Abstract
Insertions in the protease (PR) region of human immunodeficiency virus (HIV) represent an interesting mechanism of antiviral resistance against HIV PR inhibitors (PIs). Here, we demonstrate the improved ability of a phosphonate-containing experimental HIV PI, GS-8374, relative to that of other PIs, to effectively inhibit patient-derived recombinant HIV strains bearing PR insertions and numerous other mutations. We correlate enzyme inhibition with the catalytic activities of corresponding recombinant PRs in vitro and provide a biochemical and structural analysis of the PR-inhibitor complex.
TEXT
Human immunodeficiency virus (HIV) protease (PR), which processes Gag and Gag-Pol polyprotein precursors into functional enzymes and structural proteins, is indispensable for the formation of mature viral particles (1). HIV PR is therefore a major target for anti-HIV treatments (reviewed in references 2, and 3 and others), and nine PR inhibitors (PIs) have been approved by the U.S. Food and Drug Administration for treatment of HIV-infected patients. However, evolution of drug-resistant PR variants remains a major issue in effective long-term clinical use of PIs (4, 5). PI-selected mutations include amino acid substitutions as well as insertions. Insertions of 1 to 6 amino acids have been detected at various sites in the viral PR sequence, such as the regions between codons 17 and 18, 22 and 25, 31 and 32, 35 and 38, 70 and 71, and 95 and 96 (6–9). Surveys indicate that the prevalence of insertions in the PR coding regions of HIV-positive patients ranges between 0.1% and 4.5% (8), with most insertions detected in the region between amino acids 33 and 39. Position 35 seems to be most prone to insertions (6, 10, 11).
We recently characterized the role of E35EE and L33LL amino acid insertions in antiviral resistance. In vitro characterization confirmed that these insertions contribute to viral resistance in most of the clinically used PIs (7). Recently, Gilead Sciences reported the design and profiling of GS-8374, a novel phosphonate-containing PI that exhibits potent inhibitory activity against a large panel of PI-resistant viruses (12). GS-8374, a diethylphosphonate derivative of TMC-126 (13, 14), exhibits favorable in vitro pharmacological properties and a resistance profile that is superior to all clinically approved PIs and to structurally similar compounds lacking a phosphonate moiety.
In this work, we set out to analyze the interaction of GS-8374 with drug-resistant PR variants containing amino acid insertions. We also aimed to uncover the structural basis for the ability of GS-8374 to effectively inhibit these rare but clinically relevant drug-resistant PR variants.
Compounds.
GS-8374, (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl (2S,3R)-1-(4-((diethoxyphosphoryl)methoxy)phenyl)-3-hydroxy-4-(N-isobutyl-4-methoxyphenylsulfonamido) butan-2-ylcarbamate), was synthesized at Gilead Sciences. Atazanavir (ATV), lopinavir (LPV), darunavir (DRV), nelfinavir (NFV), and amprenavir (APV) were isolated by reverse-phase high-performance liquid chromatography from their therapeutic formulations. TMC-126 and brecanavir were kindly provided by Gilead Sciences.
In vitro drug susceptibility analysis: relative Ki values.
We analyzed the in vitro kinetics of the inhibition of resistant PR variants with and without insertions by several PIs, including three investigational compounds (for sequences and kinetic characterization of the PRs, see Table 1). Patient-derived PR coding regions were amplified from recombinant viral clones as previously described (7). PR1 contains the E35EE insertion and 12 amino acid substitutions, while PR3 contains the L33LL insertion combined with 15 substitutions (Table 1). To characterize the specific role of the insertions in PI resistance, we prepared two additional PR variants (PR2 and PR4) with matching amino acid substitutions, but without the E35EE or L33LL insertions, by ligation of two PCR products using previously described primers and procedures (7). In addition, to dissect the effect of the insertions alone on the PI resistance profile, recombinant protease variants harboring amino acid insertions E35EE [WT(35)] and L33LL [WT(33)] in the backbone of the wild-type protease were prepared.
TABLE 1.
Ki values for the inhibition of PR mutants by clinically approved and investigational inhibitorsa
| HIV-1 protease (recombinant virus) | Amino acid substitutions and/or insertionb |
Ki in nM (EC50in nM)c |
||||||
|---|---|---|---|---|---|---|---|---|
| ATV | LPV | BCV | APV | DRV | TMC-126 | GS-8374 | ||
| Wild type (HXB2) | 0.044 ± 0.017 (2.7 ± 2.1) | 0.032 ± 0.005 (12 ± 4) | 0.004 ± 0.0033 (ND) | 0.075 ± 0.02 (33 ± 5) | 0.0017 ± 0.003 (2.0 ± 0.1) | 0.010 ± 0.006 (1.0 ± 0.4) | 0.010 ± 0.007 (3.3 ± 1.2) | |
| WT(35) | E35EE | 0.012 ± 0.002 (ND) | 0.049 ± 0.049 (ND) | 0.025 ± 0.021 (ND) | 0.21 ± 0.02 (ND) | 0.003 ± 0.002 (ND) | 0.080 ± 0.064 (ND) | 0.001 ± 0.0008 (ND) |
| PR1, E35EE (VPR1) | L10F, I13V, G16A, K20M, V32I, E35EE, K43T, M46V, I47V, I54 M, I64V, A71V, V82A | 1.3 ± 0.2 (7.0 ± 3.6) | 13 ± 2 (>1,000) | 0.41 ± 0.15 (ND) | 67 ± 5 (1,300 ± 100) | 2.1 ± 0.2 (46 ± 32) | 0.49 ± 0.004 (16 ± 6) | 0.11 ± 0.01 (4.0 ± 3.0) |
| PR2 (VPR2) | L10F, I13V, G16A, K20M, V32I, K43T, M46V, I47V, I54 M, I64V, A71V, V82A | 0.56 ± 0.10 (3.0 ± 1.0) | 1.8 ± 0.4 (550 ± 520) | 0.07 ± 0.03 (ND) | 14 ± 2 (290 ± 140) | 0.44 ± 0.19 (8.0 ± 7.8) | 0.083 ± 0.009 (3.9 ± 2.2) | 0.063 ± 0.030 (1.0 ± 0.0) |
| WT(33) | L33LL | 0.026 ± 0.043 (ND) | 0.0006 ± 0.0004 (ND) | 0.010 ± 0.018 (ND) | 0.23 ± 0.09 (ND) | 0.019 ± 0.013 (ND) | 0.029 ± 0.014 (ND) | 0.071 ± 0.005 (ND) |
| PR3, L33LL (VPR3) | L10I, I13V, K20I, L33LL, M36I, M46I, I54V, K55R, Q58E, I62V, L63P, C67F, A71V, G73S, V82A, L90M | 5.6 ± 0.5 (220 ± 7) | 2.8 ± 0.5 (>1,000) | 0.007 ± 0.011 (ND) | 1.7 ± 0.2 (140 ± 10) | 0.15 ± 0.01 (4.0 ± 2.6) | 0.22 ± 0.02 (2.3 ± 1.7) | 0.16 ± 0.02 (2.3 ± 1.2) |
| PR4 (VPR4) | L10I, I13V, K20I, M36I, M46I, I54V, K55R, Q58E, I62V, L63P, C67F, A71V, G73S, V82A, L90M | 2.6 ± 0.8 (370 ± 140) | 0.88 ± 0.14 (970 ± 240) | 0.0021 ± 0.0093 (ND) | 1.4 ± 0.1 (200 ± 40) | 0.016 ± 0.013 (3.7 ± 0.6) | 0.11 ± 0.05 (1.2 ± 0.6) | 0.074 ± 0.005 (2.0 ± 0.1) |
PIs included atazanavir (ATV), lopinavir (LPV), brecanavir (BCV), amprenavir (APV), darunavir (DRV), TMC-126, and GS-8374.
Amino acid substitutions with respect to the HIV-1 sequence database consensus B sequence (http://www.hiv.lanl.gov). Insertions are in bold.
Inhibition constants were determined by spectrophotometric assay at pH 5.6. Numbers in parentheses are antiviral EC50s for the corresponding recombinant viruses determined from at least three independent measurements. The antiviral activities of the PIs was determined using a multicycle HIV-1 cytopathic assay in the MT-2 T cell line. All values are means ± SD. ND, not determined.
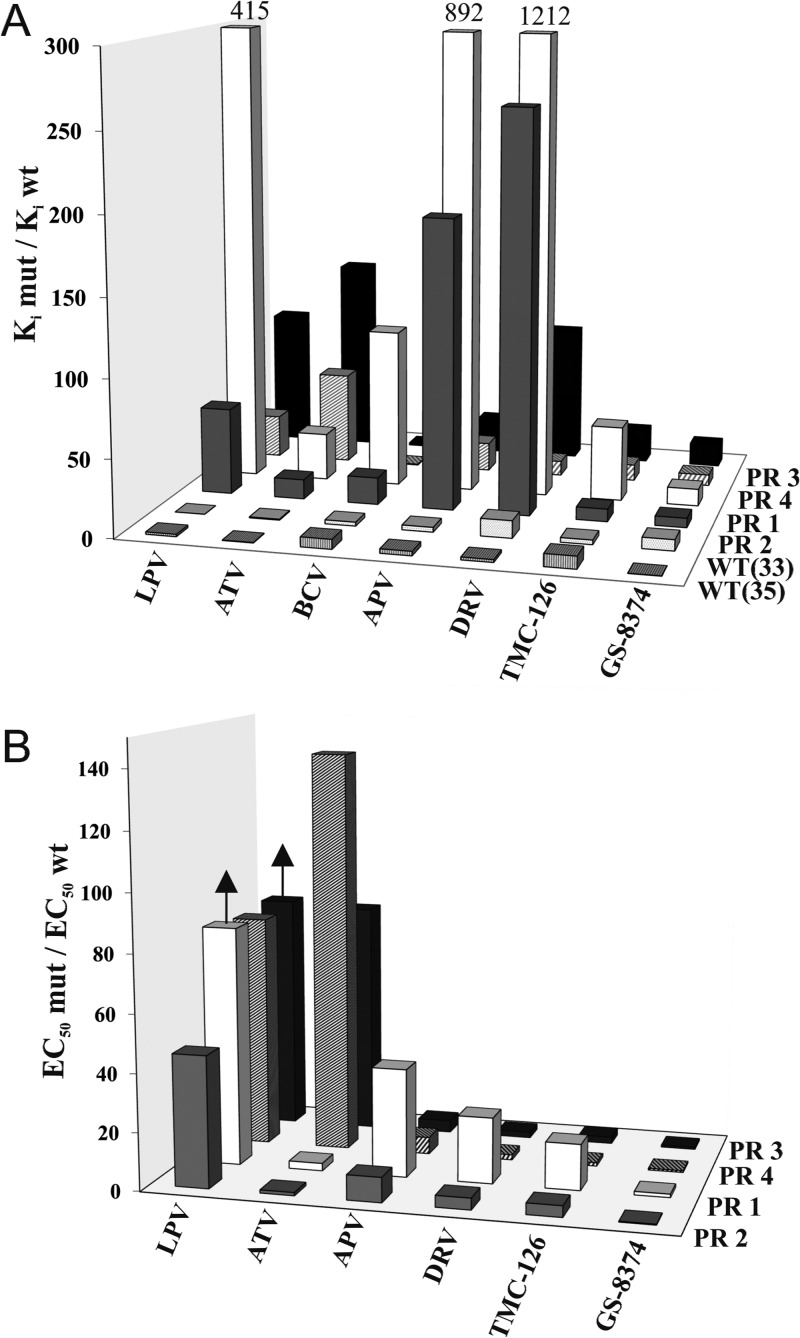
All PR variants were overexpressed in Escherichia coli and purified to homogeneity using established protocols (15). We analyzed their catalytic activities, which show a 3- to 5-fold decrease in kcat and an approximately 3-fold increase in Km, leading to a 5- to 10-fold decrease in overall catalytic efficiency relative to the WT enzyme (data not shown). We also determined inhibition constants (Ki) for four clinically used inhibitors, including LPV, APV, ATV, and DRV, and for three experimental compounds, GS-8374, TMC-126, and BCV (Table 1), by a spectrophotometric assay using a chromogenic peptide substrate (16). BCV was a bis-tetrahydrofuran-containing an investigational PI discontinued after phase 2 studies in HIV-infected patients that exhibited a favorable resistance profile against a large panel of patient-derived PI-resistant viruses (17), and therefore it was used as a comparative control along with TMC-126, the parent compound of GS-8374 lacking the phoshonate moiety (18). The potency of GS-8374 and TMC-126 to effectively inhibit multiple-drug-resistant PR species is reflected by the low relative inhibition values (i.e., ratios of Ki values for mutant and WT enzymes) of all PR variants in the presence of these inhibitors. Comparison of the relative inhibition data for PR1 (E35EE) and PR2 (without the insertion) revealed that the E35EE mutation decreases the sensitivity of the enzyme to the inhibition by all compounds tested except GS-8374 (Fig. 1A). A similar, albeit less pronounced effect on the PR sensitivity to inhibitors was seen with the L33LL insertion (compare PR3 and PR4 in Fig. 1A). Interestingly, the insertions alone without background PR mutations confer very little resistance to any of the tested PIs, and their contribution to the total resistant phenotype could be observed only in combination with multiple other mutations in PR. This is consistent with the lack of reports on the emergence of therapy-induced mutant HIV variants that would contain the sequence insertions alone in the absence of any other mutations (19).
FIG 1.
(A) Relative Ki values of PIs for tested mutant PRs normalized to the wild-type protease at pH 5.6. (B) Relative resistance of corresponding recombinant mutant HIV-1 variants as determined by a cytopathic virus replication assay in the MT-2 T cell line. The arrows indicate that the relative resistance is equal to or higher than the presented value (compare the actual values in Table 1). EC50, 50% effective concentration.
Phenotypic susceptibility of HIV strains containing PR insertion mutations to PIs.
The impact of PR insertions on phenotypic susceptibility to PIs was analyzed using an antiviral assay with corresponding recombinant viruses. The antiviral activity of PIs was tested in PR1- and PR3-containing HIV recombinant strains and in genotypically matched HIV strains without the corresponding PR insertions (PR2 and PR4). HIV-1 variants containing PR insertions were prepared from patient-derived clinical samples (7). Viral RNA was isolated from plasma according to the method described by Boom et al. (20) and used to reverse-transcribe and amplify PR and the C terminus of Gag. The cDNA amplicons were cloned into an HXB2 reference strain to yield recombinant viruses VPR1 and VPR3. Site-directed mutants VPR2 and VPR4, which lack the PR insertions, were generated as described previously (7). HIV-1 wild-type HXB2 served as a control strain susceptible to all tested PIs. In general, the relative antiviral resistance values (i.e., mutant EC50/wild-type EC50) correlated with the relative enzyme inhibition values obtained with purified recombinant PRs (compare Fig. 1A and B; see also Table 1). VPR1 mutations conferred a high-level resistance to all PIs except ATV, which showed only a moderate (2.7-fold) loss of activity against VPR1. In contrast, GS-8374 exhibited equal activities against both WT and VPR1. In comparison, the L33LL PR insertion showed a less pronounced effect on the susceptibility of VPR3 to the PIs. Notably, the presence of either of the two PR insertions had a minimal effect on the susceptibility of the HIV mutant strains to GS-8374.
X-ray structure analysis.
The crystal structures of PR1 and PR2 in complex with GS-8374 were determined by molecular replacement using protein coordinates from PR1 and PR2 in complex with LPV (PDB ID no. 2RKG and 2RKF [7]) and refined using diffraction data to 2.2 Å and 2.05 Å resolution, respectively (see Fig. 2B and C). Atomic coordinates and structure factors have been deposited in the Protein Data Bank under ID no. 4M8Y and 4M8X. Both crystals exhibited a hexagonal symmetry and an identical space group (P61), with one PR dimer in the asymmetric unit. Structures were refined with two inhibitor molecules bound in alternative orientations related by a 180° rotation with 50% relative occupancy.
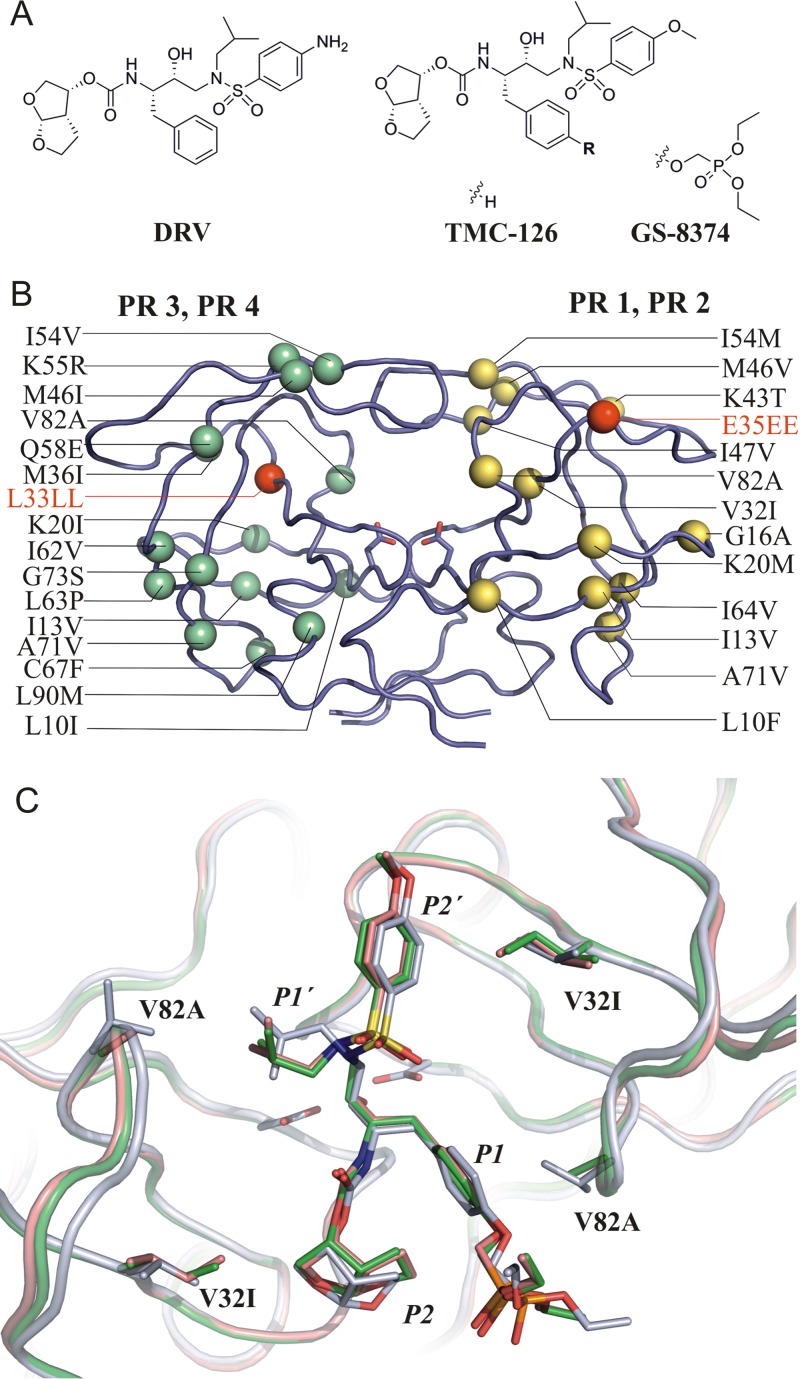
FIG 2.
(A) Structures of GS-8374 and other bis-tetrahydrofuran-containing PIs used in this study. (B) Positions of mutations in PR variants used for structural studies. Each monomer of PR is used to depict mutations in the PR1/PR2 and PR3/PR4 variants in yellow and green, respectively. Insertion mutations are highlighted in red. Mutated residues are represented by their Cα atoms (spheres), and active- site aspartates are shown as sticks; inhibitor bound to the active site was omitted from the figure. (C) Superposition of GS-8374 bound to wild-type PR (gray), PR1 (green), and PR2 (pink). Mutated residues are shown in stick representation; the flap region (residues 46 to 56), which covers the active site from the top, is omitted for clarity.
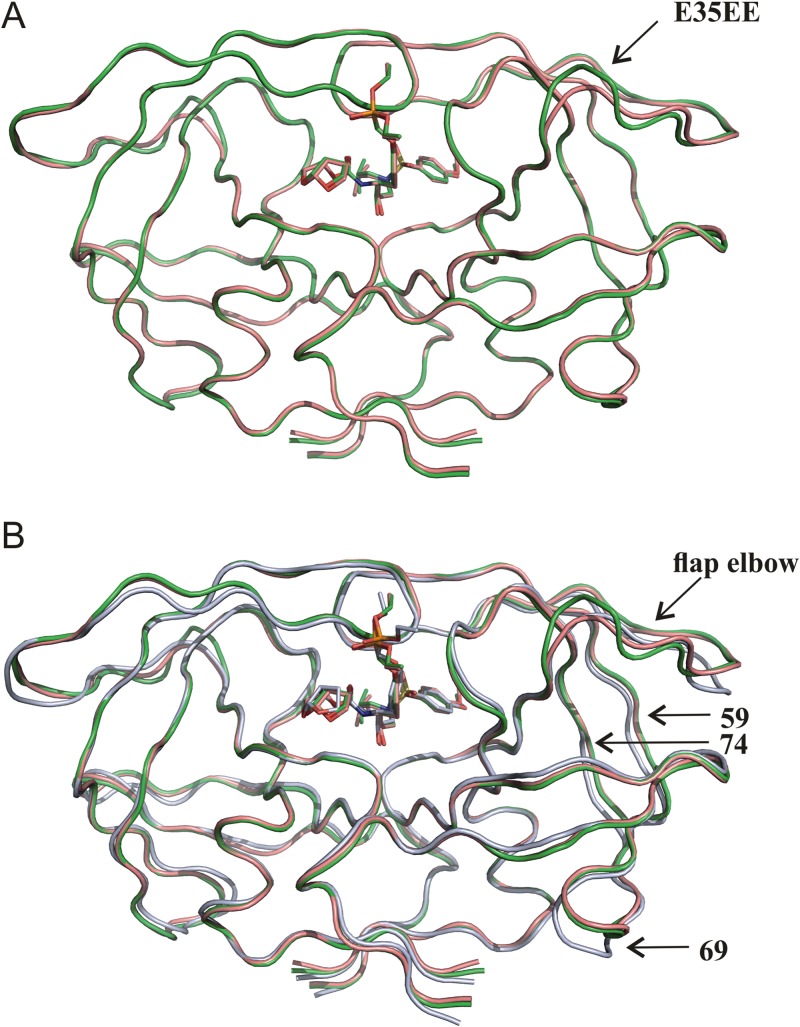
As previously seen in structures of PR1 and PR2 in complex with LPV (PDB ID no. 2RKG and 2RKF [7]), the E35EE insertion in PR1 causes structural changes in the flap elbow region (residues 34 to 38) (Fig. 3A). A comparison of the PR1 and PR2 structures with the structure of wild-type PR in complex with GS-8374 (PDB ID no. 2I4W [18]) revealed structural changes in inhibitor binding induced by the presence of mutations. The root mean square deviations (RMSDs) for superposition of the wild-type structure with PR1 and PR2 are 0.807 and 0.805 Å, respectively. Major structural differences (with an RMSD of >1 Å) for PR variants compared to the wild-type enzyme are located within the flap elbow (residues 34 to 38) and in regions around residues 59 to 74 (Fig. 3B). A detailed inspection of inhibitor binding to the mutated PR variants and wild-type PR revealed a displacement of GS-8374 in the active site of the mutated enzymes (see Fig. 2C). The V32I and I47V mutations change the shape of the S2 and S2′ pockets and consequently lead to adjustments of the P2 and P2′ substituent positions. We also observed significant differences for the P1 and P1′ groups. The V82A mutation enlarged the S1′ pocket, causing a significant change in the conformation of the P1′ isobutyl substituent. The P1 aromatic ring and the phosphonate group were rotated by about 20° relative to their positions in wild-type PR. The rotation of the phosphonate group is likely induced by a change in the molecular surface at the opening of the substrate binding tunnel caused by the V82A mutation.
FIG 3.
(A) Superposition of structures of PR1 (green) and PR2 (pink) in complex with GS-8374. The structures are very similar, with an RMSD of 0.378 Å for superposition of the corresponding Cα atoms. (B) Superposition of PR1 (green) and PR2 (pink) structures with the structure of the wild-type PR (gray) in complex with GS-8374.
The ability of GS-8374 to exploit the effectively larger active- site volume of a mutant PR was established by analysis of the crystal structure of the PR I84V/L90M mutant in complex with the inhibitor (PDB ID no. 2I4X [18]). This effect was termed solvent anchoring, since the solvent-exposed phosphonate group was shown to be crucial for an effective adaptation of the inhibitor to structural changes in the active site of mutated enzymes. The crystal structures of PR1 and PR2 presented here also demonstrate substantial adjustment of GS-8374 in the active site of heavily mutated PR variants (containing 12 mutations and one insertion). In addition, we also observed an adjustment of the solvent-exposed phosphonate group (the solvent anchor) of GS-8374. This ability of the inhibitor to adjust its position and conformation to efficiently fill the enlarged pockets of the mutant active-site cavity together with the additional interaction at the opening of the substrate binding tunnel (Fig. 2C) can explain the ability of GS-8374 to maintain the inhibitory potency against PR1 and PR2.
In conclusion, this study further expanded the characterization of the resistance profile of the prototype phosphonate-containing PI GS-8374 and demonstrated the ability of the compound to effectively inhibit HIV variants exhibiting significant resistance to most clinically used PIs due to multiple PR substitutions and unique flap insertions. Biochemical characterization and crystallographic analysis of the mutant PR enzymes provided further mechanistic and molecular insight explaining the ability of GS-8374 to effectively inhibit these unique resistant PR variants.
Protein Data Bank accession numbers.
Atomic coordinates and structure factors for GS-8374 have been deposited in the Protein Data Bank under ID no. 4M8Y and 4M8X.
ACKNOWLEDGMENTS
We thank Hillary Hoffman for critical proofreading of the manuscript.
This work was supported by the Grant Agency of the Czech Republic (P207/11/1798) and in part by research projects RVO 68378050 and 61388963 awarded by the Academy of Sciences of the Czech Republic and by EU OPPC program CZ.2.16/3.1.00/24016.
Footnotes
Published ahead of print 26 December 2013
REFERENCES
- 1.Kohl NE, Emini EA, Schleif WA, Davis LJ, Heimbach JC, Dixon RAF, Scolnick EM, Sigal IS. 1988. Active human immunodeficiency virus protease is required for viral infectivity. Proc. Natl. Acad. Sci. U. S. A. 85:4686–4690. 10.1073/pnas.85.13.4686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wlodawer A, Vondrasek J. 1998. Inhibitors of HIV-1 protease: a major success of structure-assisted drug design. Annu. Rev. Biophys. Biomol. Struct. 27:249–284. 10.1146/annurev.biophys.27.1.249 [DOI] [PubMed] [Google Scholar]
- 3.Pokorna J, Machala L, Rezacova P, Konvalinka J. 2009. Current and novel inhibitors of HIV protease. Viruses 1:1209–1239. 10.3390/v1031209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menendez-Arias L. 2013. Molecular basis of human immunodeficiency virus type 1 drug resistance: overview and recent developments. Antiviral Res. 98:93–120. 10.1016/j.antiviral.2013.01.007 [DOI] [PubMed] [Google Scholar]
- 5.Fun A, Wensing AMJ, Verheyen J, Nijhuis M. 2012. Human immunodeficiency virus gag and protease: partners in resistance. Retrovirology 9:63. 10.1186/1742-4690-9-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amiel C, Charpentier C, Desire N, Bonnard P, Lebrette M-G, Weiss L, Pialoux G, Schneider V. 2011. Long-term follow-up of 11 protease inhibitor (PI)-naive and PI-treated HIV-infected patients harbouring virus with insertions in the HIV-1 protease gene. HIV Med. 12:138–144. 10.1111/j.1468-1293.2010.00862.x [DOI] [PubMed] [Google Scholar]
- 7.Kozisek M, Saskova KG, Rezacova P, Brynda J, van Maarseveen NM, de Jong D, Boucher CA, Kagan RM, Nijhuis M, Konvalinka J. 2008. Ninety-nine is not enough: molecular characterization of inhibitor resistant human immunodeficiency virus type 1 protease mutants with insertions in the flap region. J. Virol. 82:5869–5878. 10.1128/JVI.02325-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen JHK, Wong KH, Chan KC, Lam HY, Yuen KY, Cheng VCC, Yam WC. 2008. Molecular epidemiology and divergence of HIV type 1 protease codon 35 inserted strains among treatment-naive patients in Hong Kong 2008. AIDS Res. Hum. Retroviruses 24:537–542. 10.1089/aid.2007.0231 [DOI] [PubMed] [Google Scholar]
- 9.Paolucci S, Baldanti F, Dossena L, Gerna G. 2006. Amino acid insertions at position 35 of HIV-1 protease interfere with virus replication without modifying antiviral drug susceptibility. Antiviral Res. 69:181–185. 10.1016/j.antiviral.2005.12.005 [DOI] [PubMed] [Google Scholar]
- 10.Pereira-Vaz J, Duque V, Trindade L, Saraiva-Da-Cunh J, Melico-Silvestre A. 2009. Detection of the protease codon 35 amino acid insertion in sequences from treatment-naive HIV-1 subtype C infected individuals in the Central Region of Portugal. J. Clin. Virol. 46:169–172. 10.1016/j.jcv.2009.06.019 [DOI] [PubMed] [Google Scholar]
- 11.Grotto RMT, Corvino SM, Munhoz LDR, Ghedini CG, Pardini MIDC. 2011. A first case of protease codon 35 amino acid insertion in a HIV-1 subtype B sequence detected in the Bauru Region, State of Sao Paulo, Brazil: case report. Rev. Soc. Bras. Med. Trop. 44:392–394. 10.1590/S0037-868220110003000027 [DOI] [PubMed] [Google Scholar]
- 12.Callebaut C, Stray K, Tsai L, Williams M, Yang ZY, Cannizzaro C, Leavitt SA, Liu XH, Wang K, Murray BP, Mulato A, Hatada M, Priskich T, Parkin N, Swaminathan S, Lee W, He GX, Xu LH, Cihlar T. 2011. In vitro characterization of GS-8374, a novel phosphonate-containing inhibitor of HIV-1 protease with a favorable resistance profile. Antimicrob. Agents Chemother. 55:1366–1376. 10.1128/AAC.01183-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh AK, Chapsal BD, Weber IT, Mitsuya H. 2008. Design of HIV protease inhibitors targeting protein backbone: an effective strategy for combating drug resistance. Acc. Chem. Res. 41:78–86. 10.1021/ar7001232 [DOI] [PubMed] [Google Scholar]
- 14.Ghosh AK, Pretzer E, Cho H, Hussain KA, Duzgunes N. 2002. Antiviral activity of UIC-PI, a novel inhibitor of the human immunodeficiency virus type 1 protease. Antiviral Res. 54:29–36. 10.1016/S0166-3542(01)00209-1 [DOI] [PubMed] [Google Scholar]
- 15.Kozisek M, Bray J, Rezacova P, Saskova K, Brynda J, Pokorna J, Mammano F, Rulisek L, Konvalinka J. 2007. Molecular analysis of the HIV-1 resistance development: enzymatic activities, crystal structures, and thermodynamics of nelfinavir-resistant HIV protease mutants. J. Mol. Biol. 374:1005–1016. 10.1016/j.jmb.2007.09.083 [DOI] [PubMed] [Google Scholar]
- 16.Weber J, Mesters JR, Lepsik M, Prejdova J, Svec M, Sponarova J, Mlcochova P, Skalicka K, Strisovsky K, Uhlikova T, Soucek M, Machala L, Stankova M, Vondrasek J, Klimkait T, Kräusslich H-G, Hilgenfeld R, Konvalinka K. 2002. Unusual binding mode of an HIV-1 protease inhibitor explains its potency against multi-drug-resistant virus strains. J. Mol. Biol. 324:739–754. 10.1016/S0022-2836(02)01139-7 [DOI] [PubMed] [Google Scholar]
- 17.Hazen R, Harvey R, Ferris R, Craig C, Yates P, Griffin P, Miller J, Kaldor I, Ray J, Samano V, Furfine E, Spaltenstein A, Hale M, Tung R, St Clair M, Hanlon M, Boone L. 2007. In vitro antiviral activity of the novel, tyrosyl-based human immunodeficiency virus (HIV) type 1 protease inhibitor brecanavir (GW640385) in combination with other antiretrovirals and against a panel of protease inhibitor-resistant HIV. Antimicrob. Agents Chemother. 51:3147–3154. 10.1128/AAC.00401-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cihlar T, He GX, Liu X, Chen JM, Hatada M, Swaminathan S, McDermott MJ, Yang ZY, Mulato AS, Chen X, Leavitt SA, Stray KM, Lee WA. 2006. Suppression of HIV-1 protease inhibitor resistance by phosphonate-mediated solvent anchoring. J. Mol. Biol. 363:635–647. 10.1016/j.jmb.2006.07.073 [DOI] [PubMed] [Google Scholar]
- 19.Winters MA, Merigan TC. 2005. Insertions in the human immunodeficiency virus type 1 protease and reverse transcriptase genes: clinical impact and molecular mechanisms. Antimicrob. Agents Chemother. 49:2575–2582. 10.1128/AAC.49.7.2575-2582.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boom R, Sol C, Gerrits Y, De Boer M, Wertheim-van Dillen P. 1999. A highly sensitive assay for detection and quantitation of human cytomegalovirus DNA in serum and plasma by PCR and electrochemiluminescence. J. Clin. Microbiol. 37:1489–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]