ABSTRACT
The liver bile acids transporter sodium taurocholate cotransporting polypeptide (NTCP) is responsible for the majority of sodium-dependent bile salts uptake by hepatocytes. NTCP also functions as a cellular receptor for viral entry of hepatitis B virus (HBV) and hepatitis D virus (HDV) through a specific interaction between NTCP and the pre-S1 domain of HBV large envelope protein. However, it remains unknown if these two functions of NTCP are independent or if they interfere with each other. Here we show that binding of the pre-S1 domain to human NTCP blocks taurocholate uptake by the receptor; conversely, some bile acid substrates of NTCP inhibit HBV and HDV entry. Mutations of NTCP residues critical for bile salts binding severely impair viral infection by HDV and HBV; to a lesser extent, the residues important for sodium binding also inhibit viral infection. The mutation S267F, corresponding to a single nucleotide polymorphism (SNP) found in about 9% of the East Asian population, renders NTCP without either taurocholate transporting activity or the ability to support HBV or HDV infection in cell culture. These results demonstrate that molecular determinants critical for HBV and HDV entry overlap with that for bile salts uptake by NTCP, indicating that viral infection may interfere with the normal function of NTCP, and bile acids and their derivatives hold the potential for further development into antiviral drugs.
IMPORTANCE Human hepatitis B virus (HBV) and its satellite virus, hepatitis D virus (HDV), are important human pathogens. Available therapeutics against HBV are limited, and there is no drug that is clinically available for HDV infection. A liver bile acids transporter (sodium taurocholate cotransporting polypeptide [NTCP]) critical for maintaining homeostasis of bile acids serves as a functional receptor for HBV and HDV. We report here that the NTCP-binding lipopeptide that originates from the first 47 amino acids of the pre-S1 domain of the HBV L protein blocks taurocholate transport. Some bile salts dose dependently inhibit HBV and HDV infection mediated by NTCP; molecular determinants of NTCP critical for HBV and HDV entry overlap with that for bile acids transport. This work advances our understanding of NTCP-mediated HBV and HDV infection in relation to NTCP's physiological function. Our results also suggest that bile acids or their derivatives hold potential for development into novel drugs against HBV and HDV infection.
INTRODUCTION
Infection with hepatitis B virus (HBV) remains a major public health problem. Although an effective vaccine is available, there are about a million new infections yearly and about 240 million chronic infections worldwide (1). HDV is a satellite virus of HBV, and its propagation requires the envelope of HBV (2). Globally, there are 15 million people infected with HDV (2). The current available therapeutics against HBV are limited to the immune modulator interferon-α and viral reverse transcription inhibitors, while there is no drug clinically available for the treatment of HDV infection.
We identified sodium taurocholate cotransporting polypeptide (NTCP) as a cellular receptor for human HBV and HDV viral entry (3). Multiple lines of evidence support that NTCP is likely a dominant receptor for HBV and HDV (3–5). The expression of NTCP correlates with the susceptibility of the target cells, and reducing the expression of NTCP markedly inhibits HBV and HDV infection on known susceptible cells. Exogenous expression of human NTCP, but not mouse NTCP, renders multiple mammalian cell lines susceptible to HDV infection regardless of their tissue or species origin, while HBV infection, which is known to depend on hepatic factors for replication, is efficiently achieved in HepG2 cells complemented with human or treeshrew NTCP. Replacing a few amino acids of crab-eating monkey (amino acids [aa] 157 to 165) or mouse NTCP (aa 84 to 87) with their human counterparts converted these NTCPs to functional receptors for HBV and HDV, respectively. Thus, HepG2 cells complemented with human NTCP provide a valuable and convenient in vitro cell culture system for increasing our understanding of the mechanism of viral entry and for the development of novel antiviral drugs.
Human NTCP (SLC10A1) is a multiple-transmembrane protein that is predominantly expressed at the basolateral membrane of hepatocytes. As a key bile salt transporter, the primary role of NTCP in vivo is to transport bile salts from the portal blood into hepatocytes, a process known to be of vital importance in maintaining homeostasis of bile acids (6, 7). NTCP has a preference for glycine- and taurine-conjugated bile salts over their unconjugated counterparts, and the affinities are often higher for dihydroxy bile salts than for trihydroxy bile salts (8). NTCP is responsible for the majority of hepatic influx of conjugated bile salts (9) and also for the transportation of some drugs and xenobiotics (7).
Entry of both HBV and HDV is mediated by HBV envelopes, which comprise large, medium, and small (L, M, and S) envelope proteins. These are all multiple transmembrane proteins; they differ only at the N-terminal regions and share the same C-terminal S domain (10, 11). The S protein constitutes the major component of the virions and mediates virus attachment to target cells via cell surface heparan sulfate proteoglycan (12–14), whereas the specific interaction between NTCP and the pre-S1 domain of the HBV large envelope protein is essential for HBV and HDV viral infection on target cells (3).
In this study, we examined NTCP-mediated HBV and HDV viral infections in relation to the receptor's physiological role of transporting bile acids. We found that the HBV pre-S1 domain efficiently blocked bile salts substrate uptake of NTCP. Conversely, bile salts substrates inhibited HBV and HDV infection to various extents. Mutagenesis studies showed residues of NTCP critical for bile acids binding contributed to efficient viral infections, as did the residues important for sodium binding, albeit to a lesser extent. Of note, the S267F mutation, corresponding to a single nucleotide polymorphism (SNP) (15) and that is found in 9% of East Asian population, impaired taurocholate uptake and the ability of NTCP to support HBV and HDV infections. These results deepened our understanding of the interaction between the HBV envelope protein and NTCP and provided evidence that bile acids and their derivatives might be useful in blocking HBV and HDV infections.
MATERIALS AND METHODS
Cell culture.
The human hepatocellular carcinoma cell line HepG2 was obtained from the American Type Culture Collection (ATCC); the human hepatocellular carcinoma cell line Huh-7 was from the Cell Bank of Type Culture Collection, Chinese Academy of Sciences. These cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) supplemented with 10% fetal bovine serum (FBS; Gibco) at 37°C in a 5% CO2 humidified incubator with regular passage of every 2 to 3 days. A HepG2-NTCP stable cell line expressing human NTCP was generated from HepG2 cells and maintained in DMEM supplemented with 10% FBS and 500 μg/ml G418. HepaRG cells were purchased from Biopredic International (Rennes, France) and were cultured according to the product manual. Primary Tupaia belangeri hepatocytes (PTHs) were obtained from anesthetized Tupaia by using a two-step perfusion method and cultured as described previously (3). The above cell lines and primary cells were plated on collagen (BD)-coated plates or dishes and cultured in PTH maintenance medium (PMM) for 24 h before viral infection, peptide binding, substrate uptake, and other experimental procedures (3).
Peptide, antibodies, and other reagents.
Fluorescein isothiocyante (FITC)-labeled pre-S1 peptide was derived from the pre-S1 domain of HBV (C-type; GenBank accession number EU554535.1) containing the first 59 residues, an amino-terminal myristoylation modification, with FITC conjugated to the carboxyl terminal. The myr(+)47 peptide contained residues 2 to 47 of the pre-S1 domain of HBV (D type; GenBank accession number U95551.1) with N-terminal myristoylation; the myr(+)47-N9K peptide was the same as myr(+)47 except that bore an asparagine (N)-to-lysine (K) mutation at residue 9. The myr(−)47 peptide is the same as myr(+)47 but without the modification of myristoylation at the amino terminus. All these peptides were synthesized by SunLight peptides (Beijing, China). 1C10 is a mouse monoclonal antibody (MAb) that recognizes the HBV core protein; 4G5 is a mouse MAb that specifically targets the HDV delta antigens. Both MAbs were developed by conventional hybridoma technology in our lab. 1D4 is a mouse MAb that specifically recognizes the C-9 tag (TETSQVAPA) and was purchased from Santa Cruz Biotechnology. A quantitative real-time PCR kit and reverse transcription reagents were purchased from TaKaRa Inc. (Beijing, China). Enzyme-linked immunosorbent assay (ELISA) kits for HBeAg and HBsAg detection were from Wantai Pharm Inc. (Beijing, China). alamarBlue for quantitative analysis of cell viability was purchased from Life Technologies. 3H-labeled taurocholate with an activity of 5.0 Ci/mmol (0.185 TBq/mmol) and liquid scintillation cocktail (Ultima Gold XR) were purchased from PerkinElmer. EZ-Link sulfosuccinimidyl-6-(biotin-amido)hexanoate (sulfo-NHS-LC-biotin) was from Thermo Scientific. Streptavidin-coupled magnetic beads (Dynabeads MyOne streptavidin T1) were purchased from Life Technologies. Bile acids and their derivatives were purchased from Sigma-Aldrich.
Virus production, purification, and concentration with PEG 8000.
HDV and HBV were produced as previously described (3, 4). For HDV production, two plasmids are required. One plasmid (pCMV-HDV3.0) contains a head-to-tail trimer of 1.0× HDV cDNA (genotype I; GenBank accession number AF425644.1) under the control of a cytomegalovirus (CMV) promoter and was used for the generation of HDV RNPs. The other plasmid (pUC-HBV-LMS) contains nucleotides 2431 to 1990 of HBV (genotype D; GenBank accession number U95551.1) and is based on pUC18 plasmid, used for the expression of HBV envelope proteins. Huh-7 cells were first transfected with pCMV-HDV3.0, cultured and propagated for at least 13 days, and then transfected with pUC-HBV-LMS. The culture medium was changed to PMM at 4 to 6 h after transfection, and virus-containing cell culture supernatant was collected at day 3 and day 6 posttransfection. For HBV production, a plasmid containing 1.05 copies of the HBV genome (genotype D; GenBank accession number U95551.1) under the control of a CMV promoter was transfected into Huh-7 cells. The medium was changed to PMM posttransfection, and virus-containing supernatant was collected at day 3 and day 6 posttransfection, centrifuged, and stored at −80°C. In some cases, HBV and HDV were precipitated by using 8% polyethylene glycol 8000 (PEG 8000) at 4°C for 6 to 12 h and then purified by centrifugation at 4°C, 12,000 rpm, for 20 min. The pellet was resuspended in PMM or Ringer's solution with Na+ or choline+.
NTCP expression constructs and mutants.
Human NTCP (hNTCP), mouse NTCP (mNTCP), and crab-eating monkey NTCP (mkNTCP) were cloned from cDNA from primary human hepatocytes, primary mouse hepatocytes, and macaque hepatocytes, respectively. These NTCP constructs all contained a C-9 tag at the carboxyl terminus and were cloned into a mammalian cell expression vector, pcDNA6. NTCP mutants were generated by PCR mutagenesis using reverse complementary primers containing the changes. All sequences of NTCP variants were confirmed by DNA sequencing.
FITC–pre-S1 peptide-binding assay.
HepG2-NTCP cells or HepG2 cells transfected with plasmids expressing NTCP or its variants were cultured in PMM for about 24 h and then incubated with 400 nM FITC-labeled pre-S1peptide (FITC–pre-S1) at 37°C for about 1 h in the William's E medium. In some cases, FITC–pre-S1 peptide binding was conducted in Na+ or choline+ Ringer's solution with the components and for the times indicated below. The images were captured and analyzed by using a Nikon Eclipse Ti fluorescence microscope.
[3H]taurocholate uptake assay.
The [3H]taurocholate uptake assay was conducted following a protocol previously described, with minor modifications (5). In brief, NTCP-expressing HepG2 cells were cultured in PMM for about 24 h and then were treated with the indicated chemicals, rinsed once with Na+ or choline+ Ringer's solution (the concentration of Na+ or choline+ was 145 mM unless otherwise noted). For the substrate uptake assay, about 5 × 10e4 cells were generally incubated with 1 μM [3H]taurocholate dissolved in Na+ Ringer's solution for 15 min at 37°C unless otherwise mentioned. Subsequently, cells were washed once in phosphate-buffered saline (PBS) and lysed in 100 μl of 1% Triton X-100 in H2O for 5 min at room temperature. The lysate was transferred into liquid scintillation tubes and mixed with 900 μl liquid scintillation cocktail (Ultima Gold XR; PerkinElmer). Liquid scintillation counting was performed on a PerkinElmer 1450 LSC liquid scintillation counter and luminescence counter.
HBV and HDV infection of NTCP-expressing HepG2 cells.
HepG2 cells were transfected with plasmids expressing NTCPs or their variants by using Lipofectamine 2000 (Invitrogen) following the manufacturer's instructions. In some cases, cells were cotransfected with wild-type or human NTCP S267F mutant or with the pcDNA6 plasmid at a 1:1 ratio. Cells were cultured in PMM for 24 h after transfection and then inoculated with at a multiplicity of genome equivalents (MGE) of 200 for HBV or 500 MGE for HDV in the presence of 5% PEG 8000 in PMM at 37°C for about 24 h. For the infection inhibition assay, the reagents were either treated during the infection or at other time points as indicated. For experiments analyzing the effect of sodium on viral infection, the inoculation was conducted in Na+ or choline+ Ringer's solution with different Na+ concentrations, as indicated, at room temperature for about 4 h and then replenished with fresh PMM culture medium.
ELISA for HBV viral antigens.
In general, supernatants from infected cells expressing NTCP and their variants were collected at 3, 5, and 7 days postinfection (p.i.). The secreted HBeAg and HBsAg levels in the culture medium were measured using 50 μl and 100 μl medium, respectively, in an ELISA from a commercial kit from Wantai Pharm Inc. (Beijing, China).
Immunofluorescence microscopy analysis of intracellular antigens.
On the indicated days postinfection, HDV-infected cells were fixed with 100% methanol at room temperature for 10 min, intracellular delta antigen was stained with 5 μg/ml of FITC-conjugated 4G5, and nuclei were stained blue with 4′,6-diamidino-2-phenylindole (DAPI). Images were collected by using a fluorescence microscope (Nikon); a representative picture is shown. For HBV infection, infected cells were washed with PBS twice and fixed in 3.7% paraformaldehyde (PFA) at room temperature for 10 min. Subsequently, cells were permeabilized with 0.5% Trition X-100–PBS for 10 min at room temperature, blocked with 3% bovine serum albumin at 37°C for 1 h, and then incubated with 5 μg/ml mouse MAb 1C10, which recognizes HBcAg, followed by staining with FITC-conjugated secondary antibody. Nuclei were stained blue with DAPI. Images of the stained cells were obtained with an Eclipse Ti fluorescence microscope (Nikon).
Analysis of total and surface protein expression levels of NTCP and its variants.
HepG2 cells transfected with hNTCP or its variants were cultured in PMM for 24 h after transfection. A water-soluble and membrane-impermeable reagent, sulfo-NHS-LC-biotin (Pierce) was used for labeling cell surface proteins. The NHS-activated biotins can react efficiently with primary amino groups (-NH2) in the side chains of lysine (K) residues and the N terminus of each polypeptide to form stable amide bonds, and as long as the cell remains intact, only primary amines exposed on the surface will be biotinylated. To evaluate the cell surface expression of human NTCP and its derivatives with a C-9 tag, about 1.6×106 transfected cells were washed three times with PBS and then surface biotinylated by incubating the cells with freshly dissolved sulfo-NHS-LC-biotin (Pierce) reagent (2 mM) in PBS (pH 8.0) on ice for 1 h. The cells were subsequently washed three times with PBS containing 100 mM glycine to remove excess biotin reagent and by-products. The biotinylated cells were then lysed in 600 μl RIPA buffer (20 mM Tris, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% NP40, and 1× protease inhibitor cocktail). The protein concentration was determined in the Bio-Rad DC protein assay according to the manufacturer's instructions. Surface-biotinylated NTCP in the cell lysate with about 160 μg total cellular protein was pulled down by about 300 μg streptavidin T1 Dynabeads (Invitrogen). After extensive washing with 1× RIPA buffer, the captured NTCP proteins were eluted by boiling for 5 min in 1× SDS-PAGE protein sample buffer and treatment with peptide-N-glycosidase F (PNGase F; New England BioLabs) at 37°C for 1 h. The treated samples were separated by SDS-PAGE and analyzed by Western blotting with anti-C9 MAb (1D4), which recognizes the C-9 tag fused at the carboxyl terminus of NTCPs. For determination of NTCP total expression levels, wild-type or mutant NTCP plasmid-transfected cells were lysed with 1× RIPA buffer and treated with PNGase F at 24 h posttransfection. The treated cell lysates, each containing ∼8 μg of total cellular protein, were subjected to SDS-PAGE without boiling, followed by Western blotting with 1D4. The expression level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control.
RESULTS
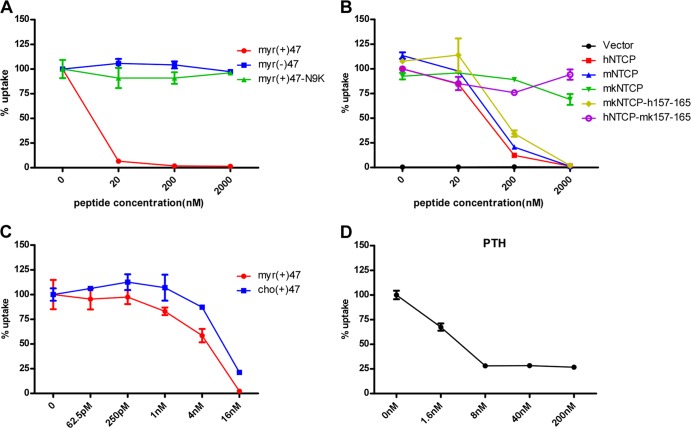
Binding of the HBV pre-S1 lipopeptide to NTCP impairs taurocholate uptake.
Binding of the HBV pre-S1 domain to NTCP mediates viral entry of HBV and HDV (3). We were interested in the consequence of the binding, in particular, whether it interferes with the physiological function of NTCP in transporting its natural substrates. To test this, a 3H-labeled taurocholate uptake inhibition assay was performed to evaluate the effect of pre-S1 binding on NTCP's transporting function. Remarkably, a myristoylated pre-S1 peptide containing the N-terminal 47 amino acids potently inhibited [3H]taurocholate uptake in HepG2 cells stably expressing human NTCP (HepG2-NTCP) at a concentration less than 20 nM, whereas neither myristoylated pre-S1 peptide carrying a non-NTCP-binding mutation, N9K, nor the pre-S1 peptide lacking the N-terminal acylation showed inhibition even at the highest concentration tested (2 μM) (Fig. 1A). In addition, the myristoylated pre-S1 peptide blocked [3H]taurocholate transport in HepG2 cells transiently transfected with hNTCP, mNTCP, and mkNTCP mutant mkNTCP-h157-165, with residues replaced by human counterparts between aa 157 and 165, but not wild-type mkNTCP or the human NTCP mutant hNTCP-mk157-165, with aa 157 to 165 replaced by the monkey counterpart (Fig. 1B). These results were consistent with our previously published results indicating that the pre-S1 lipopeptide can bind to hNTCP, mNTCP, and mkNTCP-h157-165 but not wild-type mkNTCP or hNTCP-mk157-165 (3, 5). Interestingly, the same peptide with a cholesterol modification at the N-terminal amino acid could also inhibit [3H]taurocholate uptake in HepG2-NTCP in a dose-dependent manner (Fig. 1C). This was in line with the finding that the cholesterol-modified peptide can inhibit HBV infection (data not shown) (16). Moreover, the pre-S1 lipopeptide inhibited [3H]taurocholate uptake in primary PTH cells, yet the substrate uptake was not reduced to the background level, as it was on HepG2-NTCP cells (Fig. 1D). These results indicated that binding of pre-S1 domain to NTCP interferes with bile acids transporting by NTCP. On the other hand, as shown in Fig. 1B, in the absence of pre-S1 lipopeptide, hNTCP, mNTCP, mkNTCP, hNTCP-mk157-165, and mkNTCP-h157-165 all supported taurocholate transport efficiently, indicating that NTCP can tolerate changes within the aa 157 to 165 region for taurocholate transport. Consistently, altering two residues, S162 and L163, which are conserved in human, mouse, and monkey NTCP, to alanine reduced HBV infection mediated by human NTCP but did not hamper the receptor's function in transporting taurocholate (data not shown). Considering that the viral infection susceptibility but not taurocholate transporting activity is critically affected by the structure or identity of the residues within this region of NTCP, aa 157 to 165 may be a useful target for selectively blocking viral infection with no or minimal interference of bile salts transport.
FIG 1.
Binding of the HBV pre-S1 domain to NTCP impairs taurocholate uptake. (A) Inhibition of [3H]taurocholate uptake by HBV pre-S1 lipopeptide. A [3H]taurocholate uptake assay was conducted with HepG2-hNTCP cells (HepG2 cells stably expressing human NTCP) pretreated with the indicated concentrations of HBV pre-S1 lipopeptide or its derivatives at 37°C for 2 h. myr(+)47, myristoylated pre-S1 peptide containing the first N-terminal 47 amino acids of the L protein of HBV; myr(−)47, the pre-S1 peptide without myristoylation modification; myr(+)47-N9K, myristoylated pre-S1 peptide with an asparagine (N)-to-arginine (K) mutation at residue 9. Cellular uptake of [3H]taurocholate in the absence of any peptide was set to 100%. (B) Inhibition of [3H]taurocholate uptake by myr(+)47 in HepG2 cells transiently transfected with NTCPs of different species. Cellular uptake of [3H]taurocholate by human NTCP when treated with dimethyl sulfoxide (DMSO) was set to 100%. (C) Inhibition of [3H]taurocholate uptake by cholesterol or myristoyl-modified pre-S1 peptides in HepG2-NTCP cells. Uptake of [3H]taurocholate by cells treated with DMSO was set to 100%. (D) Inhibition of [3H]taurocholate uptake by myristoylated pre-S1 peptide in primary Tupaia hepatocytes.
Substrates of NTCP inhibited pre-S1 lipopeptide binding to NTCP and viral infection by HBV and HDV.
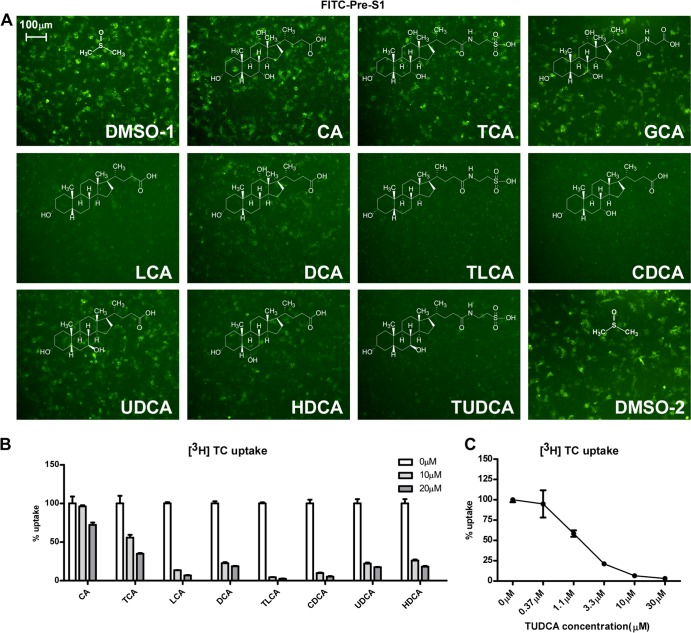
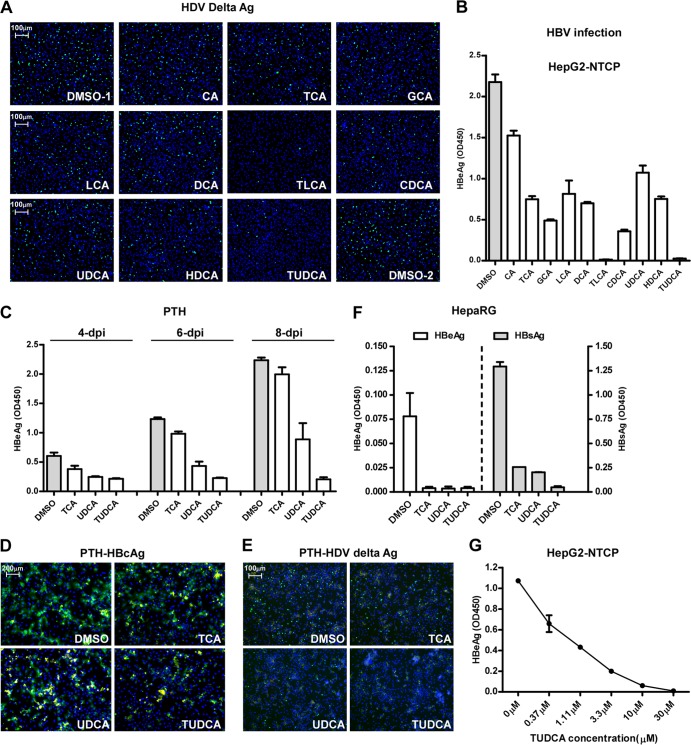
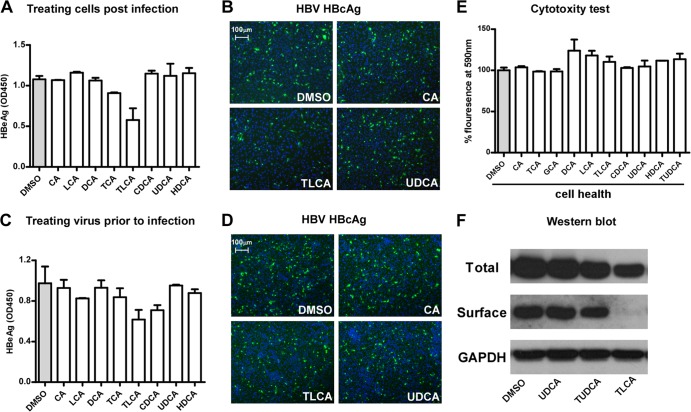
We next examined whether substrates of NTCP could inhibit pre-S1 lipopeptide binding to the receptor. Primary or secondary bile salts with or without modifications, including cholic acid (CA), taurocholic acid (TCA), glycocholic acid (GCA), lithocholic acid (LCA), deoxycholic acid (DCA), taurolithocholic acid (TLCA), chenodeoxycholic acid (CDCA), ursodeoxycholic acid (UDCA), hyodeoxycholic acid (HDCA), and tauroursodeoxycholic acid (TUDCA), were incubated with HepG2-NTCP cells, and the binding of a FITC-labeled pre-S1 peptide to the receptor was subsequently examined by using fluorescence microscopy. The bile acids inhibited the peptide binding to NTCP with various efficiencies (Fig. 2A) that were in parallel with their abilities in competing with [3H]taurocholate transport (Fig. 2B and C). We then investigated whether these bile salts could inhibit viral infection by HBV and HDV. HepG2-NTCP cells were inoculated with HDV in the presence of bile salts for 24 h, and then the infection was examined 6 days post-viral inoculation. Consistent with the results indicating that bile acids block pre-S1 lipopeptide binding to NTCP, these substrates of NTCP reduced HDV infection, as indicated by staining of the intracellular HDV delta antigen of infected cells (Fig. 3A). We further evaluated the abilities of these bile salts to inhibit HBV infection. The levels of secreted HBeAg decreased when HepG2-NTCP cells were inoculated with HBV viruses in the presence of the indicated bile salts, with TUDCA being the most potent among all bile sates tested (Fig. 3B and G). In addition to HepG2-NTCP cells, HDV and/or HBV infection of the PTH and HepaRG cells was also inhibited by the treatment with bile salts at the indicated concentrations (Fig. 3C to F). Of note, HBV infection was not affected when the bile salts were incubated with HepG2-NTCP cells for 24 h after the viral inoculation (Fig. 4A and B), and the bile salts did not exhibit significant cytotoxicity when coincubated with the cells (Fig. 4E). To test whether bile salts damaged virus integrity, virus was incubated with bile salts for 24 h, precipitated with 8% PEG 8000 to remove the bile salts, and then examined for its infectivity in HepG2-NTCP cells. The infectivity of HBV treated with the bile acids was not impaired; thus, the bile salts were not destructive to the virus per se at the concentrations tested (Fig. 4C and D). Among the bile acids tested, TLCA but neither UDCA nor TUDCA markedly reduced protein surface expression of NTCP (Fig. 4F). Together, these results indicated that the inhibitory effects of most bile acids on viral infection can be attributed mainly to direct interference of NTCP binding with the viral envelope.
FIG 2.
Bile salts blocked the interaction between the pre-S1 peptide and NTCP. (A) Inhibition of FITC-conjugated pre-S1 peptide binding by different bile salts. FITC–pre-S1 peptide (400 nM) was incubated with NTCP-transfected HepG2 cells for 1 h in the presence of the indicated bile salt (20 μM). The binding efficiency of the pre-S1 peptide was analyzed via a fluorescence microscope. CA, cholic acid; GCA, glycocholic acid; LCA, lithocholic acid; DCA, deoxycholic acid; TCA, taurocholic acid; TLCA, taurolithocholic acid; CDCA, chenodeoxycholic acid; UDCA, ursodeoxycholic acid; HDCA, hyodeoxycholic acid; TUDCA, tauroursodeoxycholic acid. (B) Competition for [3H]taurocholate uptake by other bile salts. The indicated bile salts (at 10 μM or 20 μM) were examined. Uptake efficiency is presented as the percent uptake compared with the dimethyl sulfoxide group. (C) The ability of TUDCA to inhibit [3H]taurocholate uptake by HepG2-NTCP cells. TUDCA at the indicated concentration was present in the medium during the entire [3H]taurocholate uptake process.
FIG 3.
Bile acids inhibited HBV and HDV infection. (A and B) Inhibition of HDV (A) and HBV (B) infection in HepG2-NTCP cells in the presence of bile salts. HepG2-NTCP cells were infected with HDV at 37°C for 24 h in the presence of 5% PEG 8000 and 20 μM of each of the indicated bile salts except for LCA (tested at 5 μM). The intracellular HDV delta antigen was stained with mouse monoclonal antibody 4G5 at day 8 p.i. The level of secreted HBeAg in the culture medium collected at day 7 p.i. was measured with an ELISA kit. (C to E) Inhibition of HDV and HBV infection in primary Tupaia hepatocytes by 20 μM TCA, UDCA, or TUDCA. PTHs were infected with HDV or HBV in the presence of 20 μM bile salt. For HBV infection, the level of secreted HBeAg in the culture medium was evaluated at 3, 5, and 7 days p.i. (C). On day 8 p.i., HBV core antigen of the infected cells were stained with MAb 1C10 (green) (D). For HDV, intracellular HDV delta antigen of the infected cells at day 8 p.i. were stained with MAb 4G5 (green) (E). The yellow fluorescence in panels D and E stands for the autofluorescence signal from dead cells, which existed in both the green and red channels. (F) Inhibition of HBV infection of human hepatoma HepaRG cells in the presence of 25 μM bile salts. The levels of HBeAg and HBsAg in the culture medium at 6 days p.i. were measured via an ELISA. (G) Dose-dependent inhibition of HBV infection by TUDCA in HepG2-NTCP cells. The secreted HBeAg at 6 days p.i. was measured in an ELISA.
FIG 4.
Analysis of the direct effects of bile salts on HepG2-NTCP cells and HBV. (A and B) HBV infection of HepG2-NTCP cells that were treated with bile salts post-HBV inoculation. HepG2-NTCP cells were inoculated with HBV at 37°C for 24 h in the presence of 5% PEG 8000. After the inoculation, cells were washed and then incubated with different bile salts at the indicated concentrations for another 24 h, and then culturing of cells was continued in PMM. HBeAg in the culture medium collected at 7 days p.i. was measured in an ELISA (A). On day 8 p.i., HBcAg in the infected cells treated with the indicated bile salts was stained with anticore MAb 1C10 (green), and the nuclei were stained with DAPI (blue) (B). (C and D) Infection of HepG2-NTCP cells treated with bile salts prior to HBV inoculation. The viruses were incubated with 20 μM bile salts for 24 h, then precipitated with 8% PEG 8000 to remove the bile salts, followed by resuspending in PMM. The treated viruses were then used to inoculate HepG2-NTCP cells for infection. The secreted HBeAg in the culture medium collected at 7 days p.i. was measured in an ELISA (C). On day 8 p.i., HBcAg in the infected cells was stained green with 1C10 MAb, and the nuclei were stained with DAPI (blue) (D). (E) Cytotoxicy analysis of the bile salts for HepG2-NTCP cells. HepG2-NTCP cells were treated with 20 μM bile salts (5 μM for LCA) in PMM for 24 h in the presence of 5% PEG 8000. Cells were then incubated with 10% alamarBlue (Invitrogen) in PMM for 1 h. Cellular health was evaluated based on fluorescence absorbance at 590 nm. The absorbance of dimethyl sulfoxide-treated cells was set to 100%. (F) Total and surface expression levels of NTCP from HepG2-NTCP cells in response to the indicated bile salt treatments.
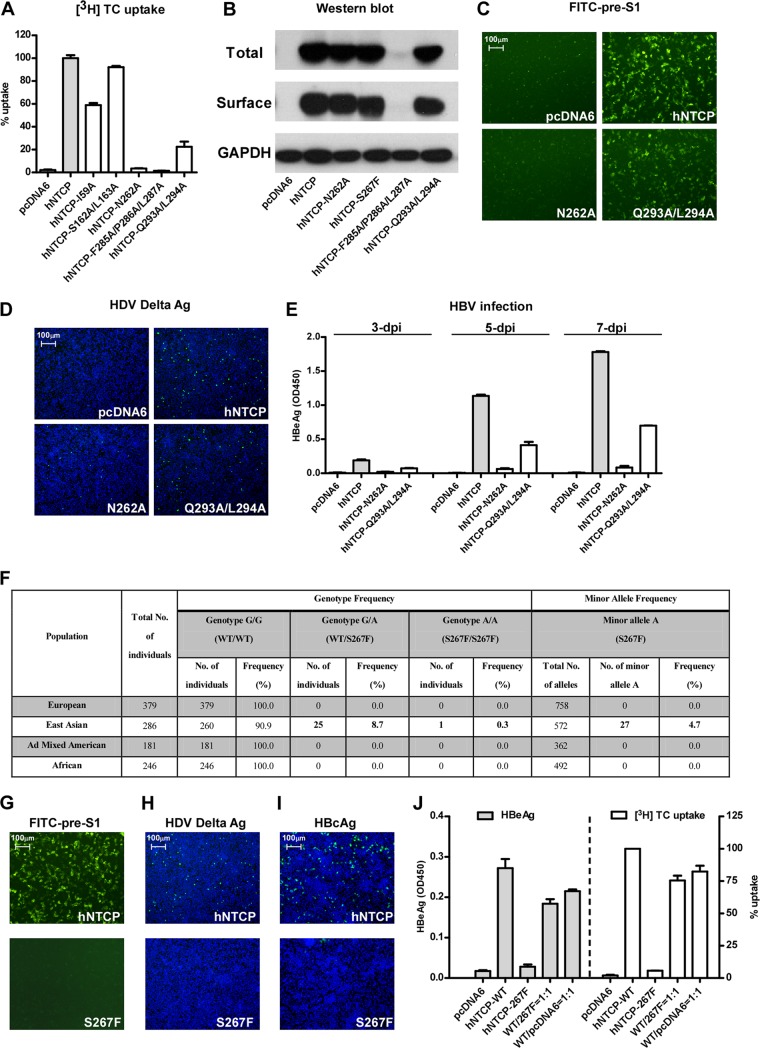
Mutations of bile salt-binding sites on hNTCP reduced viral infection by HDV and HBV.
Based on previously published mapping studies and the crystal structure of a bile acid transporter, ASBTNM (17–19), we introduced a series of mutations to the predicted bile salt-binding sites on hNTCP. As shown in Fig. 5A, the [3H]taurocholate transporting activities of NTCP variants were either abolished (N262A) or severely impaired (Q293A/L294A). We then assessed the total and surface expression levels of the NTCP mutants. Compared to wild-type hNTCP, all the tested mutant hNTCPs exhibited similar levels of total and surface expression on HepG2 cells, except for the variant with three mutations, F285A/P286A/L287A (Fig. 5B). Remarkably, the binding activity of FITC–pre-S1 lipopeptide to HepG2 cells expressing either of these two mutants, in particular N262A, was also markedly reduced (Fig. 5C). Consistent with the pre-S1 peptide-binding activity, the two mutant-transfected HepG2 cells showed marked loss of susceptibility for HDV (Fig. 5D) and HBV (Fig. 5E). These results demonstrated that the putative bile acids-binding sites on NTCP may overlap with the pre-S1-binding sites on the receptor; thus, mutations that reduce substrate binding also inhibit viral infection by HDV and HBV. We further investigated if two previously reported SNPs of hNTCP had an impact on HBV/HDV infection. The two SNPs lead to two amino acid changes, A64T and S267F, which can result in functional alterations of human NTCP in transporting bile acids substrates (20). The hNTCP variant S267F shows a minor allele frequency of 4.7% in the East Asian population, with 8.7% of this population having the heterozygous genotype and 0.3% being homozygous for the minor allele (Fig. 5F). We found that the S267F but not the A64T variant (data not shown) dramatically reduced [3H]taurocholate transporting activity. Moreover, the S267F variant could neither bind to the FITC–pre-S1 peptide (Fig. 5G) nor support HBV or HDV infection (Fig. 5H and I). Regardless, greater than 70% viral infection and [3H]taurocholate uptake were observed when the S267F variant was cotransfected with wild-type NTCP at a ratio of 1:1, indicating that heterozygous individuals may also be susceptible to HBV and HDV infection (Fig. 5J).
FIG 5.
NTCP mutants, corresponding to the residues important for bile salts binding/uptake, inhibited viral infections by HDV and HBV. (A) [3H]taurocholate uptake efficiency of HepG2 cells complemented with various NTCP mutants. HepG2 cells were transfected with NTCP mutants, followed by further culturing in PMM for 24 h before examination of their [3H]taurocholate uptake efficiency. (B) Total and surface expression levels of NTCP mutants in HepG2 cells. HepG2 cells were transfected with the indicated NTCP variants and then cultured in PMM for 24 h. Cells were then lysed by RIPA buffer, and the total and surface expression levels were analyzed by Western blotting. The expression level of GAPDH was used as an internal control. (C) FITC–pre-S1 binding efficiency of NTCP variants. HepG2 cells transfected as described for panel A were incubated with 400 nM FITC–pre-S1 at 37°C for 2 h, and the images were captured with a fluorescence microscope. (D) HDV infection efficiency for HepG2 cells transfected with NTCP variants. The intracellular delta antigen was stained with MAb 4G5 at 8 days p.i. (E) HBV infection of HepG2 cells expressing NTCP variants. The levels of HBeAg in the culture medium from HBV-infected HepG2 cells expressing the indicated NTCP variants were measured at 3, 5, and 7 days p.i. (F) Genotype frequency and allele frequency of the SNP that causes the NTCP S267F variant in different populations; the variant is caused by a single-nucleotide G-to-A change (rs2296651). The SNP is located at chromosome 14:70245193 (Ensembl Homo sapiens version 73.37) and corresponds to a C-to-T change in codon 267 (from TCC to TTC) on the reverse strand encoding human NTCP. According to data from the 1000 Genomes Project (http://www.1000genomes.org), in East Asia the heterozygous genotype frequency is 8.7%, and the minor allele homozygous genotype frequency is 0.3%. No SNP at this position is observed in other populations. The genotype distribution in East Asian population conforms to a Hardy-Weinberg equilibrium, which was verified by a chi-square test (P = 0.22). WT, wild type; Ad mixed, populations with recent ancestry from two or more genetically separated populations. (G) FITC–pre-S1 binding efficiency of NTCP in cells complemented with wild-type NTCP or a NTCP-S267F variant. (H and I) HDV and HBV infection efficiencies on HepG2 cells transfected with NTCP or NTCP-S267F. The intracellular delta antigen in the infected cells was stained with MAb 4G5 at 8 days p.i. (H). HBcAg was evaluated based on intracellular HBcAg at 8 days p.i. (I). (J) HBV infection efficiency and [3H]taurocholate uptake efficiency of HepG2 cells transfected with plasmid carrying wild-type (WT) NTCP, NTCP variant S267F, pcDNA6, or a mixture of the wild-type NTCP with either the S267F variant or pcDNA6 plasmid at a ratio of 1:1. The total amount of DNA for all the transfections was 1 μg DNA for ∼1.7 × 105 HepG2 cells. The level of secreted HBeAg at 6 days p.i. was measured in an ELISA (left), and the [3H]taurocholate uptake efficiency at 24 h posttransfection was determined by liquid scintillation (wild-type NTCP was set to 100%) (right).
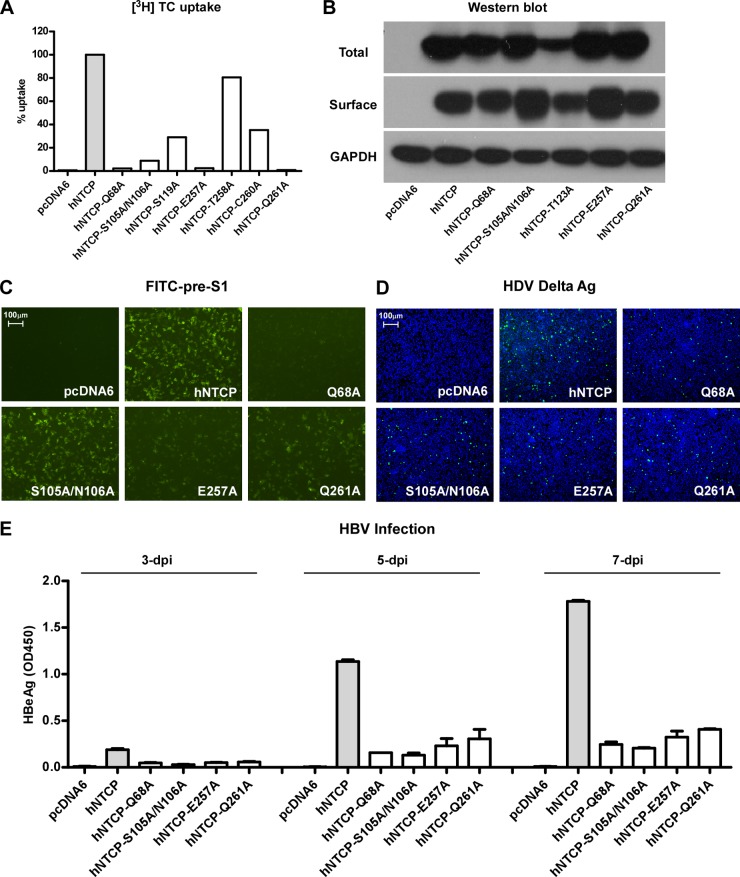
Mutations in the sodium-binding sites of hNTCP interfere with HBV and HDV infection.
We further examined whether the Na+-binding sites of hNTCP were also involved in HBV and HDV infection. Mutations were introduced to the sodium-binding sites reported previously or those predicted based on ASBTNM structure information (17). The mutants Q68A, S105A/N106A, E257A, and Q261A resulted in 95 to 99% reduction of [3H]taurocholate uptake (Fig. 6A). The reduced uptake was unlikely due to their negative effects on NTCP expression, as the levels of total and surface expression of these variants in HepG2 cells were largely comparable to that of the wild-type NTCP (Fig. 6B). As for the binding activities of these NTCP mutants to FITC–pre-S1 lipopeptide, the Q68A and E257A mutants markedly blocked pre-S1 binding, whereas the S105A/N106A or Q261A mutants affected binding with pre-S1 to a reduced level (Fig. 6C). We then tested HBV and HDV infection of HepG2 cells transfected with these NTCP variants. The viral infections by HDV, as indicated by intracellular delta antigen staining (Fig. 6D), and by HBV, as assessed by supernatant HBeAg levels (Fig. 6E), were also reduced; however, the reduction was much less than with the mutants responsible for bile acids binding, such as N262A and S267F (Fig. 5). These results indicated that, unlike the bile acids-binding site that is indispensable for viral infections, sodium-binding sites of NTCP contribute to, but may not directly involved in, pre-S1 binding or viral infection.
FIG 6.
NTCP mutants corresponding to the residues important for sodium binding inhibit viral infections by HDV and HBV. (A) [3H]taurocholate uptake efficiency of HepG2 cells complemented with NTCP variants. (B) Western blot analysis of total and surface expression levels of the NTCP variants in HepG2 cells. The expression level of GAPDH was used as an internal control. (C) FITC–pre-S1–binding efficiency in HepG2 cells expressing NTCP variants. (D and E) HDV and HBV infection efficiencies in HepG2 cells expressing the NTCP variants. The intracellular HDV delta antigen in infected cells was stained by 4G5 at 8 days p.i. (D). Secreted HBeAg in the culture medium at 3, 5, and 7 days p.i. was measured in an ELISA (E).
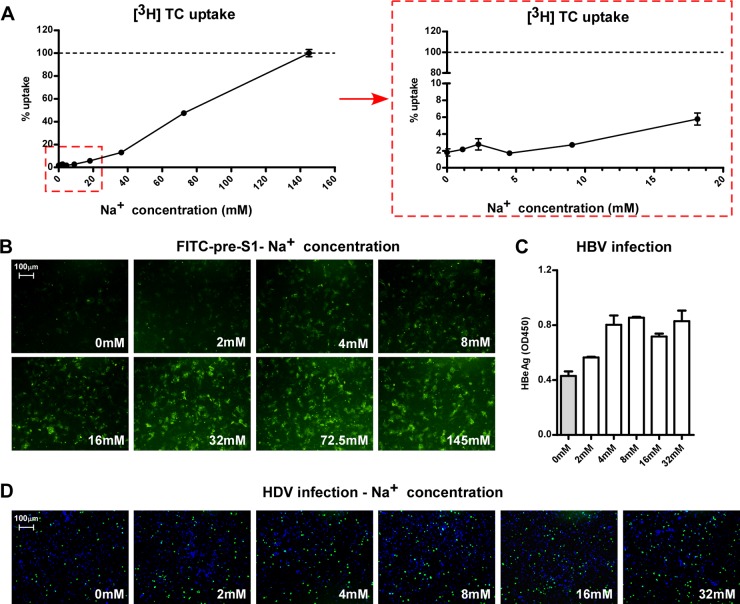
Effect of extracellular Na+ concentration on the binding of pre-S1 to NTCP and viral infection of NTCP-expressing HepG2 cells.
NTCP is a hepatic Na+-bile acid symporter. It utilizes an electrochemical gradient of sodium between the extracellular and intracellular compartments to cotransport one molecule of bile salt together with two sodium ions into hepatocytes (21). In mammals, extracellular fluid usually contains 145 mM Na+, while the concentration of intracellular Na+ is about 12 mM (22). As shown in Fig. 7A, the [3H]taurocholate uptake efficiency of NTCP correlated well with the sodium concentration in the testing solution. The transporting efficiency was less than 5% in 10 mM Na+ but increased in response to extracellular Na+ concentrations up to the normal level (Fig. 7A). In contrast, FITC–pre-S1 binding was readily detectable in solutions containing concentrations as low as 4 to 8 mM Na+; 32 mM Na+ was sufficient for achieving pre-S1 binding comparable to that observed with regular extracellular Na+ concentrations (Fig. 7B). These results showed that, different from the sodium concentration dependency for transporting monoanionic substrates, such as [3H]taurocholate, NTCP binding to HBV pre-S1 is largely independent on the sodium gradient between the extracellular and intracellular compartments. Hence, an extracellular Na+ concentration close to the intracellular level was sufficient for NTCP binding to FITC–pre-S1 lipopeptide. Similarly, incubating HepG2-NTCP cells with HBV and HDV in a solution containing a Na+ concentration close to the intracellular level resulted in viral infections comparable to those under regular conditions (Fig. 7. C and D), indicating that the inward flow of sodium current across cell membranes may not be required for the viral entry mediated by NTCP.
FIG 7.
Effects of extracellular Na+ concentration on the binding of pre-S1 to NTCP and viral infections. (A) Uptake of [3H]taurocholate in Ringer's solution containing increasing concentrations of Na+. The [3H]taurocholate uptake assay was conducted with HepG2-NTCP cells in Ringer's solution for 15 min (left). The level of uptake at 145 mM was set to 100%. (Right) Uptake efficiency in response to Na+ concentrations lower than 20 mM. (B) FITC–pre-S1 peptide-binding efficiency in different Na+ concentrations. HepG2 cells transfected with NTCP were incubated with 400 nM FITC–pre-S1 peptide in Ringer's solution containing different concentrations of Na+ for 10 min. Images were taken using a fluorescence microscope after extensive washing. (C and D) Na+ binding is required for efficient HBV and HDV infection. HBV and HDV were precipitated by 8% PEG 8000, resuspended in choline+ Ringer's solution, and then the Na+ concentration was adjusted as indicated. The viruses were then used to inoculate HepG2-NTCP cells for 6 h. HBV infection was assessed based on secreted HBeAg at 6 days p.i. in an ELISA (C). HDV infection was visualized by staining of the intracellular delta antigen at 8 days p.i. (D).
DISCUSSION
Both HBV and HDV primarily infect the liver, an organ central to metabolism, including maintenance of bile homeostasis in vivo. Bile salts, a major component of bile, are produced by hepatocytes. They aid in digestion via emulsification of lipids in the small intestine and also play other important physiological roles (23). The bile salts overall pool in humans is about 3 to 4 g and is mainly maintained by recycling of bile salts via enterohepatic circulation, which cycles 6 to 8 times between the liver and the small intestine per day. More than 90% of the bile salts in the intestine are absorbed and transported back to the liver, where hepatocytes take up the bile salts again. During this process, NTCP, as a hepatocyte sinusoidal membrane-localized transporter, plays a critical role and is responsible for the majority of sodium-dependent bile salts uptake (7). In light of this important physiological function of NTCP, we investigated whether viral infection by HBV and HDV affected the receptor's transporting function and vice versa.
We first examined whether HBV pre-S1 peptide binding to human NTCP affects its substrate transporting function via an [3H]taurocholate uptake assay. Our data showed that [3H]taurocholate uptake through NTCP could be specifically blocked by the myristoylated pre-S1 peptide of the L protein, but not its nonmyristoylated form or the myristoylated peptide with an asparagine-to-lysine mutation at residue 9 (N9K). Meanwhile, consistent with the myristoylated pre-S1 peptide not binding to crab-eating monkey NTCP, uptake of [3H]taurocholate by monkey NTCP was not impaired by the lipopeptide; however, a monkey NTCP variant that bore residues 157 to 165 from the human NTCP counterpart and supported the pre-S1 lipopeptide binding and viral infection could be effectively blocked by the peptide for [3H]taurocholate uptake. The binding of the pre-S1 domain with NTCP is a critical early entry step for HBV and HDV infection. Our data showed that pre-S1 lipopeptide binding blocked the physiological function of NTCP in bile salt transport; this raised an intriguing question regarding whether HBV and HDV infection through the receptor interferes with bile salts homeostasis and thereby plays a role in the pathogenesis of viral hepatitis and carcinogenesis in HBV/HDV-infected patients, in particular, those patients exhibiting high viral titers.
Conversely, we found that bile acids could block infection by HBV and HDV in susceptible cells, including HepG2-NTCP cells, primary treeshrew hepatocytes, and differentiated HepaRG cells. The inhibiting efficiency correlated well with their abilities to reduce pre-S1 lipopeptide binding to NTCP. The reduced viral infection in the presence of bile acids was not due to the amphiphilic nature of bile acids, nor to direct binding of bile acids with the pre-S1 region of the virus, because preincubation of the virus with most bile acids did not lead to a reduction in viral infectivity. A taurine conjugate form of ursodeoxycholic acid (TUDCA) was found to be a potent inhibitor for viral infections by HBV and HDV. Intriguingly, several clinical observations have indicated that long-term bile salt treatment, in particular that with UDCA, leads to improvement of liver function in hepatitis B patients (24, 25). In addition to bile acids and their derivatives, NTCP's substrates also include steroidal hormones, such as estrone-3-sulfate and dehydroepiandrosterone (DHEAS), bromosulphthalein, and some drugs, such as cyclosporine A, ketoconazole, irbesartan, and doxazosin (7). Although not all NTCP substrates inhibited viral infection by HBV or HDV, those with viral infection-inhibiting activities are potential candidates for further development into novel anti-HBV/HDV drugs by blocking viral entry.
As the binding of pre-S1 to NTCP and viral infections could be blocked by bile acids, we further examined if the key residues for substrate binding are involved in the interaction between NTCP and the HBV pre-S1 domain. To test this, we generated a set of human NTCP variants, into which mutations were introduced into residues previously indicated to be involved in the interaction with sodium ions or with bile salts (17–19). The transporting efficiencies of these variants were examined in a [3H]taurocholate uptake assay, and those variants that showed a significant defect in [3H]taurocholate uptake were further analyzed in pre-S1 peptide-binding and infection assays. The results showed that their pre-S1 lipopeptide-binding efficiencies and abilities in supporting viral infection were largely correlated with their bile salts uptake efficiency. NTCP mutants bearing amino acid changes at sites critical for bile acids binding, N262A and Q293A/L294A, radically lost both the bile salts uptake activity and the ability to support pre-S1 peptide binding and viral infection; NTCP mutants bearing amino acid changes at sites critical for sodium binding, Q68A and E257A, were nearly 100% deficient in transporting bile acids and supported pre-S1 peptide binding and viral infection with much lower efficiencies. These results suggest that residues important for both the sodium binding and the bile acid binding may involve an interaction between NTCP and the HBV or HDV envelope L protein. However, it appeared that the residues critical for bile acids binding are more involved in the interaction with the pre-S1 domain of the L protein. Of note, the S267F mutant of hNTCP, which is a missense mutation caused by an SNP of human NTCP that is found in about 9% of individuals from East Asia, was previously reported to result in a defect in transporting bile acids when the variant allele is expressed in HepG2 cells (15). Interestingly, we found in this study that this mutation also led to a loss of the ability to interact with the pre-S1 lipopeptide or to support viral infection by HBV and HDV in cells expressing this variant. However, HepG2 cells cotransfected with wild-type NTCP and the S267F variant at a 1:1 ratio supported HBV viral infection with an efficiency greater than 70%. Compared to that with HepG2 cells expressing wild-type NTCP, it is reasonable to speculate that individuals bearing a single allele but not homozygous for the NTCP S267F substitution are still susceptible to the viral infections. The reason that the NTCP S267F mutation occurs in 9% of the East Asian population is currently unknown; further investigations are needed to elucidate the influence of this NTCP SNP on HBV infection at a population level.
Without structural data for NTCP, it is difficult to determine how the binding of the pre-S1 domain to NTCP blocks bile acid uptake. Our results showing the mutual inhibition between pre-S1 binding and substrate binding/transport of NTCP along with the mutagenesis data suggest that bile salts and the pre-S1 lipopeptide may directly compete for the overlapping binding site(s) on NTCP. On the other hand, NTCP may be able to adopt different conformations depending on its posttranslational modification status, membrane localization, oligomerization, interactions with other cellular proteins or the pre-S1 lipopeptide, and/or the presence of its natural substrate in the environment. It is reasonable to speculate that the conformation of NTCP favorable for substrate binding and that for virus binding may mutually affect each other.
ACKNOWLEDGMENTS
We thank Liping Wei from NIBS/Peking University for her help on the analysis of human NTCP SNPs. H.Y. was supported by a postdoctoral fellowship from Merck Sharp & Dohme (MDS) R&D China.
This work was supported by the Ministry of Science and Technology, China (2010CB530101 and 2011CB812501), the National Science and Technology Major Project, China (2013ZX09509102), and the Science and Technology Bureau of the Beijing Municipal Government.
Footnotes
Published ahead of print 3 January 2014
REFERENCES
- 1.Ott JJ, Stevens GA, Groeger J, Wiersma ST. 2012. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine 30:2212–2219. 10.1016/j.vaccine.2011.12.116 [DOI] [PubMed] [Google Scholar]
- 2.Hughes SA, Wedemeyer H, Harrison PM. 2011. Hepatitis delta virus. Lancet 378:73–85. 10.1016/S0140-6736(10)61931-9 [DOI] [PubMed] [Google Scholar]
- 3.Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, Fu L, Song M, Chen P, Gao W, Ren B, Sun Y, Cai T, Feng X, Sui J, Li W. 2012. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife 1:e00049. 10.7554/eLife.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhong G, Yan H, Wang H, He W, Jing Z, Qi Y, Fu L, Gao Z, Huang Y, Xu G, Feng X, Sui J, Li W. 2013. Sodium taurocholate cotransporting polypeptide mediates woolly monkey hepatitis B virus infection of Tupaia hepatocytes. J. Virol. 87:7176–7184. 10.1128/JVI.03533-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan H, Peng B, He W, Zhong G, Qi Y, Ren B, Gao Z, Jing Z, Song M, Xu G, Sui J, Li W. 2013. Molecular determinants of hepatitis B and D virus entry restriction in mouse sodium taurocholate cotransporting polypeptide. J. Virol. 87:7977–7991. 10.1128/JVI.03540-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagenbuch B, Meier PJ. 1994. Molecular cloning, chromosomal localization, and functional characterization of a human liver Na+/bile acid cotransporter. J. Clin. Invest. 93:1326–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stieger B. 2011. The role of the sodium-taurocholate cotransporting polypeptide (NTCP) and of the bile salt export pump (BSEP) in physiology and pathophysiology of bile formation. Handb. Exp. Pharmacol. 2011(201):205–259. 10.1007/978-3-642-14541-4_5 [DOI] [PubMed] [Google Scholar]
- 8.Kramer W, Stengelin S, Baringhaus KH, Enhsen A, Heuer H, Becker W, Corsiero D, Girbig F, Noll R, Weyland C. 1999. Substrate specificity of the ileal and the hepatic Na(+)/bile acid cotransporters of the rabbit. I. Transport studies with membrane vesicles and cell lines expressing the cloned transporters. J. Lipid Res. 40:1604–1617 [PubMed] [Google Scholar]
- 9.Kullak-Ublick GA, Stieger B, Meier PJ. 2004. Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology 126:322–342. 10.1053/j.gastro.2003.06.005 [DOI] [PubMed] [Google Scholar]
- 10.Seeger C, Mason WS. 2000. Hepatitis B virus biology. Microbiol. Mol. Biol. Rev. 64:51–68. 10.1128/MMBR.64.1.51-68.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nassal M. 1996. Hepatitis B virus morphogenesis. Curr. Top. Microbiol. Immunol. 214:297–337 [DOI] [PubMed] [Google Scholar]
- 12.Schulze A, Gripon P, Urban S. 2007. Hepatitis B virus infection initiates with a large surface protein-dependent binding to heparan sulfate proteoglycans. Hepatology 46:1759–1768. 10.1002/hep.21896 [DOI] [PubMed] [Google Scholar]
- 13.Leistner CM, Gruen-Bernhard S, Glebe D. 2008. Role of glycosaminoglycans for binding and infection of hepatitis B virus. Cell Microbiol. 10:122–133. 10.1111/j.1462-5822.2007.01023.x [DOI] [PubMed] [Google Scholar]
- 14.Sureau C, Salisse J. 2013. A conformational heparan sulfate binding site essential to infectivity overlaps with the conserved hepatitis B virus a-determinant. Hepatology 57:985–994. 10.1002/hep.26125 [DOI] [PubMed] [Google Scholar]
- 15.Ho RH, Leake BF, Roberts RL, Lee W, Kim RB. 2004. Ethnicity-dependent polymorphism in Na+-taurocholate cotransporting polypeptide (SLC10A1) reveals a domain critical for bile acid substrate recognition. J. Biol. Chem. 279:7213–7222. 10.1074/jbc.M305782200 [DOI] [PubMed] [Google Scholar]
- 16.Glebe D, Urban S, Knoop EV, Cag N, Krass P, Grun S, Bulavaite A, Sasnauskas K, Gerlich WH. 2005. Mapping of the hepatitis B virus attachment site by use of infection-inhibiting preS1 lipopeptides and tupaia hepatocytes. Gastroenterology 129:234–245. 10.1053/j.gastro.2005.03.090 [DOI] [PubMed] [Google Scholar]
- 17.Hu NJ, Iwata S, Cameron AD, Drew D. 2011. Crystal structure of a bacterial homologue of the bile acid sodium symporter ASBT. Nature 478:408–411. 10.1038/nature10450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun AQ, Balasubramaniyan N, Chen H, Shahid M, Suchy FJ. 2006. Identification of functionally relevant residues of the rat ileal apical sodium-dependent bile acid cotransporter. J. Biol. Chem. 281:16410–16418. 10.1074/jbc.M600034200 [DOI] [PubMed] [Google Scholar]
- 19.Zahner D, Eckhardt U, Petzinger E. 2003. Transport of taurocholate by mutants of negatively charged amino acids, cysteines, and threonines of the rat liver sodium-dependent taurocholate cotransporting polypeptide Ntcp. Eur. J. Biochem. 270:1117–1127. 10.1046/j.1432-1033.2003.03463.x [DOI] [PubMed] [Google Scholar]
- 20.Pan W, Song IS, Shin HJ, Kim MH, Choi YL, Lim SJ, Kim WY, Lee SS, Shin JG. 2011. Genetic polymorphisms in Na+-taurocholate co-transporting polypeptide (NTCP) and ileal apical sodium-dependent bile acid transporter (ASBT) and ethnic comparisons of functional variants of NTCP among Asian populations. Xenobiotica 41:501–510. 10.3109/00498254.2011.555567 [DOI] [PubMed] [Google Scholar]
- 21.Hagenbuch B, Meier PJ. 1996. Sinusoidal (basolateral) bile salt uptake systems of hepatocytes. Semin. Liver Dis. 16:129–136 [DOI] [PubMed] [Google Scholar]
- 22.Nelson D, Cox M. 2000. Lehninger principles of biochemistry, 3rd ed, p 389–436 Worth Publishers, New York, NY [Google Scholar]
- 23.Esteller A. 2008. Physiology of bile secretion. World J. Gastroenterol. 14:5641–5649. 10.3748/wjg.14.5641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen W, Liu J, Gluud C. 2003. Bile acids for viral hepatitis. Cochrane Database Syst. Rev. 2003(2):CD003181. 10.1002/14651858.CD003181 [DOI] [PubMed] [Google Scholar]
- 25.Galsky J, Bansky G, Holubova T, Konig J. 1999. Effect of ursodeoxycholic acid in acute viral hepatitis. J. Clin. Gastroenterol. 28:249–253 [DOI] [PubMed] [Google Scholar]