Abstract
The increased prevalence of multidrug-resistant bacterial pathogens motivated us to attempt to enhance the therapeutic efficacy of bacteriophages. The therapeutic application of phages as antibacterial agents was impeded by several factors: (i) the failure to recognize the relatively narrow host range of phages; (ii) the presence of toxins in crude phage lysates; and (iii) a lack of appreciation for the capacity of mammalian host defense systems, particularly the organs of the reticuloendothelial system, to remove phage particles from the circulatory system. In our studies involving bacteremic mice, the problem of the narrow host range of phage was dealt with by using selected bacterial strains and virulent phage specific for them. Toxin levels were diminished by purifying phage preparations. To reduce phage elimination by the host defense system, we developed a serial-passage technique in mice to select for phage mutants able to remain in the circulatory system for longer periods of time. By this approach we isolated long-circulating mutants of Escherichia coli phage lambda and of Salmonella typhimurium phage P22. We demonstrated that the long-circulating lambda mutants also have greater capability as antibacterial agents than the corresponding parental strain in animals infected with lethal doses of bacteria. Comparison of the parental and mutant lambda capsid proteins revealed that the relevant mutation altered the major phage head protein E. The use of toxin-free, bacteria-specific phage strains, combined with the serial-passage technique, may provide insights for developing phage into therapeutically effective antibacterial agents.
Full text
PDF

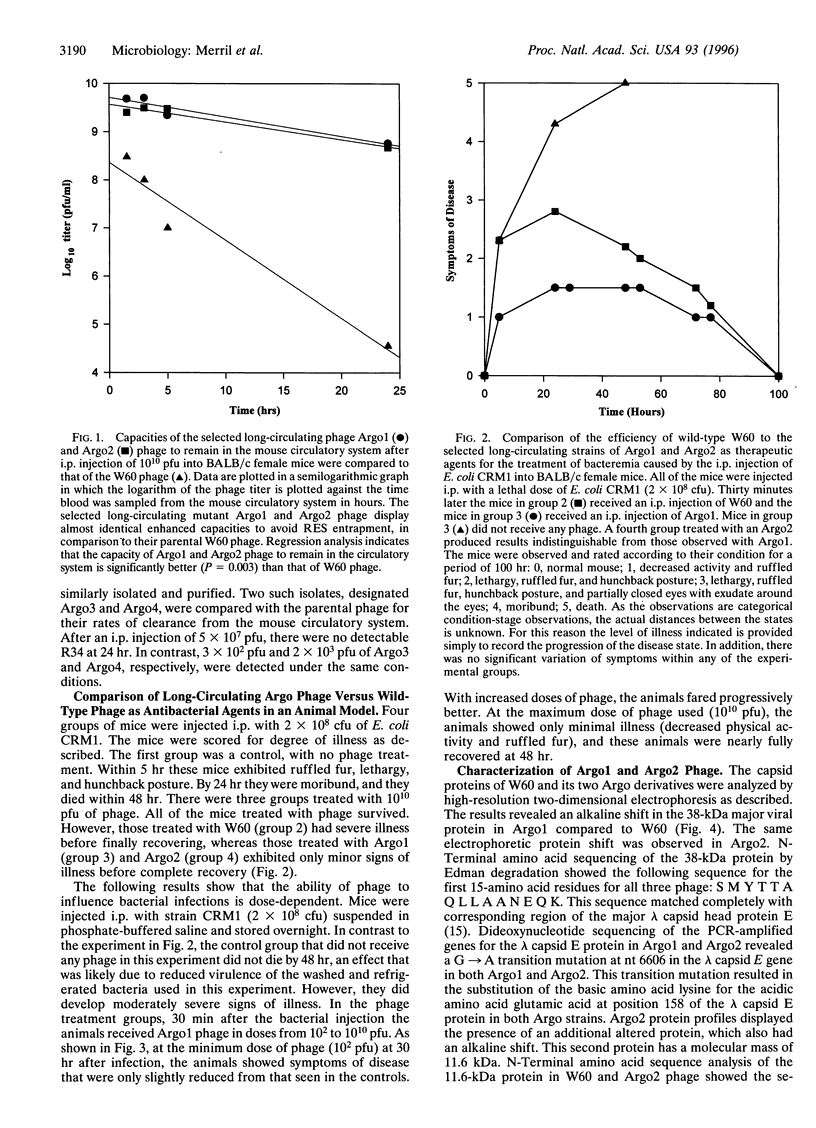
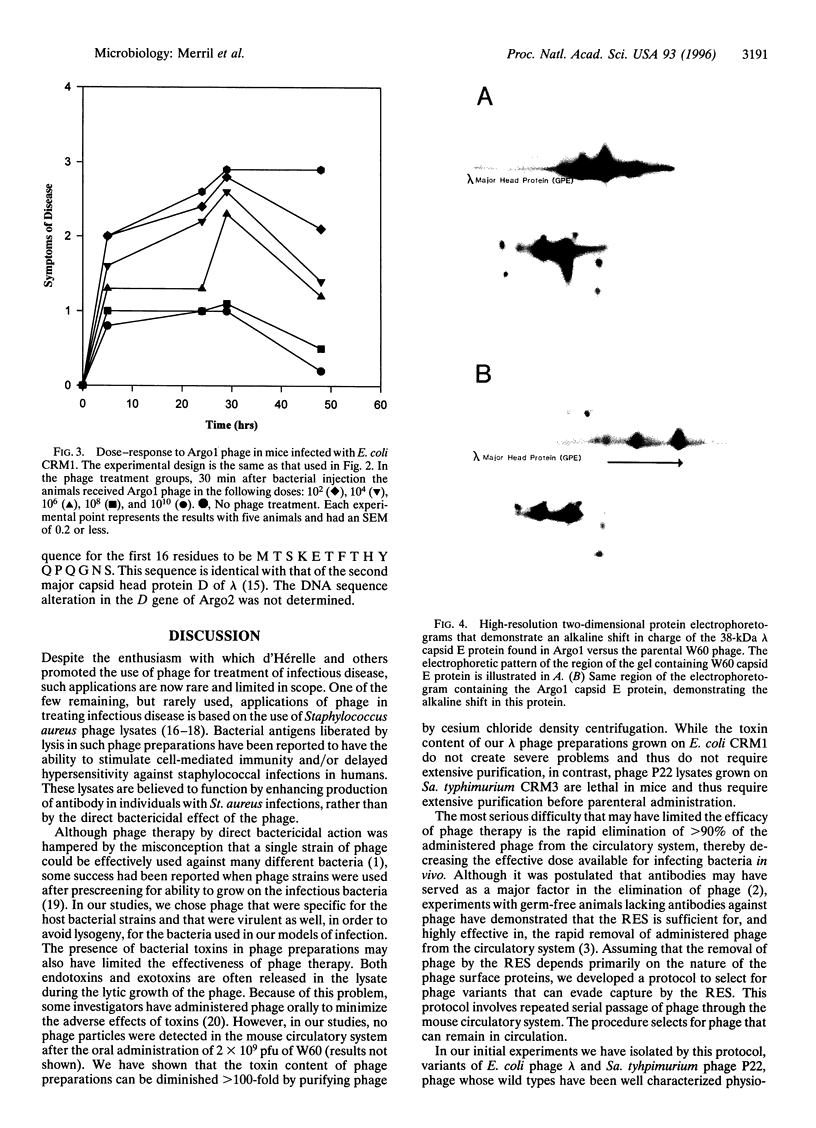

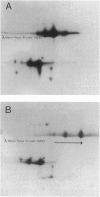
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjellqvist B., Pasquali C., Ravier F., Sanchez J. C., Hochstrasser D. A nonlinear wide-range immobilized pH gradient for two-dimensional electrophoresis and its definition in a relevant pH scale. Electrophoresis. 1993 Dec;14(12):1357–1365. doi: 10.1002/elps.11501401209. [DOI] [PubMed] [Google Scholar]
- Dean J. H., Silva J. S., McCoy J. L., Chan S. P., Baker J. J., Leonard C., Herberman R. B. In vitro human reactivity to staphylococcal phage lysate. J Immunol. 1975 Oct;115(4):1060–1064. [PubMed] [Google Scholar]
- Geier M. R., Trigg M. E., Merril C. R. Fate of bacteriophage lambda in non-immune germ-free mice. Nature. 1973 Nov 23;246(5430):221–223. doi: 10.1038/246221a0. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Hughes G. J., Frutiger S., Paquet N., Ravier F., Pasquali C., Sanchez J. C., James R., Tissot J. D., Bjellqvist B., Hochstrasser D. F. Plasma protein map: an update by microsequencing. Electrophoresis. 1992 Sep-Oct;13(9-10):707–714. doi: 10.1002/elps.11501301150. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Merril C. R., Switzer R. C., Van Keuren M. L. Trace polypeptides in cellular extracts and human body fluids detected by two-dimensional electrophoresis and a highly sensitive silver stain. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4335–4339. doi: 10.1073/pnas.76.9.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd S., Shayegani M. Delayed-type hypersensitivity to S. aureus and its uses. Ann N Y Acad Sci. 1974 Jul 31;236(0):244–251. doi: 10.1111/j.1749-6632.1974.tb41495.x. [DOI] [PubMed] [Google Scholar]
- SALMON G. G., Jr, SYMONDS M. STAPHAGE LYSATE THERAPY IN CHRONIC STAPHYLOCOCCAL INFECTIONS. J Med Soc N J. 1963 May;60:188–193. [PubMed] [Google Scholar]
- Slopek S., Weber-Dabrowska B., Dabrowski M., Kucharewicz-Krukowska A. Results of bacteriophage treatment of suppurative bacterial infections in the years 1981-1986. Arch Immunol Ther Exp (Warsz) 1987;35(5):569–583. [PubMed] [Google Scholar]