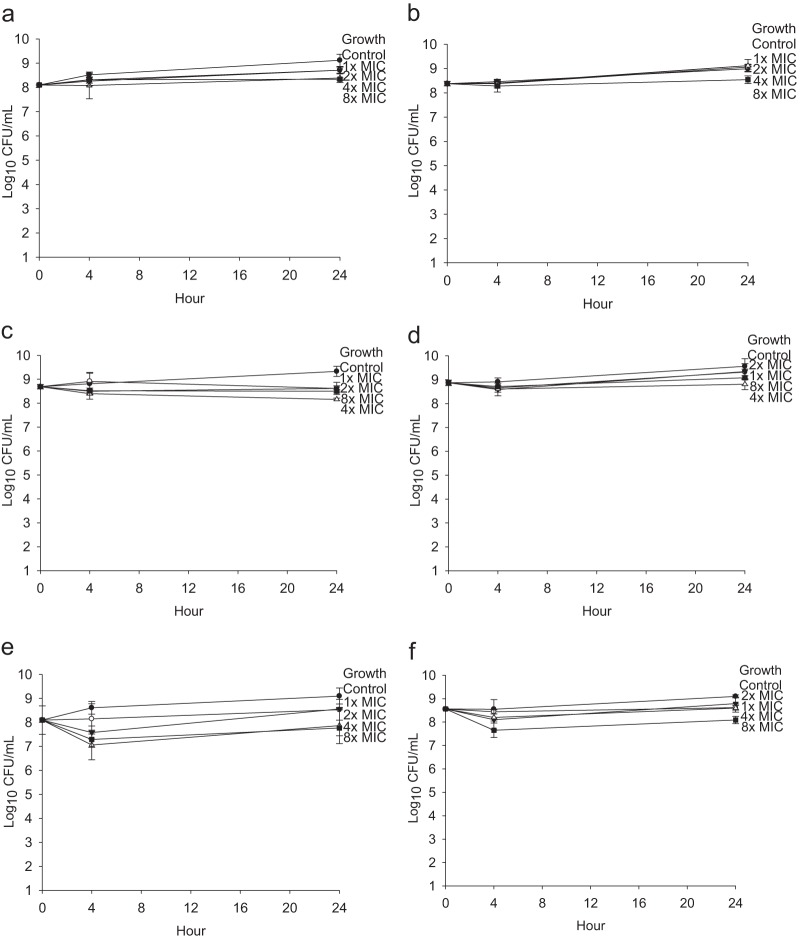
In formed-biofilm time-kill studies (simulating treatment) for PAO1, combination therapy at 4 times the MIC was significantly more active at 4 h than therapy with polymyxin E alone at 4 times the MIC (mean difference [MD] = −1.34; 95% confidence interval [CI], −2.4 to −0.3 log10 CFU/ml; P = 0.004) and 8 times the MIC (MD = −1.45; 95% CI, −2.5 to −0.4 log10 CFU/ml; P = 0.001). Similarly, combination therapy at 8 times the MIC was significantly more active at 4 h than therapy with polymyxin E alone at 8 times the MIC (MD = −1.23; 95% CI, −2.3 to −0.2 log10 CFU/ml; P = 0.01). However, indifferent activity was observed at 24 h. Similarly, for 25668, combination therapy at 8 times the MIC was significantly more active than therapy with polymyxin E alone at 4 h (MD = −1.06; 95% CI, −1.7 to −0.4 log10 CFU/ml; P < 0.001). However, indifferent activity was observed at 24 h. Once biofilm is formed, both single-agent and combination antibiotics resulted in inactivity or indifference (Fig. 3).
FIG 3.
Time kill against biofilm-formed P. aeruginosa. Data shown represent the results for tobramycin against P. aeruginosa PAO1 (a) and 25668 (b), polymyxin E against planktonic P. aeruginosa PAO1 (c) and 25668 (d), and the combination of tobramycin and polymyxin E against P. aeruginosa PAO1 (e) and 25668 (f). Results are presented as means ± standard deviations.
In addition to medical grade PVC ETTs, we assayed time kill using commercially available PVC coupons (16–18). A similar trend of bactericidal activity was demonstrated at 24 h with greater than 4 times the MIC of tobramycin (average decrease of >3.03 log10 CFU/ml) and with greater than 2 times the MIC of polymyxin E (average decrease of >3.1 log10 CFU/ml), but indifference was noted when the combination of tobramycin and polymyxin E was evaluated at 2, 4, and 8 times the MIC (average decrease of >3.21 log10 CFU/ml). ANOVA showed that there was a significant difference between substrates and CFU/ml at 4 h (MD = 0.08; 95% CI, 2.5 to 3.7 log10 CFU/ml; P = 0.041) (Table 1). Multiple-regression analysis demonstrated that there was a significant association between CFU/ml and the substrate at 4 h (partial eta squared [eta] = 0.493; P < 0.001) and at 24 h (eta = 0.208; P < 0.001). The overall model fit was R2 = 0.954.
TABLE 1.
Comparison of changes at 4 h from 0 h growth control between endotracheal tube and polyvinyl chloride coupons
| Concn | Avg change (log10 CFU/ml ± SD)a |
|||||
|---|---|---|---|---|---|---|
| Tobramycin |
Polymyxin E |
Tobramycin + polymyxin-E |
||||
| ETT | PVC coupon | ETT | PVC coupon | ETT | PVC coupon | |
| 1× MIC | −2.81 ± 0.04 | −2.82 ± 0.12 | −3.44 ± 0.43 | −1.77 ± 0.04 | −3.36 ± 0.46 | −4.09 ± 0.10 |
| 2× MIC | −3.97 ± 0.21 | −0.71 ± 0.01 | −3.92 ± 0.03 | −2.86 ± 0.10 | −3.99 ± 0.10 | −4.24 ± 0.03 |
| 4× MIC | −3.97 ± 0.21 | −1.23 ± 0.03 | −3.92 ± 0.03 | −3.16 ± 1.2 | −3.99 ± 0.10 | −4.24 ± 0.03 |
| 8× MIC | −3.97 ± 0.21 | −1.06 ± 0.03 | −3.92 ± 0.03 | −3.49 ± 1.28 | −3.99 ± 0.10 | −4.24 ± 0.03 |
ETT, endotracheal tube; PVC, polyvinyl chloride.
DISCUSSION
Ventilator-associated pneumonia, a common nosocomial infection often caused by bacteria that produce biofilm, results in increases in morbidity, medical costs, and multidrug-resistant organisms (2, 3, 30–33). In one study, adult patients with VAP were hospitalized longer (38 versus 13 days; P < 0.01), mortality rates were higher (50% versus 34%; P < 0.01), and hospital costs were greater ($70,568 versus $21,620; P < 0.01) than were seen with uninfected ventilated patients, with estimated VAP-attributable costs of $11,897 (33). However, limited diagnostic criteria and modification of ETTs make VAP prevention particularly challenging and difficult, especially for neonates and children (2).
In children, reintubation and tracheostomy insertion create the additional risk of damaging their small and fragile airway; therefore, reintubation or tracheostomy after a standard duration of intubation is not routinely practiced. Thus, the longer the ETTs remain in patients due to prolonged mechanical ventilation, the more likely biofilms are to develop and adhere (34–36). This bacterial accumulation of biofilms on ETTs may become dislodged during simple routine care such as suctioning or due to ventilation airflow. Bacteria and biofilm that break off become planktonic and seed further in the airway, causing more-complicated pneumonia (8, 37).
One controversial approach to treatment of VAP is “selective decontamination of the digestive tract” with broad-spectrum intravenous (IV) antimicrobials (38, 39). However, IV prophylaxis is not widely accepted due to fear of creating antibiotic-resistant strains among VAP pathogens. In the pediatric population, one of the most common VAP pathogens is P. aeruginosa, accounting for 17% to 25% of VAP cases (2, 4, 5). Our model is most consistent with the practice of direct instillation of liquid antimicrobial agents through the ETT as prophylaxis against or treatment of VAP caused by P. aeruginosa compared to inhalation of nebulized antibiotics (40). Instillation treatments pose less risk of systemic toxicity than IV administration because antimicrobial agents can be delivered locally using ETTs or tracheostomy tubes in children and neonates. Moreover, instillation can deliver drug directly to the site of pneumonia whereas nebulized drug may adsorb on the ETT, permeate into the ETT wall, or remain in the proximal airway. Therefore, our study model using ETT chips is useful to help understand the effects of tobramycin and polymyxin E, alone or in combination, to treat VAP caused by P. aeruginosa.
In our study, we examined P. aeruginosa growth with or without the presence of medical grade polyvinyl chloride (PVC) ETT to evaluate the bactericidal effects of two antibiotics under the conditions of VAP. We found that under in vitro conditions, the bactericidal effect of tobramycin or polymyxin E monotherapy required greater than 2 times the MIC at 24 h for the prebiofilm condition (prevention). However, antibiotics demonstrated different levels of activity against the two different strains. For PAO1, tobramycin monotherapy and the combination approach were equally active for killing. For 25668, the combination therapy was more active than monotherapy for killing at 24 h (Fig. 2); this finding may be related to the biofilm-forming abilities of each bacterium.
Our study also demonstrated that two of the antibiotics tested either in monotherapy or in combination showed inactivity against or indifference to both Pseudomonas strains once biofilm was formed on ETTs (Fig. 3). This is in contrast to the conclusions drawn by Herrmann et al. using a 96-peg Calgary biofilm device in vitro showing that combination therapy with colistin-tobramycin was superior to monotherapy against Pseudomonas biofilm (41).
Many in vitro studies have used commercially available PVC coupons, which have different characteristics with respect to texture and flexibility (based on the plasticizer content compared to medical grade PVC ETTs). We hypothesized that bacterial colonies would form differently on commercially available PVC coupons compared to medical grade PVC ETTs. To capture Pseudomonas growth in relation to different material surfaces more accurately, we studied the same antibiotic therapy against Pseudomonas PAO1 using both PVC coupons and PVC ETTs. There was a significant association between CFU/ml and substrate at 4 and 24 h (Table 1); thus, the results showed the importance of utilizing the same device material to mimic VAP conditions to evaluate antibiotic activity on biofilm.
In conclusion, neither single nor combination therapy with tobramycin and/or polymyxin E demonstrated killing activity once Pseudomonas biofilm was already formed on ETTs; however, no antagonism was noted. Bactericidal effects against preformed biofilm (simulating prevention) in the presence of ETTs suggest that surveillance cultures could identify pathogens prior to biofilm formation and could allow prophylactic or targeted approaches to therapy, especially when Pseudomonas is a potential pathogen. In addition, this study demonstrated the importance of material choice in an in vitro time-kill study. Further investigation could incorporate wild-type strains as well as clinically feasible treatment options for VAP in children.
ACKNOWLEDGMENTS
This study was funded by PCCSDP (Pediatric Critical Care Scientist Development Program), through NIH 5K12HD047349-07, and supported by the Department of Pediatrics, Hasbro Children's Hospital, Rhode Island Hospital.
Footnotes
Published ahead of print 30 December 2013
REFERENCES
- 1.Srinivasan R, Asselin J, Gildengorin G, Wiener-Kronish J, Flori HR. 2009. A prospective study of ventilator-associated pneumonia in children. Pediatrics 123:1108–1115. 10.1542/peds.2008-1211 [DOI] [PubMed] [Google Scholar]
- 2.Foglia E, Meier MD, Elward A. 2007. Ventilator-associated pneumonia in neonatal and pediatric intensive care unit patients. Clin. Microbiol. Rev. 20:409–425. 10.1128/CMR.00041-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stockwell JA. 2007. Nosocomial infections in the pediatric intensive care unit: affecting the impact on safety and outcome. Pediatr. Crit. Care Med. 8:S21–S37. 10.1097/01.PCC.0000257486.97045.D8 [DOI] [PubMed] [Google Scholar]
- 4.Weber DJ, Rutala WA, Sickbert-Bennett EE, Samsa GP, Brown V, Niederman MS. 2007. Microbiology of ventilator-associated pneumonia compared with that of hospital-acquired pneumonia. Infect. Control Hosp. Epidemiol. 28:825–831. 10.1086/518460 [DOI] [PubMed] [Google Scholar]
- 5.Babcock H, Zack JE, Garrison T, Trovillion E, Kollef MH, Fraser VJ. 2003. Ventilator-associated pneumonia in a multi-hospital system: differences in microbiology by location. Infect. Control Hosp. Epidemiol. 24:853–858. 10.1086/502149 [DOI] [PubMed] [Google Scholar]
- 6.Costerton J, Montanaro L, Arciola CR. 2005. Biofilm in implant infections: its production and regulation. Int. J. Artif. Organs 28:1062–1068 [DOI] [PubMed] [Google Scholar]
- 7.Talsma SS. 2007. Biofilms on medical devices. Home Healthc. Nurse 25:589–594. 10.1097/01.NHH.0000296117.87061.14 [DOI] [PubMed] [Google Scholar]
- 8.Adair C, Gorman SP, Feron BM, Byers LM, Jones DS, Goldsmith CE, Moore JE, Kerr JR, Curran MD, Hogg G, Webb CH, McCarthy GJ, Milligan KR. 1999. Implications of endotracheal tube biofilm for ventilator-associated pneumonia. Intensive Care Med. 25:1072–1076. 10.1007/s001340051014 [DOI] [PubMed] [Google Scholar]
- 9.Estes RJ, Meduri GU. 1995. The pathogenesis of ventilator-associated pneumonia: I. mechanisms of bacterial transcolonization and airway inoculation. Intensive Care Med. 21:365–383 [DOI] [PubMed] [Google Scholar]
- 10.Gil-Perotin S, Ramirez P, Marti V, Sahuquillo JM, Gonzalez E, Calleja I, Menendez R, Bonastre J. 2012. Implications of endotracheal tube biofilm in ventilator-associated pneumonia response: a state of concept. Crit. Care 16:R93. 10.1186/cc11357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inglis TJ, Millar MR, Jones JG, Robinson DA. 1989. Tracheal tube biofilm as a source of bacterial colonization of the lung. J. Clin. Microbiol. 27:2014–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorman S, McGovern JG, Woolfson AD, Adair CG, Jones DS. 2001. The concomitant development of poly(vinyl chloride)-related biofilm and antimicrobial resistance in relation to ventilator-associated pneumonia. Biomaterials 22:2741–2747. 10.1016/S0142-9612(01)00017-5 [DOI] [PubMed] [Google Scholar]
- 13.Zavascki A, Li J, Nation RL, Superti SV, Barth AL, Lutz L, Ramos F, Boniatti MM, Goldani LZ. 2009. Stable polymyxin B susceptibility to Pseudomonas aeruginosa and Acinetobacter spp. despite persistent recovery of these organisms from respiratory secretions of patients with ventilator-associated pneumonia treated with this drug. J. Clin. Microbiol. 47:3064–3065. 10.1128/JCM.01035-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goyal K, Gautam V, Ray P. 2012. Doripenem vs meropenem against Pseudomonas and Acinetobacter. Indian J. Med. Microbiol. 30:350–351. 10.4103/0255-0857.99502 [DOI] [PubMed] [Google Scholar]
- 15.Kiem S, Schentag JJ. 2006. Relationship of minimal inhibitory concentration and bactericidal activity to efficacy of antibiotics for treatment of ventilator-associated pneumonia. Semin. Respir. Crit. Care Med. 27:51–67. 10.1055/s-2006-933674 [DOI] [PubMed] [Google Scholar]
- 16.Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A. 1999. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 37:1771–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garey K, Vo QP, Lewis RE, Saengcharoen W, LaRocco MT, Tam VH. 2009. Increased bacterial adherence and biomass in Pseudomonas aeruginosa bacteria exposed to clarithromycin. Diagn. Microbiol. Infect. Dis. 63:81–86. 10.1016/j.diagmicrobio.2008.09.007 [DOI] [PubMed] [Google Scholar]
- 18.Hadi R, Vickery K, Deva A, Charlton T. 2010. Biofilm removal by medical device cleaners: comparison of two bioreactor detection assays. J. Hosp. Infect. 74:160–167. 10.1016/j.jhin.2009.10.023 [DOI] [PubMed] [Google Scholar]
- 19.Tarquinio K, Confreda K, Shurko J, LaPlante K. 2012. Abstr. 52nd Intersci. Conf. Antimicrob. Agents Chemother., poster E-790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray T, Kazmierczak BI. 2008. Pseudomonas aeruginosa exhibits sliding motility in the absence of type IV pili and flagella. J. Bacteriol. 190:2700–2708. 10.1128/JB.01620-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray T, Okegbe C, Gao Y, Kazmierczak BI, Motterlini R, Dietrich LE, Bruscia EM. 2012. The carbon monoxide releasing molecule CORM-2 attenuates Pseudomonas aeruginosa biofilm formation. PLoS One 7:e35499. 10.1371/journal.pone.0035499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klausen M, Heydorn A, Ragas P, Lambertsen L, Aaes-Jørgensen Molin AS, Tolker-Nielsen T. 2003. Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol. Microbiol. 48:1511–1524. 10.1046/j.1365-2958.2003.03525.x [DOI] [PubMed] [Google Scholar]
- 23.Seil JT, Rubien NM, Webster TJ, Tarquinio KM. 2011. Comparison of quantification methods illustrates reduced Pseudomonas aeruginosa activity on nanorough polyvinyl chloride. J. Biomed. Mater. Res. B Appl. Biomater. 98:1–7. 10.1002/jbm.b.31821 [DOI] [PubMed] [Google Scholar]
- 24.LaPlante K, Sakoulas G. 2009. Evaluating aztreonam and ceftazidime pharmacodynamics with Escherichia coli in combination with daptomycin, linezolid, or vancomycin in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 53:4549–4555. 10.1128/AAC.00180-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clinical and Laboratory Standards Institute 2013. Performance standards for antimicrobial susceptibility testing M100-S23, 23rd informational supplement ed. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 26.NCCLS 1999. Methods for determining bactericidal activity of antimicrobial agents; approval guideline M26-A, 1st ed. National Committee for Clinical Laboratory Standards, Wayne, PA [Google Scholar]
- 27.LaPlante K, Rybak MJ. 2004. Impact of high-inoculum Staphylococcus aureus on the activities of nafcillin, vancomycin, linezolid, and daptomycin, alone and in combination with gentamicin, in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 48:4665–4672. 10.1128/AAC.48.12.4665-4672.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merritt J, Kadouri DE, O'Toole GA. 2005. Growing and analyzing static biofilms. Curr. Protoc. Microbiol. Chapter 1:Unit 1B.1. 10.1002/9780471729259.mc01b01s00 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LaPlante K, Rybak MJ. 2004. Clinical glycopeptide-intermediate staphylococci tested against arbekacin, daptomycin, and tigecycline. Diagn. Microbiol. Infect. Dis. 50:125–130. 10.1016/j.diagmicrobio.2004.06.014 [DOI] [PubMed] [Google Scholar]
- 30.Chastre J, Fagon JY. 2002. Ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 165:867–903. 10.1164/ajrccm.165.7.2105078 [DOI] [PubMed] [Google Scholar]
- 31.Fagon J, Chastre J, Domart Y, Trouillet JL, Gibert C. 1996. Mortality due to ventilator-associated pneumonia or colonization with Pseudomonas or Acinetobacter species: assessment by quantitative culture of samples obtained by a protected specimen brush. Clin. Infect. Dis. 23:538–542. 10.1093/clinids/23.3.538 [DOI] [PubMed] [Google Scholar]
- 32.Elward A, Warren DK, Fraser VJ. 2002. Ventilator-associated pneumonia in pediatric intensive care unit patients: risk factors and outcomes. Pediatrics 109:758–764. 10.1542/peds.109.5.758 [DOI] [PubMed] [Google Scholar]
- 33.Warren D, Shukla SJ, Olsen MA, Kollef MH, Hollenbeak CS, Cox MJ, Cohen MM, Fraser VJ. 2003. Outcome and attributable cost of ventilator-associated pneumonia among intensive care unit patients in a suburban medical center. Crit. Care Med. 31:1312–1317. 10.1097/01.CCM.0000063087.93157.06 [DOI] [PubMed] [Google Scholar]
- 34.Levine SA, Niederman MS. 1991. The impact of tracheal intubation on host defenses and risks for nosocomial pneumonia. Clin. Chest Med. 12:523–543 [PubMed] [Google Scholar]
- 35.Feldman C, Kassel M, Cantrell J, Kaka S, Morar R, Goolam Mahomed A, Philips JI. 1999. The presence and sequence of endotracheal tube colonization in patients undergoing mechanical ventilation. Eur. Respir. J. 13:546–551. 10.1183/09031936.99.13354699 [DOI] [PubMed] [Google Scholar]
- 36.Gibbs K, Holzman IR. 2012. Endotracheal tube: friend or foe? Bacteria, the endotracheal tube, and the impact of colonization and infection. Semin. Perinatol. 36:454–461. 10.1053/j.semperi.2012.06.008 [DOI] [PubMed] [Google Scholar]
- 37.Bauer TT, Torres A, Ferrer R, Heyer CM, Schultze-Werninghaus G, Rasche K. 2002. Biofilm formation in endotracheal tubes. Association between pneumonia and the persistence of pathogens. Monaldi Arch. Chest Dis. 57:84–87 [PubMed] [Google Scholar]
- 38.Bonten MJ. 2002. Strategies for prevention of hospital-acquired pneumonia: oral and selective decontamination of the gastrointestinal tract. Semin. Respir. Crit. Care Med. 23:481–488. 10.1055/s-2002-35719 [DOI] [PubMed] [Google Scholar]
- 39.van Essen E, de Jonge E. 2011. Selective decontamination of the digestive tract (SDD): is the game worth the candle? Semin. Respir. Crit. Care Med. 32:236–242. 10.1055/s-0031-1275536 [DOI] [PubMed] [Google Scholar]
- 40.Brown RB, Kruse JA, Counts GW, Russell JA, Christou NV, Sands ML. 1990. Double-blind study of endotracheal tobramycin in the treatment of gram-negative bacterial pneumonia. The Endotracheal Tobramycin Study Group. Antimicrob. Agents Chemother. 34:269–272. 10.1128/AAC.34.2.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herrmann G, Yang L, Wu H, Song Z, Wang H, Hoiby N, Ulrich M, Molin S, Riethmuller J, Doring G. 2010. Colistin-tobramycin combinations are superior to monotherapy concerning the killing of biofilm Pseudomonas aeruginosa. J. Infect. Dis. 202:1585–1592. 10.1086/656788 [DOI] [PubMed] [Google Scholar]