Abstract
During a survey in the year 2010, a novel phlebovirus was isolated from the Rousettus leschenaultii species of bats in western India. The virus was identified by electron microscopy from infected Vero E6 cells. Phylogenic analysis of the complete genome showed its close relation to severe fever with thrombocytopenia syndrome (SFTS) and Heartland viruses, which makes it imperative to further study its natural ecology and potential as a novel emerging zoonotic virus.
TEXT
Global surveillance for emerging infectious diseases has led to the isolation of numerous viruses from a variety of bat species, which include pathogenic and nonpathogenic zoonotic and arboviruses (1–4). A large number of zoonotic viruses, such as rabies virus, Mapueravirus, Menangle and Tioman viruses, filoviruses, Lake Victoria Marburg virus (MARV), and Ebola virus (EBOV), have been isolated from bats (3, 4). Several other novel viruses, like Catu virus, Guama virus, Nepuyo virus, Mojui dos Campos virus (genus Orthobunyavirus), KaengKhoi virus (genus Phlebovirus), and Bangui virus (ungrouped), have also been isolated from wild caught bats (5).
After the initial confirmation of Nipah virus during an encephalitis outbreak with high case fatality that occurred in Siliguri, in northeastern India (6), surveillance for zoonotic viruses was initiated in India by the National Institute of Virology (NIV), Pune. In this communication, we report the isolation and genetic characterization of a novel phlebovirus, Malsoor virus (named after Malsoor village), isolated from Rousettus species of bats from Mahabaleshwar, Maharashtra State, India.
Geographically, the study area (lat 17.9217, long 73.6556) was in the mountain terrain of the Western Ghats that run north-south along the western coast of India and have an average elevation of 1,353 meters. The bats were harvested and necropsies were carried out in field laboratories under appropriate biosafety conditions. Liver and spleen tissues of bats were homogenized together and used for virus isolation in Vero E6 cells as described earlier (7, 8). Cytopathic effect (CPE) was observed in inoculated cells on the 4th day of the 3rd passage. Typically, CPE included rounding of cells, shrinking, and progressive cell detachment (Fig. 1). Cytopathic effect was seen in material from two different bats, suggesting the possibility of two virus isolates, which were designated NIV 1050639 and NIV 1050650.
FIG 1.
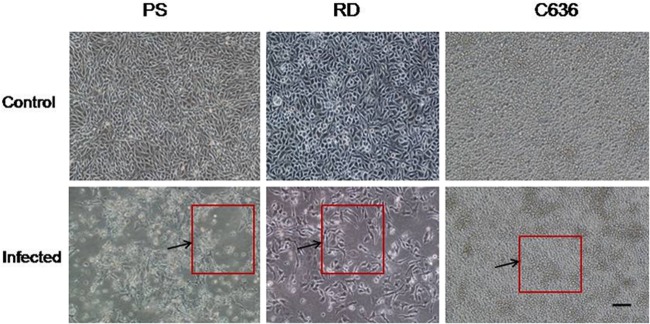
Susceptibility of different cell lines to Malsoor virus based on cytopathic effect (CPE) with 10× magnification. Cells were infected at a multiplicity of infection of 10. The areas of CPE were seen as microfoci of clearing in PS cells showing cellular changes (lower left panel) and distinct rounding of RD cells showing cellular changes on the 3rd p.i.d. (lower middle panel). There was no cytopathic effect in C6/36 cells on the 4th p.i.d. (lower right panel). The cultures were monitored for CPE until the 7th p.i.d. The arrows and the boxes outlined in red indicate areas of CPE. The magnification bar for all photomicrographs represents 1 μm.
Cell culture supernatants of Vero E6-infected cells showing CPE were examined by negative-staining transmission electron microscopy (TEM). A morphologically typical bunyavirus showing mostly enveloped spherical virions having an average size distribution of 75 ± 5 nm (Fig. 2) could be observed in both isolates. The envelope projections were double fringed and consistent with the morphology of the family Bunyaviridae, as described earlier (9).
FIG 2.
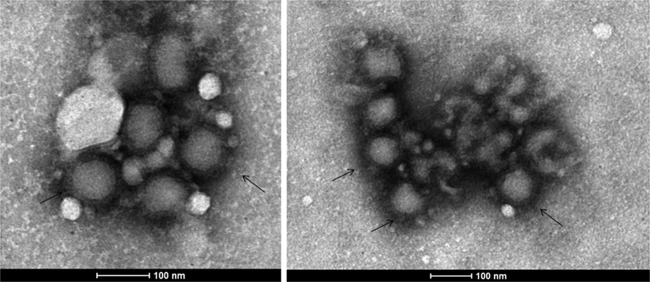
Representative transmission electron micrographs showing a typical negative stain showing bunyavirus particles in Vero CCL81 cells at the 4th p.i.d.. Scale bars are integrated into the micrograph. The arrows indicate representative bunyavirus particles showing distinct envelopes.
Based on electron microscopy findings, genus-specific reverse transcriptase PCRs (RT-PCRs) were carried out as described earlier for Orthobunyavirus, Phlebovirus, Hantavirus, and Nairovirus among the family Bunyaviridae (10–13). Phlebovirus-specific RT-PCR amplified the partial S and L segments. Amplification of complete segments was carried out using genus-specific conserved primers as described earlier and newly designed primers derived from Rift Valley fever virus (RVFV) for amplification of the S gene (SVFS-A Fwd, 5′-ACA CAA AGC TCC CTA G-3′, and SVFS-A Rev, 5′-ACA CAA AGA CCC CCT AG-3′), L segment (SVFL-A Fwd, 5′-ACA CAA AGG CGC CCA ATC-3′, and SVFL-A Rev, 5′-ACA CAA AGA CCG CCC A-3′), and M segment (SVMF, 5′-ACA CAA AGA CGG TGC-3′, and SVFMR, 5′-ACA CAA AGA CCG GTG C-3′) (14, 15). Initial sequencing of the L, M, and S genes was performed using PCR primers. Further sequences obtained were used to design additional primers, and published primers for severe fever with thrombocytopenia syndrome (SFTS) and Heartland viruses were also used to cover the complete genome (16, 17). Cyclic sequencing, purification, and sequencing of products were carried out at as defined earlier, using an ABI 3100 genetic analyzer (7, 8).
The nucleotide sequences obtained were edited and converted into amino acids for determining the open reading frames (ORFs) and molecular weights of the putative proteins (Kodon Software, USA). Genus-specific primers amplified 3,460 nucleotides of M segment, 6,408 nucleotides of L segment, and 1,785 nucleotides of S segment of both phleboviruses. The L genome ORF codes for the RNA polymerase with 2,026 amino acids (aa). The M segment codes for the glycoprotein with 1,075 aa. The 1,785 nucleotides of the small segment, the S segment, contained two open reading frames in opposing orientations and coded for 243 aa of NP and 297 aa of NSs.
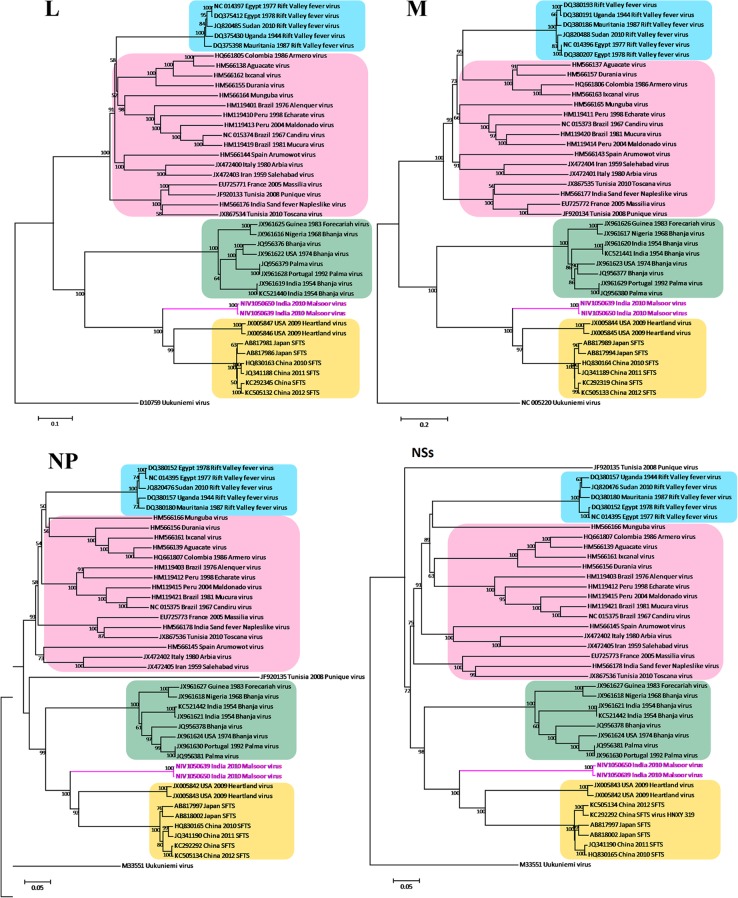
Phylogenetic analysis of the three segments of the two genomes of the new isolates was performed with 975 phlebovirus sequences available in GenBank as of August 25, 2013 (Fig. 3). Coding regions of genes were aligned using MEGA version 5.2, and phylogenetic trees were constructed using the neighbor-joining (NJ) algorithm. Distances were calculated using a p-distance model with 1,000 bootstrap replicates (18). The percent nucleotide identity (PNI) and percent amino acid identity (PAI) values were calculated as pairwise p-distances.
FIG 3.
Phylogenetic analysis of N, NSs, M, and L segments of Malsoor virus. The phleboviruses and GenBank sequences used for phylogentic analysis of M, L, and S segments are as follows: Aguacate virus (HM566137, HM566138, HM566139), Alenquer virus (HM119402, HM119401, HM119403), Arbia virus (JX472401, JX472400, JX472402), Armero virus (HQ661806, HQ661805, HQ661807), Arumowot virus (HM566143, HM566144, HM566145), Bhanja virus (JQ956377, JQ956376, JQ956378), Bhanja virus (JX961617, JX961616, JX961618), Bhanja virus (JX961620, JX961619, JX961621), Bhanja virus (JX961623, JX961622, JX961624), Bhanja virus (KC521441, KC521440, KC521442), Candiru virus (NC_015373, NC_015374, NC_015375), Durania virus (HM566157, HM566155, HM566156), Echarate virus (HM119411, HM119410, HM119412), Forecariah virus (JX961626, JX961625, JX961627), Heartland virus (JX005844, JX005846, JX005842), Heartland virus (JX005845, JX005847, JX005843), Ixcanal virus (HM566163, HM566162, HM566161), Maldonado virus (HM119414, HM119413, HM119415), Massilia virus (EU725772, EU725771, EU725773), Mucura virus (HM119420, HM119419, HM119421), Munguba virus (HM566165, HM566164, HM566166), Palma virus (JQ956380, JQ956379, JQ956381), Palma virus (JX961629, JX961628, JX961630), phlebovirus JS24 (HQ830164, HQ830163, HQ830165), phlebovirus WCH/97/HN/China/2011 (JQ341189, JQ341188, JQ341190), Punique virus (JF920134, JF920133, JF920135), Rift Valley fever virus (RVFV) (DQ380186, DQ375398, DQ380180), RVFV (DQ380207, DQ375412, DQ380152), RVFV (DQ380193, DQ375430, DQ380157), RVFV (JQ820488, JQ820485, JQ820476), RVFV (NC_014396, NC_014397, NC_014395), Salehabad virus (JX472404, JX472403, JX472405), sand fever Naples-like virus (HM566177, HM566176, HM566178), severe fever with thrombocytopenia syndrome virus (SFTSV) (AB817989, AB817981, AB817997), SFTSV (AB817994, AB817986, AB818002), SFTSV (KC505133, KC505132, KC505134), SFTSV HNXY 319 (KC292319, KC292345, KC292292), Toscana virus (JX867535, JX867534, JX867536), and outgroup Uukuniemi virus (M33551, M17417, D10759).
We then studied the susceptibility of different cell types from mice and Aedes mosquitoes to the Malsoor virus isolates. Several mammalian cell lines, baby hamster kidney (BHK-21), rhabdomyosarcoma (RD), porcine stable kidney (PS), Vero CCL81 clone, and Pipistrellus bat, maintained at 37°C and one mosquito cell line (C6/36) maintained at 28°C were infected with virus at a multiplicity of infection of 10. The cultures were monitored for CPE until the 7th postinfection day (p.i.d.). The nature of CPE in the Vero CCL81 cells appeared by the 4th p.i.d. as rounded and floating cells, and the cell sheet degenerated by the 6th p.i.d. The PS, RD, and BHK-21 cells also showed a similar CPE by the 4th p.i.d. (Fig. 1; see also Fig. S1 in the supplemental material). Virus growth was detected by an indirect immune fluorescence assay (IFA) using antisera against the virus raised in mice. All the cell lines studied except C6/36 and Pipistrellus bat cells showed evidence of virus susceptibility. RT-PCR was also performed on these infected cells (see Fig. S2, panels A and B, in the supplemental material) using the phlebovirus-specific RT-PCR primers Phlebopoly 1292F (5′-GTG CTG CTG CCA ATA TGC TA-3′) and Phlebopoly 3243R (5′-ATC CCA GTT TCT GTT TGG ATG TAT-3′), which were designed based on the L gene for detecting replication of Malsoor virus. PCR positivity with amplification of 2.0 kb was observed in all the cells except C6/36 and Pipistrellus bat cells. The Vero CCL81 cell line infected with the Malsoor virus had a titer of approximately 105.5 50% tissue culture infective doses (TCID50)/ml on the 4th p.i.d.
The three mouse groups (0 to 1 day old) were either inoculated with one of the virus isolates (two groups) or left uninoculated (control group) and were observed for 15 days for sickness and mortality. No mortality was observed in any of the groups. Antisera against Malsoor virus was raised in mice as described earlier (19).
The susceptibility of female Aedes aegypti mosquitoes (laboratory-reared colony in the NIV, Pune) to Malsoor virus was studied using an earlier-described method (20, 21). No positivity was recorded in mosquitoes infected with virus.
Phlebovirus amplicons were sequenced, and BLAST results suggested the highest homology with known sequences of phleboviruses close to Heartland and SFTS viruses. Further, the nucleotide and deduced amino acid sequences of the S, M, and L segments of Malsoor virus were compared with the corresponding genes of previously identified phleboviruses and phylogenetic analysis showed grouping of Malsoor virus with SFTS, Bhanja, and Palma viruses. Importantly, it showed closer clustering with Heartland virus and with SFTS group viruses of Japanese and Chinese origins (Fig. 3).
In the past, a number of bunyaviruses have been isolated from India. While some of these viruses, like Bhanja virus, Batai/Chittoor virus, Nairobi sheep disease virus (NSDV)/Ganjam virus, Sathuperi virus, sandfly fever Naples and Sicilian viruses, Palma virus, Ingwavuma virus, and Thottapalayam virus, have shown serosignatures in humans and animals (22–25), the role of many of these viruses in causing human diseases remains incompletely understood. Importantly, human infections with Ganjam virus have been reported in laboratory workers involved in the isolation of this virus (26). Recently, activity of the pathogenic Crimean-Congo hemorrhagic fever virus (CCHFV), a risk group 4 agent responsible for human infections with high mortality, has been documented from western India (27). Another highly pathogenic agent, Nipah virus, a member of the genus Henipavirus, family Paramyxoviridae, was detected from Pteropus bats in India, Cambodia, and Thailand (8, 28, 29). Subsequently, China, Bangladesh, Malaysia, and India have witnessed many outbreaks of Nipah virus-associated encephalitis (6, 30, 31). Moreover, the demographic factors affecting a large population movement from Bangladesh, which has a high level of activity of Nipah virus in areas bordering India, brings into focus the urgent need to initiate studies on viruses present in wild bats.
Here, we describe a novel phlebovirus, named Malsoor virus after Malsoor village, located in a region where the bats were collected. Importantly, this is the first report of the isolation of a phlebovirus from Rousettus bat species. Although this novel virus was seen to infect several different mammalian cell types, experimental studies with ticks would be important, as its close “cousins,” the SFTS and Heartland viruses, are both tick borne. Moreover, this study also rationalizes the importance of coordinated application of virology techniques like cell culture isolation and electron microscopy in identifying novel viruses, consistent with earlier reports (32).
The importance of this finding is further highlighted by the close genetic relatedness of Malsoor virus with SFTS and Heartland viruses (16, 17), both of which have caused severe human diseases elsewhere, which makes it imperative to further study the host distribution, possible human exposure, and detailed ecobiology of this novel phlebovirus in India.
Nucleotide sequence accession numbers.
The GenBank accession numbers assigned to the NIV 1050639 and NIV 1050650 strains are KF186494 to KF186496 and KF186497 to KF186499 for the S, M, and L segments.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Principal Chief Conservator of forests, Maharashtra, for kind permission to collect samples from bats in Mahabaleshwar, Maharashtra, India, and Rajen Lakra and Uttam K. Shende for laboratory work. We are grateful to Stuart Nichol, Jon Towner, and Brian Amman, Viral Special Pathogens Branch, Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, GA, USA, for training provided for capturing the bats and processing of samples for the survey of bats. We also acknowledge S. V. Gopale, G. K. Chopade, and M. Holeppanavar for their assistance in the field.
Footnotes
Published ahead of print 3 January 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.02617-13.
REFERENCES
- 1.Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T. 2006. Bats: important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 19:531–545. 10.1128/CMR.00017-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halpin K, Hyatt AD, Plowright RK, Epstein JH, Daszak P, Field HE, Wang L, Daniels PW. 2007. Emerging viruses: coming in on a wrinkled wing and a prayer. Henipavirus Ecology Research Group. Clin. Infect. Dis. 44:711–717. 10.1086/511078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pavri KM, Singh KR, Hollinger FB. 1971. Isolation of a new parainfluenza virus from a frugivorous bat, Rousettus leschenaulti, collected at Poona, India. Am. J. Trop. Med. Hyg. 20:125–130 [DOI] [PubMed] [Google Scholar]
- 4.Mackenzie JS, Field HE, Guyatt KJ. 2003. Managing emerging diseases borne by fruit bats (flying foxes), with particular reference to henipaviruses and Australian bat lyssavirus. J. Appl. Microbiol. 94:59S–69S. 10.1046/j.1365-2672.94.s1.7.x [DOI] [PubMed] [Google Scholar]
- 5.Newman SH, Field H, Epstein J, de Jong C. (ed). 2011. Investigating the role of bats in emerging zoonoses: balancing ecology, conservation and public health interest. FAO animal production and health manual no. 12. Food and Agriculture Organization of the United Nations, Rome, Italy: http://www.fao.org/docrep/014/i2407e/i2407e00.pdf [Google Scholar]
- 6.Chadha MS, Comer JA, Lowe L, Rota PA, Rollin PE, Bellini WJ. 2006. Nipah virus-associated encephalitis outbreak, Siliguri, India. Emerg. Infect. Dis. 12:235–240. 10.3201/eid1202.051247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raut CG, Yadav PD, Towner JS, Amman BR, Erickson BR, Cannon DL, Sivaram A, Basu A, Nichol ST, Mishra AC, Mourya DT. 2012. Isolation of a novel adenovirus from Rousettus leschenaultii bats from India. Intervirology 55:488–490. 10.1159/000337026 [DOI] [PubMed] [Google Scholar]
- 8.Yadav PD, Raut CG, Shete A, Mishra AC, Towner JS, Nichol ST, Mourya DT. 2012. Detection of Nipah virus RNA in fruit bat (Pteropus giganteus) from India. Am. J. Trop. Med. Hyg. 87:576–578. 10.4269/ajtmh.2012.11-0416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madeley CR, Fields AN. 1989. Bunyamwera virus, p 207 In Virus morphology, 2nd ed. Churchill Livingstone, London, United Kingdom [Google Scholar]
- 10.Lambert AJ, Lanciotti RS. 2008. Molecular characterization of medically important viruses of the genus Orthobunyavirus. J. Gen. Virol. 89:2580–2585. 10.1099/vir.0.2008/002253-0 [DOI] [PubMed] [Google Scholar]
- 11.Puthavathana P, Lee HW, Kang CY. 1992. Typing of hantaviruses from five continents by polymerase chain reaction. Virus Res. 26:1–14. 10.1016/0168-1702(92)90142-V [DOI] [PubMed] [Google Scholar]
- 12.Lambert AJ, Lanciotti RS. 2009. Consensus amplification and novel multiplex sequencing method for S segment species identification of 47 viruses of the Orthobunyavirus, Phlebovirus, and Nairovirus genera of the family Bunyaviridae. J. Clin. Microbiol. 47:2398–2404. 10.1128/JCM.00182-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu F, Chen H, Travassos da Rosa AP, Tesh RB, Xiao SY. 2007. Phylogenetic relationships among sandfly fever group viruses (Phlebovirus: Bunyaviridae) based on the small genome segment. J. Gen. Virol. 88:2312–2319. 10.1099/vir.0.82860-0 [DOI] [PubMed] [Google Scholar]
- 14.Charrel RN, Moureau G, Temmam S, Izri A, Marty P, Parola P, da Rosa AT, Tesh RB, de Lamballerie X. 2009. Massilia virus, a novel Phlebovirus (Bunyaviridae) isolated from sandflies in the Mediterranean. Vector Borne Zoonotic Dis. 9:519–530. 10.1089/vbz.2008.0131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bird BH, Khristova ML, Rollin PE, Ksiazek TG, Nichol ST. 2007. Complete genome analysis of 33 ecologically and biologically diverse Rift Valley fever virus strains reveals widespread virus movement and low genetic diversity due to recent common ancestry. J. Virol. 81:2805–2816. 10.1128/JVI.02095-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu B, Liu L, Huang X, Ma H, Zhang Y. 2011. Metagenomic analysis of fever, thrombocytopenia and leukopenia syndrome (FTLS) in Henan Province, China: discovery of a new bunyavirus. PLoS Pathog. 7:e1002369. 10.1371/journal.ppat.1002369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMullan LK, Folk SM, Kelly AJ, MacNeil A, Goldsmith CS, Metcalfe MG, Batten BC, Albarino CG, Zaki SR, Rollin PE, Nicholson WL, Nichol ST. 2012. A new phlebovirus associated with severe febrile illness in Missouri. N. Engl. J. Med. 367:834–841. 10.1056/NEJMoa1203378 [DOI] [PubMed] [Google Scholar]
- 18.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ilkal MA, Dhanda V, Rodrigues JJ, Mohan Rao CV, Mourya DT. 1984. Xenodiagnosis of laboratory acquired dengue infection by mosquito inoculation & immunofluorescence. Indian J. Med. Res. 79:587–590 [PubMed] [Google Scholar]
- 20.Padbidri VS, Mourya DT, Dhanda V. 1982. Multiplication of Coxiella burnetii in Aedes aegypti. Indian J. Med. Res. 76:185–189 [PubMed] [Google Scholar]
- 21.Sivaram A, Barde P, Gokhale M, Singh DK, Mourya DT. 2010. Evidence of co-infection of chikungunya and densonucleosis viruses in C6/36 cell lines and laboratory infected Aedes aegypti (L.) mosquitoes. Parasit. Vectors 3:95. 10.1186/1756-3305-3-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wanzeller A, Diniz J, Gomes M, Cruz A, Soares M, De Souza W, Da Rosa A, Vasconcelos P. 2002. Ultrastructural, antigenic and physicochemical characterization of the Mojuí dos Campos (Bunyavirus) isolated from bat in the Brazilian Amazon region. Mem. Inst. Oswaldo Cruz (Rio de Janeiro) 97:307–311. 10.1590/S0074-02762002000300005 [DOI] [PubMed] [Google Scholar]
- 23.Shah KV, Work TH. 1969. Bhanja virus: a new arbovirus from ticks Haemaphysalis intermedia Warburton and Nuttall, 1909, in Orissa, India. Indian J. Med. Res. 57:793–798 [PubMed] [Google Scholar]
- 24.Dilcher M, Alves MJ, Finkeisen D, Hufert F, Weidmann M. 2012. Genetic characterization of Bhanja virus and Palma virus, two tick-borne phleboviruses. Virus Genes 45:311–315. 10.1007/s11262-012-0785-y [DOI] [PubMed] [Google Scholar]
- 25.Yadav PD, Sudeep AB, Mishra AC, Mourya DT. 2012. Molecular characterization of Chittoor (Batai) virus isolates from India. Indian J. Med. Res. 136:792–798 [PMC free article] [PubMed] [Google Scholar]
- 26.Yadav PD, Vincent MJ, Khristova M, Kale C, Nichol ST, Mishra AC, Mourya DT. 2011. Genomic analysis reveals Nairobi sheep disease virus to be highly diverse and present in both Africa, and in India in the form of the Ganjam virus variant. Infect. Genet. Evol. 11:1111–1120. 10.1016/j.meegid.2011.04.001 [DOI] [PubMed] [Google Scholar]
- 27.Yadav PD, Raut CG, Patil DY, Majumdar TD, Mourya DT. 26 June 2013. Crimean-Congo hemorrhagic fever: current scenario in India. Proc. Natl. Acad. Sci. India B Biol. Sci. 10.1007/s40011-013-0197-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reynes JM, Counor D, Ong S, Faure C, Seng V, Molia S, Walston J, Georges-Courbot MC, Deubel V, Sarthou JL. 2005. Nipah virus in Lyle's flying foxes, Cambodia. Emerg. Infect. Dis. 11:1042–1047. 10.3201/eid1107.041350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wacharapluesadee S, Lumlertdacha B, Boongird K, Wanghongsa S, Chanhome L, Rollin P, Stockton P, Rupprecht CE, Ksiazek TG, Hemachudha T. 2005. Bat Nipah virus, Thailand. Emerg. Infect. Dis. 11:1949–1951. 10.3201/eid1112.050613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu VP, Hossain MJ, Parashar UD, Ali MM, Ksiazek TG, Kuzmin I, Niezgoda M, Rupprecht C, Bresee J, Breiman RF. 2004. Nipah virus encephalitis reemergence, Bangladesh. Emerg. Infect. Dis. 10:2082–2087. 10.3201/eid1012.040701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chua KB. 2003. Nipah virus outbreak in Malaysia. J. Clin. Virol. 26:265–275. 10.1016/S1386-6532(02)00268-8 [DOI] [PubMed] [Google Scholar]
- 32.Goldsmith CS, Ksiazek TG, Rollin PE, Comer JA. 2013. Cell culture and electron microscopy for identifying viruses in diseases of unknown cause. Emerg. Infect. Dis. 19:886–891. 10.3201/eid1906.130173 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.