ABSTRACT
Seasonal influenza causes substantial morbidity and mortality because of efficient human-to-human spread. Rarely, zoonotic strains of influenza virus spread to humans, where they have the potential to mediate new pandemics with high mortality. We studied systemic viral spread after intranasal infection with highly pathogenic avian influenza virus (H5N1 [A/Viet Nam/1203/2004]) in ferrets with or without prior pandemic H1N1pdm09 (A/Mexico/4108/2009) or H3N2 (A/Victoria/361/2011) infection. After intranasal challenge with H5N1 influenza virus, naive ferrets rapidly succumbed to systemic infection. Animals challenged with H5N1 influenza virus greater than 3 months after recovering from an initial H1N1pdm09 infection survived H5N1 virus challenge and cleared virus from the respiratory tract 4 days after infection. However, a prolonged low-level infection of hematopoietic elements in the small bowel lamina propria, liver, and spleen was present for greater than 2 weeks postinfection, raising the potential for reassortment of influenza genes in a host infected with multiple strains of influenza. Animals previously infected with an H3N2 influenza virus succumbed to systemic disease and encephalitis after H5N1 virus challenge. These results indicate prior infection with different seasonal influenza strains leads to radically different protection from H5N1 challenge and fatal encephalitis.
IMPORTANCE Seasonal influenza is efficiently transmitted from human to human, causing substantial morbidity and mortality. Rarely, zoonotic strains of influenza virus spread to humans, where they have the potential to mediate new pandemics with high mortality. Infection of naive ferrets with H5N1 avian influenza virus causes a rapid and lethal systemic disease. We studied systemic H5N1 viral spread after infection of ferrets with or without prior exposure to either of two seasonal influenza virus strains, H1N1 and H3N2. Ferrets previously infected with H1N1 survive H5N1 challenge while those previously infected with H3N2 die of encephalitis. However ferrets protected from lethal H5N1 infection develop persistent low-level infection of the small intestine, liver, or spleen, providing a nidus for future viral strain recombination. The mechanism by which prior infection with specific strains of seasonal influenza virus protect from lethal H5N1 challenge needs to be elucidated in order to design effective immunization and treatments.
INTRODUCTION
Seasonal influenza virus infection causes mild to moderate disease symptoms in humans, but periodically, pandemic influenza viruses emerge after reassortment with zoonotic sources, causing catastrophic disease. Over the past century, four influenza pandemics have been recorded following direct adaptation of avian viruses or after reassortment of various combinations of influenza virus genes originating from human, swine, or fowl (1; reviewed in references 2–7). In 1997, there was an outbreak in humans of a novel influenza A virus subtype, H5N1 (A/HK/97), in Southeast Asia (1, 8, 9). Although H5N1 influenza viruses have infected only a few individuals with severely limited human-to-human spread (10, 11), confirmed H5N1 influenza virus infections have been highly lethal, with a mortality rate of approximately 60% (12). Human H5N1 outbreaks have sporadically arisen over the past 15 years, with approximately 600 documented human infections to date (12). H5N1 influenza viruses that have emerged since 2003 contain single-amino-acid substitutions in the NS1 protein and are even more virulent than the original 1997 strain (10, 13).
In avian populations, H5N1 influenza virus infection causes a broad spectrum of disease (reviewed in references 14 and 15). In ground fowl (Galliformes), an initial pneumonic infection with highly pathogenic strains is accompanied by widespread systemic infection (including lymph nodes, spleen, liver, myocardium, fat, pancreas, and brain). In waterfowl (Anseriformes), infection with highly pathogenic strains is more limited and is focused on the respiratory tract and gastrointestinal system.
Infection with highly pathogenic H5N1 can be lethal for mammals, but the outcome of infection depends upon many host and viral factors. Aerosol, intranasal, intratracheal, and intragastric routes are all effective means of virus inoculation (16–19). However, lung disease can vary dramatically depending upon the route of delivery and host species (severe disease is observed in mice [20, 21], while nonhuman primates exhibit milder disease [22, 23]). For many reasons, ferrets are the animal model of choice to study influenza virus infection in humans (24, 25). Ferrets are relatively inexpensive to maintain, have a similar distribution of sialic acid receptor linkages (26) conferring similar patterns of viral attachment in the respiratory tract (27), are naturally infected with human influenza virus, and develop similar clinical and pathological disease.
Since over the course of their lives humans are infected with many different strains of influenza virus, it would be expected that heterosubtypic immunity would modulate the response to infection with emergent strains. Bodewes et al. (28) recently studied the role of heterosubtypic immunity in modulating H5N1 virus infections. Infection with the H3N2 influenza virus (A/Brisbane/10/2007) 1 month prior to H5N1 influenza (A/Ind/5/2005) challenge protected ferrets from lethal disease. This protection correlated with T cell proliferation after exposure to whole inactivated influenza A/Ind/5/2005 virus antigen. However, vaccination with an H3N2 subunit vaccine was not sufficient to induce protective heterosubtypic immunity.
To model the human condition where H5N1 virus infection most likely occurs months to years after seasonal influenza virus infection, we infected ferrets with either a 2009 pandemic H1N1 (H1N1pdm09) or H3N2 influenza virus isolate and allowed the ferrets to recover. Three to five months later, these same ferrets were challenged with an H5N1 influenza virus. Prior infection with H1N1pdm09 influenza virus protected ferrets from H5N1 challenge; however, this protection was not in the form of sterilizing immunity. H5N1 virus was detected in the spleen, small bowel, and other organs for greater than 2 weeks. Prior infection with H3N2 influenza virus did not confer the same heterosubtypic immunity, and all animals died within 6 days of H5N1 virus challenge. Heterosubtypic immunity plays an important role in modulating susceptibility to acute H5N1 influenza virus-induced disease.
MATERIALS AND METHODS
Animals and infections.
Female influenza virus-naive Fitch ferrets (Mustela putorius furo, 6 to 12 months of age) were purchased from Marshall Farms and determined to be negative for antibody to circulating influenza A (H1N1 and H3N2) and B viruses. Ferrets were healthy but exhibited low-grade chronic periportal hepatitis. Lymphocytic hepatitis is common in ferrets but is underdiagnosed because it is usually subclinical (29). While some investigators believe that mild periportal lymphocytic infiltrates are normal in ferrets (30), it can be associated with coronavirus infection (29). Ferrets were pair housed and provided with Teklad Global ferret diet (Harlan Teklad, Madison, WI) and fresh water ad libitum. Ferrets were first infected intranasally with either H1N1pdm09 virus A/Mexico/4108/2009 (Mex/09) or A/California/07/2009 (CA/09) or with H3N2 virus A/Victoria/361/2011 (Vic/11) or A/Perth/16/2009 (1 × 106 PFU) and allowed to recover and seroconvert. Animals were monitored daily for adverse events, including weight loss, elevated temperature, low activity level, and nasal discharge. Serum was collected at 14 days postinfection (DPI). At 90 or more days, ferrets were challenged intranasally with 500 μl of phosphate-buffered saline (PBS) containing 5 × 103 PFU H5N1 virus A/Viet Nam/1203/2004 (VN/04). Animals were monitored daily for weight loss and clinical illness (i.e., inactivity, lethargy, sneezing, nasal discharge, and hunched back). Animals were randomly assigned to be sacrificed at 2, 3, 4, 6, 8, 14, 16, 18, or 23 DPI or euthanized if their clinical condition (e.g., loss of >20% body weight) required humane sacrifice. A summary of infection groups and time points of humane sacrifice is listed in Table 1. After inoculation, nasal washes were collected after instilling 3 ml of PBS into the nares of anesthetized ferrets. All highly pathogenic wild-type H5N1 influenza virus studies were performed under high-containment biosafety level 3 enhanced conditions (BSL3+). The University of Pittsburgh Institutional Animal Care and Use Committee approved all experiments, which were in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals (31), the Animal Welfare Act, and the CDC/NIH's Biosafety in Microbiological and Biomedical Laboratories guide (32). Individual ferret infection information is detailed in Tables S1 and S2 in the supplemental material.
TABLE 1.
Summary of groupsa
| Group | Primary influenza strain | Length of time between infections (mo) | Second influenza strain | DPI after last strain inoculation |
|---|---|---|---|---|
| Naive | NA | NA | NA | NA |
| H1N1 | CA/09 | NA | NA | 2, 3, 5, 7, 14 |
| H5N1 | VN/04 | NA | NA | 2, 4, 5, 6, 7 |
| H1N1/H5N1 | Mex/09 | >2 | VN/04 | 2, 4, 6, 18 |
| H3N2/H5N1 | Perth/09, Vic/11 | >3 | VN/04 | 2, 4, 6 |
| H3N2/H5N1 | Vic/11 | 1 | VN/04 | 7, 16, 23 |
Ferrets were divided into 6 groups and sacrificed at the time points listed in the last column. See Table S1 in the supplemental material for further information. Abbreviations: CA/09, A/California/07/2009; VN/04, A/Viet Nam/1203/2004; Mex/09, A/Mexico/4108/2009; Perth/09, A/Perth/16/2009; Vic/11, A/Victoria/361/2011; NA, not applicable.
Tissue.
After serum was collected, necropsies were performed following transcardial perfusion. Trachea, lung, liver, kidney, spleen, small bowel, and brain were harvested. Tissues were either snap frozen or immersion fixed in 10% buffered formalin for histological analysis. After 14 days of fixation, tissue was paraffin embedded and 6-μm-thick sections were prepared for histopathological analysis.
Plaque assay.
Nasal wash virus titers were used to assess viral burden using a plaque assay (33, 34). Briefly, Madin-Darby canine kidney (MDCK) cells (ATCC, Manassas, VA) were plated at 5 × 105 cells per well of a 6-well plate. Nasal washes were diluted (1 × 101 to 1 × 106), overlaid on MDCK cells in 100 μl Iscove's modified Dulbecco minimum essential medium (iDMEM), and incubated 1 h. Virus-containing medium was removed and 2 ml Leibovitz's L-15 medium (Lonza, Walkersville, MD) plus 0.8% agarose was added to each well for 96 h. Agarose was removed and cells were fixed with 10% buffered formalin. Plates were stained with 1% crystal violet for 15 min, washed with distilled water, and dried. Plaques were counted to calculate PFU/ml.
Serology.
Sera were tested for total antibody titer to the corresponding hemagglutinin (HA) used to infect ferrets by enzyme-linked immunosorbent assay (ELISA) (35). Briefly, high-binding, 96-well polystyrene plates (Costar, Lowell, MA) were coated with 50 ng/well of recombinant HA derived from the viral strain used for infection. After blocking, serum samples were serially diluted 2-fold and added to plates for 1 h at room temperature. Wells were washed and then incubated with horseradish peroxidase (HRP)-linked species-specific antibody against IgG for 1 h at room temperature. The HRP was developed with 3,3′,5,5′-tetramethylbenzidine substrate (Sigma-Aldrich, St. Louis, MO) in the dark for 20 min.
ELISAs for neuraminidase and hemagglutinin stalk region.
ELISAs were used to assess total antibody titer and IgG isotype titer to neuraminidase (NA) and the stalk regions of HA. High-binding, 96-well polystyrene plates (Costar, Lowell, MA) were coated overnight with 50 ng/well recombinant NA or chimeric HA proteins. NA coating antigens were derived from viral isolate A/Thailand/1(KAN-1)/2004. Chimeric HA molecules with an HA globular head region from an H6 strain and an H1 HA stalk region were provided by Peter Palese and Florian Krammer (36). Plates were blocked with 5% milk diluted in PBS with 0.05% Tween 20. Serum samples were diluted in blocking buffer and added to plates. Serum was 2-fold serially diluted and allowed to incubate for 1 h at room temperature. After washing, an HRP-linked antibody against IgG (Southern Biotech, Birmingham, AL) diluted in blocking buffer was added to the wells. Plates were incubated for 1 h at room temperature followed by washing. The HRP was developed in the dark with TMB substrate (Sigma-Aldrich) for 15 min and then the reaction was stopped with 2N H2SO4. Optical densities at a wavelength of 450 nm (OD450) were read by a spectrophotometer (BioTek, Winooski, VT) and endpoint dilution titers were determined. Endpoint titers were determined as the reciprocal dilution of the last well that had an OD450 above the mean OD450 plus two standard deviations of naive animal sera.
Immunohistochemistry and lectin histochemistry.
Immunostaining was performed as described before (37). Formalin-fixed paraffin-embedded (FFPE) sections containing systemic organs or brain were stained using rabbit antiserum against influenza A virus or antibodies against specific markers of cell lineage, including glial fibrillary acidic protein (Dako, Carpinteria, CA), cytokeratin (AE1/AE3; Dako), and microtubule-associated protein-2 (SMI52; Covance, Princeton, NJ). For lectin histochemistry to detect ferret macrophages (38), sections were incubated with biotin-conjugated Griffonia (Bandeiraea) simplicifolia isolectin B4 (Vector Laboratories, Burlingame, CA) instead of antibodies.
In situ hybridization.
Sense and antisense templates were generated from a 259-bp segment of influenza A virus matrix protein. 35S-Labeled riboprobes were synthesized using a Riboprobe in vitro transcription system (Promega, Madison, WI). Hybridization was performed on deparaffinized FFPE tissue sections of brain and systemic organs as described previously (39, 40). The influenza riboprobe did not hybridize to noninfected tissue. Control riboprobes for West Nile virus (WNV) RNA hybridized to WNV-infected tissues but not to influenza virus-infected tissues. Influenza virus in situ hybridization (ISH) foci were scored as follows: 0, no definitive signal; 1, occasional focus; 2, focus in most fields; 3, more than one focus per field. Abundances of ISH hybridization varied for each organ, with brain>lung>liver>spleen>small intestine.
PCR.
RNA was extracted from scrolls of FFPE using a QuickExtract FFPE RNA extraction kit (Epicentre Biotechnologies, Madison, WI) according to the manufacturer's recommendations. Aliquots of RNA were diluted to generate cDNA using a RETROscript kit (Life Technologies, Carlsbad, CA). Previously reported primers and TaqMan probes for the avian H5N1 influenza A virus matrix gene (41) and ferret GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (42) were used to amplify nucleic acids with a 2× TaqMan Gene Expression Master mix (Life Technologies) and detected using the Applied Biosystems StepOne real-time PCR system.
RESULTS
H1N1pdm09 virus infection in naive ferrets is predominantly a submucosal gland and bronchial infection.
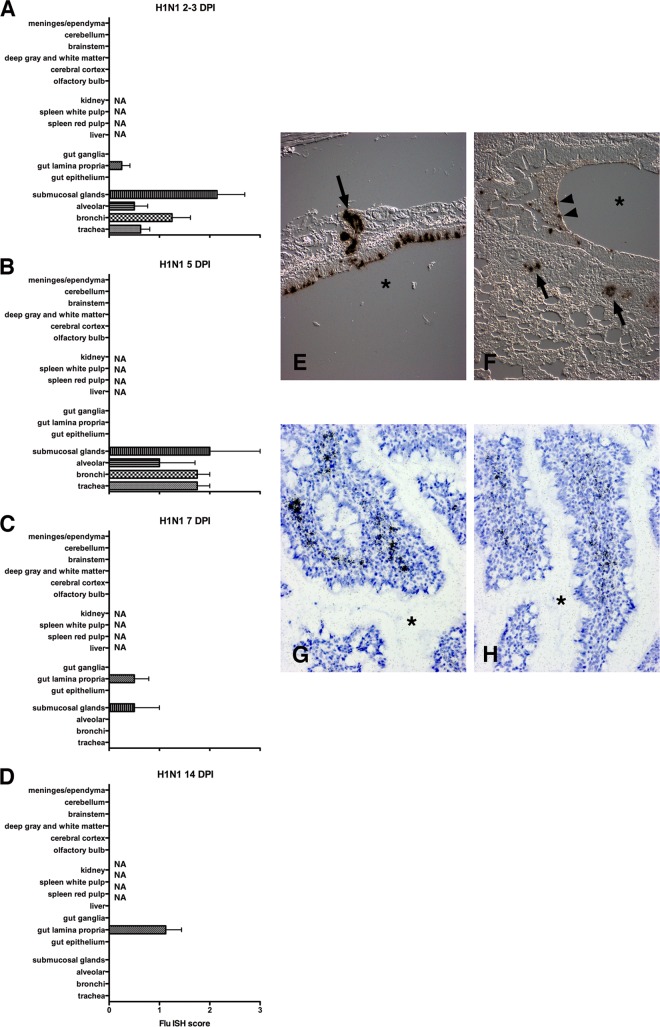
Ferrets were infected intranasally with 1 × 106 PFU CA/09, monitored daily for clinical illness, and sacrificed at 2, 3, 5, 7, and 14 DPI. To visualize viral replication, ISH for the influenza A virus matrix protein gene (Flu ISH) was performed on fixed lung, small bowel, and brain tissues at each time point (Table 2). Shortly after infection (2 to 3 DPI), ferrets showed robust tracheal epithelium and bronchial submucosal gland infections by ISH (Fig. 1A and Table 2). Influenza virus-infected cells peaked at 5 DPI with predominant bronchial and tracheal epithelium and submucosal gland infections (Fig. 1B, E, and F). Respiratory tract infection was limited to occasional submucosal glands on 7 DPI (Fig. 1C) and was absent by 14 DPI (Fig. 1D). At 2 to 7 DPI, lamina propria of the small bowel exhibited occasional foci of infected cells in 4 of 12 animals (Table 2). By 14 DPI, influenza virus-infected lamina propria cells were observed in all animals examined despite clearance of lung infection (Fig. 1D, G, and H and Table 2).
TABLE 2.
Distribution of viral infection in various organs as assessed by ISHa
| Groupb | DPIc | No. of ferretsd | No. of ferrets that exhibited influenza virus RNA ine: |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trachea | Bronchi | Alveoli | Submucosal glands | Small bowel |
Liver | Spleen |
Brain |
Kidney | |||||||||||
| Epithelia | Lamina propria | Ganglia | Red pulp | White pulp | Olfactory bulb | Cerebral cortex | Deep gray and white matter | Brainstem | Cerebellum | Meninges and ependyma | |||||||||
| Naive | NA | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NA |
| H1N1 | 2 | 4 | 3 | 3 | 2 | 2 | 0 | 0 | 0 | NA | NA | NA | 0 | 0 | 0 | 0 | 0 | 0 | NA |
| 3 | 4 | 2 | 3 | 1 | 3 | 0 | 2 | 0 | NA | NA | NA | 0 | 0 | 0 | 0 | 0 | 0 | NA | |
| 5 | 4 | 4 | 4 | 2 | 4 | 0 | 0 | 0 | NA | NA | NA | 0 | 0 | 0 | 0 | 0 | 0 | NA | |
| 7 | 4 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | NA | NA | NA | 0 | 0 | 0 | 0 | 0 | 0 | NA | |
| 14 | 4 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | NA | NA | NA | 0 | 0 | 0 | 0 | 0 | 0 | NA | |
| H5N1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NA |
| 4 | 2 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | NA | |
| 5 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | NA | |
| 6 | 4 | 1 | 0 | 3 | 0 | 0 | 1 | 0 | 4 | 0 | 2 | 2/3 | 2/3 | 2/3 | 3/3 | 3/3 | 1/3 | NA | |
| 7 | 4 | 2 | 1 | 4 | 0 | 0 | 2 | 0 | 4 | 0 | 2 | 2/3 | 3/3 | 3/3 | 3/3 | 3/3 | 1/3 | NA | |
| H1N1/H5N1 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NA |
| 4 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0/2 | 0/2 | 0 | 0 | 0 | 0 | 0 | 0 | NA | |
| 6 | 3 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NA | |
| 18 | 14 | 0 | 0 | 0 | 0 | 0 | 14 | 0 | 5 | 12 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | NA | |
| H3N2/H5N1 | 2 | 4 | 1 | 1 | 3 | 0 | 0 | 2 | 1 | 3 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 5 | 1 | 1 | 3 | 0 | 0 | 1 | 0 | 5 | 1 | 5 | 3 | 3 | 1 | 3 | 2 | 1 | 1 | |
| 6 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 1 | 2 | 1 | 2 | 2 | 0 | 2 | 0 | |
| 7 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | NA | |
Abbreviations: ISH, in situ hybridization; NA, not available.
Viral infection groups.
Number of days postinfection that the animals were sacrificed.
Number of animals studied in each group at each time point.
See Table S2 in the supplemental material for data on individual animals.
FIG 1.
Histograms delineate the average ISH score for different regions of organs 2 to 3 (A), 5 (B), 7 (C), and 14 (D) days after primary H1N1pdm09 infection. In the first 5 days of infection, the majority of the viral infection was limited to lung with predominant infection of bronchial epithelium and submucosal glands. By 7 DPI most of the lung infection had cleared except for occasional submucosal glands. A mild focal infection in the small bowel was limited to the lamina propria. NA, tissue not available. (E) ISH on paraffin section from trachea at 5 DPI. ISH demonstrates influenza virus RNA (dark grains) in tracheal epithelium and submucosal glands (arrow). The asterisk is in the tracheal lumen. (Differential interference contrast [DIC] without counterstain.) (F) ISH on paraffin section from lung at 5 DPI demonstrates influenza virus RNA in bronchial epithelium, necrotic debris (arrowheads), and submucosal glands (arrows). The asterisk is in the bronchial lumen. (DIC without counterstain.) (G and H) ISH on paraffin sections from small bowel at 14 DPI demonstrates influenza virus RNA (dark grains) in lamina propria. The asterisk is in the bowel lumen between villi. For panels G and H, tissues were counterstained with hematoxylin. Scoring: 0 = no definitive signal, 1 = occasional focus, 2 = focus in most fields, 3 = more than one focus per field.
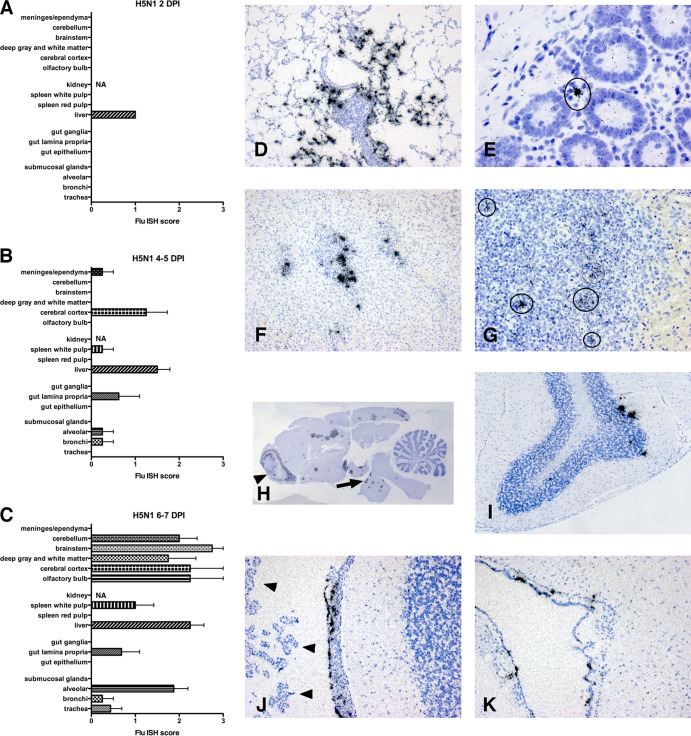
H5N1 (VN/04) virus infection is lethal and spreads to small bowel, liver, spleen, and brain.
Ferrets were infected intranasally with 103 to 104 PFU VN/04. Animals displayed lethargy, nasal discharge, increased temperature, and sneezing, by 4 DPI. All ferrets showed progressive rapid weight loss (see Fig. 3), necessitating humane sacrifice by 7 DPI. Virus replication was detected in the lungs as early as 1 DPI (6.7 × 104 PFU/g tissue) and peaked at 3 DPI (5.5 ×108 PFU/g tissue), remaining high until 7 DPI. Viral replication was visualized using Flu ISH on lung, small bowel, liver, spleen, and brain sections (Table 2). Influenza virus RNA was first detected in the liver at 2 DPI, but surprisingly, no infected cells were detected in the lung (Fig. 2A). By 4 to 5 DPI, mild to moderate influenza virus infection was observed in all organs examined (Fig. 2B). Mild focal infection was detected in the lungs (Fig. 2D). Liver showed moderate infection, predominantly in regions of periportal inflammation (Fig. 2F). The spleen exhibited influenza virus RNA associated with cells in the white pulp (Fig. 2G). One ferret demonstrated infection of ependymal cells lining the ventricles (Fig. 2K) and cells within the leptomeninges (Fig. 2J), suggesting viral dissemination through the cerebrospinal fluid. Ferrets surviving to 6 to 7 DPI showed abundant influenza virus RNA, especially in lung alveoli (7/8), liver (8/8), and brain (8/8) (Fig. 2C and Table 2). Some ferrets showed abundant infection of the olfactory cortex compatible with dissemination through the olfactory epithelium; however, foci of infection throughout the cerebral cortex and deep gray matter were compatible with hematogenous spread (Fig. 2H). Some ferrets also exhibited infected cells in the Purkinje layer of the cerebellum (Fig. 2I). Occasional infected cells in the lamina propria of the small bowel were also observed in 3 of 8 ferrets at this acute time point (Fig. 2E).
FIG 3.
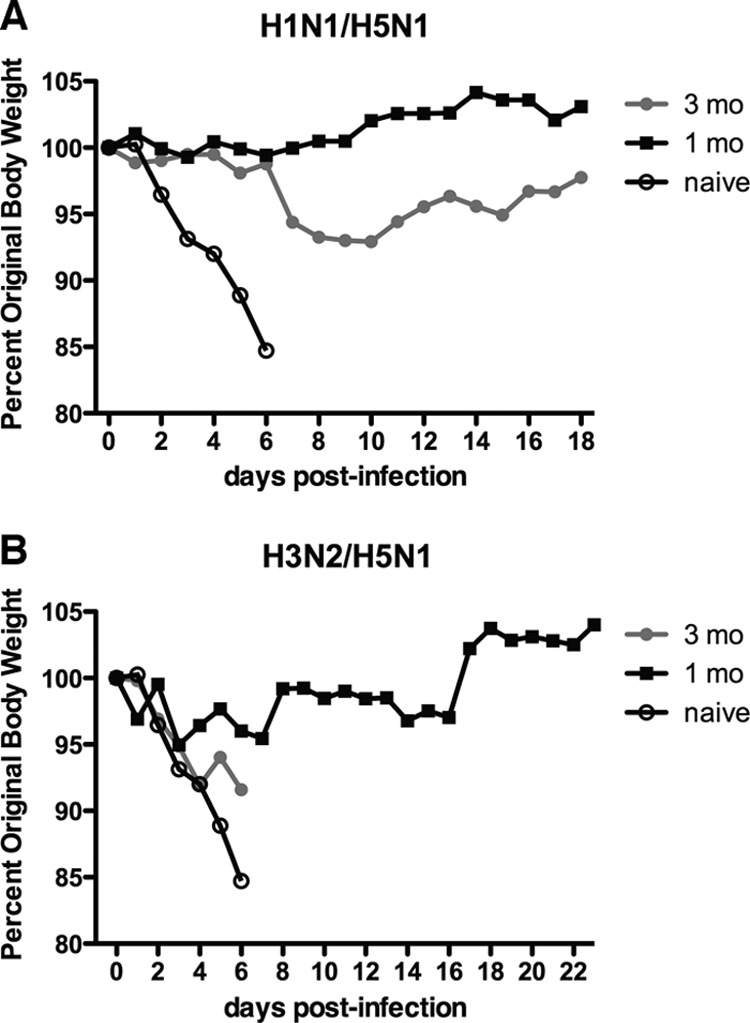
Weight loss in ferrets challenged with H5N1 after exposure to seasonal influenza virus strains. Ferrets were first infected with H1N1pdm09 (Mex/09) (A) or H3N2 (Vic/11) (B) virus, allowed to recover for 1 (black squares) or 3 (gray circles) months, and then challenged with H5N1 (VN/04) virus along with a group of naive ferrets (open circles). Weight loss was assessed daily following H5N1 viral challenge. Ferrets that lost substantial amounts of body weight and were in extremis were euthanized. Ferrets challenged after 1 month of recovery from H1N1pdm09 infection did not experience weight loss, while those challenged 3 months after H1N1 infection showed weight loss after 6 DPI. For H3N2 virus preexposure, ferrets challenged 3 months later showed severe weight loss, while those infected with H5N1 virus 1 month later showed minimal weight loss followed by weight recovery.
FIG 2.
Histograms delineate the average ISH score for different regions of organs 2 (A), 4 to 5 (B), and 6 to 8 (C) days after primary H5N1 infection. In the first 2 days after infection, influenza virus RNA was not widely observed by ISH. At 2 DPI, no infected cells were detected in the lung and a mild infection was detected in the liver. By 4 to 5 DPI, influenza RNA was readily detected in a broad range of organs, including brain, spleen, liver, small bowel lamina propria, and occasionally in the lung. During terminal stages, 6 to 7 DPI, virus was more abundant in many organs, especially the brain. (D to G) ISH for viral RNA in paraffin sections from different systemic organs. (D) Lung at 4 DPI demonstrates influenza virus RNA mostly in lung alveoli. (E) Small bowel at 7 DPI demonstrates influenza virus RNA in lamina propria cells between glandular epithelium. (F) Liver at 5 DPI shows infected cells in regions of hematopoiesis and periportal inflammation. (G) Spleen at 4 DPI shows infected cells (circles) mostly in follicles of white pulp. (H to K) ISH for viral RNA in paraffin sections of the brain at 5 to 6 DPI. (H) Whole mount preparation shows abundant infection of olfactory cortex (arrowhead) and, in multiple sites throughout the cerebral cortex, deep gray matter and brainstem (arrow). (I) Cerebellum shows multiple infected cells in the Purkinje layer. (J and K) Ependymal cells lining the ventricles and cells within the leptomeninges demonstrate viral RNA hybridization while choroid plexus (arrowheads) shows no hybridization. All ISH slides were counterstained with hematoxylin. Scoring: 0 = no definitive signal, 1 = occasional focus, 2 = focus in most fields, 3 = more than one focus per field.
Prior infection with H1N1pdm09 virus confers protection against lethal H5N1 virus infection but is associated with prolonged infection of small bowel, liver, and spleen.
Ferrets were first infected intranasally with 1 × 106 PFU Mex/09 and then given a 2- to 3-month recovery period. Animals infected for 3 months or longer showed an HA titer to Mex/09 HA of 1:160 to 1:1280 (see Table S1 in the supplemental material). After the recovery period, ferrets were challenged intranasally with 1 × 103 PFU VN/04. Ferrets did not exhibit weight loss during the first 6 DPI but lost ∼5% of body weight between 6 and 8 DPI without significant recovery during the remainder of the study (Fig. 3A). Viral replication was assessed at 2, 4, 6, and 18 DPI using Flu ISH (Table 2). Early after VN/04 infection, rare infected cells were detected in the small bowel lamina propria and liver (Fig. 4A and F). At time points when naive animals necessitated humane sacrifice, influenza virus RNA in VN/04-challenged animals was limited to rare cells in the lung alveoli, small bowel lamina propria, and liver (Fig. 4B and D). At 18 DPI, virus was absent in the lung but persisted in hematopoietic elements in the small bowel lamina propria (14/14), liver (5/14), and splenic white (3/14) and red (12/14) pulp (Fig. 4C, E, G, H, and I and Table 2). Viral RNA was not detected in the brain at any time point. These results indicate that infection with H1N1pdm09 influenza virus conferred protection against lethal H5N1 virus infection and H5N1 virus-induced encephalitis, but the protection was not complete, with persistent infection in the small bowel, spleen, and liver at least to 18 DPI.
FIG 4.
Ferrets were initially infected with H1N1pdm09 (Mex/09) and then 2 to 3 months later challenged with H5N1 as described in the text. Histograms delineate the average ISH score for different regions of organs at 2 (A), 4 to 6 (B), and 18 (C) days after H5N1 challenge. In the first 2 days after challenge, influenza virus RNA is limited to small bowel lamina propria and regions of periportal hepatitis. At 4 to 6 DPI, ISH demonstrates little viral infection, which is limited to liver, lamina propria, and occasional alveolar cells. At 18 DPI, ISH demonstrates limited viral RNA in hematopoietic elements of the spleen, liver, and lamina propria. At 6 (D) and 18 (E) DPI, infected cells (circled) can be detected focally in the small bowel lamina propria. At 2 (F) and 18 (G) DPI, infected cells (circled) can be seen in liver in regions of periportal hepatitis. At 18 DPI, infected cells (circled) can be detected in splenic white (H) and red (I) pulp. All ISH slides were counterstained with hematoxylin. Scoring: 0 = no definitive signal, 1 = occasional focus, 2 = focus in most fields, 3 = more than one focus per field.
To determine whether the cross-protection elicited by H1N1pdm09 viral infection was conferred by antibodies against NA or the conserved head and stalk/stem regions of HA, we performed ELISA on sera of the protected ferrets. No detectable antibodies against NA were observed. However, antibodies recognizing NA were detected in serum samples from mice vaccinated with purified NA protein and not detected in serum samples from mice vaccinated with purified HA only. Likewise, no detectable antibodies against the stalk regions of HA were detected using chimeric HA molecules consisting of mismatched globular head and stalk regions from different subtypes. HA molecules with a globular head region from an H6 virus were used with a stalk region from the H1N1 virus, which allowed for detection of specific antibodies to the H1N1 portion of the HA molecule. As a control, HA proteins containing all H1N1 regions were used. Following infection with H1N1pdm09 virus, all sera elicited antibodies that bound the full H1N1 HA protein (data not shown). However, no detectable antibodies bound to the H6/H1 head/stalk HA molecule, indicating that all the polyclonal sera recognized the globular head region of the H1 HA protein. Using chimeras specific for H3 stalk and H5 stalk, we observed similar results with H3N2- and H5N1-specific antisera from ferrets following infection with these viruses.
Prior infection with H3N2 virus does not protect against lethal H5N1 virus infection.
Ferrets were first infected intranasally with 1 × 106 PFU Vic/11 and then given a >3-month recovery period, at which time animals had a 1:20 to 1:160 hemagglutination inhibition (HAI) titer to Vic/11 (see Table S1 in the supplemental material). After the recovery period, ferrets were challenged intranasally with 1 × 103 PFU VN/04. All challenged ferrets experienced weight loss similar to that of naive animals infected with H5N1 virus and required humane sacrifice by 7 DPI (Fig. 3B). Mild infection in the lung, small bowel, liver, and spleen was readily observed at 2 DPI (Fig. 5A, F, and G). By 4 DPI, mild to moderate infection was seen throughout the body (Fig. 5B, H, and I), with widespread, multifocal infection of the brain (Fig. 5L). One ferret with an HA titer to Vic/11 HA below the threshold of detection (1:20) required humane sacrifice at 4 DPI and exhibited viral RNA in kidney glomeruli (Fig. 5K). Lung and small bowel infections were cleared by 6 DPI (Fig. 5C), but abundant liver and brain influenza viral RNA was detected in all animals (Fig. 5J, M, and N). These results show that previous infection with a seasonal H3N2 virus strain did not provide long-term protection from lethal H5N1 virus-induced encephalitis.
FIG 5.
Ferrets were initially infected with H3N2 and then 3 months later challenged with H5N1 as described in the text. (A to C) Histograms delineate the average ISH score for different regions of studied organs at 2 (A), 4 (B), and 6 (C) days after H5N1 challenge. (D and E) Histograms delineating ISH scores for a separate group of H3N2-infected ferrets that were challenged 1 month later with H5N1. (D) Score of one ferret at 7 days after H5N1 virus challenge. (E) Average score at 16 to 23 days after H5N1 virus challenge. (F to N) In the first 2 days after challenge (>3 months after H3N2 infection), influenza virus RNA was observed in hematopoietic elements of spleen, lamina propria, and liver, in addition to lung, where it was mostly identified in alveoli. Occasional infected cells (circled) were detected in the spleen red pulp at 2 DPI (F), small bowel lamina propria at 2 DPI (G), and lung at 4 DPI (H). More abundant infection was observed in spleen follicle centers of white pulp at 4 DPI (I), periportal hepatitis regions of the liver at 6 DPI (J), and in one animal kidney glomeruli at 4 DPI (K). The brain showed prominent infection in multiple foci of cerebral cortex at 4 DPI (L), ependyma at 6 DPI (M), and olfactory cortex at 6 DPI (N). All ISH slides were counterstained with hematoxylin. Scoring: 0 = no definitive signal, 1 = occasional focus, 2 = focus in most fields, 3 = more than one focus per field.
If the window of recovery after Vic/11 infection was shortened to 1 month, approximately 80% of ferrets subsequently challenged with H5N1 were protected from lethal H5N1 encephalitis (Fig. 3B, 5D and E, and Table 2). However, one ferret in this group succumbed to H5N1 virus infection at 7 DPI with abundant lung, liver, and meningeal infections (Fig. 5D). The majority of ferrets lost about 5% of their body weight after H5N1 virus challenge but began to gain weight at approximately 10 DPI (Fig. 3B). Influenza virus RNA associated with cells in the liver and to a lesser extent in the splenic white pulp was detected in these ferrets at 16 to 23 DPI (Fig. 5E). Thus, prior H3N2 virus exposure provided short-term protection from lethal H5N1 infection in the majority of animals.
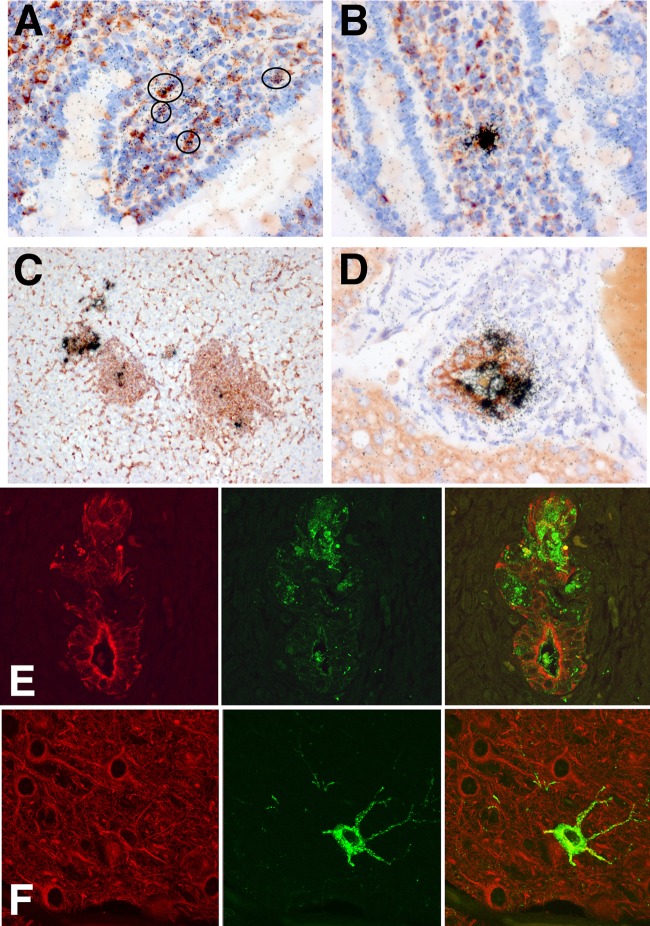
Macrophages, epithelial cells, and neurons are infected with H5N1 virus.
To define the lineage of influenza virus-infected hematopoietic cells in the small bowel lamina propria, liver, and spleen, we performed double-labeling experiments. Using a combination of Flu ISH and staining with Griffonia simplicifolia isolectin B4, a lectin that binds to ferret macrophages, demonstrated colocalization of ISH grains and macrophages in the lamina propria (Fig. 6A and B), liver (Fig. 6C), and spleen (data not shown). CD3 immunohistochemistry in conjunction with Flu ISH did not show colocalization (data not shown). In addition to macrophage infection in the liver, cytokeratin immunohistochemistry, a marker of bile duct epithelium, showed colocalization with influenza RNA (Fig. 6D) and influenza protein (Fig. 6E). However, in the brain, the predominant infected cell type was neurons (Fig. 6F), with some animals showing ependymal cell infection. Some macrophages in the vicinity of dying and necrotic neurons showed colabeling with influenza virus RNA.
FIG 6.
To better define the lineages of infected cells, we stained paraffin sections with cell-specific markers in conjunction with ISH for influenza viral RNA. (A to C) Sections stained with Griffonia simplicifolia lectin that binds to ferret macrophages. (D) Section stained with anti-cytokeratin antibody that binds to epithelial cells. (A and B) Small bowel sections show infected cells (circled) in the lamina propria that display both peroxidase reaction product (red) and ISH grains (black), indicating infection of lamina propria macrophages. (C) Liver section demonstrates infected macrophage elements in regions of periportal hepatitis. (D) Some bile duct epithelial cells labeled for cytokeratin (red) also hybridize with the influenza virus probe (black grains). (E and F) Double-label immunofluorescent images illustrate inflamed liver bile duct epithelia stained for cytokeratin (red) that colocalize with H5 HA protein (green) (E) and infected neurons that stain with microtubule-associated protein-2 (red) and influenza virus H5 HA protein (green) (F).
Liver infection shows three distinct, not mutually exclusive, patterns.
The ferrets in this study, irrespective of influenza inoculation, showed low-grade periportal hepatitis with mild to moderate immune cell infiltrate. As stated above, the liver showed both macrophage (Fig. 6C) and bile duct epithelium infections (Fig. 6D and E). Interestingly, the acute and persistent influenza virus RNA detected in the liver localized to these collections of hematopoietic cells with three patterns of infection. The first pattern demonstrated infection of macrophages and epithelia in inflamed periportal ducts (Fig. 7A and B). The second pattern exhibited infection of cells in the center and perimeter of inflammatory nodules (Fig. 7C and D). These two patterns were not mutually exclusive and could be found within the same liver. The last pattern showed multifocal areas of infected cells surrounding regions of hepatic necrosis (Fig. 7D and E). This pattern was observed only in acute H5N1 infection in ferrets previously infected with H3N2. In addition, some ferrets demonstrated scattered infected macrophages throughout the parenchyma.
FIG 7.
Ferrets supplied for these experiments had a low-grade chronic hepatitis independent of influenza virus infection. With H5N1 challenge, animals showed 3 patterns of influenza-related hepatitis: acute periportal duct inflammation (A and B), severe chronic inflammatory nodules (C and D), and multifocal hepatic necrosis (E and F). In the first pattern, a hematoxylin and eosin (H&E)-stained section of the liver (A) shows acute bile duct inflammation, while ISH on a sequential section (B) demonstrates severe H5N1 infection of inflamed periportal ducts. In the second pattern, an H&E-stained section (C) illustrates a large nodule of chronic inflammatory cells that on ISH (D) shows numerous H5N1-infected cells both in the center and at the perimeter. An H&E-stained section (E) of the third pattern shows central necrosis with ISH (F) demonstrating a perimeter of infected cells surrounding the central necrosis.
Influenza immunohistochemistry verified infection of small bowel, liver, and spleen.
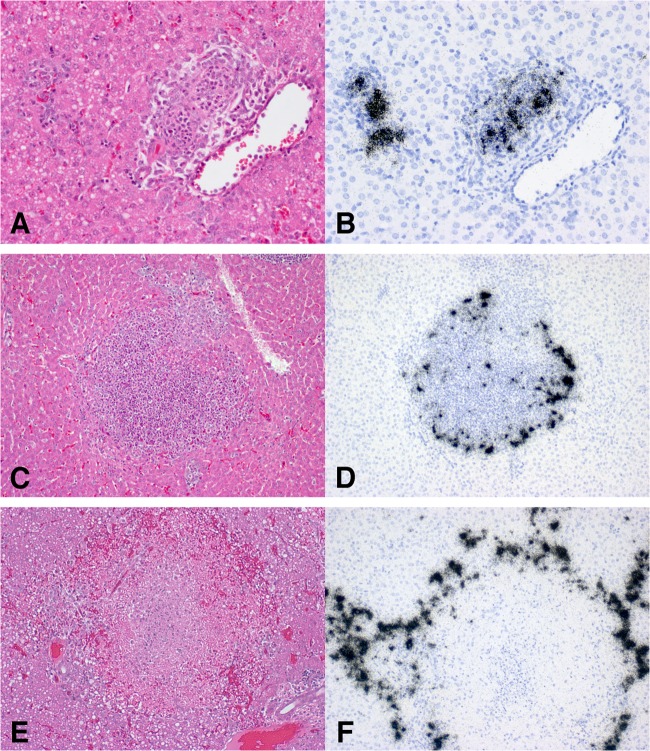
To support the ISH observations using additional methods, we visualized influenza virus protein using H5 HA immunohistochemistry and detected influenza virus RNA by RT-PCR using RNA extracted from FFPE tissue. In ferrets previously infected with H1N1pdm09 virus, cells expressing H5 HA protein 18 days after H5N1 challenge were detected in the small bowel lamina propria (Fig. 8A and B), liver (data not shown), and spleen (Fig. 8C). Although the sensitivity of immunohistochemistry for influenza virus is lower than that of Flu ISH, it is clear that the two methods demonstrated the same tissue distribution of infection. Using RT-PCR of RNA extracted from FFPE tissues, influenza virus matrix gene RNA was detected in lung, brain, and liver samples of ferrets during primary infection with H5N1 virus with the same relative tissue distribution as the ISH results. As with immunohistochemistry, RT-PCR was less sensitive than ISH and two samples with low levels of infection by ISH (one liver and one lung) were below the limit of detection by RT-PCR. The low levels of small bowel and liver expression observed in H5N1-infected ferrets that were previously infected with H1N1pdm09 virus were also below the limits of detection by RT-PCR.
FIG 8.
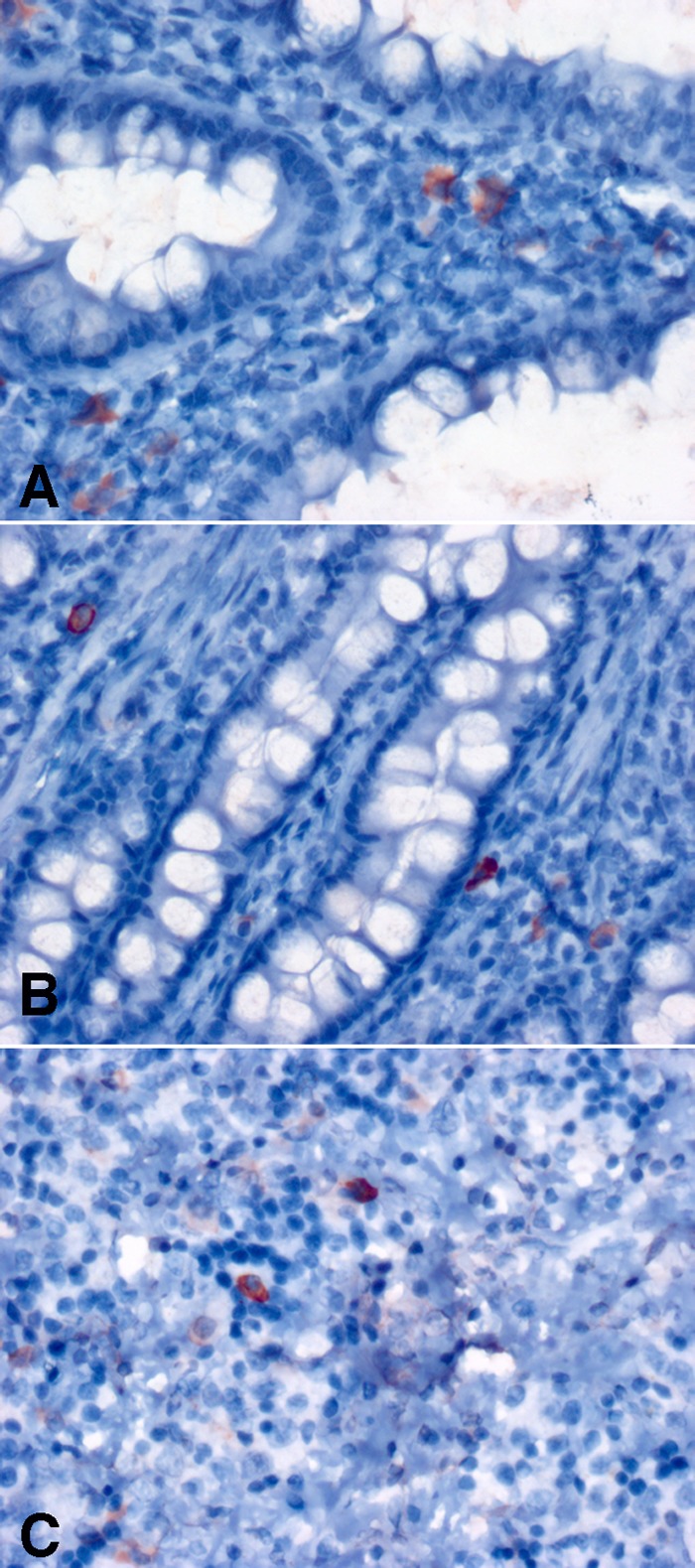
Influenza virus H5 HA immunohistochemistry in ferrets initially infected with H1N1pdm09 (Mex/09) and then challenged with H5N1. At 18 days after challenge with H5N1, the small bowel lamina propria from two separate ferrets (A and B) and spleen white pulp (C) show occasional infected cells (red) in a distribution and frequency similar to those of infected cells observed with ISH. (Counterstained with hematoxylin.)
DISCUSSION
Highly pathogenic avian influenza (HPAI) viruses cause lethal disease in a large fraction of infected mammals, including humans. While frequently associated with severe respiratory disease, HPAI infection is also notable for more widespread systemic disease, including gastroenteritis and encephalitis (8, 43, 44). H5N1 RNA has been detected in feces of human patients with diarrhea and at autopsy, although it is unclear whether influenza virus disseminates to the gastrointestinal tract or is a site of initial infection (45–47). Rodent animal models of lethal H5N1 virus infection illustrate systemic dissemination to a variety of organs, including the brain. Although H5N1 virus infection of humans also shows substantial mortality, history of seasonal influenza exposure is associated with heterosubtypic immunity that might be able to moderate H5N1 virus infection. We used a ferret model to show that a history of previous seasonal influenza virus infection influences the course of H5N1 virus influenza disease. Because of their size, ease of manipulation, distribution of sialic acid receptor linkages, pattern of viral attachment to the respiratory mucosa, and similarities with human clinical symptoms and lung physiology, ferrets are ideally suited for influenza virus pathogenesis studies (24–27).
Primary infection of ferrets with seasonal influenza viruses has been studied extensively (24, 48–51). Our findings of H1N1pdm09 influenza virus respiratory infection in ferrets substantially agree with previous reports where viral clearance was documented by PFU assays (49, 52, 53). Previous reports have also detected H1N1pdm09 in the intestinal tract (53), as we have shown using in situ hybridization; however, we discovered a more persistent infection of hematopoietic cells in the small bowel. While the low level of virus detected by ISH did not appear to mediate clinical symptoms, such a prolonged infection may have broader implications for the environment. Multiple strains of influenza simultaneously circulate throughout the world. The longer an individual host is infected with one strain, the greater the opportunity for a second primary infection by a different strain. Simultaneous infection of an individual host with multiple strains of influenza viruses raises the potential for reassortment of influenza virus genes and the generation of new pandemic strains (54–57). It will be interesting to test whether multiple strains of influenza A virus can infect these hematopoietic elements simultaneously.
The nature of the persistent hematopoietic infection appeared to be different from the acute infection observed in bronchial epithelium and neurons. With our ISH protocols, acutely infected cells showed a strong ISH signal after 5 days of emulsion exposure. However, the persistent infection of hematopoietic cells was just detectable after 5 days and achieved a more pronounced signal after 9 days of exposure. This difference in ISH signals implies differential kinetics of viral expression and replication depending upon the type of cell infected and the tissue microenvironment. By cell morphology and double-label immunofluorescence, we show that the hematopoietic cells in the intestinal tract, spleen, and liver infected by H5N1 virus are consistent with a macrophage lineage. Because the livers of all animals exhibit mild periportal hepatitis, a feature considered by some investigators to be normal in the ferret (30), the hepatitis may provide an additional site of infectible host macrophages. Recent evidence demonstrated that the HA gene of some, but not all, HPAI H5N1 influenza viruses conferred the ability to productively infect alveolar macrophages and macrophage cell lines by allowing viral ribonucleoprotein entrance into the macrophage nucleus (58). In our study, both negative and positive viral RNA were detected by ISH in lamina propria, spleen, and liver macrophages, indicating viral transcription.
Prior infection with seasonal influenza virus confers various degrees of heterosubtypic immunity to subsequent challenges with new strains. This immunity is specific to influenza and not conferred by infection with unrelated respiratory viruses (e.g., respiratory syncytial virus [RSV]) (59). Using a similar model to what we described here, Bodewes et al. observed different results in ferrets challenged with H5N1 virus after previous H3N2 virus infection (28). In their studies, ferrets infected with an H3N2 influenza virus 1 month prior to H5N1 challenge were protected from lethal disease. We saw similar protection with a 1-month gap between the two infections; however, when we waited 5 months between initial infection and HPAI challenge, we discovered dramatically different survival. We chose this longer interval to more closely model the human history of influenza virus exposure and avoid issues associated with residual activation of the innate immune system temporarily providing antigen-independent protection from subsequent infection (60–62). Heterosubtypic immunity in our delayed model was conferred only by specific seasonal influenza virus strains (H1N1pdm09) and not by H3N2 virus strains.
Why does heterosubtypic immunity to H1N1 protect from H5N1 virus challenge? While ferrets infected with H3N2 had lower HAI titers (mean, 1:96.4; range, 1:20 to 1:160) than ferrets infected with H1N1pdm09 (mean, 1:1040; range, 1:160 to 1:2,560), HAI titers of 1:40 are considered to be protective. This suggests that differences in the character of the immune response rather than the magnitude of the response mediate the different outcomes and could be due to differences in induction of antibody or T cell immunity to viral proteins.
Phylogenetically, H1N1 and H5N1 viruses are closely related influenza virus subtypes belonging to Group 1 influenza viruses, while H3N2 strains belong to Group 2 influenza viruses and are more distant phylogenetically (63). Traditionally, heterosubtypic immunity to influenza virus has been hypothesized to be mediated by T cells targeting nuclear and matrix proteins. Why prior H1N1 virus infection protects the host from lethal H5N1 virus infection and H3N2 does not may be related to antigenic similarities between the HA1 and HA5 stalk, eliciting cross-protective humoral immunity. We tested this hypothesis but found no detectable antibodies to the HA1 or HA5 stalk region elicited by H1N1pdm09 infection. However, antibodies recognizing the globular head portion of the H1N1pdm09 HA protein were readily detected. Another hypothesis was tested to determine whether specific antibodies elicited against NA after H1N1pdm09 infection might cross-react with the NA of H5N1. Others have reported that immunization with NA from pre-H1N1pdm09 pandemic strains afforded partial protection to mice (64) and ferrets (65) challenged with H5N1 virus approximately 1 month later. However, no NA antibodies were detected following H1N1pdm09 infection in the ferrets presented in this manuscript. Studies are under way to dissect whether the cross-protection is mediated by cellular or mucosal immunity, using our longer gap between initial H1N1pdm09 infection and H5N1 challenge.
The heterosubtypic immunity elicited with initial H1N1pdm09 virus infection was not sterilizing for subsequent H5N1 virus challenge. Secondary H5N1 disease was clinically mild or absent and was limited to a mild alveolar infection in some animals and minimal systemic hematopoietic infection in the majority of animals. As stated above, the prolonged persistence of the infection might have significant implications for environmental mixing of different viral strains.
H5N1 is endemic in avian populations across Asia and the Middle East. Despite frequent contact with infected poultry, transmission to humans is relatively rare (66). This is likely to be mediated largely by H5N1 viral traits (e.g., genetic composition of HA and basic polymerase 2 [26, 67]) and the limited binding of H5 HA to human-type sialic acid-linked receptor proteins (26, 67) in the upper respiratory tract. However, close and prolonged exposure to infected poultry (or infected humans) is a substantial risk factor for H5N1 infection, but the disproportionate infection of children and young adults suggests prior infections with influenza A virus strains might confer protection to exposed individuals, as suggested in our study of ferrets. Serological evidence shows H5N1 infection can be subclinical with 4 to 10% of exposed poultry workers testing positive for H5 antibodies (66, 68). However, severely limited human-to-human spread of H5N1 is likely to be mediated by other factors, such as viral traits (26, 67), since ferret-to-ferret transmission of wild-type H5N1 is not readily observable in influenza virus-naive animals. It would be interesting to learn if prior influenza virus exposure influences airborne transmission of mutant H5N1 viruses such as those shown to achieve ferret-to-ferret transmission (67).
Significant concern has been raised about the potential of vaccination programs to diminish heterosubtypic immunity and leave segments of the population vulnerable to infection with avian influenza viruses (69, 70). Revelation of the limits of conventional immunization paradigms argues for better understanding of the mechanism of protection and searching for new vaccination paradigms that provide protection beyond the respiratory system.
Supplementary Material
ACKNOWLEDGMENTS
We thank Mark Stauffer, Dana Weber, Arlene Carbone-Wiley, and Brooke Paul for their valuable technical assistance.
Footnotes
Published ahead of print 26 December 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.01840-13.
REFERENCES
- 1.Claas EC, Osterhaus AD, van Beek R, De Jong JC, Rimmelzwaan GF, Senne DA, Krauss S, Shortridge KF, Webster RG. 1998. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet 351:472–477. 10.1016/S0140-6736(97)11212-0 [DOI] [PubMed] [Google Scholar]
- 2.Domínguez-Cherit G, Namendys-Silva SA, de la Torre A, Macias AE, Cordova-Villalobos JA. 2010. H1N1 influenza pandemic of 2009 compared with other influenza pandemics: epidemiology, diagnosis, management, pulmonary complications, and outcomes. Curr. Infect. Dis. Rep. 12:204–210. 10.1007/s11908-010-0097-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morens DM, Taubenberger JK, Fauci AS. 2009. The persistent legacy of the 1918 influenza virus. N. Engl. J. Med. 361:225–229. 10.1056/NEJMp0904819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimmer SM, Burke DS. 2009. Historical perspective—emergence of influenza A (H1N1) viruses. N. Engl. J. Med. 361:279–285. 10.1056/NEJMra0904322 [DOI] [PubMed] [Google Scholar]
- 5.Smith GJ, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, Ma SK, Cheung CL, Raghwani J, Bhatt S, Peiris JS, Guan Y, Rambaut A. 2009. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature 459:1122–1125. 10.1038/nature08182 [DOI] [PubMed] [Google Scholar]
- 6.Medina RA, Garcia-Sastre A. 2011. Influenza A viruses: new research developments. Nat. Rev. Microbiol. 9:590–603. 10.1038/nrmicro2613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watanabe Y, Ibrahim MS, Suzuki Y, Ikuta K. 2012. The changing nature of avian influenza A virus (H5N1). Trends Microbiol. 20:11–20. 10.1016/j.tim.2011.10.003 [DOI] [PubMed] [Google Scholar]
- 8.de Jong JC, Claas EC, Osterhaus AD, Webster RG, Lim WL. 1997. A pandemic warning? Nature 389:554. 10.1038/39221, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Subbarao K, Klimov A, Katz J, Regnery H, Lim W, Hall H, Perdue M, Swayne D, Bender C, Huang J, Hemphill M, Rowe T, Shaw M, Xu X, Fukuda K, Cox N. 1998. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science 279:393–396. 10.1126/science.279.5349.393 [DOI] [PubMed] [Google Scholar]
- 10.Ungchusak K, Auewarakul P, Dowell SF, Kitphati R, Auwanit W, Puthavathana P, Uiprasertkul M, Boonnak K, Pittayawonganon C, Cox NJ, Zaki SR, Thawatsupha P, Chittaganpitch M, Khontong R, Simmerman JM, Chunsutthiwat S. 2005. Probable person-to-person transmission of avian influenza A (H5N1). N. Engl. J. Med. 352:333–340. 10.1056/NEJMoa044021 [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Feng Z, Shu Y, Yu H, Zhou L, Zu R, Huai Y, Dong J, Bao C, Wen L, Wang H, Yang P, Zhao W, Dong L, Zhou M, Liao Q, Yang H, Wang M, Lu X, Shi Z, Wang W, Gu L, Zhu F, Li Q, Yin W, Yang W, Li D, Uyeki TM, Wang Y. 2008. Probable limited person-to-person transmission of highly pathogenic avian influenza A (H5N1) virus in China. Lancet 371:1427–1434. 10.1016/S0140-6736(08)60493-6 [DOI] [PubMed] [Google Scholar]
- 12.WHO/PED. 2013. Cumulative number of confirmed human cases for avian influenza A (H5N1) reported to WHO, 2003–2013. World Health Organization, Geneva, Switzerland: http://www.who.int/influenza/human_animal_interface/EN_GIP_20130201CumulativeNumberH5N1cases.pdf [Google Scholar]
- 13.Spesock A, Malur M, Hossain MJ, Chen LM, Njaa BL, Davis CT, Lipatov AS, York IA, Krug RM, Donis RO. 2011. The virulence of 1997 H5N1 influenza viruses in the mouse model is increased by correcting a defect in their NS1 proteins. J. Virol. 85:7048–7058. 10.1128/JVI.00417-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuiken T, van den Brand J, van Riel D, Pantin-Jackwood M, Swayne DE. 2010. Comparative pathology of select agent influenza A virus infections. Vet. Pathol. 47:893–914. 10.1177/0300985810378651 [DOI] [PubMed] [Google Scholar]
- 15.Cardona CJ, Xing Z, Sandrock CE, Davis CE. 2009. Avian influenza in birds and mammals. Comp. Immunol. Microbiol. Infect. Dis. 32:255–273. 10.1016/j.cimid.2008.01.001 [DOI] [PubMed] [Google Scholar]
- 16.Bodewes R, Kreijtz JH, van Amerongen G, Fouchier RA, Osterhaus AD, Rimmelzwaan GF, Kuiken T. 2011. Pathogenesis of influenza A/H5N1 virus infection in ferrets differs between intranasal and intratracheal routes of inoculation. Am. J. Pathol. 179:30–36. 10.1016/j.ajpath.2011.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipatov AS, Kwon YK, Pantin-Jackwood MJ, Swayne DE. 2009. Pathogenesis of H5N1 influenza virus infections in mice and ferret models differs according to respiratory tract or digestive system exposure. J. Infect. Dis. 199:717–725. 10.1086/596740 [DOI] [PubMed] [Google Scholar]
- 18.Gustin KM, Belser JA, Wadford DA, Pearce MB, Katz JM, Tumpey TM, Maines TR. 2011. Influenza virus aerosol exposure and analytical system for ferrets. Proc. Natl. Acad. Sci. U. S. A. 108:8432–8437. 10.1073/pnas.1100768108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lednicky JA, Hamilton SB, Tuttle RS, Sosna WA, Daniels DE, Swayne DE. 2010. Ferrets develop fatal influenza after inhaling small particle aerosols of highly pathogenic avian influenza virus A/Vietnam/1203/2004 (H5N1). Virol. J. 7:231. 10.1186/1743-422X-7-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishimura H, Itamura S, Iwasaki T, Kurata T, Tashiro M. 2000. Characterization of human influenza A (H5N1) virus infection in mice: neuro-, pneumo- and adipotropic infection. J. Gen. Virol. 81:2503–2510 http://vir.sgmjournals.org/content/81/10/2503.long [DOI] [PubMed] [Google Scholar]
- 21.Garigliany MM, Habyarimana A, Lambrecht B, Van de Paar E, Cornet A, van den Berg T, Desmecht D. 2010. Influenza A strain-dependent pathogenesis in fatal H1N1 and H5N1 subtype infections of mice. Emerg. Infect. Dis. 16:595–603. 10.3201/eid1604.091061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rimmelzwaan GF, Kuiken T, van Amerongen G, Bestebroer TM, Fouchier RA, Osterhaus AD. 2001. Pathogenesis of influenza A (H5N1) virus infection in a primate model. J. Virol. 75:6687–6691. 10.1128/JVI.75.14.6687-6691.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giles BM, Crevar CJ, Carter DM, Bissel SJ, Schultz-Cherry S, Wiley CA, Ross TM. 2012. A computationally optimized hemagglutinin virus-like particle vaccine elicits broadly reactive antibodies that protect nonhuman primates from H5N1 infection. J. Infect. Dis. 205:1562–1570. 10.1093/infdis/jis232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maher JA, DeStefano J. 2004. The ferret: an animal model to study influenza virus. Lab Anim. 33:50–53. 10.1038/laban1004-50 [DOI] [PubMed] [Google Scholar]
- 25.Belser JA, Katz JM, Tumpey TM. 2011. The ferret as a model organism to study influenza A virus infection. Dis. Model Mech. 4:575–579. 10.1242/dmm.007823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herfst S, Schrauwen EJ, Linster M, Chutinimitkul S, de Wit E, Munster VJ, Sorrell EM, Bestebroer TM, Burke DF, Smith DJ, Rimmelzwaan GF, Osterhaus AD, Fouchier RA. 2012. Airborne transmission of influenza A/H5N1 virus between ferrets. Science 336:1534–1541. 10.1126/science.1213362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Riel D, Munster VJ, de Wit E, Rimmelzwaan GF, Fouchier RA, Osterhaus AD, Kuiken T. 2007. Human and avian influenza viruses target different cells in the lower respiratory tract of humans and other mammals. Am. J. Pathol. 171:1215–1223. 10.2353/ajpath.2007.070248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bodewes R, Kreijtz JH, Geelhoed-Mieras MM, van Amerongen G, Verburgh RJ, van Trierum SE, Kuiken T, Fouchier RA, Osterhaus AD, Rimmelzwaan GF. 2011. Vaccination against seasonal influenza A/H3N2 virus reduces the induction of heterosubtypic immunity against influenza A/H5N1 virus infection in ferrets. J. Virol. 85:2695–2702. 10.1128/JVI.02371-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewington JH. 2000. Ferret husbandry, medicine, and surgery. Butterworth-Heinemann, Oxford, United Kingdom [Google Scholar]
- 30.Fox JG. 1998. Biology and diseases of the ferret, 2nd ed. Williams & Wilkins, Baltimore, MD [Google Scholar]
- 31.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC [Google Scholar]
- 32.U.S. Department of Health and Human Services. 2009. Biosafety in microbiological and biomedical laboratories, 5th ed. Department of Health and Human Services, Washington, DC [Google Scholar]
- 33.Tobita K. 1975. Permanent canine kidney (MDCK) cells for isolation and plaque assay of influenza B viruses. Med. Microbiol. Immunol. 162:23–27. 10.1007/BF02123574 [DOI] [PubMed] [Google Scholar]
- 34.Tobita K, Sugiura A, Enomote C, Furuyama M. 1975. Plaque assay and primary isolation of influenza A viruses in an established line of canine kidney cells (MDCK) in the presence of trypsin. Med. Microbiol. Immunol. 162:9–14. 10.1007/BF02123572 [DOI] [PubMed] [Google Scholar]
- 35.Giles BM, Bissel SJ, Craigo JK, Dealmeida DR, Wiley CA, Tumpey TM, Ross TM. 2012. Elicitation of anti-1918 influenza virus immunity early in life prevents morbidity and lower levels of lung infection by 2009 pandemic H1N1 influenza virus in aged mice. J. Virol. 86:1500–1513. 10.1128/JVI.06034-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krammer F, Pica N, Hai R, Margine I, Palese P. 2013. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J. Virol. 87:6542–6550. 10.1128/JVI.00641-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bissel SJ, Wang G, Ghosh M, Reinhart TA, Capuano S, III, Stefano Cole K, Murphey-Corb M, Piatak M, Jr, Lifson JD, Wiley CA. 2002. Macrophages relate presynaptic and postsynaptic damage in simian immunodeficiency virus encephalitis. Am. J. Pathol. 160:927–941. 10.1016/S0002-9440(10)64915-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson JK, Berman NE. 1996. A transient phase of cell death in the developing medial forebrain of the perinatal ferret. Brain Res. Dev. Brain Res. 94:159–165. 10.1016/S0165-3806(96)80007-1 [DOI] [PubMed] [Google Scholar]
- 39.Fallert BA, Reinhart TA. 2002. Improved detection of simian immunodeficiency virus RNA by in situ hybridization in fixed tissue sections: combined effects of temperatures for tissue fixation and probe hybridization. J. Virol. Methods 99:23–32. 10.1016/S0166-0934(01)00378-0 [DOI] [PubMed] [Google Scholar]
- 40.Bissel SJ, Giles BM, Wang G, Olevian DC, Ross TM, Wiley CA. 2012. Acute murine H5N1 influenza A encephalitis. Brain Pathol. 22:150–158. 10.1111/j.1750-3639.2011.00514.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X, Zhao J, Tang S, Ye Z, Hewlett I. 2010. Viremia associated with fatal outcomes in ferrets infected with avian H5N1 influenza virus. PLoS One 5:e12099. 10.1371/journal.pone.0012099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakata M, Itou T, Sakai T. 2009. Quantitative analysis of inflammatory cytokines expression in peripheral blood mononuclear cells of the ferret (Mustela putorius furo) using real-time PCR. Vet. Immunol. Immunopathol. 130:88–91. 10.1016/j.vetimm.2008.12.009 [DOI] [PubMed] [Google Scholar]
- 43.Gambotto A, Barratt-Boyes SM, de Jong MD, Neumann G, Kawaoka Y. 2008. Human infection with highly pathogenic H5N1 influenza virus. Lancet 371:1464–1475. 10.1016/S0140-6736(08)60627-3 [DOI] [PubMed] [Google Scholar]
- 44.Gu J, Xie Z, Gao Z, Liu J, Korteweg C, Ye J, Lau LT, Lu J, Zhang B, McNutt MA, Lu M, Anderson VM, Gong E, Yu AC, Lipkin WI. 2007. H5N1 infection of the respiratory tract and beyond: a molecular pathology study. Lancet 370:1137–1145. 10.1016/S0140-6736(07)61515-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beigel JH, Farrar J, Han AM, Hayden FG, Hyer R, de Jong MD, Lochindarat S, Nguyen TK, Nguyen TH, Tran TH, Nicoll A, Touch S, Yuen KY, Writing Committee of the World Health Organization Consultation on Human Influenza A/H5 2005. Avian influenza A (H5N1) infection in humans. N. Engl. J. Med. 353:1374–1385. 10.1056/NEJMra052211 [DOI] [PubMed] [Google Scholar]
- 46.Uiprasertkul M, Puthavathana P, Sangsiriwut K, Pooruk P, Srisook K, Peiris M, Nicholls JM, Chokephaibulkit K, Vanprapar N, Auewarakul P. 2005. Influenza A H5N1 replication sites in humans. Emerg. Infect. Dis. 11:1036–1041. 10.3201/eid1107.041313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, Chau TN, Hoang DM, Chau NV, Khanh TH, Dong VC, Qui PT, Cam BV, Ha do Q, Guan Y, Peiris JS, Chinh NT, Hien TT, Farrar J. 2006. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 12:1203–1207. 10.1038/nm1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maines TR, Jayaraman A, Belser JA, Wadford DA, Pappas C, Zeng H, Gustin KM, Pearce MB, Viswanathan K, Shriver ZH, Raman R, Cox NJ, Sasisekharan R, Katz JM, Tumpey TM. 2009. Transmission and pathogenesis of swine-origin 2009 A(H1N1) influenza viruses in ferrets and mice. Science 325:484–487. 10.1126/science.1177238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rowe T, Leon AJ, Crevar CJ, Carter DM, Xu L, Ran L, Fang Y, Cameron CM, Cameron MJ, Banner D, Ng DC, Ran R, Weirback HK, Wiley CA, Kelvin DJ, Ross TM. 2010. Modeling host responses in ferrets during A/California/07/2009 influenza infection. Virology 401:257–265. 10.1016/j.virol.2010.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith H, Sweet C. 1988. Lessons for human influenza from pathogenicity studies with ferrets. Rev. Infect. Dis. 10:56–75. 10.1093/clinids/10.1.56 [DOI] [PubMed] [Google Scholar]
- 51.Belser JA, Szretter KJ, Katz JM, Tumpey TM. 2009. Use of animal models to understand the pandemic potential of highly pathogenic avian influenza viruses. Adv. Virus Res. 73:55–97. 10.1016/S0065-3527(09)73002-7 [DOI] [PubMed] [Google Scholar]
- 52.Huang SS, Banner D, Fang Y, Ng DC, Kanagasabai T, Kelvin DJ, Kelvin AA. 2011. Comparative analyses of pandemic H1N1 and seasonal H1N1, H3N2, and influenza B infections depict distinct clinical pictures in ferrets. PLoS One 6:e27512. 10.1371/journal.pone.0027512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Munster VJ, de Wit E, van den Brand JM, Herfst S, Schrauwen EJ, Bestebroer TM, van de Vijver D, Boucher CA, Koopmans M, Rimmelzwaan GF, Kuiken T, Osterhaus AD, Fouchier RA. 2009. Pathogenesis and transmission of swine-origin 2009 A(H1N1) influenza virus in ferrets. Science 325:481–483. 10.1126/science.1177127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma W, Kahn RE, Richt JA. 2009. The pig as a mixing vessel for influenza viruses: human and veterinary implications. J. Mol. Genet. Med. 3:158–166 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2702078/ [PMC free article] [PubMed] [Google Scholar]
- 55.Scholtissek C, Burger H, Kistner O, Shortridge KF. 1985. The nucleoprotein as a possible major factor in determining host specificity of influenza H3N2 viruses. Virology 147:287–294. 10.1016/0042-6822(85)90131-X [DOI] [PubMed] [Google Scholar]
- 56.Ma W, Lager KM, Vincent AL, Janke BH, Gramer MR, Richt JA. 2009. The role of swine in the generation of novel influenza viruses. Zoonoses Public Health 56:326–337. 10.1111/j.1863-2378.2008.01217.x [DOI] [PubMed] [Google Scholar]
- 57.Khiabanian H, Trifonov V, Rabadan R. 2009. Reassortment patterns in swine influenza viruses. PLoS Curr. 1:RRN1008. 10.1371/currents.RRN1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cline TD, Karlsson EA, Seufzer BJ, Schultz-Cherry S. 2013. The hemagglutinin protein of highly pathogenic H5N1 influenza viruses overcomes an early block in the replication cycle to promote productive replication in macrophages. J. Virol. 87:1411–1419. 10.1128/JVI.02682-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kreijtz JH, Bodewes R, van den Brand JM, de Mutsert G, Baas C, van Amerongen G, Fouchier RA, Osterhaus AD, Rimmelzwaan GF. 2009. Infection of mice with a human influenza A/H3N2 virus induces protective immunity against lethal infection with influenza A/H5N1 virus. Vaccine 27:4983–4989. 10.1016/j.vaccine.2009.05.079 [DOI] [PubMed] [Google Scholar]
- 60.Hammerbeck DM, Burleson GR, Schuller CJ, Vasilakos JP, Tomai M, Egging E, Cochran FR, Woulfe S, Miller RL. 2007. Administration of a dual toll-like receptor 7 and toll-like receptor 8 agonist protects against influenza in rats. Antiviral Res. 73:1–11. 10.1016/j.antiviral.2006.07.011 [DOI] [PubMed] [Google Scholar]
- 61.Yager EJ, Szaba FM, Kummer LW, Lanzer KG, Burkum CE, Smiley ST, Blackman MA. 2009. γ-Herpesvirus-induced protection against bacterial infection is transient. Viral Immunol. 22:67–72. 10.1089/vim.2008.0086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abe T, Takahashi H, Hamazaki H, Miyano-Kurosaki N, Matsuura Y, Takaku H. 2003. Baculovirus induces an innate immune response and confers protection from lethal influenza virus infection in mice. J. Immunol. 171:1133–1139 http://www.jimmunol.org/content/171/3/1133.long [DOI] [PubMed] [Google Scholar]
- 63.Russell RJ, Kerry PS, Stevens DJ, Steinhauer DA, Martin SR, Gamblin SJ, Skehel JJ. 2008. Structure of influenza hemagglutinin in complex with an inhibitor of membrane fusion. Proc. Natl. Acad. Sci. U. S. A. 105:17736–17741. 10.1073/pnas.0807142105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sandbulte MR, Jimenez GS, Boon AC, Smith LR, Treanor JJ, Webby RJ. 2007. Cross-reactive neuraminidase antibodies afford partial protection against H5N1 in mice and are present in unexposed humans. PLoS Med. 4:e59. 10.1371/journal.pmed.0040059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rockman S, Brown LE, Barr IG, Gilbertson B, Lowther S, Kachurin A, Kachurina O, Klippel J, Bodle J, Pearse M, Middleton D. 2013. Neuraminidase-inhibiting antibody is a correlate of cross-protection against lethal H5N1 influenza virus in ferrets immunized with seasonal influenza vaccine. J. Virol. 87:3053–3061. 10.1128/JVI.02434-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Kerkhove MD, Mumford E, Mounts AW, Bresee J, Ly S, Bridges CB, Otte J. 2011. Highly pathogenic avian influenza (H5N1): pathways of exposure at the animal-human interface, a systematic review. PLoS One 6:e14582. 10.1371/journal.pone.0014582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Imai M, Watanabe T, Hatta M, Das SC, Ozawa M, Shinya K, Zhong G, Hanson A, Katsura H, Watanabe S, Li C, Kawakami E, Yamada S, Kiso M, Suzuki Y, Maher EA, Neumann G, Kawaoka Y. 2012. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 486:420–428. 10.1038/nature10831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bridges CB, Lim W, Hu-Primmer J, Sims L, Fukuda K, Mak KH, Rowe T, Thompson WW, Conn L, Lu X, Cox NJ, Katz JM. 2002. Risk of influenza A (H5N1) infection among poultry workers, Hong Kong, 1997–1998. J. Infect. Dis. 185:1005–1010. 10.1086/340044 [DOI] [PubMed] [Google Scholar]
- 69.Bodewes R, Kreijtz JH, Baas C, Geelhoed-Mieras MM, de Mutsert G, van Amerongen G, van den Brand JM, Fouchier RA, Osterhaus AD, Rimmelzwaan GF. 2009. Vaccination against human influenza A/H3N2 virus prevents the induction of heterosubtypic immunity against lethal infection with avian influenza A/H5N1 virus. PLoS One 4:e5538. 10.1371/journal.pone.0005538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bodewes R, Fraaij PL, Kreijtz JH, Geelhoed-Mieras MM, Fouchier RA, Osterhaus AD, Rimmelzwaan GF. 2012. Annual influenza vaccination affects the development of heterosubtypic immunity. Vaccine 30:7407–7410. 10.1016/j.vaccine.2012.04.086 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.