Abstract
Francisella tularensis, the causative agent of tularemia, modulates the host immune response to gain a survival advantage within the host. One mechanism of immune evasion is the ability of F. tularensis to induce the synthesis of the small lipid mediator prostaglandin E2 (PGE2), which alters the host T cell response making the host more susceptible to Francisella growth. PGE2 is synthesized by a tightly regulated biosynthetic pathway following stimulation. The synthesis of PGE2 begins with the liberation of arachidonic acid (AA) from membrane phospholipids by cytosolic phospholipase A2 (cPLA2). AA is subsequently converted to the unstable intermediate PGH2 by cyclooxygenase-2 (COX-2), and PGH2 undergoes an isomerization reaction to generate PGE2. Our objective was to identify F. tularensis-activated host signaling pathways that regulate the activity of the enzymes in the PGE2-biosynthetic pathway. In this study, we show that cPLA2, p38 mitogen-activated protein kinase (MAPK), and Janus kinase 3 (JAK3) signaling are necessary for F. tularensis-induced PGE2 production. Inhibition of JAK3 activity reduced the phosphorylation of cPLA2 and COX-2 protein levels. In addition, JAK3 regulates cPLA2 phosphorylation independent of transcription. Moreover, p38 MAPK activity is required for F. tularensis-induced COX-2 protein synthesis, but not for the phosphorylation of cPLA2. This research highlights a unique signaling axis in which JAK3 and p38 MAPK regulate the activity of multiple enzymes of the PGE2-biosynthetic pathway in macrophages infected with F. tularensis.
INTRODUCTION
Francisella tularensis is a highly virulent Gram-negative facultative intracellular bacterium and the causative agent of tularemia. The inhalation of as few as 10 F. tularensis organisms is capable of causing pneumonic tularemia (1). Pneumonic tularemia is the most severe form of tularemia, with a case fatality rate of 30% if untreated (1, 2). F. tularensis is classified by the Centers for Disease Control and Prevention as a category A select agent due to its low infectious dose, its ease of dissemination, and the high morbidity and mortality rate associated with the disease. There is currently no FDA-approved vaccine.
F. tularensis invades and replicates within numerous host cell types. The ability of F. tularensis to invade and replicate within macrophages is paramount to its survival within the host, as F. tularensis mutants that fail to replicate in macrophages are avirulent (3). However, modulation of the host immune response by F. tularensis is also important to bacterial survival within the host (4). Numerous mechanisms of F. tularensis-mediated immune evasion have been identified (reviewed in reference 5). F. tularensis can inactivate complement, modify its lipopolysaccharide (LPS) structure to minimize recognition by TLR4, disrupt NADPH assembly to inhibit a respiratory burst, induce the synthesis of anti-inflammatory mediators by the host, and alter macrophage differentiation. One immune evasion mechanism we identified is the ability of both F. tularensis subsp. tularensis Schu S4 and F. tularensis subspecies holarctica LVS (live vaccine strain) to induce the synthesis of prostaglandin E2 (PGE2) from infected macrophages (6, 7). Prostaglandins are a family of small lipid mediators that have both anti- and proinflammatory functions depending on the cellular context (8, 9). F. tularensis LVS-induced PGE2 downregulates major histocompatibility complex (MHC) class II expression on infected macrophages via a ubiquitin-dependent mechanism (10). F. tularensis LVS-induced PGE2 also blocks T cell proliferation and skews the T cell response away from a Th1- to a Th2-like response in vitro (7). Proinflammatory Th1 cells that secrete gamma interferon (IFN-γ) and/or tumor necrosis factor alpha (TNF-α) are critical for both clearance of F. tularensis from the host and the generation of long-term immunity (11). Blocking the synthesis of PGE2 by administering the cyclooxygenase inhibitor indomethacin increases the number of F. tularensis-specific IFN-γ+ T cells and decreases bacterial burden in the lungs of mice infected with F. tularensis LVS (12). These results suggest that the ability of F. tularensis to stimulate the synthesis of PGE2 provides the organism with a growth advantage within the host.
PGE2 is not stored within host cells but rather is synthesized by a cell through a tightly regulated biosynthetic pathway. Induction of the canonical inducible PGE2-biosynthetic pathway requires the liberation of arachidonic acid (AA) from cell membrane phospholipids by cytosolic phospholipase A2 (cPLA2) (13, 14). Free AA is then oxidized to PGH2 by cyclooxygenase-2 (COX-2). PGH2 is isomerized to PGE2 by a terminal prostaglandin E synthase. COX-2 is essential for F. tularensis-induced PGE2 synthesis. However, the importance of cPLA2 in the production of PGE2 in macrophages infected with F. tularensis is unknown. In this study, we determined that cPLA2 activity is critical for the synthesis of PGE2 in macrophages infected with F. tularensis.
Different regulatory processes control each enzyme of the inducible PGE2-biosynthetic pathway. Phosphorylation of cPLA2 on Ser505 by mitogen-activated protein kinases (MAPKs) results in increased enzymatic activity of cPLA2 (15). COX-2 is regulated at the transcriptional and posttranscriptional levels (16); thus, an increase in COX-2 protein levels typically correlates with an increase in activity. Though the PGE2-biosynthetic pathway has been extensively studied, the eukaryotic signaling pathways that regulate the activation of cPLA2 and COX-2 are poorly understood, particularly within the context of bacterial infection. The objective of this study was to identify macrophage signaling pathways that mediate the activity of the key enzymes in the PGE2-biosynthetic pathway and ultimately the synthesis of PGE2 in macrophages infected with F. tularensis.
In the present study, we demonstrate that increased COX-2 protein levels, cPLA2 phosphorylation, and PGE2 synthesis in F. tularensis LVS-infected murine macrophages are dependent on Janus kinase 3 (JAK3). Importantly, JAK3 regulates the phosphorylation of cPLA2 through a transcription-independent mechanism. Additionally, we demonstrate that p38 MAPK activity is necessary for enhanced COX-2 protein levels but not for increased phosphorylation of cPLA2 in macrophages infected with F. tularensis. To our knowledge, we provide the first evidence that JAK3 can positively regulate the PGE2-biosynthetic pathway.
MATERIALS AND METHODS
Bacteria.
F. tularensis LVS (ATCC 29684; American Type Culture Collection) was used in this study. Bacteria were grown on brain heart infusion (BHI) agar supplemented with 1% hemoglobin and 1% IsoVitaleX. To prepare bacterial inoculations, bacteria were removed from an overnight lawn grown on BHI agar and resuspended in sterile phosphate-buffered saline (PBS) at an optical density at 600 nm (OD600) of 1 (equivalent to 5 × 109 CFU/ml). Appropriate dilutions were made in sterile phosphate-buffered saline (PBS) to obtain the desired bacterial inoculum. To determine if the pharmacological inhibitors used in this study affected F. tularensis LVS growth in broth, we performed a growth assay in a 96-well plate in the absence or presence of 50 μM JANEX-1, 15 μM SB203580, or 5 μM pyrrophenone using a Spectramax 190 plate reader. OD600 readings were taken every 5 min for 12 h.
Mice.
Female C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor, ME). All animals used in this study were maintained under specific-pathogen-free conditions in the American Association for Laboratory Animal Care (AALAC)-accredited Louisiana State University Health Sciences Center (LSUHSC) animal medicine facilities. All work was approved by LSUHSC Animal Care and Use Committee (ACUC).
Generation and culture of bone marrow-derived macrophages.
Bone marrow cells from female C57BL/6J mouse femurs were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 30% L-cell conditioned medium, 20% fetal bovine serum (Atlas Biologicals), 1% l-glutamine (HyClone), 1% penicillin-streptomycin (ATCC), 1% sodium pyruvate (HyClone), and 0.2% sodium bicarbonate for 6 days at 37°C and 5% CO2. Sixteen hours prior to F. tularensis inoculation of bone marrow-derived macrophages (BMDMs), the medium was replaced with antibiotic-free RPMI 1640 medium (HyClone) supplemented with 1% l-glutamine (HyClone), 1% sodium pyruvate (HyClone), 0.1% beta-mercaptoethanol, and 10% fetal bovine serum (Atlas Biologicals). On the day of inoculation, nonadherent cells were removed by a PBS wash, and BMDMs were removed from petri dishes by treatment with 1 mM EDTA in PBS (pH 7.6).
Cell culture.
The RAW264.7 murine macrophage-like cell line was obtained from the American Type Culture Collection (Manassas, VA) and maintained in DMEM supplemented with 1% l-glutamine (HyClone), 1% penicillin-streptomycin (ATCC), 1% sodium pyruvate (HyClone), 0.1% beta-mercaptoethanol, and 10% fetal bovine serum (Atlas Biologicals). Sixteen hours prior to F. tularensis inoculation, the medium was replaced with antibiotic-free RPMI (HyClone) supplemented with 1% l-glutamine (HyClone), 1% sodium pyruvate (HyClone), 0.1% beta-mercaptoethanol, and 10% fetal bovine serum (Atlas Biologicals).
Stimulation of macrophages with F. tularensis LVS, interleukin 4 (IL-4), or lipopolysaccharide (LPS).
Stimulation was carried out in either a 96-well plate (2 × 105 cells/well), a 24-well plate (1.5 × 106 cells/well), or a 12-well plate (106 cells/well). Macrophages were allowed to adhere for 2 h prior to their stimulation with 100 ng/ml LPS, 30 ng/ml IL-4 or F. tularensis LVS at a multiplicity of infection (MOI) of 200:1 (bacteria to macrophages). One hour prior to stimulation, macrophages were treated with specific inhibitor compounds. After treatment of macrophages with the particular stimuli, the plate was spun for 5 min at 300 × g to allow closer contact and more efficient infection by F. tularensis LVS. The plate was maintained in an incubator at 37°C and 5% CO2 throughout the experiment. For experiments in which spent medium was collected at 24 h postinoculation to determine the concentration of PGE2, F. tularensis LVS was coincubated with macrophages for 2 h and then macrophages were treated with 50 μg/ml gentamicin for 1 h to kill extracellular bacteria. The macrophages were then washed twice with fresh antibiotic-free RPMI 1640 medium. Antibiotic-free RPMI 1640 medium was added to the macrophages and collected at 24 h postinoculation. For experiments in which cellular lysates were collected beyond 2 h postinoculation, macrophages were treated with penicillin-streptomycin to kill intracellular F. tularensis at 2 h postinoculation.
Pharmacological inhibitors.
The inhibitors and the final concentration of each inhibitor used in this study were 5 μM pyrrophenone (Cayman Chemical), 15 μM SB203580 (Calbiochem), 50 μM JANEX-1 (Cayman Chemical), 10 μM ruxolitinib (Cayman Chemical), 50 μM AG490 (Calbiochem), and 2 μg/ml actinomycin D (Sigma). All inhibitors were dissolved in DMSO. Working concentrations of 100× of all inhibitors were made in complete medium, and 1/100 of the 100× inhibitor solution was added to the appropriate wells to get the inhibitor to the final concentration used throughout this study. Inhibitors were added 1 h prior to inoculation and were maintained throughout the experiment.
Live/dead fixable dead-cell stain.
A total of 106 macrophages were put into a 15-ml conical tube. The cells were treated 1 h after being put into the conical tube with 5 μM pyrrophenone, 15 μM SB203580, or 50 μM JANEX-1. One hour after inhibitor treatment, the cells were either uninfected or inoculated with F. tularensis LVS at an MOI of 200:1. At 2 h postinoculation, cells were treated with 50 μg/ml gentamicin for 1 h and then washed once with fresh antibiotic-free RPMI 1640 medium. At 20 h postinoculation, the cells were washed once with PBS and resuspended in PBS, and 1 μl of live/dead yellow fluorescent reactive dye (Life Technologies) was added to each conical tube for 30 min at room temperature protected from light. Cells were washed twice with PBS and resuspended in 500 μl fluorescence-activated cell sorting (FACS) fixative. The percentage of live and dead cells was determined by flow cytometry.
Lentiviral delivery of short-hairpin RNA (shRNA).
Short-hairpin RNAs (shRNAs) directed toward JAK3 were delivered into RAW264.7 macrophages using Mission lentiviral transduction particles (Sigma-Aldrich). Lentiviral particles were used to deliver JAK3 shRNA into RAW264.7 macrophages. Twenty-four hours after addition of the lentivirus, macrophages were put under puromycin selection (5 μg/ml) for 48 h to select for cells expressing the JAK3 shRNA. To confirm that JAK3 gene expression was reduced in these cells, we examined the expression levels of JAK3 mRNA by RT-PCR. Briefly, RNA was extracted from cells following the RNA STAT-60 protocol for RNA extraction. cDNA was synthesized from DNA-free RNA using a cDNA synthesis kit (Bio-Rad). Samples of cDNA were subjected to reverse transcription-PCR (RT-PCR) using specific primers for JAK3 with hypoxanthine phosphoribosyltransferase (HPRT) as an internal control. The primer sequences used for amplifying JAK3 were 5′-GGCGTGGCGGTTAGTAAAGAA-3′ (forward) and 5′-CCCCCTATCTAGTCTCACCCT-3′ (reverse), and those for HPRT were 5′-CGTCTTGCTCGAGATGTGATG-3′ (forward) and 5′-TTTATAGCCCCCCTTGAGCAC-3′ (reverse).
Bacterial uptake and intracellular replication of F. tularensis LVS in macrophages.
A total of 105 RAW264.7 macrophages were plated in each well of a 96-well plate. We followed the protocol described above for stimulation of macrophages with F. tularensis LVS in the presence of 15 μM SB203580, 50 μM JANEX-1, and 5 μM pyrrophenone. To determine bacterial uptake and replication, infected macrophages were lysed at 6 h postinoculation (uptake) and 24 h postinoculation (replication) with PBS containing 0.5% SDS. Ten-fold serial dilutions were performed in PBS, and dilutions were plated on chocolate agar plates. The plates were incubated for 2 days at 37°C in 5% CO2, and colonies were counted and CFU/ml were calculated.
Western blot analyses.
At the appropriate time points, host cells were lysed based on the number of live cells. A ratio of 10 μl of lysis buffer to 2 × 105 live host cells was used throughout this study. The denaturing lysis buffer used in this study was 1× NuPage LDS sample buffer containing 100 mM dithiothreitol (DTT) (Life Technologies), 1× protease inhibitor cocktail (Thermo Scientific), 1× phosphatase inhibitor cocktail 1 (Sigma-Aldrich), and 1× phosphatase inhibitor cocktail 3 (Sigma-Aldrich). To minimize the potential dilution effect bacterial protein could have on the measurement of host protein levels, we loaded equivalent numbers of lysed host cells per sample rather than equivalent amounts of protein. Samples were sonicated and boiled prior to gel loading. A total of 10 μl of whole-cell lysate was loaded per well of the gel. Proteins in cell lysates were separated by 4 to 12% SDS-PAGE (Life Technologies) utilizing a Life Technologies XCell SureLock electrophoresis chamber system at constant amperage (50 mA). Proteins were transferred onto an Immobilon-FL transfer membrane (Millipore) using a semidry blotter run at a constant 20 V for 1 h at room temperature. Membranes were blocked in LiCor blocking buffer (LiCor Biosciences) and then incubated with primary antibodies against alpha/beta tubulin (1:2,000), p38 MAPK (1:1,000), phospho-p38 MAPK (Thr180/Tyr182) (1:1,000), phospho-STAT6 (Tyr641) (1:1,000) (all from Cell Signaling Technology), COX-2 (1:500) (Cayman Chemical Company), cPLA2 (1:1,000), or phospho-cPLA2 (Ser505) (1:500) (both from Santa Cruz Biotechnology). Membranes were washed in PBS containing 0.1% Tween-20 and then incubated in anti-rabbit IgG (IRDye 680 LT) (1:20,000) secondary antibody (LiCor Biosciences). Bands were detected using the LiCor Odyssey infrared imaging system. Band densities were determined using the LiCor Odyssey infrared imaging system software. For each sample, we normalized the band intensity of the protein of interest for that sample to the tubulin loading control for that same sample by using the following equation: band intensity of protein of interest/band intensity of tubulin. Once we had normalized the band intensity for the protein of interest in each sample, we then used these normalized values to make comparisons between samples to determine changes in protein levels of our proteins of interest.
PGE2 ELISA.
PGE2 concentration in supernatants was determined using a commercially available PGE2 enzyme-linked immunosorbent assay (ELISA) kit (ENZO Life Sciences, Farmingdale, NY) according to the manufacturer's protocol.
Statistical analyses.
Data were analyzed using one-way analysis of variance (ANOVA) followed by Dunnett's test. Western blot protein intensities were first normalized to those of infected untreated samples and then analyzed to remove technical variability associated with Western blot analysis. The GraphPad Prism 5.0 software program was used for analysis. Statistical significance was determined by a P value of <0.05.
RESULTS
Cytosolic phospholipase A2 is required for F. tularensis LVS-induced PGE2 synthesis by macrophages.
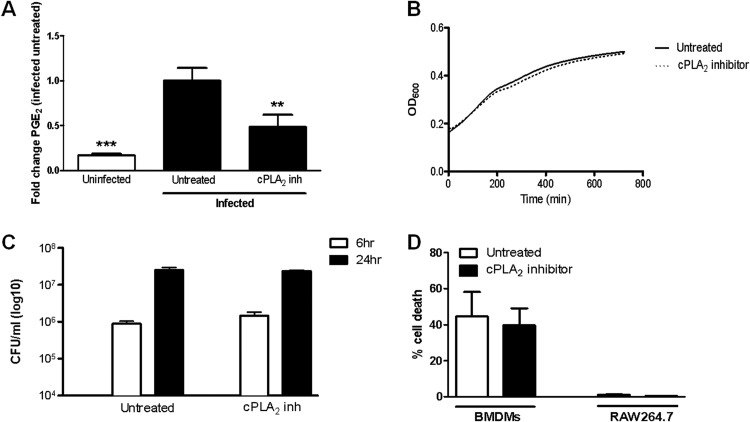
We previously demonstrated that F. tularensis induces the synthesis of PGE2 by infected macrophages (6, 7). It is unknown if cPLA2 is required for the synthesis of PGE2 by macrophages infected with F. tularensis. Therefore, we wanted to examine if cPLA2 activity is necessary for the production of PGE2 by macrophages infected with F. tularensis. Bone marrow-derived macrophages (BMDMs) were treated with the well-characterized cPLA2 inhibitor pyrrophenone 1 h prior to being inoculated with F. tularensis LVS at a multiplicity of infection (MOI) of 200:1 (bacteria to macrophages). At 24 h postinoculation, supernatants were collected, and the concentration of PGE2 was determined by ELISA. Treatment with pyrrophenone significantly reduced F. tularensis LVS-induced PGE2 synthesis (Fig. 1A), suggesting that cPLA2 activity is required for increased PGE2 synthesis by macrophages infected with F. tularensis. To ensure that the decrease in PGE2 production observed with pyrrophenone treatment was not due to an adverse effect of the inhibitor on the bacteria or the macrophages, we examined F. tularensis LVS growth in broth, macrophage uptake of LVS, intramacrophage growth of LVS, and macrophage viability with pyrrophenone treatment. Treatment with pyrrophenone did not prevent LVS growth in broth culture (Fig. 1B), macrophage uptake of LVS, or intramacrophage growth of LVS in RAW264.7 macrophages (Fig. 1C). Lastly, we did not observe decreased cell viability in F. tularensis-infected RAW264.7 macrophages or BMDMs treated with pyrrophenone (Fig. 1D). These data strongly imply that cPLA2 is necessary for the synthesis of PGE2 by macrophages infected with F. tularensis.
FIG 1.
Inhibition of cPLA2 significantly decreases F. tularensis LVS-induced PGE2 synthesis by macrophages. BMDMs were either untreated or pretreated for 1 h with 5 μM pyrrophenone (cPLA2 inhibitor) prior to inoculation with F. tularensis LVS at an MOI of 200:1. The inhibitor was maintained throughout the experiment. At 24 h postinoculation, supernatants were collected, and PGE2 concentration was determined by ELISA. The results are the means and standard errors of the means (SEM) from four independent experiments. (B) F. tularensis LVS was grown in BHI-IsoVitaleX broth in a 96-well plate in the absence or presence of 5 μM pyrrophenone for 12 h, with OD600 readings taken every 5 min. Results are representative of three independent experiments. (C) RAW264.7 macrophages were either untreated or pretreated for 1 h with 5 μM pyrrophenone prior to inoculation with F. tularensis LVS at an MOI of 200:1. Cells were lysed at 6 h and 24 h postinoculation to determine the number of viable bacteria (CFU). (D) RAW264.7 macrophages and BMDMs were either untreated or pretreated for 1 h with 5 μM pyrrophenone prior to inoculation with F. tularensis LVS at an MOI of 200:1. At 20 h postinoculation, a live/dead staining was performed. The results are the percent cell death from three independent experiments. Statistical differences (**, P ≤ 0.01; ***, P ≤ 0.001) compared with infected untreated controls were determined by Dunnett's multiple comparison test.
F. tularensis infection of macrophages increases COX-2 protein levels, cPLA2 phosphorylation, and PGE2 synthesis.
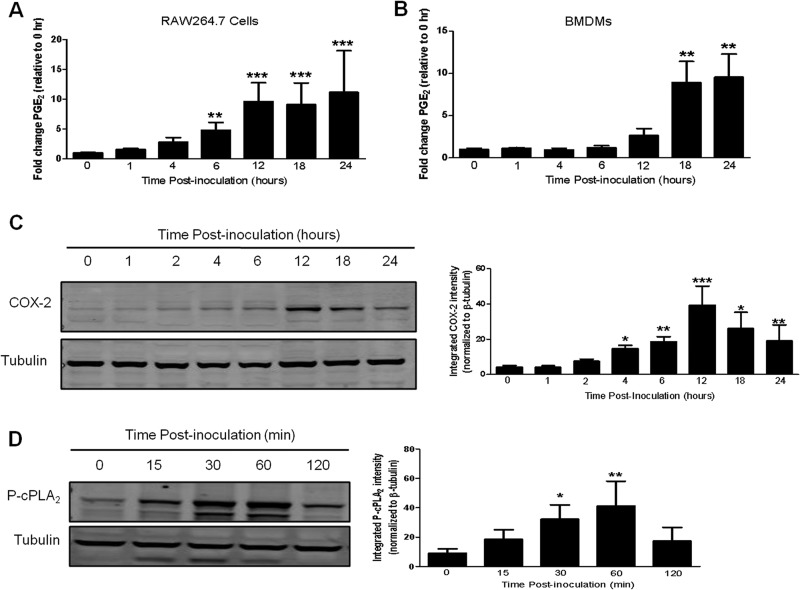
The kinetics of PGE2 synthesis following infection of macrophages with F. tularensis has not been examined. Determining the kinetics of PGE2 synthesis will allow us to more easily investigate the eukaryotic signaling pathways responsible for regulating PGE2 production in F. tularensis-infected macrophages. Therefore, we performed a time course experiment to determine when the concentration of PGE2 begins to increase in F. tularensis-infected macrophages. RAW264.7 cells and BMDMs were inoculated with F. tularensis LVS at an MOI of 200:1. At 0, 1, 4, 6, 12, 18, and 24 h postinoculation, supernatants were collected, and the concentration of PGE2 in each sample was determined by ELISA. In RAW264.7 macrophages, we observed a significant increase in PGE2 synthesis by 6 h postinoculation (Fig. 2A). In contrast, infected BMDMs did not synthesize a significant amount of PGE2 until 18 h postinoculation (Fig. 2B). One would hypothesize that an increase in the synthesis of PGE2 would correlate with increased activation of the enzymes in the PGE2-biosynthetic pathway. To test this hypothesis, we examined the kinetics of COX-2 protein synthesis and cPLA2 phosphorylation.
FIG 2.
F. tularensis infection of macrophages increases PGE2 synthesis, COX-2 protein levels, and cPLA2 phosphorylation. (A and B) RAW264.7 macrophages (A) or BMDMs (B) were inoculated with LVS at an MOI of 200:1. At 0, 1, 4, 6, 12, 18, and 24 h postinoculation, supernatants were collected, and PGE2 concentrations were determined. The data are the means and SEM from three independent experiments. (C) RAW264.7 macrophages were inoculated with LVS at an MOI of 200:1. At 0, 1, 2, 4, 6, 12, 18, and 24 h postinoculation, whole-cell lysates were collected and analyzed for COX-2 protein levels by Western blotting (left). The band intensities (right) were measured in each experiment using LiCor Odyssey infrared imaging system software and were normalized to the loading control (tubulin). The graph shows the band intensities from three independent experiments. (D) BMDMs were inoculated with LVS at an MOI of 200:1. At 0, 15, 30, 60, and 120 min postinoculation whole-cell lysates were collected and analyzed for cPLA2 phosphorylated on Ser505 by Western blotting (left). The band intensities (right) were measured in each experiment using LiCor Odyssey infrared imaging system software and were normalized to the loading control (tubulin).The graph shows the band intensities from three independent experiments. Statistical differences (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001) compared to the 0-h sample were determined by Dunnett's multiple comparison test.
COX-2 is tightly regulated at the transcriptional and posttranscriptional levels and there is no evidence of posttranslational modifications regulating COX-2 activity. Therefore, an increase in the level of COX-2 protein within a cell correlates with an increase in activity. To determine COX-2 protein levels in F. tularensis-infected macrophages, RAW264.7 macrophages were inoculated with LVS at an MOI of 200:1. The RAW264.7 murine macrophage cell line was used in these experiments because the level of COX-2 protein in BMDMs is too low to be detected by Western blotting. At 0, 1, 4, 6, 12, 18, and 24 h postinoculation, whole-cell lysates were collected and examined for COX-2 protein levels by Western blotting. COX-2 protein levels increased in a time-dependent manner with a significant increase in COX-2 protein levels occurring at 4 h postinoculation and peaking at 12 h postinoculation (Fig. 2C).
cPLA2 is regulated posttranslationally by phosphorylation on Ser505. Therefore, an increase in cPLA2 phosphorylation on Ser505 indicates an increase in cPLA2 activity (17). We demonstrated that cPLA2 activity is required for F. tularensis-induced PGE2 synthesis (Fig. 1A). To determine if cPLA2 is phosphorylated in macrophages infected with F. tularensis, BMDMs were inoculated with LVS at an MOI of 200:1 and whole-cell lysates were collected at 0, 15, 30, 60, and 120 min postinoculation. Whole-cell lysates were subjected to Western blot analysis using an antibody that recognizes cPLA2 phosphorylated on Ser505. F. tularensis induced a time-dependent increase in cPLA2 phosphorylation on Ser505 with significant phosphorylation of cPLA2 occurring as early as 30 min postinoculation and peaking at 60 min postinoculation (Fig. 2D). The level of cPLA2 phosphorylation returns to uninfected levels by 2 h postinoculation. These data show that F. tularensis infection of macrophages increases cPLA2 phosphorylation and COX-2 protein levels which are required for the synthesis of PGE2.
JAK3 and p38 MAPK activities are required for F. tularensis-induced PGE2 synthesis by primary murine macrophages.
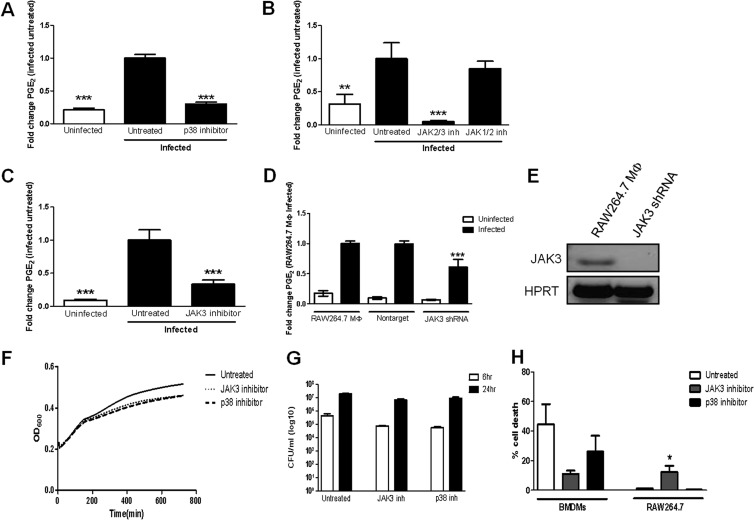
p38 MAPK regulates PGE2 synthesis in nonmacrophage cells, including lung epithelial cells infected with Haemophilus influenzae (18). F. tularensis stimulates the phosphorylation and activation of p38 MAPK following infection of macrophages (19). This led us to hypothesize that p38 MAPK is involved in regulating PGE2 synthesis by macrophages infected with F. tularensis. To test this hypothesis, we utilized a well-characterized inhibitor of p38 MAPK, SB203580 (20). BMDMs were treated with 15 μM SB203580 prior to inoculation with LVS at an MOI of 200:1. At 24 h postinoculation, cell supernatants were collected, and PGE2 concentration was determined by ELISA. Treatment with SB203580 significantly decreased F. tularensis-induced PGE2 synthesis compared to infected untreated macrophages (Fig. 3A). Similar results were observed in F. tularensis-infected RAW264.7 macrophages (data not shown). These data suggest that p38 MAPK activity is necessary for the synthesis of PGE2 by macrophages infected with F. tularensis.
FIG 3.
Inhibition of JAK3 and p38 MAPK decreases F. tularensis LVS-induced PGE2 synthesis by macrophages. BMDMs were either untreated or pretreated for 1 h with 15 μM SB203580 (p38 MAPK inhibitor) (A), 50 μM AG490 (JAK2/3 inhibitor) (B), 400 nM ruxolitinib (JAK1/2 inhibitor) (B), or 50 μM JANEX-1 (JAK3 inhibitor) (C) prior to inoculation with F. tularensis LVS at an MOI of 200:1. At 24 h postinoculation, supernatants were collected, and PGE2 concentration was determined by ELISA. (D) RAW264.7 macrophages and nontarget (NT) shRNA- or JAK3 shRNA-expressing macrophages were inoculated with F. tularensis LVS at an MOI of 200:1. At 24 h postinoculation, supernatants were collected, and PGE2 concentration was determined by ELISA. Results are the means and SEM from nine (A), three (B), five (C), and five (D) independent experiments. (E) RT-PCR was performed using cDNA synthesized from RNA extracted from wild-type RAW264.7 macrophages and RAW264.7 macrophages expressing JAK3 shRNA. (F) F. tularensis LVS was grown in BHI-IsoVitaleX broth in a 96-well plate in the absence or presence of 15 μM SB203580 or 50 μM JANEX-1 for 12 h, with OD600 readings taken every 5 min. Results are representative of three independent experiments. (G) RAW264.7 macrophages were either untreated or pretreated for 1 h with 15 μM SB203580 or 50 μM JANEX-1 prior to inoculation with F. tularensis LVS at an MOI of 200:1. Cells were lysed at 6 h and 24 h postinoculation to determine the number of viable bacteria (CFU) on chocolate agar medium. (H) RAW264.7 macrophages and BMDMs were either untreated or pretreated for 1 h with 15 μM SB203580 or 50 μM JANEX-1 prior to inoculation with F. tularensis LVS at an MOI of 200:1. At 20 h postinoculation, a live/dead staining was performed. The results are the means from three independent experiments. Statistical differences (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001) compared with infected untreated or wild-type macrophages (D) were determined by Dunnett's multiple comparison test.
Janus kinases (JAKs) can regulate the synthesis of PGE2 in human colonic epithelial cells and human endometrial cancer cells (21, 22). We wanted to determine if JAKs were necessary for F. tularensis-induced PGE2 synthesis in macrophages. In mammals, there are four members of the JAK family of tyrosine kinases: JAK1, JAK2, JAK3, and tyrosine kinase 2 (Tyk2). To identify which if any JAKs are necessary for F. tularensis-induced PGE2 synthesis in macrophages, we utilized pharmacological inhibitors of multiple members of the JAK family of tyrosine kinases. Treatment of BMDMs with AG490 (JAK2/JAK3 inhibitor) significantly reduced the synthesis of PGE2 by F. tularensis-infected macrophages. Conversely, treatment with ruxolitinib (JAK1/JAK2 inhibitor) did not decrease PGE2 production (Fig. 3B). These data suggested to us that JAK3 is the primary JAK involved in regulating the synthesis of PGE2 by F. tularensis-infected macrophages. To test this idea, we utilized a well-characterized inhibitor of JAK3, JANEX-1 (23). BMDMs were treated with 50 μM JANEX-1 prior to inoculation with LVS at an MOI of 200:1. At 24 h postinoculation, supernatants were collected, and PGE2 concentration was determined by ELISA. Treatment with JANEX-1 markedly reduced F. tularensis-induced PGE2 synthesis by macrophages (Fig. 3C). These data were recapitulated in the RAW264.7 macrophage cell line (data not shown). These data suggest that JAK3 activity is necessary for the synthesis of PGE2 by macrophages infected with F. tularensis.
We determined that treatment with JANEX-1 or SB203580 did not significantly affect LVS growth in broth (Fig. 3D), macrophage uptake of LVS, or intramacrophage growth of LVS in RAW264.7 cells (Fig. 3E). Treatment of RAW264.7 macrophages with JANEX-1 did result in a slight increase in cell death (up from ∼5% to ∼12%) (Fig. 3F). We do not believe that this slight increase in cell death in JANEX-1-treated RAW264.7 cells can account for the significant decrease in PGE2 production in infected macrophages. Alternatively, treatment of F. tularensis-infected BMDMs with JANEX-1 or SB203580 actually enhanced cell survival of BMDMs (Fig. 3F). These data confirm our view that macrophage cell death is not the cause of decreased PGE2.
Due to the potential for off-target effects of JANEX-1, we wanted to confirm that JAK3 was required for F. tularensis-induced PGE2 synthesis via a genetic approach. We utilized lentivirus-delivered vectors to express JAK3 short-hairpin RNA (shRNA) to generate RAW264.7 macrophage cell lines with reduced JAK3 gene expression. As we had difficulty in detecting JAK3 protein levels, we decided to demonstrate JAK3 depletion by examining JAK3 gene expression. To verify that JAK3 gene expression was in fact reduced in our cells expressing JAK3 shRNA, we performed reverse transcription-PCR (RT-PCR) using primers specific to the JAK3 gene. We observed a decrease in JAK3 transcript levels in our JAK3 shRNA-expressing cells (Fig. 3G). Cells expressing JAK3 shRNA were inoculated with F. tularensis LVS at an MOI of 200:1. At 24 h postinoculation, supernatants were collected, and a PGE2 ELISA was performed to determine the concentration of PGE2. In cells expressing JAK3 shRNA, we observed a significant decrease in PGE2 production compared to wild-type RAW264.7 macrophages (Fig. 3H). A nontarget (NT) shRNA vector control plasmid was expressed as a negative control.
Unfortunately, we observed that the JAK3-depleted cells quickly recovered their ability to synthesize PGE2 in response to F. tularensis after cell culture passage due to some unknown compensatory mechanism. Because of this compensation, it was not possible to generate enough JAK3-depleted macrophages to recapitulate the data from all of the experiments performed throughout this study in the JAK3-depleted macrophages. However, since we performed six independent lentiviral transfections to generate JAK3-depleted macrophages that all resulted in the same phenotype upon initial infection, decreased PGE2 synthesis, this strongly suggests to us that JAK3 is involved in F. tularensis-induced PGE2 production. Taken together, these data demonstrate that JAK3 activity is critical for the synthesis of PGE2 by F. tularensis-infected macrophages.
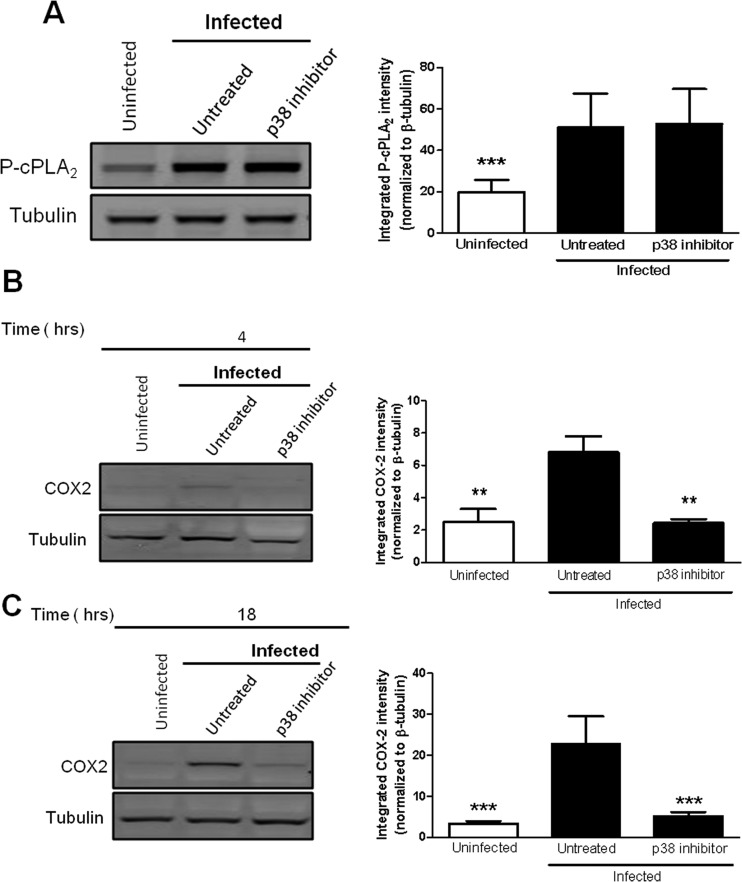
p38 MAPK activity is required for increased COX-2 protein levels, but not for enhanced phosphorylation of cPLA2 in macrophages infected with F. tularensis.
p38 MAPK has been reported to regulate both the phosphorylation of cPLA2 and COX-2 protein levels in macrophages (24). We first investigated if p38 MAPK was required for the phosphorylation of cPLA2 in macrophages infected with F. tularensis. BMDMs were treated with 15 μM SB203580 prior to inoculation with LVS at an MOI of 200:1. Whole-cell lysates were collected at 1 h postinoculation, and a Western blot analysis was performed to determine the level of cPLA2 phosphorylated on Ser505. Inhibition of p38 MAPK did not alter the phosphorylation state of cPLA2 (Fig. 4A), suggesting that p38 MAPK activity is not required for the phosphorylation of cPLA2 in F. tularensis-infected macrophages. We next examined if p38 MAPK controlled COX-2 protein levels in macrophages infected with F. tularensis. RAW264.7 macrophages were untreated or treated with the p38 MAPK inhibitor SB203580 prior to inoculation with LVS at an MOI of 200:1. Whole-cell lysates were collected at 4 h and 18 h postinoculation to allow us to examine COX-2 protein levels at an early time point as well as a late time point. We found that inhibition of p38 MAPK resulted in a significant decrease in COX-2 protein levels at both 4 h and 18 h postinoculation (Fig. 4B and C). These data demonstrate that p38 MAPK activity is necessary for F. tularensis-induced COX-2 protein synthesis in macrophages. Overall, these data suggest that p38 MAPK activity contributes to the synthesis of PGE2 in F. tularensis-infected macrophages by increasing COX-2 protein levels but not by increasing the phosphorylation of cPLA2.
FIG 4.
Inhibition of p38 MAPK decreases COX-2 protein levels but not cPLA2 phosphorylation. (A) BMDMs were either untreated or pretreated with 15 μM SB203580 (p38 MAPK inhibitor) for 1 h prior to inoculation with LVS at an MOI of 200:1. At 1 h postinoculation, whole-cell lysates were collected and analyzed for cPLA2 phosphorylated on Ser505 by Western blotting (left). The band intensities (right) were measured in each experiment using LiCor Odyssey infrared imaging system software and were normalized to the loading control (tubulin). The graph presents data from three independent experiments. (B and C) RAW264.7 cells were either untreated or pretreated with 15 μM SB203580 for 1 h prior to inoculation with LVS at an MOI of 200:1. At 2 h postinoculation, the cells were incubated in RPMI containing penicillin-streptomycin. At 4 h (B) and 18 h (C) postinoculation, whole-cell lysates were collected and analyzed for COX-2 protein levels by Western blotting (left). The band intensities (right) were measured in each experiment and were normalized to the loading control tubulin. The graphs present data from three independent experiments. Statistical differences (**, P ≤ 0.01; ***, P ≤ 0.001) compared to infected untreated controls were determined by Dunnett's multiple comparison test.
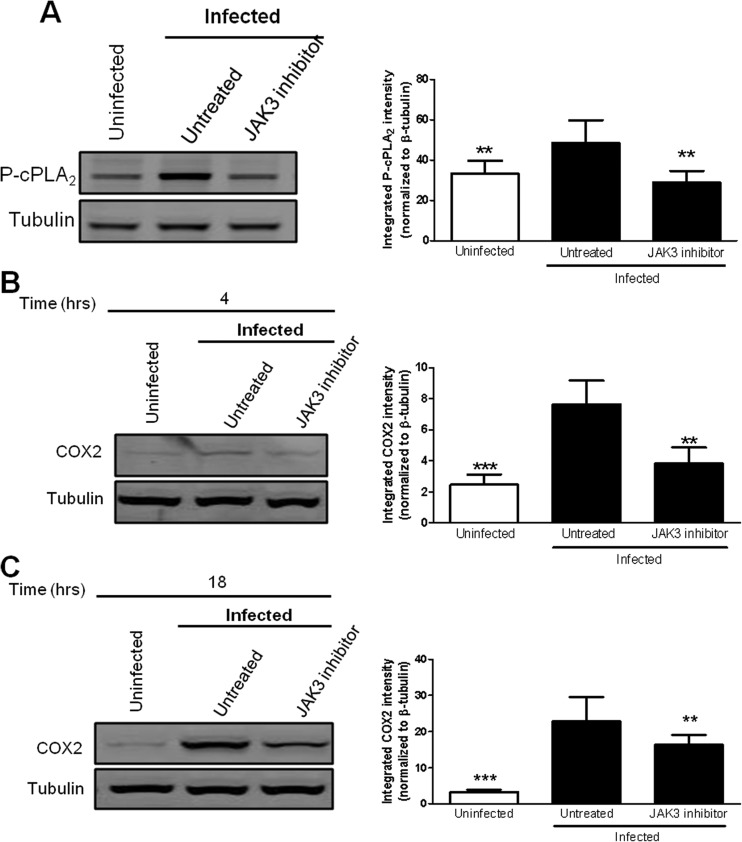
JAK3 activity is necessary for both increased COX-2 protein levels and cPLA2 phosphorylation in macrophages infected with F. tularensis.
JAKs are capable of mediating cPLA2 expression and activation in response to growth factors and interferon in vascular smooth muscle cells and HeLa cells (25, 26). Therefore, we first wanted to determine if JAK3 activity is necessary for the phosphorylation of cPLA2 in macrophages infected with F. tularensis. BMDMs were untreated or treated with 50 μM JANEX-1 prior to inoculation with LVS at an MOI of 200:1. Whole-cell lysates were collected at 1 h postinoculation and examined for cPLA2 phosphorylation on Ser505 by Western blotting. Treatment with JANEX-1 significantly decreased cPLA2 phosphorylation on Ser505 (Fig. 5A), suggesting that JAK3 signaling is important for F. tularensis-induced cPLA2 phosphorylation.
FIG 5.
Inhibition of JAK3 decreases COX-2 protein levels and phosphorylation of cPLA2 in macrophages infected with F. tularensis. (A) BMDMs were either untreated or pretreated with 50 μM JANEX-1 (JAK3 inhibitor) for 1 h prior to inoculation with LVS at an MOI of 200:1. At 1 h postinoculation, whole-cell lysates were collected and analyzed for cPLA2 phosphorylated on Ser505 by Western blotting (left). The band intensities (right) were measured in each experiment using LiCor Odyssey infrared imaging system software and were normalized to the loading control (tubulin). The graph presents data from eight independent experiments. (B and C) RAW264.7 macrophages were either untreated or pretreated with 50 μM JANEX-1 (JAK3 inhibitor) for 1 h prior to inoculation with LVS at an MOI of 200:1. After 2 h, the cells were incubated in RPMI containing penicillin-streptomycin. At 4 h (B) and 18 h (C) postinoculation, whole-cell lysates were collected and analyzed for COX-2 protein levels by Western blotting (left). The band intensities (right) were measured in each experiment and were normalized to the loading control (tubulin). The graphs present data from seven (B) and six (C) independent experiments. Statistical differences (**, P ≤ 0.01; ***, P ≤ 0.001) compared to infected untreated controls were determined by Dunnett's multiple comparison test.
JAKs are capable of regulating COX-2 protein levels by regulating COX-2 gene expression (27, 28). Therefore, we next wanted to determine if JAK3 activity was necessary for the increased COX-2 protein levels in macrophages infected with F. tularensis. RAW264.7 macrophages were untreated or treated with 50 μM JANEX-1 prior to inoculation with LVS at an MOI of 200:1. Whole-cell lysates were collected at 4 h and 18 h postinoculation and COX-2 protein levels were determined by Western blotting. Treatment with JANEX-1 significantly decreased COX-2 protein levels at 4 h and 18 h postinoculation (Fig. 5B and C), suggesting that JAK3 activity is important for COX-2 protein synthesis in F. tularensis-infected macrophages. Treatment of macrophages with JANEX-1 decreased COX-2 protein levels to a greater extent at 4 h postinoculation than 18 h postinoculation, suggesting that other signaling pathways that are less reliant on JAK3 could be regulating COX-2 protein synthesis at later time points postinfection. Future work will be necessary to understand the mechanism behind this differential regulation of COX-2 protein synthesis. Together, these data demonstrate that JAK3 is important for regulating multiple steps of the PGE2-biosynthetic pathway, including the phosphorylation of cPLA2 and COX-2 protein synthesis in F. tularensis-infected macrophages.
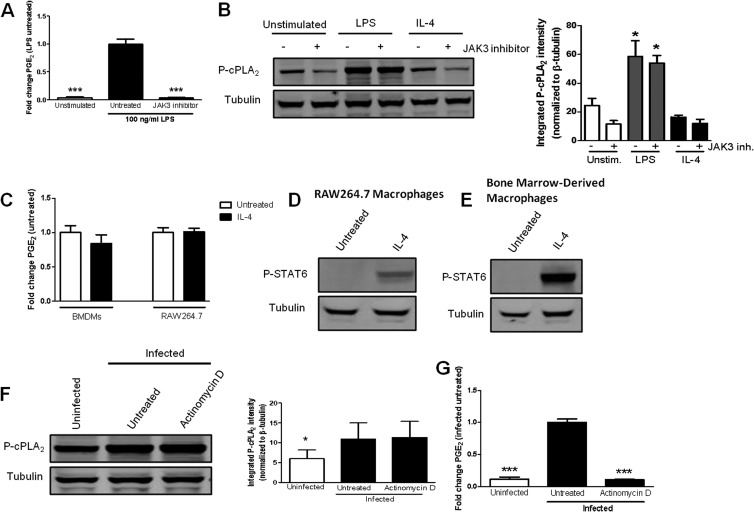
JAK3 regulation of cPLA2 phosphorylation is not universal for agonist-induced PGE2 synthesis in macrophages.
We were intrigued by the data suggesting that JAK3 stimulates the phosphorylation of cPLA2 in F. tularensis-infected macrophages. To the best of our knowledge, JAK3 has not been documented to induce the phosphorylation of cPLA2 in any cell type. JAKs are best characterized for their ability to regulate gene transcription through the phosphorylation and activation of members of the signal transducer and activator of transcription (STAT) family of transcription factors (29). However, the fact that JAK3 activity is necessary for the phosphorylation of cPLA2 at 1 h postinoculation suggests that JAK3 stimulates cPLA2 phosphorylation independently of transcription. To test this hypothesis, BMDMs were untreated or treated with the transcription inhibitor actinomycin D prior to inoculation with LVS at an MOI of 200:1. Whole-cell lysates were collected at 1 h postinoculation, and a Western blot analysis was performed to examine cPLA2 phosphorylation on Ser505. Treatment with actinomycin D did not decrease F. tularensis-induced phosphorylation of cPLA2 (Fig. 6A). To confirm that actinomycin D was functional, we examined F. tularensis-induced PGE2 production by macrophages treated with actinomycin D. The synthesis of PGE2 requires the generation of new proteins (e.g., COX-2) and is therefore dependent on transcription. As expected, treatment with actinomycin D significantly decreased PGE2 synthesis compared to infected untreated samples (Fig. 6B). These data indicate that transcription is essential for F. tularensis-induced PGE2 synthesis and that the phosphorylation of cPLA2 in macrophages infected with F. tularensis occurs via a transcription-independent mechanism. Together, these results suggest that JAK3 regulates the phosphorylation of cPLA2 by a mechanism that is independent of STAT proteins. Furthermore, it suggests that JAK3 activity is critical for the phosphorylation of cPLA2 either directly or indirectly via an intermediate serine kinase, since JAK3 is a tyrosine kinase. Future work will focus on defining the mechanism of JAK3 phosphorylation of cPLA2 in macrophages infected with F. tularensis.
FIG 6.
Inhibition of JAK3 does not decrease cPLA2 phosphorylation in macrophages stimulated with LPS or IL-4. (A) BMDMs were either untreated or pretreated with 50 μM JANEX-1 (JAK3 inhibitor) for 1 h prior to stimulation with 100 ng/ml LPS. JANEX-1 was maintained throughout the experiment. At 24 h postinoculation, supernatants were collected, and PGE2 concentration was determined by ELISA. The results are the means and SEM from three independent experiments. (B) BMDMs were either untreated or pretreated with 50 μM JANEX-1 (JAK3 inhibitor) for 1 h prior to stimulation with 100 ng/ml LPS or 30 ng/ml IL-4. After 1 h, whole-cell lysates were collected and analyzed for cPLA2 phosphorylated on Ser505 by Western blotting (left). The band intensities (right) were measured in each experiment and were normalized to the loading control (tubulin). The graph presents data from three independent experiments. (C) BMDMs were either untreated or treated with 30 ng/ml IL-4. At 24 h postinoculation, supernatants were collected, and PGE2 concentration was determined by ELISA. The results are means and SEM from three independent experiments. RAW264.7 macrophages (D) or BMDMs (E) were untreated or treated with 30 ng/ml IL-4. At 1 h postinoculation, whole-cell lysates were collected and analyzed for STAT6 phosphorylated on Tyr641 by Western blotting. (F) BMDMs were untreated or pretreated with 2 μg/ml actinomycin D for 1 h prior to inoculation with LVS at an MOI of 200:1. At 1 h postinoculation, whole-cell lysates were collected and analyzed for cPLA2 phosphorylated on Ser505 by Western blotting (left). The band intensities (right) were measured in each experiment and were normalized to the loading control (tubulin). The graph presents data from five independent experiments. (G) BMDMs were either untreated or pretreated for 1 h with 2 μg/ml actinomycin D (transcription inhibitor) prior to inoculation with F. tularensis LVS at an MOI of 200:1. At 24 h postinoculation, supernatants were collected and PGE2 concentration was determined by ELISA. Statistical differences (*, P ≤ 0.05; ***, P ≤ 0.001) compared to infected untreated (G) or untreated unstimulated (A and B) controls were determined by Dunnett's multiple comparison test.
We wanted to determine if JAK3-mediated phosphorylation of cPLA2 is unique to F. tularensis or if JAK3 is involved in cPLA2 phosphorylation in macrophages in response to other stimulants. To address this, BMDMs were untreated or treated with 50 μM JANEX-1 prior to stimulation with 100 ng/ml lipopolysaccharide (LPS) (a known inducer of cPLA2 phosphorylation) or 30 ng/ml interleukin-4 (IL-4) (a known activator of JAK3) (30, 31). Whole-cell lysates were collected at 1 h poststimulation and examined for cPLA2 phosphorylation on Ser505 by Western blotting. Stimulation of macrophages with LPS resulted in a significant increase in cPLA2 phosphorylation compared to unstimulated control macrophages. However, treatment with JANEX-1 did not reduce the level of phosphorylated cPLA2 in macrophages stimulated with LPS (Fig. 6C), which suggests that LPS-induced phosphorylation of cPLA2 occurs independently of JAK3. LPS is capable of inducing PGE2 synthesis from BMDMs, and treatment with JANEX-1 decreases PGE2 synthesis by macrophages stimulated with LPS (Fig. 6D). Based on these data, we believe that F. tularensis and LPS utilize distinct signal transduction pathways to induce the synthesis of PGE2. Conversely, treatment with IL-4 did not increase cPLA2 phosphorylation (Fig. 6C) or induce the synthesis of PGE2 from macrophages (Fig. 6E). We wanted to confirm that the IL-4 that we used to stimulate the macrophages activated IL-4 receptor signaling by examining the phosphorylation of STAT6 (a known downstream protein that is phosphorylated following IL-4 receptor engagement) (31). IL-4 stimulation of RAW264.7 macrophages and BMDMs resulted in the phosphorylation of STAT6 (Fig. 6F and G). These data suggest that activation of JAK3 does not always result in cPLA2 phosphorylation or PGE2 production. More importantly, these data support a unique role for JAK3 in regulating the phosphorylation of cPLA2 and the synthesis of PGE2 in F. tularensis-infected macrophages. Overall, these data suggest that F. tularensis activates a signaling pathway to stimulate the synthesis of PGE2 that is distinct from LPS. In F. tularensis-infected macrophages, JAK3 plays a novel role in regulating, likely indirectly, both the phosphorylation of cPLA2 and COX-2 protein levels, which results in increased PGE2 production.
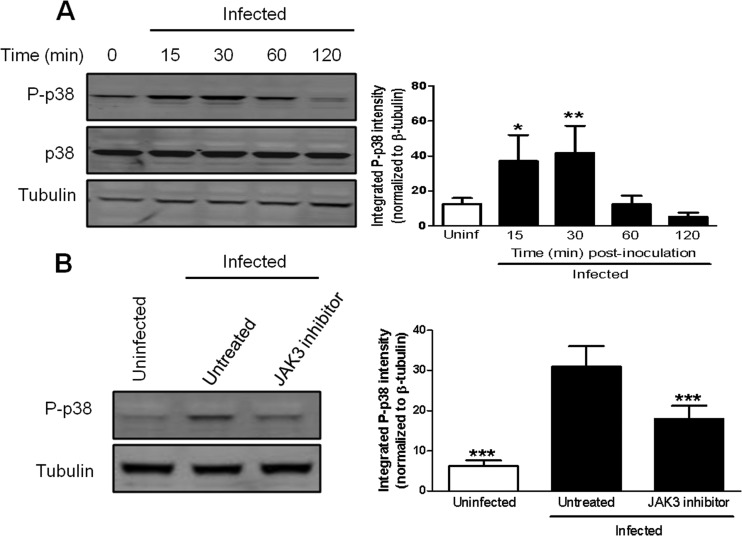
JAK3 activity is required for the phosphorylation of p38 MAPK in murine macrophages infected with F. tularensis.
Activated JAKs are capable of initiating a multitude of signaling cascades, including major signaling pathways such as JAK/STAT, PI3K/Akt, and p38 MAPK pathways (32, 33). Like activation of other MAPKs, p38 MAPK activation is regulated posttranslationally by phosphorylation. This led us to hypothesize that JAK3 acts upstream of p38 MAPK in a linear pathway and JAK3 activity is required for the phosphorylation of p38 MAPK in F. tularensis-infected macrophages. Prior to examining if JAK3 activity is necessary for the phosphorylation of p38 MAPK, we wanted to confirm the previously reported kinetics of p38 MAPK phosphorylation in macrophages infected with F. tularensis (19). BMDMs were inoculated with LVS at an MOI of 200:1, and whole-cell lysates were collected at 0, 15, 30, 60, and 120 min postinoculation. Cell lysates were examined for p38 MAPK phosphorylation on Thr180 and Tyr182 by Western blotting. There was a significant increase in p38 MAPK phosphorylation as early as 15 min postinoculation, and peak phosphorylation of p38 MAPK occurred by 30 min postinoculation (Fig. 7A). To determine if JAK3 activity was necessary for the phosphorylation of p38 MAPK in F. tularensis-infected macrophages, BMDMs were treated with 50 μM JANEX-1 prior to inoculation with LVS at an MOI of 200:1. Whole-cell lysates were collected at 30 min postinoculation and subjected to Western blot analysis to determine the levels of phosphorylated p38 MAPK. Treatment with JANEX-1 significantly decreased the phosphorylation of p38 MAPK, as shown by Western blotting (Fig. 7B). These data suggest that JAK3 is activated early by F. tularensis and acts upstream of p38 MAPK in a signaling pathway that induces the synthesis of PGE2 in macrophages.
FIG 7.
Inhibition of JAK3 decreases p38 MAPK phosphorylation in BMDMs infected with F. tularensis. (A) BMDMs were inoculated with LVS at an MOI of 200:1. At 0, 15, 30, 60, and 120 min postinoculation, whole-cell lysates were collected and analyzed for total p38 MAPK or p38 MAPK phosphorylated on Thr180 and Tyr182 by Western blotting (left). The band intensities (right) were measured in each experiment and were normalized to the loading control (tubulin). The graph presents data from four independent experiments. (B) BMDMs were either untreated or pretreated with 50 μM JANEX-1 (JAK3 inhibitor) for 1 h prior to inoculation with LVS at an MOI of 200:1. At 30 min postinoculation, whole-cell lysates were collected and analyzed for p38 MAPK phosphorylated on Thr180 and Tyr182 by Western blotting (left). The band intensities (right) were measured in each experiment and were normalized to the loading control (tubulin). The graph presents data from eight independent experiments. Statistical differences (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001) compared to infected untreated controls were determined by Dunnett's multiple comparison test.
DISCUSSION
The ability of F. tularensis to modulate and subvert the host immune response is critical to its survival within the host. F. tularensis-induced synthesis of PGE2 from infected macrophages is one of several identified mechanisms of F. tularensis immune evasion (6, 7). The signaling pathways responsible for regulating the activity of the enzymes in the PGE2-biosynthetic pathway in F. tularensis-infected macrophages are not well defined at this time. In the present study, we demonstrated that JAK3, cPLA2, and p38 MAPK signaling are required for F. tularensis LVS-induced PGE2 synthesis by infected murine macrophages. p38 MAPK activity was necessary for increased COX-2 protein levels, while JAK3 activity was necessary for the phosphorylation of cPLA2, the phosphorylation of p38 MAPK, and increased COX-2 protein levels. To the best of our knowledge, this is the first evidence of JAK3 regulating enzymes in the inducible PGE2-biosynthetic pathway in any eukaryotic cell.
The canonical inducible PGE2-biosynthetic pathway typically requires cPLA2 to liberate AA from phospholipids. Liberated AA is then oxidized and isomerized by COX-2 and terminal prostaglandin synthases to produce PGE2 (34). As seen with other pathogen-induced PGE2 synthesis, F. tularensis required cPLA2 for the induction of PGE2 synthesis by infected macrophages. F. tularensis infection resulted in a significant increase in COX-2 protein levels by 4 h postinoculation and a significant increase in detectable PGE2 by 6 h postinoculation in RAW264.7 macrophages and an increase in PGE2 production between 12 h and 18 h postinoculation in BMDMs. The phosphorylation of cPLA2 on Ser505 is functionally important for cPLA2-mediated release of AA (35). We observed peak phosphorylation of cPLA2 at 1 h postinoculation and a return to baseline phosphorylation by 2 h postinoculation. We did not observe phosphorylation of cPLA2 after 1 h postinoculation (data not shown). We also did not observe an overlap between cPLA2 phosphorylation and PGE2 synthesis. This is surprising, since these are typically closely coupled events. We take these data to suggest that cPLA2 is not the phospholipase responsible for supplying the AA that is used by the cell to synthesize PGE2 in F. tularensis-infected macrophages. It is not unreasonable to hypothesize that cPLA2 is not supplying AA, considering that phospholipase D- and phospholipase C-mediated pathways have been implicated in liberating AA (36–38). In fact, we have preliminary data that suggest that phospholipase D is responsible for liberating the AA for PGE2 synthesis (data not shown). We are currently trying to elucidate an alternative mechanism by which cPLA2 regulates PGE2 synthesis independently of liberating AA that is converted into PGE2. Overall, from these studies we were able to establish the kinetics of cPLA2 phosphorylation, COX-2 protein synthesis, and PGE2 synthesis in F. tularensis-infected macrophages. By knowing the timing of these events, we can identify the host signal transduction pathways that regulate F. tularensis-induced PGE2 synthesis in macrophages.
The p38 MAPK signaling cascade is one of the primary intracellular pathways activated in response to a variety of extracellular stimuli. Previous studies have shown that p38 MAPK can regulate agonist-induced transcription/translation of COX-2, phosphorylation of cPLA2, and PGE2 production (39). p38 MAPK is rapidly phosphorylated following infection of both murine and human macrophages with F. tularensis, and the phosphorylation of p38 MAPK begins to decrease by 2 h postinoculation (19), which we also observed. We demonstrated that p38 MAPK activity is needed for the production of PGE2 by macrophages infected with F. tularensis. This is likely due to the fact that p38 MAPK activity is necessary for COX-2 protein synthesis in F. tularensis-infected macrophages. We hypothesize that p38 MAPK either regulates the transcription of the COX-2 gene or regulates the stability of COX-2 mRNA. However, at this time, the mechanism behind p38 MAPK regulation of COX-2 gene expression in F. tularensis-infected macrophages is unknown.
Our studies uncovered an unexpected signaling pathway involving JAK3 that may be unique to F. tularensis LVS-induced PGE2 synthesis. Specifically, JAK3 activity is necessary for F. tularensis-induced PGE2 synthesis. We hypothesize that JAK3 is involved in two distinct signaling pathways that are activated by F. tularensis in murine macrophages. This hypothesis is based on the following observations: (i) JAK3 is involved in the phosphorylation of p38 MAPK and cPLA2 and increased COX-2 protein levels and (ii) p38 MAPK is involved only in COX-2 protein synthesis, not cPLA2 phosphorylation. In the first pathway, JAK3 mediates the phosphorylation of cPLA2 on Ser505. However, JAK3 is a tyrosine kinase, suggesting that an intermediate serine kinase is phosphorylated and activated directly by JAK3 and this serine kinase subsequently phosphorylates cPLA2. We are currently trying to identify the intermediate serine kinase(s) responsible for phosphorylating cPLA2 in F. tularensis-infected macrophages. The second pathway involves JAK3 phosphorylating p38 MAPK, which results in increased COX-2 protein levels. Regardless of whether there are two distinct pathways, we know that phosphorylation of cPLA2 and increased COX-2 protein levels are necessary for F. tularensis-induced PGE2 production.
JAK3 typically associates with the common γ chain (γc) subunit of cytokine receptors and becomes activated only following cytokine binding to the receptor (40–42). We showed that JAK3 is involved in the phosphorylation of p38 MAPK at 30 min postinoculation and cPLA2 phosphorylation at 1 h postinoculation. It seems unlikely that a cytokine could be synthesized, be secreted, bind to its receptor on the cell surface, and activate JAK3 in less than 30 min following F. tularensis infection. Based on this, we believe that JAK3 is not phosphorylated and activated by cytokine-mediated receptor engagement at this early time postinoculation. We propose that either F. tularensis binds a common γ chain-containing cytokine receptor or JAK3 associates with a surface receptor that functions as a pathogen pattern recognition receptor. To our knowledge, there are no studies that have shown F. tularensis binding to a cytokine receptor or JAK3 associating with a receptor that does not contain a common γc subunit. We are excited about exploring these two possibilities, and clearly, more work is required to define the mechanism of JAK3 activation in macrophages infected with F. tularensis.
Our results demonstrate for the first time a novel role for JAK3 in regulating multiple enzymes in the prostaglandin-biosynthetic pathway. We observed in F. tularensis LVS-infected murine macrophages that JAK3 is necessary for cPLA2 phosphorylation, p38 MAPK phosphorylation, increased COX-2 protein levels, and ultimately the synthesis of PGE2. We propose that F. tularensis interacts with a macrophage surface receptor that results in the activation of JAK3 and initiates an intracellular signaling cascade that increases the production of PGE2. This represents an unexpected bacterial-eukaryotic receptor interaction or the involvement of JAK3 with a host pattern recognition receptor that was previously unknown.
ACKNOWLEDGMENTS
We thank Michelle Arnold, Robert Chervanek, and Kenneth Peterson for helpful conversations and critical comments on the manuscript.
This work was supported by the NIH/NIAID grant number K22AI83373-2 and a Louisiana State University Health Sciences Center-Shreveport Grant-In-Aid.
A.M.B. and M.D.W. designed experiments; A.M.B, A.R.N., and J.D.B. performed experiments; A.M.B. and M.D.W. analyzed data and wrote the paper.
There are no conflicting financial interests among the authors.
Footnotes
Published ahead of print 16 December 2013
REFERENCES
- 1.Saslaw S, Eigelsbach HT, Prior JA, Wilson HE, Carhart S. 1961. Tularemia vaccine study. II. Respiratory challenge. Arch. Intern. Med. 107:702–714 [DOI] [PubMed] [Google Scholar]
- 2.Conlan JW, Chen W, Shen H, Webb A, KuoLee R. 2003. Experimental tularemia in mice challenged by aerosol or intradermally with virulent strains of Francisella tularensis: bacteriologic and histopathologic studies. Microb. Pathog. 34:239–248. 10.1016/S0882-4010(03)00046-9 [DOI] [PubMed] [Google Scholar]
- 3.Baron GS, Nano FE. 1998. MglA and MglB are required for the intramacrophage growth of Francisella novicida. Mol. Microbiol. 29:247–259. 10.1046/j.1365-2958.1998.00926.x [DOI] [PubMed] [Google Scholar]
- 4.Barrigan LM, Tuladhar S, Brunton JC, Woolard MD, Chen CJ, Saini D, Frothingham R, Sempowski GD, Kawula TH, Frelinger JA. 2013. Infection with Francisella tularensis LVS clpB leads to an altered yet protective immune response. Infect. Immun. 81:2028–2042. 10.1128/IAI.00207-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones CL, Napier BA, Sampson TR, Llewellyn AC, Schroeder MR, Weiss DS. 2012. Subversion of host recognition and defense systems by Francisella spp. Microbiol. Mol. Biol. Rev. 76:383–404. 10.1128/MMBR.05027-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woolard MD, Barrigan LM, Fuller JR, Buntzman AS, Bryan J, Manoil C, Kawula TH, Frelinger JA. 2013. Identification of Francisella novicida mutants that fail to induce prostaglandin E(2) synthesis by infected macrophages. Front. Microbiol. 4:16. 10.3389/fmicb.2013.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woolard MD, Wilson JE, Hensley LL, Jania LA, Kawula TH, Drake JR, Frelinger JA. 2007. Francisella tularensis-infected macrophages release prostaglandin E2 that blocks T cell proliferation and promotes a Th2-like response. J. Immunol. 178:2065–2074 http://www.jimmunol.org/content/178/4/2065.long [DOI] [PubMed] [Google Scholar]
- 8.Davies P, Bailey PJ, Goldenberg MM, Ford-Hutchinson AW. 1984. The role of arachidonic acid oxygenation products in pain and inflammation. Annu. Rev. Immunol. 2:335–357. 10.1146/annurev.iy.02.040184.002003 [DOI] [PubMed] [Google Scholar]
- 9.Takayama K, Garcia-Cardena G, Sukhova GK, Comander J, Gimbrone MA, Jr, Libby P. 2002. Prostaglandin E2 suppresses chemokine production in human macrophages through the EP4 receptor. J. Biol. Chem. 277:44147–44154. 10.1074/jbc.M204810200 [DOI] [PubMed] [Google Scholar]
- 10.Wilson JE, Katkere B, Drake JR. 2009. Francisella tularensis induces ubiquitin-dependent major histocompatibility complex class II degradation in activated macrophages. Infect. Immun. 77:4953–4965. 10.1128/IAI.00844-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elkins KL, Rhinehart-Jones TR, Culkin SJ, Yee D, Winegar RK. 1996. Minimal requirements for murine resistance to infection with Francisella tularensis LVS. Infect. Immun. 64:3288–3293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woolard MD, Hensley LL, Kawula TH, Frelinger JA. 2008. Respiratory Francisella tularensis live vaccine strain infection induces Th17 cells and prostaglandin E2, which inhibits generation of gamma interferon-positive T cells. Infect. Immun. 76:2651–2659. 10.1128/IAI.01412-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leslie CC. 2004. Regulation of the specific release of arachidonic acid by cytosolic phospholipase A2. Prostaglandins Leukot Essent Fatty Acids 70:373–376. 10.1016/j.plefa.2003.12.012 [DOI] [PubMed] [Google Scholar]
- 14.Murakami M, Kudo I. 2004. Recent advances in molecular biology and physiology of the prostaglandin E2-biosynthetic pathway. Prog. Lipid Res. 43:3–35. 10.1016/S0163-7827(03)00037-7 [DOI] [PubMed] [Google Scholar]
- 15.Lin LL, Wartmann M, Lin AY, Knopf JL, Seth A, Davis RJ. 1993. cPLA2 is phosphorylated and activated by MAP kinase. Cell 72:269–278. 10.1016/0092-8674(93)90666-E [DOI] [PubMed] [Google Scholar]
- 16.Harper KA, Tyson-Capper AJ. 2008. Complexity of COX-2 gene regulation. Biochem. Soc. Trans. 36:543–545. 10.1042/BST0360543 [DOI] [PubMed] [Google Scholar]
- 17.Qiu ZH, de Carvalho MS, Leslie CC. 1993. Regulation of phospholipase A2 activation by phosphorylation in mouse peritoneal macrophages. J. Biol. Chem. 268:24506–24513 [PubMed] [Google Scholar]
- 18.Xu F, Xu Z, Zhang R, Wu Z, Lim JH, Koga T, Li JD, Shen H. 2008. Nontypeable Haemophilus influenzae induces COX-2 and PGE2 expression in lung epithelial cells via activation of p38 MAPK and NF-kappa B. Respir. Res. 9:16. 10.1186/1465-9921-9-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Telepnev M, Golovliov I, Sjostedt A. 2005. Francisella tularensis LVS initially activates but subsequently down-regulates intracellular signaling and cytokine secretion in mouse monocytic and human peripheral blood mononuclear cells. Microb. Pathog. 38:239–247. 10.1016/j.micpath.2005.02.003 [DOI] [PubMed] [Google Scholar]
- 20.Kumar S, Jiang MS, Adams JL, Lee JC. 1999. Pyridinylimidazole compound SB 203580 inhibits the activity but not the activation of p38 mitogen-activated protein kinase. Biochem. Biophys. Res. Commun. 263:825–831. 10.1006/bbrc.1999.1454 [DOI] [PubMed] [Google Scholar]
- 21.Gao J, Tian J, Lv Y, Shi F, Kong F, Shi H, Zhao L. 2009. Leptin induces functional activation of cyclooxygenase-2 through JAK2/STAT3, MAPK/ERK, and PI3K/AKT pathways in human endometrial cancer cells. Cancer Sci. 100:389–395. 10.1111/j.1349-7006.2008.01053.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koon HW, Zhao D, Zhan Y, Rhee SH, Moyer MP, Pothoulakis C. 2006. Substance P stimulates cyclooxygenase-2 and prostaglandin E2 expression through JAK-STAT activation in human colonic epithelial cells. J. Immunol. 176:5050–5059 http://www.jimmunol.org/content/176/8/5050.long [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sudbeck EA, Liu XP, Narla RK, Mahajan S, Ghosh S, Mao C, Uckun FM. 1999. Structure-based design of specific inhibitors of Janus kinase 3 as apoptosis-inducing antileukemic agents. Clin. Cancer Res. 5:1569–1582 [PubMed] [Google Scholar]
- 24.Gijon MA, Spencer DM, Siddiqi AR, Bonventre JV, Leslie CC. 2000. Cytosolic phospholipase A2 is required for macrophage arachidonic acid release by agonists that do and do not mobilize calcium. Novel role of mitogen-activated protein kinase pathways in cytosolic phospholipase A2 regulation. J. Biol. Chem. 275:20146–20156 [DOI] [PubMed] [Google Scholar]
- 25.Flati V, Haque SJ, Williams BR. 1996. Interferon-alpha-induced phosphorylation and activation of cytosolic phospholipase A2 is required for the formation of interferon-stimulated gene factor three. EMBO J. 15:1566–1571 [PMC free article] [PubMed] [Google Scholar]
- 26.Yellaturu CR, Rao GN. 2003. Cytosolic phospholipase A2 is an effector of Jak/STAT signaling and is involved in platelet-derived growth factor BB-induced growth in vascular smooth muscle cells. J. Biol. Chem. 278:9986–9992. 10.1074/jbc.M211276200 [DOI] [PubMed] [Google Scholar]
- 27.Xuan YT, Guo Y, Zhu Y, Han H, Langenbach R, Dawn B, Bolli R. 2003. Mechanism of cyclooxygenase-2 upregulation in late preconditioning. J. Mol. Cell. Cardiol. 35:525–537. 10.1016/S0022-2828(03)00076-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu H, Liu Z, Zhou H, Dai W, Chen S, Shu Y, Feng J. 2012. JAK-STAT pathway modulates the roles of iNOS and COX-2 in the cytoprotection of early phase of hydrogen peroxide preconditioning against apoptosis induced by oxidative stress. Neurosci. Lett. 529:166–171. 10.1016/j.neulet.2012.09.013 [DOI] [PubMed] [Google Scholar]
- 29.O'Shea JJ, Holland SM, Staudt LM. 2013. JAKs and STATs in immunity, immunodeficiency, and cancer. N. Engl. J. Med. 368:161–170. 10.1056/NEJMra1202117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doerfler ME, Weiss J, Clark JD, Elsbach P. 1994. Bacterial lipopolysaccharide primes human neutrophils for enhanced release of arachidonic acid and causes phosphorylation of an 85-kD cytosolic phospholipase A2. J. Clin. Invest. 93:1583–1591. 10.1172/JCI117138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rolling C, Treton D, Pellegrini S, Galanaud P, Richard Y. 1996. IL4 and IL13 receptors share the gamma c chain and activate STAT6, STAT3 and STAT5 proteins in normal human B cells. FEBS Lett. 393:53–56. 10.1016/0014-5793(96)00835-6 [DOI] [PubMed] [Google Scholar]
- 32.Bhattacharjee A, Pal S, Feldman GM, Cathcart MK. 2011. Hck is a key regulator of gene expression in alternatively activated human monocytes. J. Biol. Chem. 286:36709–36723. 10.1074/jbc.M111.291492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birzniece V, Sata A, Ho KK. 2009. Growth hormone receptor modulators. Rev. Endocr Metab. Disord. 10:145–156. 10.1007/s11154-008-9089-x [DOI] [PubMed] [Google Scholar]
- 34.Scott KF, Bryant KJ, Bidgood MJ. 1999. Functional coupling and differential regulation of the phospholipase A2-cyclooxygenase pathways in inflammation. J. Leukoc. Biol. 66:535–541 [DOI] [PubMed] [Google Scholar]
- 35.Tucker DE, Ghosh M, Ghomashchi F, Loper R, Suram S, John BS, Girotti M, Bollinger JG, Gelb MH, Leslie CC. 2009. Role of phosphorylation and basic residues in the catalytic domain of cytosolic phospholipase A2alpha in regulating interfacial kinetics and binding and cellular function. J. Biol. Chem. 284:9596–9611. 10.1074/jbc.M807299200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Habenicht AJ, Goerig M, Grulich J, Rothe D, Gronwald R, Schettler G, Kommerell B. 1985. The phospholipase C diglyceride lipase pathway contributes to arachidonic acid release and prostaglandin E2 formation in platelet-derived growth factor stimulated Swiss 3T3 cells. Adv. Prostaglandin Thromboxane Leukot. Res. 13:37–39 [PubMed] [Google Scholar]
- 37.Ishimoto T, Akiba S, Sato T, Fujii T. 1994. Contribution of phospholipases A2 and D to arachidonic acid liberation and prostaglandin D2 formation with increase in intracellular Ca2+ concentration in rat peritoneal mast cells. Eur. J. Biochem. 219:401–406. 10.1111/j.1432-1033.1994.tb19952.x [DOI] [PubMed] [Google Scholar]
- 38.Moscat J, Herrero C, Garcia-Barreno P, Municio AM. 1986. Phospholipase C-diglyceride lipase is a major pathway for arachidonic acid release in macrophages. Biochem. Biophys. Res. Commun. 141:367–373. 10.1016/S0006-291X(86)80378-3 [DOI] [PubMed] [Google Scholar]
- 39.Noor S, Goldfine H, Tucker DE, Suram S, Lenz LL, Akira S, Uematsu S, Girotti M, Bonventre JV, Breuel K, Williams DL, Leslie CC. 2008. Activation of cytosolic phospholipase A2alpha in resident peritoneal macrophages by Listeria monocytogenes involves listeriolysin O and TLR2. J. Biol. Chem. 283:4744–4755. 10.1074/jbc.M709956200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boussiotis VA, Barber DL, Nakarai T, Freeman GJ, Gribben JG, Bernstein GM, D'Andrea AD, Ritz J, Nadler LM. 1994. Prevention of T cell anergy by signaling through the gamma c chain of the IL-2 receptor. Science 266:1039–1042. 10.1126/science.7973657 [DOI] [PubMed] [Google Scholar]
- 41.Miyazaki T, Kawahara A, Fujii H, Nakagawa Y, Minami Y, Liu ZJ, Oishi I, Silvennoinen O, Witthuhn BA, Ihle JN, Taniguchi T. 1994. Functional activation of Jak1 and Jak3 by selective association with IL-2 receptor subunits. Science 266:1045–1047. 10.1126/science.7973659 [DOI] [PubMed] [Google Scholar]
- 42.Russell SM, Johnston JA, Noguchi M, Kawamura M, Bacon CM, Friedmann M, Berg M, McVicar DW, Witthuhn BA, Silvennoinen O, Goldman AS, Schmalstieg FC, Ihle JN, O'Shea JJ, Leonard WJ. 1994. Interaction of IL-2R beta and gamma c chains with Jak1 and Jak3: implications for XSCID and XCID. Science 266:1042–1045. 10.1126/science.7973658 [DOI] [PubMed] [Google Scholar]