ABSTRACT
Respiratory syncytial virus (RSV) is the most common cause of viral lower respiratory tract infections in infants and children under the age of 5. Studies examining RSV infection in susceptible BALB/c mice indicate that both CD4 and CD8 T cells not only contribute to viral clearance but also facilitate RSV-induced disease. However, efforts to understand the mechanisms by which RSV-specific T cells mediate disease following acute RSV infection have been hampered by the lack of defined RSV-specific T cell epitopes. Using an overlapping peptide library spanning each of the RSV-derived proteins, intracellular cytokine staining for gamma interferon was utilized to identify novel RSV-specific CD4 and CD8 T cell epitopes. Five novel CD8 T cell epitopes were revealed within the RSV fusion (F) protein and glycoprotein (G). In addition, five previously unidentified CD4 T cell epitopes were discovered, including epitopes in the phosphoprotein (P), polymerase protein (L), M2-1 protein, and nucleoprotein (N). Though the initial CD4 T cell epitopes were 15 amino acids in length, synthesis of longer peptides increased the frequency of responding CD4 T cells. Our results indicate that CD4 T cell epitopes that are 17 amino acids in length result in more optimal CD4 T cell stimulation than the commonly used 15-mer peptides.
IMPORTANCE Respiratory syncytial virus (RSV) is the leading cause of hospitalization for lower respiratory tract infection in children. T cells play a critical role in clearing an acute RSV infection, as well as contributing to RSV-induced disease. Here we examined the breadth of the RSV-specific T cell response, using for the first time an overlapping peptide library spanning the entire viral genome. We identified 5 new CD4 and 5 new CD8 T cell epitopes, including a CD8 T cell epitope within the G protein that was previously believed not to elicit a CD8 T cell response. Importantly, we also demonstrated that the use of longer, 17-mer peptides elicits a higher frequency of responding CD4 T cells than the more commonly used 15-mer peptides. Our results demonstrate the breadth of the CD4 and CD8 T cell response to RSV and demonstrate the importance of using longer peptides when stimulating CD4 T cell responses.
INTRODUCTION
Respiratory syncytial virus (RSV), a single-stranded negative-sense RNA paramyxovirus, is the leading cause of hospitalizations for lower respiratory tract infections in young children (1). RSV is a ubiquitous pathogen, infecting 30 to 60% of children during their first year of life and up to 90% of children by their second year of life (2–4). Furthermore, approximately 3% of RSV-infected children require hospitalization (5). In total, it is estimated that worldwide, RSV is responsible for 3.4 million acute lower respiratory tract infections annually in children under the age of 5, resulting in up to 196,000 yearly fatalities (6). In addition to children, the elderly are also susceptible to severe RSV-induced disease (7).
BALB/c mice are susceptible to RSV infection and have been used extensively to examine the role of the immune response in mediating viral clearance and contributing to the development of immunopathology following infection. Studies in BALB/c mice have demonstrated that although both CD4 and CD8 T cells mediate viral clearance, CD4 and CD8 T cells also contribute to RSV-associated disease (8). Furthermore, several human studies have demonstrated that the increased presence of T cells is correlated to severe RSV disease in both children and the elderly (9–12).
The breadth of the CD4 and CD8 T cell responses against RSV has not been thoroughly explored. Utilizing a recombinant vaccinia virus (VacV)-based vaccination system to boost the T cell response against the RSV-derived glycoprotein (G) and fusion protein (F), two RSV-derived CD4 epitopes, G183–195 and F51–66, have been previously identified (13–16). However, upon acute RSV infection, both epitopes represent only a small fraction (i.e., 1 to 2%) of lung CD4 T cells (17). Furthermore, several RSV-specific CD8 T cell epitopes have been described. Identified epitopes include the immunodominant epitope M2-182–90 (18–20) and the subdominant epitopes M2-1127–135 (21) and F85–93 (22). Much like the previously identified CD4 T cell epitopes, these epitopes were defined utilizing a recombinant VacV immunization system (18–20, 22) or upon secondary RSV challenge (21). Furthermore, these studies utilized peptide libraries that span only the individual RSV M2-1 and F proteins (19, 21, 22). Therefore, the other 9 RSV-derived proteins have not been thoroughly examined for potential CD8 T cell epitopes.
In this study, we utilized a peptide library spanning the entire RSV proteome to identify novel CD4 and CD8 T cell epitopes. We identified 5 novel CD4 T cell epitopes and 5 novel CD8 T cell epitopes directed against RSV. Furthermore, we found that the previously identified RSV-Long CD8 T cell epitope F249–258 (23) also serves as a CD8 T cell epitope against RSV-A2. Additionally, we have demonstrated that the addition of amino acids to the commonly used 15-mer CD4 T cell epitopes to form 17-mer epitopes results in a substantial increase in the immunogenicity of the epitope.
MATERIALS AND METHODS
Mice and viruses.
BALB/cAnNCr mice between 6 and 8 weeks of age were purchased from the National Cancer Institute (Frederick, MD). Female mice were used in all experiments. The A2 strain of RSV (RSV-A2) was a gift from Barney S. Graham (National Institutes of Health, Bethesda, MD) and was propagated on HEp-2 cells (American Type Culture Collection [ATCC], Manassas, VA) (24). Mice were infected intranasally (i.n.) with 2 × 106 to 3 × 106 PFU RSV-A2 (25). Recombinant VacV expressing the RSV-derived F protein (VacV-F) was a gift from Thomas J. Braciale (University of Virginia, Charlottesville, VA) and J. L. Beeler (U.S. Food and Drug Administration, Bethesda, MD) and was propagated in BSC-40 cells (ATCC). Mice were scarified with 3 × 106 PFU of recombinant VacV-F (26). All experimental procedures utilizing mice were approved by the University of Iowa Animal Care and Use Committee.
Intracellular cytokine stain.
Spleen cells from day 8 RSV-infected mice were harvested and single-cell suspensions were generated as previously described (27). Splenocytes (1.5 × 106) were incubated with 1 μM peptide from an overlapping peptide library (15-mers overlapping every 10 amino acids), spanning the entirety of the RSV-A2-proteome (Mimotopes Pty. Ltd., Roseville, MN) in the presence of 10 μg/ml brefeldin A (BFA) (Sigma-Aldrich, St. Louis, MO) for 5 h at 37°C (24). Following stimulation, cells were surface stained with antibodies specific to CD4, CD8, and CD90.2 (Biolegend, San Diego, CA) as previously described (24). Intracellular cytokine staining (ICS) for gamma interferon (IFN-γ) (Biolegend) was performed, and cells were analyzed on a BD FACSCanto instrument (BD Bioscience, San Jose, CA). Data were analyzed using the FlowJo software program (Tree Star, Ashland, OR). Candidate epitopes were identified as having results ≥2 standard deviations (SD) over those for the no-peptide controls in at least one of two experiments.
For analysis of lung mononuclear cells, lungs were harvested from day 8 RSV-infected mice, and single-cell suspensions were generated as previously described (27). Cells (1.5 × 106) were incubated with 1 μM peptide (Mimotopes) identified in the initial screening experiments or synthesized peptides (Biosynthesis, Lewisville, TX) in the presence of 10 μg/ml BFA for 5 h at 37°C. Following stimulation, cells were surface stained with antibodies specific to CD4, CD8, and CD90.2 and stained intracellularly for IFN-γ (24). For CD107α staining, cells were incubated in the presence of 10 μg/ml monensin alone or stimulated with the newly identified peptides and CD107α antibody (BD Bioscience). All antibodies were purchased from BioLegend unless otherwise stated. Stained cells were analyzed on either a BD FACSCanto or BD LSRFortessa instrument (BD Bioscience). Data were evaluated using FlowJo software. Confirmed epitopes were identified as having results ≥2 SD over those for the no-peptide controls in at least two of three experiments or ≥1 SD in all three experiments. In some experiments, the fold increase of IFN-γ+ CD4 T cells stimulated by 17-mer peptides versus results with their respective 15-mer peptide was determined by the following calculation: (% of cells responding to 17-mer peptides − % of cells for BFA control)/(% of cells responding to 15-mer peptides − % of cells for BFA control).
Splenic dendritic cell isolation and dendritic cell immunization.
BALB/c mice were given an intraperitoneal injection with 5 × 106 B16 cells expressing Fms-related tyrosine kinase 3 ligand (kindly provided by Martin Prlic and Michael J. Bevan). Fourteen to seventeen days following injection, mice were administered 2 μg of lipopolysaccharide (LPS) intravenously to mature the dendritic cells (DCs), and spleens were harvested 16 h later. Spleens were digested with DNase and collagenase for 20 min at 37°C with shaking (100 rpm), and mononuclear cells were isolated by pressing the tissue through a wire mesh screen (Cellector; Bellco Glass, Inc., Vineland, NJ). Red blood cells were lysed with NH4Cl and washed, and cells were stained with CD11c-phycoerythrin (PE) (Biolegend). Cells were incubated with anti-PE microbeads (Miltenyi Biotec, Auburn, CA), and splenic DCs were isolated by positive selection using an AutoMACS instrument. Following isolation, splenic DCs were resuspended in 2 parts supplemented RPMI 1640 to 1 part B16-Flt3L-conditioned medium plus recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF) (1,000 U/ml) and incubated with 2 μM G271–285 peptide for 2 h at 37°C with shaking (100 rpm). Following peptide pulse, splenic DCs were washed and resuspended in saline, and 5 × 105 peptide-pulsed splenic DCs were subsequently injected intravenously into naive BALB/c mice.
MHC class I restriction assay.
Mice were immunized with 3 × 106 PFU VacV-F by scarification at the base of the tail (26). Twenty-one days following immunization, mice were infected with RSV. Six days following RSV infection, lungs were harvested and single-cell suspensions were generated as described above. Cells were stimulated at a 1:1 effector-to-stimulator (E:S) ratio with peptide-pulsed parent L929 cells or Kd-, Ld- or Dd-expressing cells (T4.3.3; kindly provided by Ted H. Hansen, Washington University, St. Louis, MO) for 5 h at 37°C. Following stimulation, cells were surface stained with monoclonal antibodies (MAbs) specific to CD4, CD8, and CD90.2. ICS for IFN-γ (Biolegend) was performed, and cells were analyzed using a BD LSRFortessa instrument (24). Data were evaluated using FlowJo software.
In some experiments, DC-primed mice (described above) were infected with RSV 8 days following immunization with splenic DC plus G271–285. Five days following RSV infection, lungs were harvested and lung single-cell suspensions were generated as described above. Cells were stimulated with peptide-pulsed cell lines as described above. Following stimulation, cells were surface stained with MAbs specific to CD4, CD8, and CD90.2. ICS for IFN-γ (Biolegend) was performed, and cells were analyzed using a BD LSRFortessa instrument (24). Data were evaluated using FlowJo software.
Prediction of major histocompatibility complex class I epitopes.
Following confirmation of the major histocompatibility complex (MHC) class I restriction, the MHC class I binding predictions of each library epitope were made using the immune epitope database (IEDB) analysis resource consensus tool (28), which combines predictions from artificial neural networks (ANN), also known as NetMHC (29, 30), stabilized matrix method (SMM), (31) and comblib (32). Utilizing this method, we predicted that the following were the core CD8 T cell epitopes: F32–40, F52–59, F163–171, and F167–175. However, the best match predicted for the G271–285 epitope was a 13-mer peptide. Therefore, this epitope was not synthesized. The consensus core epitopes were synthesized (Biosynthesis) and utilized to stimulate lung CD8 T cells from day 8 RSV-infected mice as described previously (24). Following stimulation, cells were surface stained for CD8 and CD90.2, and an ICS for IFN-γ was performed. Stained cells were subsequently analyzed on a BD LSRFortessa instrument, and data were evaluated using FlowJo software (24).
RESULTS
Identification of candidate CD4 and CD8 T cell epitopes against RSV-A2.
A set of 889 15-mer peptides, overlapping every 10 amino acids, spanning the entire RSV-A2 proteome was generated. To identify candidate RSV-derived CD4 and CD8 T cell epitopes, splenocytes isolated from day 8 RSV-infected BALB/c mice were stimulated with individual peptides from the RSV peptide library. Reactivity was determined by ICS for IFN-γ, and candidate CD4 and CD8 T cell epitopes were identified as having results ≥2 SD above those for the no-peptide controls (0.18% and 0.26%, respectively).
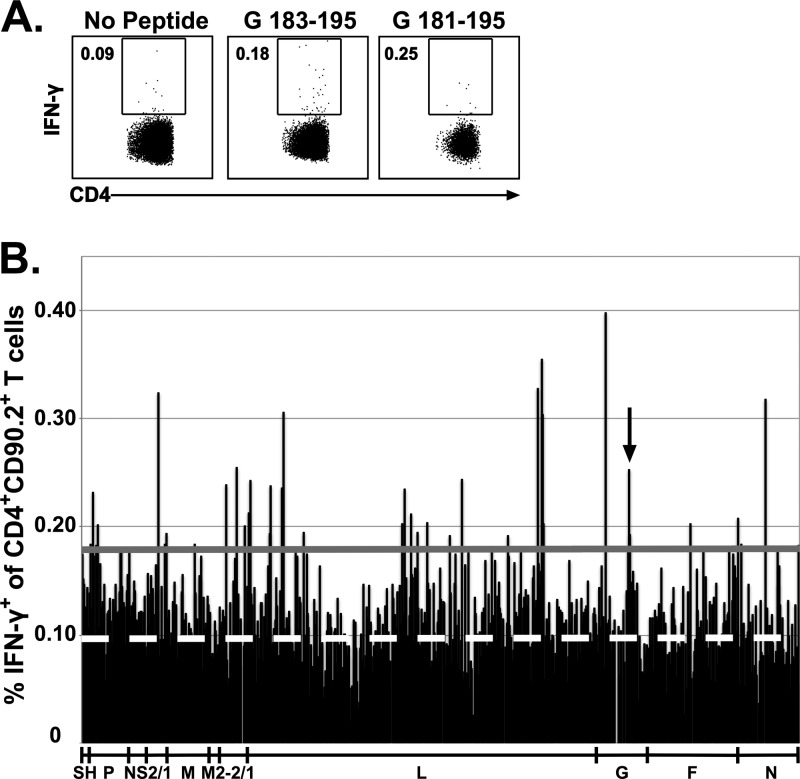
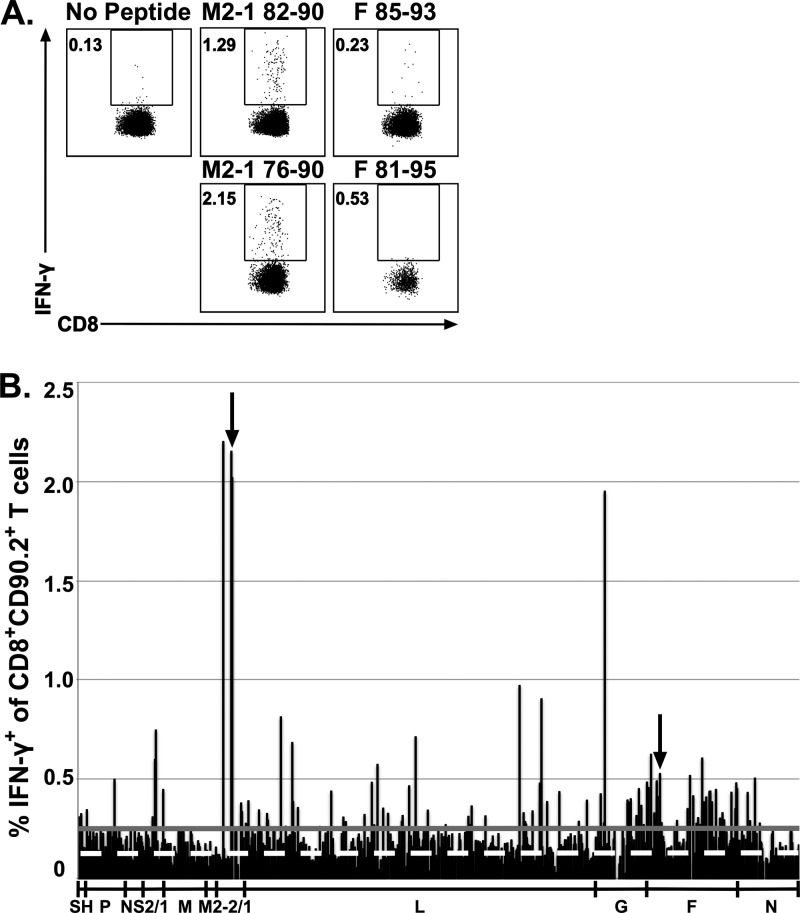
To confirm the efficacy of the experimental design, we determined if the previously identified immunodominant CD4 T cell epitope G183–195 and its respective peptide within the peptide library, G181–195, met the ≥2-SD threshold (Fig. 1A). Similarly, we determined if the CD8 T cell immunodominant epitope M2-182–90 and the subdominant epitope F85–93 and their respective peptides within the peptide library (M2-181–90 and F81–95) also met the ≥2-SD threshold (Fig. 2A). The G183–195 and M2-182–90 CD4 and CD8 T cell epitopes, respectively, and their respective epitopes within the peptide library all stimulated responses that exceeded the 2-SD threshold (Fig. 1A and 2A, respectively). Furthermore, although the optimal F85–93 subdominant CD8 T cell epitope elicited a response slightly under the 2-SD threshold, the library peptide containing this epitope (F81–95) stimulated a response that exceeded the 2-SD threshold. Overall, these data indicate that this approach is sufficient to detect RSV-derived CD4 and CD8 T cell epitopes. Utilizing this approach, we identified 85 candidate peptides that stimulated IFN-γ production by splenic CD4 T cells (Fig. 1B) and 141 candidate peptides that stimulated IFN-γ production by splenic CD8 T cells (Fig. 2B).
FIG 1.
Identification of candidate RSV CD4 T cell epitopes. BALB/c mice were infected i.n. with RSV, and spleens were harvested 8 days following infection. Splenocytes were incubated with BFA alone or stimulated with individual peptides from a peptide library spanning the entire RSV proteome. (A) Dot plots show the frequency of IFN-γ+ CD90.2+ CD4+ T cells elicited by the previously defined G183–195 epitope and its corresponding peptide in the peptide library, G181–195. (B) The bar graph depicts the CD4 T cell reactivity against all individual peptides. The dashed white line represents the average for stimulation with BFA alone, and the solid gray line represents the 2-SD threshold for positivity. An arrow denotes the previously identified G183–195 epitope. Data are from one of two experiments, with 15 to 16 mice pooled per experiment.
FIG 2.
Identification of candidate RSV CD8 T cell epitopes. BALB/c mice were infected i.n. with RSV, and spleens were harvested 8 days following infection. Splenocytes were incubated with BFA alone or stimulated with individual peptides from a peptide library spanning the entire RSV proteome. (A) Dot plots show the frequency of IFN-γ+ CD90.2+ CD8+ T cells elicited by the previously defined M2-182–90 and F85–93 epitopes and their corresponding peptides in the peptide library, M2-181–95 and F81–95. (B) The bar graphs depicts the CD8 T cell reactivity against all individual peptides. The dashed white line represents the average for stimulation with BFA alone, and the solid gray line represents the 2-SD threshold for positivity. Arrows denote previously identified CD8 T cell epitopes. Data are from one of two experiments, with 15 to 16 mice pooled per experiment.
Confirmation of CD4 T cell and CD8 T cell epitopes.
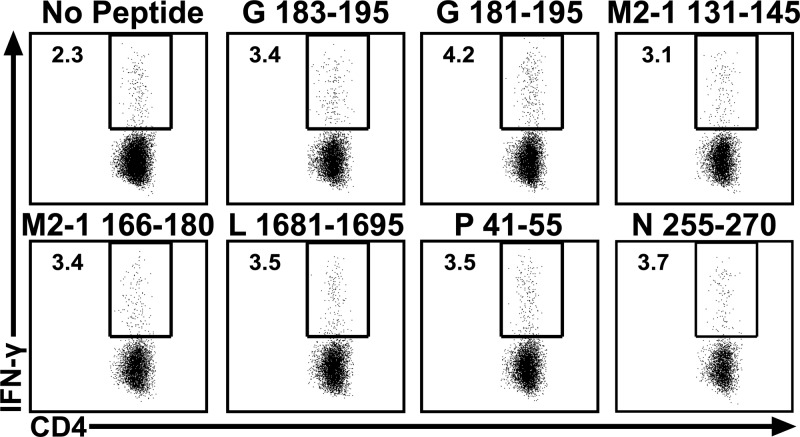
To verify the candidate CD4 T cell epitopes, lung mononuclear cells were isolated from RSV-infected BALB/c mice and were stimulated with the candidate peptides identified in the initial screen (Fig. 1B). A total of 6 peptides, including the peptide containing the previously described G183–195 epitope, stimulated IFN-γ production by CD4 T cells (Fig. 3). The newly identified CD4 T cell epitopes target multiple RSV-derived proteins, including the L protein (L1681–1695), M2-1 protein (M2-1126–140/M2-1131–145 and M2-1166–180), N (N251–265/N256–270), and P (P36–50/P41–55). Although the majority of the CD4 T cells responding to acute RSV-A2 infection are IFN-γ-producing Th1 cells, RSV has been demonstrated to induce both Th2 and Th17 responses in mice (25, 33–36). To determine if any of the newly identified CD4 T cell epitopes elicit a Th2 or Th17 cytokine response following acute RSV-A2 infection, production of interleukin 5 (IL-5), IL-13, and IL-17A by CD4 T cells was evaluated following stimulation with each of the newly identified CD4 peptides. We did not observe a significant population of CD4 T cells that produced any of these cytokines following peptide stimulation (data not shown).
FIG 3.
Identification of novel CD4 T cell epitopes. BALB/c mice were infected i.n. with RSV. Lung mononuclear cells were incubated with BFA alone or stimulated with candidate epitopes (Fig. 1B). Dot plots show the frequency of IFN-γ+ CD90.2+ CD4+ T cells for epitopes that met the criteria for a positive CD4 T cell epitope. Data are from one of three experiments, with 15 mice pooled per experiment.
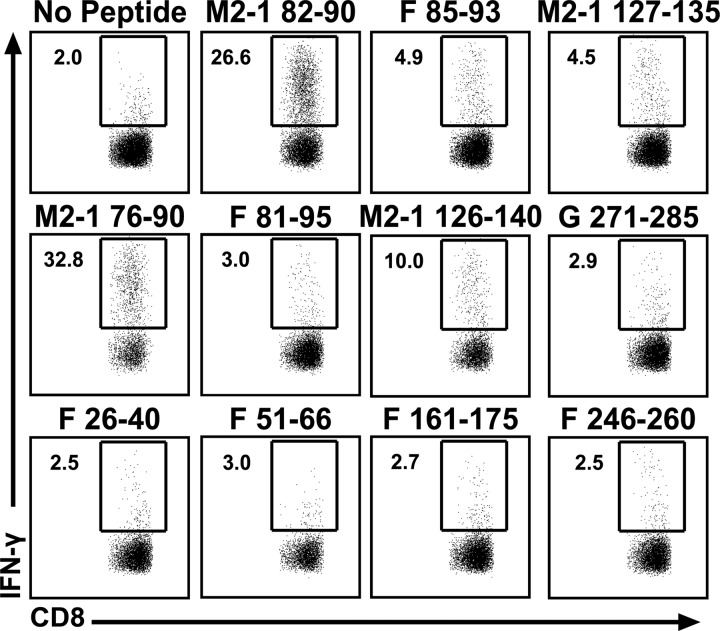
Similarly, CD8 T cell epitopes were verified by stimulating CD8 T cells from day 8 RSV-infected lungs with the candidate peptides identified in Fig. 2B. Four peptides containing novel CD8 T cell epitopes stimulated IFN-γ production from lung CD8 T cells (Fig. 4). As expected, the library peptides containing the previously described CD8 T cell epitopes M2-182–90, M2-1127–135, and F85–93 also stimulated IFN-γ production by CD8 T cells (18–20, 22). In addition, the peptide containing the previously identified RSV-Long epitope F249–258 (F246–260) stimulated IFN-γ production by CD8 T cells following RSV-A2 infection (23). The amino acid sequence in this region is conserved between the two virus strains, indicating that this peptide serves as a CD8 T cell epitope against both RSV-A2 and RSV-Long infection. Compared to that of CD4 T cells, the CD8 T cell specificity against RSV was more restricted, targeting only the M2-1 protein (M2-182–90 and M2-1127–135), G (G271–285), and the F protein (F26–40, F46–60/F51–66, F81–95/F86–100, F161–175, and F246–260).
FIG 4.
Identification of novel CD8 T cell epitopes. BALB/c mice were infected i.n. with RSV. Lung mononuclear cells were incubated with BFA alone or stimulated with candidate epitopes (Fig. 2B). Dot plots show the frequency of IFN-γ+ CD90.2+ CD8+ T cells for epitopes that met the criteria for a positive CD8 T cell epitope. Data are from one of three experiments, with 15 mice pooled per experiment.
MHC class I restriction of CD8 T cell epitopes.
To determine the MHC class I restriction profile of the newly identified CD8 T cell epitopes, we utilized cell lines expressing H-2Kd, H-2Ld, or H-2Dd to stimulate CD8 T cells. Given the small frequency of CD8 T cells responding to the newly identified CD8 T cell epitopes following acute RSV infection, we utilized a recombinant VacV expressing the RSV F protein to boost the frequency of F-specific CD8 T cells (26). Following VacV-F immunization, mice were infected with RSV and lungs were harvested at day 6 postinfection. CD8 T cells were stimulated with peptide-pulsed L929 fibroblast cell lines expressing H-2Kd, H-2Ld, or H-2Dd, and IFN-γ production was examined by ICS. As previously described, the F85–93 and F249–258 epitopes were H-2Kd restricted (Fig. 5A; F81-95- and F246–260, respectively) (22, 23). Furthermore, the newly identified epitopes directed against the F protein (F26–40, F46–60, and F161–175) were also all H-2Kd restricted (Fig. 5A).
FIG 5.
Determination of MHC class I restriction. (A) BALB/c mice were primed with VacV-F. Twenty-one days following priming, mice were infected i.n. with RSV. CD8 T cells were stimulated with peptide-pulsed MHC class I-expressing cells line (1:1 E:S ratio). Graphs depict the frequency of IFN-γ+ CD90.2+ CD8+ T cells. Data are from one of two experiments. (B) BALB/c mice were primed with G271–285-pulsed DCs. Eight days following priming, mice were infected i.n. with RSV. CD8 T cells were stimulated with peptide-pulsed MHC class I-expressing cells line (1:1 E:S ratio). Graphs depict frequencies of IFN-γ+ CD90.2+ CD8+ T cells. *, significantly increased (P < 0.05) compared to results for the parent cell line. **, significantly increased (P < 0.01) compared to results for the parent cell line. ***, significantly increased (P < 0.001) compared to results for the parent cell line. All statistical analysis was done by ANOVA with Dunnett's posttest comparing each group to the parent cell line. Data are from one of two experiments, with 4 mice per experiment.
Consistent with previous data, VacV-G immunization failed to prime a CD8 T cell response (37–39). Therefore, we utilized a low-inflammation DC immunization protocol to boost the G271–285-specific CD8 T cell response (40). Eight days following immunization with G271–285-pulsed DCs, mice were infected with RSV. Lung mononuclear cells were isolated at day 5 postinfection and subsequently stimulated with peptide-pulsed L929 fibroblast cell lines expressing H-2Kd, H-2Ld, or H-2Dd. IFN-γ production was examined by ICS to determine reactivity. In contrast to the RSV F-derived epitopes, the G271–285 epitope was H-2Ld restricted (Fig. 5B), thus making G271–285 the only RSV-derived epitope described to date that is not H-2Kd restricted.
Confirmation of minimal core CD8 T cell epitopes.
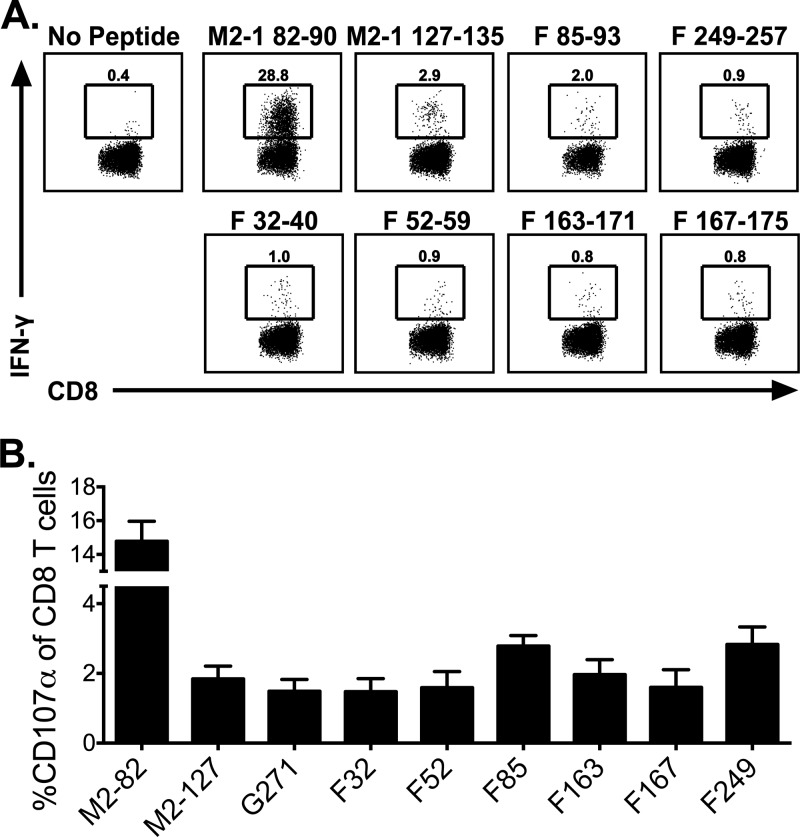
Following determination of the MHC class I restriction of the CD8 T cell epitopes, the minimal core epitope sequence was predicted utilizing the IEDB analysis resource (28). Using this database, we predicted the minimal sequences for the F26–40 and F46–60 epitopes: F32–40 and F52–59, respectively. Furthermore, F161–175 contained two predicted minimal epitopes: F163–171 and F167–175. These core epitopes were synthesized and tested for confirmation. All four minimal core epitopes were confirmed to be stimulatory using ICS (Fig. 6A). Furthermore, the F249–257 minimal core epitope induced IFN-γ production from CD8 T cells following RSV-A2 infection, demonstrating that this peptide serves as a CD8 T cell epitope against both RSV-A2 and RSV-Long (Fig. 6A). In addition to IFN-γ production, CD107α expression by CD8 T cells was evaluated following stimulation with all of the newly identified epitopes (Fig. 6B). Stimulation with all peptides induced CD107α expression on CD8 T cells, indicating that these T cells were capable of undergoing degranulation. Considering these confirmed epitopes along with the previously identified M2-182–90, M2-1127–135, and F85–93 epitopes and the identified G271–285 epitope (Fig. 4), there are now a total of 8 RSV-derived CD8 T cell epitopes (Table 1).
FIG 6.
Confirmation of minimal core CD8 T cell epitopes. BALB/c mice were infected i.n. with RSV. Lung mononuclear cells were incubated with BFA alone or stimulated with M2-182–90, M2-1127–135, F85–93, F249–257, F32–40, F52–59, F163–171, and F167–175. For CD107α staining, cells were either incubated with monensin alone or stimulated with peptides in the presence of CD107α antibodies. (A) Dot plots depict the frequency of IFN-γ+ CD90.2+ CD8+ T cells. (B) Graph shows the frequency of CD107α+ CD90.2+ CD8+ T cells following stimulation. Data are representative from one of three experiments with 4 to 5 mice per experiment (A) or compiled data from two independent experiments (n = 8) (B).
TABLE 1.
CD8 T cell epitopes for RSV-A2 in BALB/c micea
| RSV protein | Amino acid positions | Peptide sequence | MHC restriction |
|---|---|---|---|
| M2-1 | 82–90 | SYIGSINNI | H2-Kd |
| M2-1 | 127–135 | VYNTVISYI | H2-Kd |
| G | 271–285 | EGNPSPSQVSTTSEY | H2-Ld |
| F | 32–40 | FYQSTCSAV | H2-Kd |
| F | 52–59 | WYTSVITI | H2-Kd |
| F | 85–93 | KYKNAVTEL | H2-Kd |
| F | 163–171 | EVNKIKSAL | H2-Kd |
| F | 167–175 | IKSALLSTN | H2-Kd |
| Fb | 249–258 | TYMLTNSEL | H2-Kd |
Bold text denotes novel epitopes.
Previously identified as an RSV-Long epitope (28).
Enhancement of reactivity to CD4 T cell epitopes.
Unlike MHC class I molecules, MHC class II molecules have an open-ended binding cleft allowing for “overhang” residues when peptide is bound (41–46). These flanking residues stabilize the peptide binding to the MHC class II molecule and enhance the immunogenicity of the CD4 T cell response (47–50). Although several studies have demonstrated that peptide length can drastically alter the immunogenicity of the CD4 T cell against the respective peptide, the optimal length for MHC class II peptides is unclear (49, 51–53). We noted that extension of the G183–195 epitope by two N-terminal amino acids resulted in an ∼2-fold-increased frequency of CD4 T cells that respond to this epitope (Fig. 3). Furthermore, generation of a 17-mer peptide containing four amino acids on each side of the core resulted in a further enhancement (∼1.5-fold) of CD4 T cell reactivity toward this epitope (Fig. 7). To determine if this observation is universal among MHC class II epitopes, we utilized the IEDB database to predict the core sequence of each of the identified epitopes (Table 2) (54, 55). Of all of the epitopes examined, the P and N epitopes were predicted to bind with the highest affinity to their respective MHC class II molecule. Given that two of the largest CD4 T cell responses were directed against P41–55 and N251–265 (Fig. 3), 17-mer peptides containing four amino acids on each side of the predicted core of both peptides were synthesized. These peptides were subsequently used to stimulate lung mononuclear cells isolated from day 8 RSV-infected mice. We compared the abilities of the 17-mer epitopes, P39–55 and N252–268, to induce IFN-γ production by CD4 T cells to those of their respective 15-mer library epitopes, P41–55 and N251–265. We observed that the generation of 17-mer epitopes resulted in a ∼2-fold increase in the frequency of CD4 T cells responding to the P epitope and an ∼5-fold increase in the frequency of responding CD4 T cells for the N epitope (Fig. 7). These data suggest that 17-mer epitopes increase the frequency of responding CD4 T cells compared to that with the more commonly used 15-mer epitopes.
FIG 7.
Increased peptide length results in increased CD4 T cell reactivity. BALB/c mice were infected i.n. with RSV. Lung mononuclear cells were incubated with BFA alone or stimulated with G181–195, G181–197, P41–55, P39–55, N251–265, or N252–268. (A) Representative dot plots depict the frequency of IFN-γ+ CD4+ CD90.2+ T cells. (B) Bar graphs depict the fold increase of IFN-γ+ CD4 T cells stimulated by the 17-mer peptide over levels for their respective 15-mer peptide. Data are from two experiments, with 5 mice per experiment.
TABLE 2.
CD4 T cell epitopes for RSV-A2 in BALB/c micea
| RSV protein | Amino acid positions | Peptide sequenceb | MHC restrictionc |
|---|---|---|---|
| G | 181–197 | TCWAICKRIPNKKPGKK | I-Ed |
| F | 51–66 | GWYTSVITIELSNIKE | I-Ed |
| M2-1 | 126–145 | RVYNTVISYIESNRKNNKQT | I-Ed |
| M2-1 | 166–180 | DIHKSITINNPKEST | I-Ad |
| L | 1681–1695 | SDNTHLLTKHIRIAN | I-Ad |
| P | 39–55 | SIISVNSIDIEVTKESP | I-Ad |
| N | 252–268 | GAGQVMLRWGVLAKSVK | I-Ad |
DISCUSSION
Both CD4 and CD8 T cells contribute to RSV-induced disease following primary infection (8). Although several studies have examined the role of Th1-, Th2-, and more recently Th17-associated cytokines in mediating RSV-induced disease (9, 56, 57), the ability to identify RSV-specific CD4 T cells producing these cytokines has been hampered by the lack of defined RSV-derived epitopes. In this study, we sought to identify the specificities of both the CD4 and CD8 T cell response against RSV. Overall, we identified 5 novel CD4 T cell epitopes (Table 2) and 5 novel CD8 T cell epitopes (Table 1).
We show that the specificity of the CD4 T cell response against RSV is very broad, targeting 6 of the 11 RSV-derived proteins (G, F, L, M2-1, N, and P). However, the specificity of the CD8 T cell response against RSV is much narrower, targeting only 3 of the RSV-derived proteins (M2-1, G, and F). Furthermore, we show that the immunodominance of the CD8 T cell response is very strong, with almost a third of the total CD8 T cells in the lung being specific for M282–90. In contrast, the most immunodominant CD4 T cell epitope, G181–195, represents only 2 to 3% of the total CD4 T cells at the peak of the T cell response following RSV infection. Additionally, the CD4 T cell epitopes together account for approximately ∼10% of the CD4 T cells in the lung following RSV infection, compared to the CD8 T cell response, where the epitopes account for ∼45% of the total CD8 T cell population in the lung. A similar disparity has been observed for other viral pathogens, including lymphocytic choriomeningitis virus and VacV (58–61).
Priming of a large RSV-specific CD4 T cell response using VacV-G is associated with vaccine-enhanced disease upon subsequent RSV infection (15, 16, 38). In contrast, VacV-G immunization does not induce a CD8 T cell response (37–39). It is currently unclear why VacV-G immunization fails to elicit a CD8 T cell response. One potential explanation may be related to the ability of G to be secreted in the strain of VacV-G utilized in these studies. This secretion of G would result in cross-presentation as the major mechanism for MHC class I presentation of G-associated peptides. In contrast to G, the F protein is not secreted and therefore is processed and loaded onto MHC class I molecules via the classical MHC class I presentation pathway. In concordance with this, we found that all of the CD8 T cell epitopes against the F protein were boosted following VacV-F immunization.
Two of the newly defined CD4 T cell epitopes reside within the M2-1 protein. However, VacV-M2-1-primed mice do not exhibit a boosted response to either of the M2-1-derived CD4 T cell epitopes (data not shown). Previous studies from our laboratory have shown that priming an M2-182–90-specific memory CD8 T cell response using VacV-M2-1 plus VacV-G inhibits the expansion of G183–195-specific memory CD4 T cells upon subsequent RSV infection (26). These studies together suggest that the immunodominant CD8 T cell epitope M2-182–90 is capable of inhibiting the response to multiple CD4 T cell epitopes in a VacV-priming setting (26, 62, 63). However, it remains to be determined if the CD8 T cell inhibition of the CD4 T cell response occurs during the acute response (i.e., VacV priming) or during the memory recall response (i.e., RSV infection).
Utilizing cell lines expressing H-2Dd, H-2Kd, or H-2Ld, we were able to determine the MHC restriction of the newly identified CD8 T cell epitopes. Although all of the F-derived epitopes were H-2Kd restricted, the G271–285 epitope was H-2Ld restricted. To our knowledge, G271–285 is the only RSV-derived CD8 T cell epitope in BALB/c mice that is not H-2Kd restricted. Utilizing the IEDB database, the minimal core epitopes were predicted and subsequently tested and confirmed. In all, 5 novel RSV-A2-derived CD8 T cell epitopes were confirmed (Fig. 6). Attempts were made to identify the MHC class II restriction profiles for the identified CD4 T cell epitopes utilizing I-Ad and I-Ed transfected-fibroblast cell lines to stimulate CD4 T cells. However, the inefficiency of the fibroblast cells lines hindered our ability to accurately determine the MHC class II restriction profiles for the newly identified CD4 T cell epitopes.
Peptides presented by MHC class II molecules commonly contain “overhanging” residues (41–46) that can provide increased stability for the MHC class II peptide complex (47–50). Furthermore, these “overhanging” residues can enhance the immunogenicity of the peptide to stimulate CD4 T cells (47, 50–53). However, it is unclear what ideal peptide length is required to elicit optimal CD4 T cell responses. Our data demonstrate that the addition of amino acids to the N-terminal end of the G183–195 epitope, for G181–195, results in an ∼2-fold increase in reactivity toward this epitope (Fig. 3). Furthermore, the addition of two amino acids on the C-terminal end of the G181–195 epitope, for G181–197, resulted in a further increase in the frequency of CD4 T cells responding to the G epitope (Fig. 7). This result was not limited to the RSV G epitope, since the addition of two amino acids to the P39–55 epitope (P41–55) resulted in a 2-fold increase in the frequency of responding CD4 T cells (Fig. 7). Moreover, the addition of two amino acids to the N251–265 epitope, for N252–268, resulted in a more than 5-fold increase in the frequency of responding CD4 T cells following stimulation. Together, these data indicate that 17-mer peptides induce a higher frequency of responding CD4 T cells than the commonly used 15-mer epitopes, highlighting the importance of anchor residues and emphasizing the complexity in how MHC class II peptide length greatly influences the frequency of CD4 T cells that respond to the epitope following stimulation.
ACKNOWLEDGMENTS
We thank Stacey Hartwig for excellent technical assistance.
Research described in this publication was supported by NIAID of the National Institutes of Health under awards T32 AI007511 to D.S.M. and R01 AI063520 to S.M.V. and a Development Grant from the University of Iowa Department of Microbiology to S.M.V.
This content is solely our responsibility and does not necessarily represent the official views of the National Institutes of Health.
We have no financial conflict of interest.
Footnotes
Published ahead of print 26 December 2013
REFERENCES
- 1.Heilman CA for NIAID and WHO. 1990. Respiratory syncytial and parainfluenza viruses. J. Infect. Dis. 161:402–406. 10.1093/infdis/161.3.402 [DOI] [PubMed] [Google Scholar]
- 2.Glezen WP, Taber LH, Frank AL, Kasel JA. 1986. Risk of primary infection and reinfection with respiratory syncytial virus. Am. J. Dis. Child. 140:543–546 [DOI] [PubMed] [Google Scholar]
- 3.Hall CB, Geiman JM, Biggar R, Kotok DI, Hogan PM, Douglas GR., Jr 1976. Respiratory syncytial virus infections within families. N. Engl. J. Med. 294:414–419. 10.1056/NEJM197602192940803 [DOI] [PubMed] [Google Scholar]
- 4.Legg JP, Hussain IR, Warner JA, Johnston SL, Warner JO. 2003. Type 1 and type 2 cytokine imbalance in acute respiratory syncytial virus bronchiolitis. Am. J. Respir. Crit. Care Med. 168:633–639. 10.1164/rccm.200210-1148OC [DOI] [PubMed] [Google Scholar]
- 5.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. 1999. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA 282:1440–1446. 10.1001/jama.282.15.1440 [DOI] [PubMed] [Google Scholar]
- 6.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simoes EA, Rudan I, Weber MW, Campbell H. 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375:1545–1555. 10.1016/S0140-6736(10)60206-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. 2005. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 352:1749–1759. 10.1056/NEJMoa043951 [DOI] [PubMed] [Google Scholar]
- 8.Graham BS, Bunton LA, Wright PF, Karzon DT. 1991. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J. Clin. Invest. 88:1026–1033. 10.1172/JCI115362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins PL, Graham BS. 2008. Viral and host factors in human respiratory syncytial virus pathogenesis. J. Virol. 82:2040–2055. 10.1128/JVI.01625-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walsh EE, Peterson DR, Kalkanoglu AE, Lee FE, Falsey AR. 2013. Viral shedding and immune responses to respiratory syncytial virus infection in older adults. J. Infect. Dis. 207:1424–1432. 10.1093/infdis/jit038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson JE, Gonzales RA, Olson SJ, Wright PF, Graham BS. 2007. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod. Pathol. 20:108–119. 10.1038/modpathol.3800725 [DOI] [PubMed] [Google Scholar]
- 12.King JC, Jr, Burke AR, Clemens JD, Nair P, Farley JJ, Vink PE, Batlas SR, Rao M, Johnson JP. 1993. Respiratory syncytial virus illnesses in human immunodeficiency virus- and noninfected children. Pediatr. Infect. Dis. J. 12:733–739. 10.1097/00006454-199309000-00006 [DOI] [PubMed] [Google Scholar]
- 13.Castilow EM, Olson MR, Meyerholz DK, Varga SM. 2008. Differential role of gamma interferon in inhibiting pulmonary eosinophilia and exacerbating systemic disease in fusion protein-immunized mice undergoing challenge infection with respiratory syncytial virus. J. Virol. 82:2196–2207. 10.1128/JVI.01949-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durbin JE, Johnson TR, Durbin RK, Mertz SE, Morotti RA, Peebles RS, Graham BS. 2002. The role of IFN in respiratory syncytial virus pathogenesis. J. Immunol. 168:2944–2952 [DOI] [PubMed] [Google Scholar]
- 15.Srikiatkhachorn A, Chang W, Braciale TJ. 1999. Induction of Th-1 and Th-2 responses by respiratory syncytial virus attachment glycoprotein is epitope and major histocompatibility complex independent. J. Virol. 73:6590–6597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varga SM, Wissinger EL, Braciale TJ. 2000. The attachment (G) glycoprotein of respiratory syncytial virus contains a single immunodominant epitope that elicits both Th1 and Th2 CD4+ T cell responses. J. Immunol. 165:6487–6495 [DOI] [PubMed] [Google Scholar]
- 17.McDermott DS, Varga SM. 2011. Quantifying antigen-specific CD4 T cells during a viral infection: CD4 T cell responses are larger than we think. J. Immunol. 187:5568–5576. 10.4049/jimmunol.1102104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connors M, Kulkarni AB, Collins PL, Firestone CY, Holmes KL, Morse HC, III, Murphy BR. 1992. Resistance to respiratory syncytial virus (RSV) challenge induced by infection with a vaccinia virus recombinant expressing the RSV M2 protein (Vac-M2) is mediated by CD8+ T cells, while that induced by Vac-F or Vac-G recombinants is mediated by antibodies. J. Virol. 66:1277–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulkarni AB, Morse HC, III, Bennink JR, Yewdell JW, Murphy BR. 1993. Immunization of mice with vaccinia virus-M2 recombinant induces epitope-specific and cross-reactive Kd-restricted CD8+ cytotoxic T cells. J. Virol. 67:4086–4092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Openshaw PJ, Anderson K, Wertz GW, Askonas BA. 1990. The 22,000-kilodalton protein of respiratory syncytial virus is a major target for Kd-restricted cytotoxic T lymphocytes from mice primed by infection. J. Virol. 64:1683–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S, Miller SA, Wright DW, Rock MT, Crowe JE., Jr 2007. Tissue-specific regulation of CD8+ T-lymphocyte immunodominance in respiratory syncytial virus infection. J. Virol. 81:2349–2358. 10.1128/JVI.01910-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang J, Srikiatkhachorn A, Braciale TJ. 2001. Visualization and characterization of respiratory syncytial virus F-specific CD8+ T cells during experimental virus infection. J. Immunol. 167:4254–4260 [DOI] [PubMed] [Google Scholar]
- 23.Johnstone C, de Leon P, Medina F, Melero JA, Garcia-Barreno B, Del Val M. 2004. Shifting immunodominance pattern of two cytotoxic T-lymphocyte epitopes in the F glycoprotein of the Long strain of respiratory syncytial virus. J. Gen. Virol. 85:3229–3238. 10.1099/vir.0.80219-0 [DOI] [PubMed] [Google Scholar]
- 24.Fulton RB, Meyerholz DK, Varga SM. 2010. Foxp3+ CD4 regulatory T cells limit pulmonary immunopathology by modulating the CD8 T cell response during respiratory syncytial virus infection. J. Immunol. 185:2382–2392. 10.4049/jimmunol.1000423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss KA, Christiaansen AF, Fulton RB, Meyerholz DK, Varga SM. 2011. Multiple CD4+ T cell subsets produce immunomodulatory IL-10 during respiratory syncytial virus infection. J. Immunol. 187:3145–3154. 10.4049/jimmunol.1100764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olson MR, Hartwig SM, Varga SM. 2008. The number of respiratory syncytial virus (RSV)-specific memory CD8 T cells in the lung is critical for their ability to inhibit RSV vaccine-enhanced pulmonary eosinophilia. J. Immunol. 181:7958–7968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fulton RB, Olson MR, Varga SM. 2008. Regulation of cytokine production by virus-specific CD8 T cells in the lungs. J. Virol. 82:7799–7811. 10.1128/JVI.00840-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim YPJ, Zhu Z, Tamang D, Wang P, Greenbaum J, Lundegaard C, Sette A, Lund O, Bourne PE, Nielsen M, Peters B. 2012. Immune epitope database analysis resource. Nucleic Acids Res. 40(Web Server issue):W525–W530. 10.1093/nar/gks438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nielsen M, Lundegaard C, Worning P, Lauemoller SL, Lamberth K, Buus S, Brunak S, Lund O. 2003. Reliable prediction of T-cell epitopes using neural networks with novel sequence representations. Protein Sci. 12:1007–1017. 10.1110/ps.0239403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lundegaard C, Lamberth K, Harndahl M, Buus S, Lund O, Nielsen M. 2008. NetMHC-3.0: accurate web accessible predictions of human, mouse and monkey MHC class I affinities for peptides of length 8-11. Nucleic Acids Res. 36:W509–W512. 10.1093/nar/gkn202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peters B, Sette A. 2005. Generating quantitative models describing the sequence specificity of biological processes with the stabilized matrix method. BMC Bioinformatics 6:132. 10.1186/1471-2105-6-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sidney J, Assarsson E, Moore C, Ngo S, Pinilla C, Sette A, Peters B. 2008. Quantitative peptide binding motifs for 19 human and mouse MHC class I molecules derived using positional scanning combinatorial peptide libraries. Immunome Res. 4:2. 10.1186/1745-7580-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barends M, de Rond LG, Dormans J, van Oosten M, Boelen A, Neijens HJ, Osterhaus AD, Kimman TG. 2004. Respiratory syncytial virus, pneumonia virus of mice, and influenza A virus differently affect respiratory allergy in mice. Clin. Exp. Allergy 34:488–496. 10.1111/j.1365-2222.2004.01906.x [DOI] [PubMed] [Google Scholar]
- 34.Dakhama A, Park JW, Taube C, Joetham A, Balhorn A, Miyahara N, Takeda K, Gelfand EW. 2005. The enhancement or prevention of airway hyperresponsiveness during reinfection with respiratory syncytial virus is critically dependent on the age at first infection and IL-13 production. J. Immunol. 175:1876–1883 [DOI] [PubMed] [Google Scholar]
- 35.Mukherjee S, Lindell DM, Berlin AA, Morris SB, Shanley TP, Hershenson MB, Lukacs NW. 2011. IL-17-induced pulmonary pathogenesis during respiratory viral infection and exacerbation of allergic disease. Am. J. Pathol. 179:248–258. 10.1016/j.ajpath.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newcomb DC, Boswell MG, Reiss S, Zhou W, Goleniewska K, Toki S, Harintho MT, Lukacs NW, Kolls JK, Peebles RS., Jr 2013. IL-17A inhibits airway reactivity induced by respiratory syncytial virus infection during allergic airway inflammation. Thorax 68:717–723. 10.1136/thoraxjnl-2012-202404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pemberton RM, Cannon MJ, Openshaw PJ, Ball LA, Wertz GW, Askonas BA. 1987. Cytotoxic T cell specificity for respiratory syncytial virus proteins: fusion protein is an important target antigen. J. Gen. Virol. 68(Part 8):2177–2182. 10.1099/0022-1317-68-8-2177 [DOI] [PubMed] [Google Scholar]
- 38.Openshaw PJ, Clarke SL, Record FM. 1992. Pulmonary eosinophilic response to respiratory syncytial virus infection in mice sensitized to the major surface glycoprotein G. Int. Immunol. 4:493–500. 10.1093/intimm/4.4.493 [DOI] [PubMed] [Google Scholar]
- 39.Bangham CR, Openshaw PJ, Ball LA, King AM, Wertz GW, Askonas BA. 1986. Human and murine cytotoxic T cells specific to respiratory syncytial virus recognize the viral nucleoprotein (N), but not the major glycoprotein (G), expressed by vaccinia virus recombinants. J. Immunol. 137:3973–3977 [PubMed] [Google Scholar]
- 40.Schmidt NW, Podyminogin RL, Butler NS, Badovinac VP, Tucker BJ, Bahjat KS, Lauer P, Reyes-Sandoval A, Hutchings CL, Moore AC, Gilbert SC, Hill AV, Bartholomay LC, Harty JT. 2008. Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. Proc. Natl. Acad. Sci. U. S. A. 105:14017–14022. 10.1073/pnas.0805452105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rudensky A, Preston-Hurlburt P, Hong SC, Barlow A, Janeway CA., Jr 1991. Sequence analysis of peptides bound to MHC class II molecules. Nature 353:622–627. 10.1038/353622a0 [DOI] [PubMed] [Google Scholar]
- 42.Hunt DF, Michel H, Dickinson TA, Shabanowitz J, Cox AL, Sakaguchi K, Appella E, Grey HM, Sette A. 1992. Peptides presented to the immune system by the murine class II major histocompatibility complex molecule I-Ad. Science 256:1817–1820. 10.1126/science.1319610 [DOI] [PubMed] [Google Scholar]
- 43.Chicz RM, Urban RG, Lane WS, Gorga JC, Stern LJ, Vignali DA, Strominger JL. 1992. Predominant naturally processed peptides bound to HLA-DR1 are derived from MHC-related molecules and are heterogeneous in size. Nature 358:764–768. 10.1038/358764a0 [DOI] [PubMed] [Google Scholar]
- 44.Chicz RM, Urban RG, Gorga JC, Vignali DA, Lane WS, Strominger JL. 1993. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J. Exp. Med. 178:27–47. 10.1084/jem.178.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown JH, Jardetzky TS, Gorga JC, Stern LJ, Urban RG, Strominger JL, Wiley DC. 1993. Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature 364:33–39. 10.1038/364033a0 [DOI] [PubMed] [Google Scholar]
- 46.Fremont DH, Hendrickson WA, Marrack P, Kappler J. 1996. Structures of an MHC class II molecule with covalently bound single peptides. Science 272:1001–1004. 10.1126/science.272.5264.1001 [DOI] [PubMed] [Google Scholar]
- 47.Carson RT, Vignali KM, Woodland DL, Vignali DA. 1997. T cell receptor recognition of MHC class II-bound peptide flanking residues enhances immunogenicity and results in altered TCR V region usage. Immunity 7:387–399. 10.1016/S1074-7613(00)80360-X [DOI] [PubMed] [Google Scholar]
- 48.Sant'Angelo DB, Robinson E, Janeway CA, Jr, Denzin LK. 2002. Recognition of core and flanking amino acids of MHC class II-bound peptides by the T cell receptor. Eur. J. Immunol. 32:2510–2520. [DOI] [PubMed] [Google Scholar]
- 49.Nelson CA, Petzold SJ, Unanue ER. 1993. Identification of two distinct properties of class II major histocompatibility complex-associated peptides. Proc. Natl. Acad. Sci. U. S. A. 90:1227–1231. 10.1073/pnas.90.4.1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arnold PY, La Gruta NL, Miller T, Vignali KM, Adams PS, Woodland DL, Vignali DA. 2002. The majority of immunogenic epitopes generate CD4+ T cells that are dependent on MHC class II-bound peptide-flanking residues. J. Immunol. 169:739–749 [DOI] [PubMed] [Google Scholar]
- 51.Srinivasan M, Domanico SZ, Kaumaya PT, Pierce SK. 1993. Peptides of 23 residues or greater are required to stimulate a high affinity class II-restricted T cell response. Eur. J. Immunol. 23:1011–1016. 10.1002/eji.1830230504 [DOI] [PubMed] [Google Scholar]
- 52.Roman E, Harris DP, Jurcevic S, Ivanyi J, Moreno C. 1995. H-2-associated effects of flanking residues on the recognition of a permissive mycobacterial T-cell epitope. Immunology 86:183–189 [PMC free article] [PubMed] [Google Scholar]
- 53.O'Brien C, Flower DR, Feighery C. 2008. Peptide length significantly influences in vitro affinity for MHC class II molecules. Immunome Res. 4:6. 10.1186/1745-7580-4-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang P, Sidney J, Dow C, Mothe B, Sette A, Peters B. 2008. A systematic assessment of MHC class II peptide binding predictions and evaluation of a consensus approach. PLoS Comput. Biol. 4:e1000048. 10.1371/journal.pcbi.1000048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang P, Sidney J, Kim Y, Sette A, Lund O, Nielsen M, Peters B. 2010. Peptide binding predictions for HLA DR, DP and DQ molecules. BMC Bioinformatics 11:568. 10.1186/1471-2105-11-568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Collins PL, Melero JA. 2011. Progress in understanding and controlling respiratory syncytial virus: still crazy after all these years. Virus Res. 162:80–99. 10.1016/j.virusres.2011.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lotz MT, Peebles RS., Jr 2012. Mechanisms of respiratory syncytial virus modulation of airway immune responses. Curr. Allergy Asthma Rep. 12:380–387. 10.1007/s11882-012-0278-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mothe BR, Stewart BS, Oseroff C, Bui HH, Stogiera S, Garcia Z, Dow C, Rodriguez-Carreno MP, Kotturi M, Pasquetto V, Botten J, Crotty S, Janssen E, Buchmeier MJ, Sette A. 2007. Chronic lymphocytic choriomeningitis virus infection actively down-regulates CD4+ T cell responses directed against a broad range of epitopes. J. Immunol. 179:1058–1067 [DOI] [PubMed] [Google Scholar]
- 59.Moutaftsi M, Bui HH, Peters B, Sidney J, Salek-Ardakani S, Oseroff C, Pasquetto V, Crotty S, Croft M, Lefkowitz EJ, Grey H, Sette A. 2007. Vaccinia virus-specific CD4+ T cell responses target a set of antigens largely distinct from those targeted by CD8+ T cell responses. J. Immunol. 178:6814–6820 [DOI] [PubMed] [Google Scholar]
- 60.Harrington LE, van der Most R, Whitton JL, Ahmed R. 2002. Recombinant vaccinia virus-induced T-cell immunity: quantitation of the response to the virus vector and the foreign epitope. J. Virol. 76:3329–3337. 10.1128/JVI.76.7.3329-3337.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dow C, Oseroff C, Peters B, Nance-Sotelo C, Sidney J, Buchmeier M, Sette A, Mothe BR. 2008. Lymphocytic choriomeningitis virus infection yields overlapping CD4+ and CD8+ T-cell responses. J. Virol. 82:11734–11741. 10.1128/JVI.00435-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Olson MR, Varga SM. 2007. CD8 T cells inhibit respiratory syncytial virus (RSV) vaccine-enhanced disease. J. Immunol. 179:5415–5424 [DOI] [PubMed] [Google Scholar]
- 63.Srikiatkhachorn A, Braciale TJ. 1997. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J. Exp. Med. 186:421–432. 10.1084/jem.186.3.421 [DOI] [PMC free article] [PubMed] [Google Scholar]