Abstract
Malaria is a vector-borne disease caused by the single-cell eukaryote Plasmodium. The infectious parasite forms are sporozoites, which originate from midgut-associated oocysts, where they eventually egress and reach the mosquito hemocoel. Sporozoites actively colonize the salivary glands in order to be transmitted to the mammalian host. Whether residence in the salivary glands provides distinct and vital cues for the development of infectivity remains unsolved. In this study, we systematically compared the infectivity of Plasmodium berghei sporozoites isolated from the mosquito hemocoel and salivary glands. Hemocoel sporozoites display a lower proportion of gliding motility but develop into liver stages when added to cultured hepatoma cells or after intravenous injection into mice. Mice infected by hemocoel sporozoites had blood infections similar to those induced by sporozoites liberated from salivary glands. These infected mice display indistinguishable systemic inflammatory cytokine responses and develop experimental cerebral malaria. When used as metabolically active, live attenuated vaccine, hemocoel sporozoites elicit substantial protection against sporozoite challenge infections. Collectively, these findings show that salivary gland colonization does not influence parasite virulence in the mammalian host when sporozoites are administered intravenously. This conclusion has important implications for in vitro sporozoite production and manufacturing of whole-sporozoite vaccines.
INTRODUCTION
Malaria is caused by erythrocyte infections with obligate intracellular parasites of the genus Plasmodium. The pathogenic blood phase is preceded by a clinically silent hepatic phase, where the parasite propagates and overcomes the bottleneck during mosquito transmission (1). Sporozoites, the infectious stage of malaria parasites, are formed in midgut-associated oocysts, and they eventually egress into the mosquito hemocoel, which is the circulatory system of the mosquito (2). Sporozoites are passively transported by the slow hemolymph circulation and will eventually pass the basal lamina of salivary glands. Here, sporozoites attach, penetrate the acinar cells, and accumulate in the salivary duct, marking the final step of sporogony (3, 4). During this passage, sporozoites mature and acquire traits that are essential to colonize a new vertebrate host. When an infectious female Anopheles mosquito probes for a blood vessel, she injects most sporozoites intradermally. Salivary gland sporozoites accomplish continuous and fast gliding locomotion, transmigration of cellular barriers, invasion of hepatocytes, and formation of a replication-competent niche, the parasitophorous vacuole (4). In marked contrast, young midgut-associated sporozoites lack these abilities (5). Sporozoite maturation correlates with differential upregulation of genes that often perform vital functions in pre-erythrocytic development (6–9). This differentiation process is apparently irreversible resulting in complete loss of infectivity to salivary glands once inside (10).
The first study on the development of infectivity during the passage of sporozoites in the mosquito vector already indicated that hemocoel sporozoites display some degree of gliding locomotion, albeit considerably less than salivary gland sporozoites (5). This notion is fully supported by a recent study using automated tracking of large sporozoite populations (11). Another recent study using advanced microscopy revealed that during the process of maturation sporozoites acquire their distinct curvature, which is structured by a subpellicular network of polarized microtubules (12). However, no structural information is available yet for sporozoites in transit in the mosquito hemocoel.
Comparative analysis of infectivity of hemocoel sporozoites to the mammalian host became particularly important with the generation of Plasmodium mutant lines that displayed defects in salivary gland invasion. Direct comparison between wild-type and mutant sporozoites isolated from the mosquito hemocoel exposed either additional or no roles in other sporozoite traits. For instance, thrombospondin-related anonymous protein (TRAP), sporozoite-specific protein 6 (S6), and sporozoite invasion-associated protein 1 (SIAP-1) are critical factors for salivary gland colonization, hepatocyte invasion, and gliding locomotion (13–16). Apical membrane antigen/erythrocyte binding-like protein (MAEBL) is necessary for infection of salivary glands and hepatocytes, but dispensable for gliding motility, highlighting its critical function as a parasite adhesin (17, 18). In marked contrast, analysis of mutant hemocoel sporozoites revealed that the role(s) of several Plasmodium proteins, including cysteine modular repeat proteins 1 and 2 (CRMP1 and -2), and upregulated in oocyst sporozoites gene 3 (UOS3), are apparently restricted to salivary gland adherence and/or invasion only (7, 19). Together, in these few studies, it was noticed that hemocoel sporozoites display less continuous gliding, ranging between 6% (17) and 30% (16). Hemocoel sporozoites generally infect susceptible hosts (5, 16), although one study reported no infectivity after syringe injection of 20,000 Plasmodium berghei hemocoel sporozoites (20).
In this study, we performed a systematic comparison of the major sporozoite traits in hemocoel and salivary gland sporozoites, including liver colonization, induction of blood infection, and protective liver stage-specific immunity. We reasoned that such an analysis would also help to solve whether sporozoite virulence largely depends on homing to the salivary glands.
MATERIALS AND METHODS
Experimental animals.
All animal work was conducted in accordance with the German “Tierschutzgesetz in der Fassung vom 18. Mai 2006” (BGBl. I S. 1207), which implements the Directive 86/609/EEC from the European Union and the European Convention for the protection of vertebrate animals used for experimental and other scientific purposes. The protocol was approved by the ethics committee of the Max Planck Institute for Infection Biology and the Berlin state authorities (Landesamt für Gesundheit und Soziales (LAGeSo regulation G0469/09). C57BL/6 female mice were ordered from Charles River Laboratories.
Plasmodium life cycle.
For all experiments Plasmodium berghei parasites (strain ANKA), which constitutively express green fluorescent protein (GFP) under the EF1α promoter, were used (21). Anopheles stephensi mosquitoes were raised at 20°C in 75% humidity under a 14-h light/10-h dark cycle. Blood feeding and mosquito dissection were performed as previously described (5). Midgut-associated sporozoites were isolated 14 days after the infective blood meal. Hemocoel and salivary gland sporozoites were isolated from the same batch of mosquitoes and processed on the same day, 17 to 22 days after a blood meal (22). Hemolymph was obtained by gentle lavage with RPMI medium via the thorax of CO2-anesthetized mosquitoes after removal of the distal abdominal segment. In addition, hemocoel sporozoite preparations were carefully examined by phase-contrast microscopy for the lack of tissue debris and the presence of hemocytes.
Sporozoite gliding motility.
Eight-well chamber glass slides were precoated with RPMI medium containing 3% bovine serum albumin (BSA) for 20 min at 37°C in a humid chamber. Sporozoites were dissected in RPMI–3% BSA and incubated for 45 min at 37°C for settlement and gliding. After fixation with 4% paraformaldehyde, sporozoites and trails were detected by anti-P. berghei CSP antibody (23).
Cell traversal assay.
Twenty-four-well plates were seeded with 300,000 human hepatoma cells (Huh7) per well in Dulbecco modified Eagle complete medium (DMEM) and inoculated with 35,000 sporozoites in 300 μl of DMEM with 0.5 μg of fluorescein isothiocyanate (FITC)-dextran (Invitrogen)/μl. After centrifugation for 5 min at 3,000 rpm, the plates were incubated for either 20 or 40 min at 37°C with 5% CO2. Thereafter, the cells were treated with trypsin and resuspended in 500 μl of 1% paraformaldehyde. Quantification of dextran-positive cells was performed by fluorescence-activated cell sorting (FACS) analysis using a Fortessa cell analyzer (BD Biosciences) and FlowJo software (Tree Star).
Sporozoite cell adhesion and invasion.
For these assays, 8,000 sporozoites prepared in DMEM were added to cultured Huh7 cells. For cellular adhesion, wells were incubated for 30 min at room temperature, and the supernatant was removed to determine nonattached sporozoites in a hemocytometer. The difference to the sporozoite inoculum was considered the number of retained sporozoites. Inoculated hepatoma cells were incubated for additional 90 min at 37°C with 5% CO2 to quantify cell invasion. The protocol was slightly modified from the established two-color invasion assay (24). Briefly, cells were fixed with 4% paraformaldehyde, followed by an immunofluorescence assay using anti-P. berghei CSP antibody (23) to label extracellular sporozoites and anti-GFP antibody after cell permeabilization to detect intracellular parasites.
Plasmodium liver-stage development in vitro.
To monitor successful parasite development in hepatoma cells, Huh7 cells were infected with 6,000 hemocoel or salivary gland sporozoites isolated in DMEM. For settlement, the wells were centrifuged for 5 min at 3,000 rpm and incubated for 2 h at 37°C with 5% CO2. To stop cell invasion, the cells were washed three times with DMEM, followed by incubation for 24 h or 48 h to permit development of enumerated exoerythrocytic forms (EEFs). Cells were fixed with 4% paraformaldehyde for 10 min, followed by immunofluorescent assay with anti-P. berghei HSP70 antibody (25).
Murine infections.
Age-matched female C57BL/6 mice were infected with 5,000 sporozoites in RPMI medium. Sporozoites were injected intravenously or subcutaneously at the tail vein. Patency, i.e., the time to detection of blood-stage parasites, and parasitemia were determined by daily microscopic examination of Giemsa-stained blood films. During the analysis, development of signature symptoms of experimental cerebral malaria (ECM) was monitored. Mice were diagnosed with onset of ECM if they showed behavioral and functional abnormalities, such as ataxia, paralysis, or convulsions (26). Mice were sacrificed immediately after a diagnosis of ECM.
Determination of parasite liver load by quantitative real-time reverse transcription-PCR (RT-PCR).
A total of 5,000 sporozoites isolated in RPMI medium were syringe injected either subcutaneously or intravenously into female C57BL/6 mice. The livers of infected and control mice were isolated 42 h after infection. Organs were rinsed in phosphate-buffered saline and homogenized. Total RNA was isolated (RNeasy; Qiagen), and cDNA was synthesized (RETROscript; Ambion). Real-time PCR was performed with the ABI 7500 sequence detection system and Power SYBER green PCR master mix (Applied Biosystems) as described previously (27, 28). Gene-specific primers for P. berghei 18S rRNA (gi:160641 [forward, 5′-AAGCATTAAATAAAGCGAATACATCCTTAC-3′; reverse, 5′-GGAGATTGGTTTTGACGTTTATGTG-3′) and the mouse GAPDH gene (gi:281199965 [forward, 5′-TGAGGCCGGTGCTGAGTATGTCG-3′; reverse, 5′-CCACAGTCTTCTGGGTGGCAGTG-3′]) were used for amplification. The relative transcript abundance was determined by using the 2−ΔΔCT method.
Systemic cytokine measurement.
Plasma was isolated from mice infected by 5,000 sporozoite intravenously at the days indicated. Plasma cytokines were assayed by using a cytometric bead array (mouse inflammation kit; BD Bioscience) as described previously (29). Analysis was performed using a Fortessa cell analyzer (BD Biosciences) and FlowJo software (Tree Star).
Whole-sporozoite immunizations.
Freshly dissected hemocoel or salivary gland sporozoites were irradiated with 12,000 cGy. A total of 10,000 irradiated sporozoites were intravenously injected per immunization. Challenge experiments were carried out with 10,000 salivary gland sporozoites. Immunized animals were monitored for the presence of blood-stage parasites from day 3 onward until day 14 after challenge by daily microscopic examination of Giemsa-stained blood films. Sterile protection was defined as the complete absence of blood-stage parasites. Alternatively, the parasite load after challenge infection was quantified. Livers were isolated and homogenized 42 h after challenge infection with 10,000 sporozoites. Total RNA was extracted from samples preserved in TRIzol reagent (Invitrogen) according to the manufacturer's instructions. cDNA synthesis and real-time PCR were performed as described above.
Statistical analysis.
Statistical significance was assessed using a Mann-Whitney test or an unpaired t test, with a P value of <0.05 considered a significant difference. Survival curves were compared by using the log-rank (Mantel-Cox) test. The Kruskal-Wallis test was performed to compare the significance of dependent data. All statistical tests were computed with GraphPad Prism 5 (GraphPad Software).
RESULTS
Hemocoel sporozoites perform continuous gliding locomotion.
We initiated our analysis by comparative analysis of sporozoite gliding motility. To this end, we isolated P. berghei sporozoites from the three mosquito compartments, i.e., midgut, hemocoel, and salivary glands (see Fig. S1A in the supplemental material). Midgut-associated sporozoites were obtained from mosquitoes 14 days after an infectious blood meal, while sporozoites from hemocoel and salivary glands were dissected from the same batch of mosquitoes between days 17 and 22 after infection. Sporozoite gliding motility was analyzed by immunofluorescence using antibodies against the circumsporozoite protein (CSP), which is a surface protein that is deposited in trails by sporozoites gliding on glass slides (30). Consistent with previous findings (5, 11, 12), we observed no gliding locomotion in midgut sporozoites, whereas gliding motility by the majority (∼79%) of sporozoites that have colonized the salivary gland was vigorous and continuous (see Fig. S1B and C in the supplemental material). Sporozoites isolated from the mosquito hemocoel exhibited intermediate motility (see Fig. S1B in the supplemental material). Quantification of the proportion of hemocoel sporozoites that displayed continuous gliding locomotion in vitro revealed a substantial proportion (∼14%) with this capacity (see Fig. S1C). Together, these findings indicate that hemocoel sporozoites have, at least partially, acquired a signature of mature sporozoites, i.e., fast and continuous gliding locomotion.
Hemocoel sporozoites display a distinct impairment in cell traversal only.
In the liver, sporozoites adhere to sinusoidal cells, breach a Kupffer cell, and traverse several hepatocytes, until they ultimately reside in the final target cell (31). We tested three distinct sporozoite capacities in vitro, namely, cell adhesion, traversal, and invasion, which reflect the principal steps in successful liver colonization (Fig. 1). Previous work showed that midgut and salivary gland sporozoites display comparable cellular adhesion (14). Accordingly, in our analysis we could not distinguish salivary gland from hemocoel sporozoites in the cell adhesion assay (Fig. 1A). However, when we quantified cell traversal we observed a significant impairment in sporozoites isolated from mosquito hemocoel compared to salivary glands (Fig. 1B). This difference was no longer apparent when we quantified the proportion of intracellular parasites (Fig. 1C). Collectively, these data show that hemocoel sporozoites display normal infectivity to the final target cell, the hepatocyte, but do not efficiently traverse cells prior to productive invasion.
FIG 1.
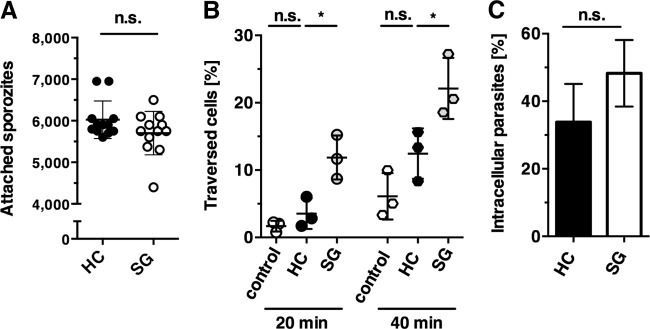
Hemocoel sporozoites display a distinct impairment of cell traversal. (A) Sporozoite adhesion to hepatoma cells. Hepatoma cells were incubated with hemocoel sporozoites (black circles; HC) or salivary gland sporozoites (white circles; SG), and adherent sporozoites were quantified. Each dot represents one sample. Shown are mean values (± the standard deviation [SD]) from three independent experiments. (B) Sporozoite traversal of hepatoma cells. Hepatoma cells were incubated with FITC-dextran either alone (white circles; control), with hemocoel sporozoites (black circles; HC), or with salivary gland sporozoites (gray circles; SG) for 20 and 40 min. Cells were fixed and analyzed by FACS to enumerate the percentage of dextran-positive cells. The results represent mean values (± the SD) of three independent experiments with three samples each. (C) Sporozoite invasion of hepatoma cells. Hepatoma cells were infected with hemocoel sporozoites (black bar, HC) or salivary gland sporozoites (white bar, SG) for 2 h. Cells were fixed and extracellular sporozoites were stained with an anti-CSP antibody, followed by permeabilization and staining with an anti-GFP antibody, in order to distinguish intracellular parasites that have invaded the cell versus attached parasites. The results represent mean values (± the SD) of three independent experiments with two samples each. n.s., not significant; *, P < 0.05 (unpaired t test).
FIG 2.
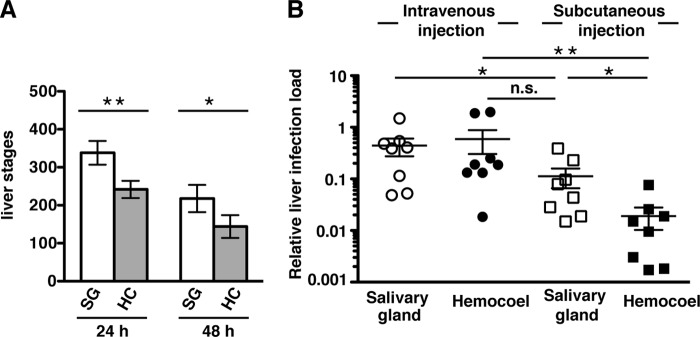
Hemocoel sporozoites establish liver infections. (A) Development of exoerythrocytic forms (EEFs) in cultured hepatoma cells. Hepatoma cells were infected with salivary gland sporozoites (white bars) or hemocoel sporozoites (gray bars) and cultured for 24 and 48 h before fixation and staining with an anti-HSP70 antibody. The results represent mean values (± the SD) of three independent experiments with duplicate or triplicate samples. *, P < 0.05; **, P < 0.01 (unpaired t test). (B) Quantification of parasite loads in the liver by real-time RT-PCR. Livers were harvested 42 h after infection of C57BL/6 mice with either salivary gland sporozoites (white) or hemocoel sporozoites (black). Sporozoites were inoculated by intravenous (circles) or subcutaneous (squares) injections. Relative expression levels of P. berghei 18S rRNA were normalized to mouse GAPDH (n = 8 for each condition). The results represent mean values (± the standard error of the mean). n.s., not significant; *, P < 0.05; **, P < 0.01 (Kruskal-Wallis test).
Liver infectivity of hemocoel sporozoites.
Previous work established that fast gliding locomotion is only required for intradermal migration and not a prerequisite for liver infectivity (32). To corroborate these findings, we first infected cultured hepatoma cells with hemocoel or salivary gland sporozoites and enumerated exoerythrocytic forms (EEFs) 24 and 48 h later (Fig. 2A). Despite the observed lower proportion of gliding motility (see Fig. S1C in the supplemental material) and transmigration (Fig. 1B), but in good agreement with normal cell adhesion (Fig. 1A) and invasion (Fig. 1C), hemocoel sporozoites were clearly able to transform into EEFs. As expected, total EEF numbers were reduced compared to salivary gland sporozoites (Fig. 2A). However, this difference was less pronounced than the observed ∼4-fold reduction in gliding motility, supporting the notion of robust invasive capacity of hemocoel sporozoites.
This finding prompted us to perform in vivo infection experiments to test liver colonization by hemocoel sporozoites (Fig. 2B). Intriguingly, the parasite burdens in livers were indistinguishable between salivary gland and hemocoel sporozoites when parasites were injected intravenously, the standard route of infection to study sporozoite-induced malaria. Apparently, incomplete maturation of hemocoel sporozoites, as observed by in vitro assays (Fig. 2A; see also Fig. S1C in the supplemental material), did not translate into detectable differences in liver colonization in vivo (Fig. 2B).
We wanted to substantiate our findings by subcutaneous sporozoite injection. This delivery route previously revealed locomotion defects in a mutant parasite line, leading to the conclusion that fast and continuous gliding locomotion is only required for intradermal migration of Plasmodium sporozoites (32). In good agreement with a reduced proportion of gliding sporozoites and cell traversal, parasite loads in the liver were significantly (P < 0.05) reduced in hemocoel sporozoite-infected compared to salivary gland-infected mice (Fig. 2B). In conclusion, in vitro and in vivo liver infection assays revealed attainment of infectivity by sporozoites before salivary gland colonization.
Hemocoel sporozoites are infectious and virulent.
We next assessed the induction and kinetics of blood-stage development and disease outcome in mice infected with hemocoel sporozoites (Fig. 3). We first monitored prepatency, i.e., the time to detection of blood-stage parasites in peripheral blood (Fig. 3A). A total of 5,000 sporozoites were injected either intravenously or subcutaneously into groups of 10 C57BL/6 mice. All mice became positive for parasitemia after an average of 3.1 days of intravenous injection with salivary gland sporozoites. When salivary gland sporozoites were injected subcutaneously or hemocoel sporozoites were injected intravenously, infected mice displayed a slight—albeit insignificant—delay in prepatency (4 days). Since all mice became infected, we also tested injection of hemocoel sporozoites into the subcutaneous layer (Fig. 3A.) Blood stages were detectable after an average of 4.9 days in all mice. This finding is in good agreement with our data on hepatic parasite load (Fig. 2B) and supports the notion that hemocoel sporozoites can be reliably used to infect mice intravenously.
FIG 3.
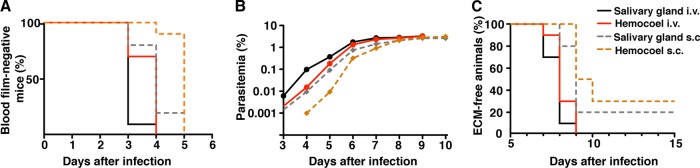
Hemocoel sporozoites retain virulence in mice. In vivo infectivity of sporozoites to C57BL/6 mice by intravenous (continuous lines) and subcutaneous (hatched lines) injections. A total of 5,000 salivary gland sporozoites (black and gray) or hemocoel sporozoites (red and orange) were injected for all experiments. Survival curves are based on cumulative data from three independent experiments (n = 10 each). (A) Kaplan-Meier analysis of the percentage of parasite-free animals over time (days after infection). Infections were monitored daily by microscopic examination of Giemsa-stained blood films. None of the differences were significant (Kruskal-Wallis test). (B) Parasitemias of blood infection. The percentage of parasite-infected erythrocytes is presented on a logarithmic scale. (C) Kaplan-Meier analysis of experimental cerebral malaria (CM) over time (days after sporozoite-induced infections). Analyses by log-rank (Mantel-Cox) test for salivary gland versus hemocoel sporozoites intravenously and subcutaneously were both not significant.
Since all mice became blood-stage positive, we could quantify the kinetics of blood infection (Fig. 3B). Notably, we observed similar growth kinetics, irrespective of the day of onset of blood infection. All mice reached the characteristic plateau, observed in P. berghei ANKA infections between days 6 and 8 after sporozoite-induced infections (Fig. 3B). Most importantly, all mice that were infected by the intravenous route, regardless of the sporozoite origin, developed signature symptoms of experimental cerebral malaria (ECM), a lethal outcome of a P. berghei ANKA infection (26, 29, 33, 34), between 7 and 9 days after infection (Fig. 3C). When sporozoites were injected subcutaneously, 2 and 3 out of 10 developed severe anemia instead of ECM for salivary gland and hemocoel sporozoite infections, respectively. Based on these findings, we conclude that hemocoel sporozoites achieve full virulence for murine infections.
Hemocoel and salivary gland sporozoites elicit similar systemic cytokine responses in the host.
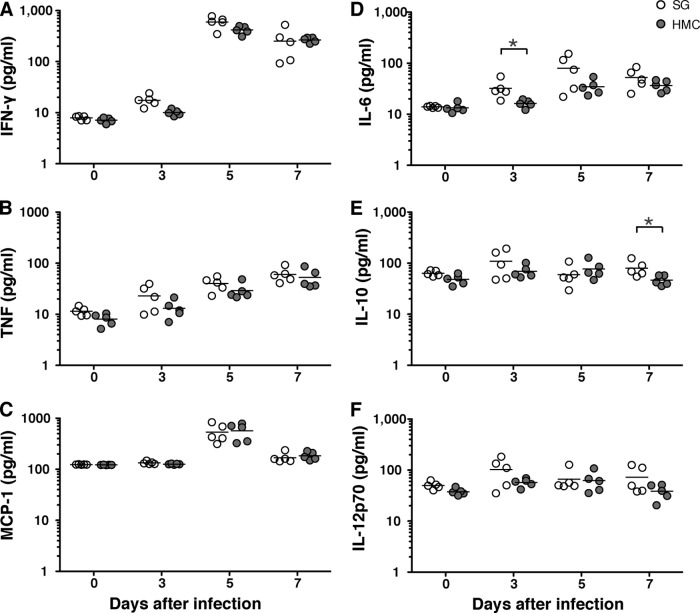
In response to infections with virulent P. berghei ANKA parasites C57BL/6 mice mount proinflammatory cytokine responses that, at least partially, contribute to disease exacerbation (29, 35–40). To compare cytokine responses in mice infected with either hemocoel or salivary gland sporozoites, we took plasma samples at days 3, 5, and 7 after infection and measured steady-state levels of signature cytokines (Fig. 4). As previously shown (29, 40), we detected a marked increase in the proinflammatory cytokine gamma interferon (IFN-γ) at day 5 after infection in both groups of mice, which were either infected with hemocoel or salivary gland sporozoites (Fig. 4A). We also noticed a gradual increase of tumor necrosis factor (TNF) in all infected mice (Fig. 4B). Similarly, monocyte chemotactic protein 1 (MCP-1) was transiently upregulated at day 5 after sporozoite infection, irrespective of the sporozoite origin (Fig. 4C). In contrast, the regulatory cytokines interleukin-6 (IL-6), IL-10, and IL-12p70 remained mostly unaffected throughout the course of infection with either salivary gland or hemocoel sporozoites (Fig. 4D to F). Collectively, hemocoel and salivary gland sporozoites elicit similar systemic cytokine responses in the infected hosts.
FIG 4.
Proinflammatory and regulatory cytokines induced by hemocoel sporozoites. C57BL/6 mice were injected intravenously with 5,000 salivary gland sporozoites (white circles) or hemocoel sporozoites (gray circles). Cytokines were determined in plasma samples. Day 0 results represent data from uninfected mice. Shown are the steady-state levels of proinflammatory cytokines: gamma interferon (IFN-γ) (A), tumor necrosis factor (TNF) (B), monocyte chemotactic protein-1 (MCP-1) (C), and the regulatory cytokines IL-6 (D), IL-10 (E), and IL-12p70 (F) (n = 5) for each time point. Assessment by Mann-Whitney test revealed nonsignificant differences between salivary gland and hemocoel sporozoites at all time points, except as follows: *, P < 0.05 for IL-6, 3 days after infection, and for IL-10, 7 days after infection.
Immunizations with irradiated hemocoel sporozoites elicit protection against reinfection.
Thus far, our data show that hemocoel sporozoites can infect the mammalian host. We therefore wanted to explore whether this sporozoite population can also be used for whole-organism vaccinations. To this end, we immunized groups of C57BL/6 mice with 10,000 irradiated sporozoites, either isolated from the hemocoel or mosquito salivary glands (Fig. 5). For challenge infections, salivary gland sporozoites were used. We used two complementary protocols to test protective efficacy: (i) quantification of parasite load in the liver 42 h after challenge infection (Fig. 5A) and (ii) microscopic examination of Giemsa-stained blood films (Fig. 5B).
FIG 5.
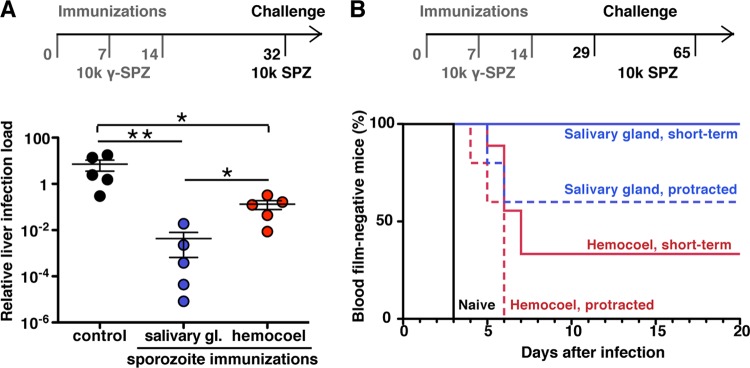
Immunization with irradiation-attenuated hemocoel sporozoites. (A) C57BL/6 mice were immunized three times at weekly intervals with 10,000 irradiated sporozoites (10k y-SPZ), isolated either from mosquito salivary glands (blue) or from the hemocoel (red). All animals were challenged with 10,000 salivary gland sporozoites (10k SPZ) 18 days after the last immunization. After 42 h, the livers were removed, and the parasite loads were quantified by real-time RT-PCR (n = 5 each; *, P < 0.05; **, P < 0.01 [Mann-Whitney test]). (B) Kaplan-Meier analysis of C57BL/6 mice that were immunized as described above. Challenge was performed either 15 days (day 29; short-term protection) or 51 days (day 65; protracted protection) after the last immunization by intravenous injection of 10,000 salivary gland sporozoites. Blood parasitemia was determined by daily microscopic examination of Giemsa-stained blood films (n = 5 for all experiments). Analysis by Kruskal-Wallis test revealed nonsignificant differences for protracted protection but P < 0.01 (**) for short-term protection.
Quantification of parasite burden in the liver after challenge infection revealed robust protection in mice immunized with irradiated hemocoel sporozoites compared to the control group that received no immunization (P < 0.05) (Fig. 5A). However, we noticed that the parasite loads in the livers of mice immunized with irradiated hemocoel sporozoites were slightly, yet significantly (P < 0.05), higher than of mice immunized with irradiated salivary gland sporozoites. Sterile protection was determined by monitoring parasitemia after the challenge infection 15 days after the last immunization (Fig. 5B). While all mice immunized with irradiated salivary gland sporozoites remained parasite-free throughout the observation period of 3 weeks, a proportion of hemocoel sporozoite-immunized mice developed parasitemia, albeit only after a significant delay of at least 3 days. When we challenged immunized mice >6 weeks after the last immunization, we observed that also a proportion (40%) of mice immunized with irradiated salivary gland sporozoites became blood stage-positive by day 6 after challenge (Fig. 5B). All mice immunized with irradiated hemocoel sporozoites developed parasitemia by day 6. However, the 3-day delay in patency compared to the control mice signifies the notion of substantial pre-erythrocytic immunity, consistent with the reduction of the parasite load in the liver (Fig. 5A). In conclusion, immunizations with hemocoel sporozoites elicit effective, albeit partial, protection against reinfections.
DISCUSSION
In this report, we show the indistinguishable progression of malaria in mice that are infected with sporozoites, which are either isolated from the mosquito hemocoel or salivary glands and syringe delivered. Intravenous injection of both sporozoite populations leads to the development of blood-stage parasites and experimental cerebral malaria with similar systemic cytokine responses. This finding also implies that an endogenous developmental program is sufficient for acquisition of the critical sporozoite traits in order to establish an infection in the vertebrate host. We show that hemocoel sporozoites display a remarkable degree of infectivity and virulence and that these features do not critically depend on the physiological environment of the salivary gland. We therefore conclude that sporozoite maturation occurs in a time-dependent manner, as was initially suggested by Jerome Vanderberg (5). However, spatial triggers, such as contact with and/or passage to salivary glands, are necessary for complete acquisition of two distinct sporozoite capacities, i.e., gliding locomotion and cell traversal. During natural transmission, the acquisition of full gliding motility is important for intradermal movement to eventually reach a blood capillary. Accordingly, bypassing the skin by intravenous injection renders hemocoel sporozoites more similar to salivary gland sporozoites.
This notion has important implications for axenic in vitro cultures that aim at reproducing sporogony of malarial parasite. Because this is the only growth and replication phase in the Plasmodium life cycle that occurs outside, yet closely associated with, host cells (2, 4), it is conceivable that infectious and immunogenic sporozoites might be produced without the need for a mosquito vector. In a landmark study, Alon Warburg and Louis Miller seeded purified P. gallinaceum ookinetes onto Matrigel and Drosophila melanogaster L2 feeder cells and obtained spherical and elongated oocysts (41). These cultured oocysts produced in many cases sporozoites, which expressed the major surface protein CSP. These findings could subsequently be confirmed for other Plasmodium species, including P. falciparum (42), P. berghei (43), and P. yoelii (44). Irrespective of the morphological and molecular signatures, the single most important criterion for successful axenic sporozoite culturing is the capacity to infect a vertebrate host. Thus far, this was reported only from P. gallinaceum sporozoites isolated from a xenohost, ookinete-injected Drosophila melanogaster (45), and from axenically cultured P. berghei sporozoites (43). In both cases, the infectivity to chicken and mice, respectively, was very low, suggestive of incomplete maturation. In order to increase efficiency of axenic sporozoite production, several improvements are critical, including high-yield in vitro generation of ookinetes (46) and improved ookinete-to-oocyst transformation (47). Developing oocysts take up lipophorin, the major insect lipoprotein in the hemocoel (48, 49), indicating that external lipid sources are important nutrients. Our results highlight sporozoite maturation after egress from oocysts as a central factor for production of infectious sporozoites. It is highly likely that additional components, as shown for phosphatidylethanolamine (48), are incorporated into sporozoites during their residence in the hemocoel. Although major biotechnological investments are required to eventually achieve axenic production of infectious sporozoites, our data provide an important stimulus toward that goal.
During the course of our studies, we noticed a correlation between a smaller proportion of continuous gliding locomotion in hemocoel sporozoites and a specific reduction in liver infectivity only when sporozoites are delivered subcutaneously. Bypassing intradermal migration by intravenous syringe inoculation resulted in high parasite burden in the liver. This finding using two developmental stages of sporozoites provides independent support for our previous experimental genetics evidence that fast and continuous gliding locomotion is only important for intradermal migration and not for hepatocyte invasion in vitro and in vivo (32). In hsp20(−) sporozoites, speed and cellular adhesion is dramatically altered, resulting in impaired natural malaria transmission but perfectly normal host cell invasion (32, 50).
We also note that ECM is not an inevitable fate when mice are infected subcutaneously. Although all mice that were subcutaneously infected with either salivary gland or hemocoel sporozoites developed high parasitemia, a proportion (∼25%) did not develop signature symptoms of ECM. It is plausible that the first parasite-host cross talk can modulate disease outcome of an infection with an otherwise virulent parasite. One recent study comparing natural and transfusion-mediated infection with Plasmodium chabaudi provided compelling evidence for virulence regulation by vector transmission (51). However, in most Plasmodium-host combinations, including human infections, parasitemia and clinical development of sporozoite- and asexual blood-stage-induced infections are indistinguishable (8, 28, 29, 40, 52, 53). Clearly, further studies are warranted to address this important issue.
Live whole-sporozoite vaccine strategies, including radiation-attenuated or genetically attenuated sporozoites and sporozoite infections under antimalarial drug cover, elicit a high degree of sterile and lasting protection against reinfections in murine models and clinical trials (8, 9, 28, 54–57). Clinical development and testing of these complementary vaccine approaches against a complex eukaryotic pathogen offer an attractive alternative to Plasmodium subunit vaccines (58, 59). Irrespective of the attenuation strategy, sporozoites are presently hand-dissected from the salivary glands of infected Anopheles mosquitoes (58). Our data on substantial protective efficacy of irradiation-arrested hemocoel sporozoites suggest that residence in salivary glands is not an absolute requirement for immunogenicity and, ultimately, vaccine efficacy. Most importantly, very recent clinical trials in human volunteers established that immunization with irradiated sporozoites needs to be performed by the intravenous route, as opposed to intradermal or subcutaneous injection, in order to elicit robust, and in some cases, sterile protection (60, 61). Our data indicate that intravenous injection of hemocoel sporozoites allows to, at least partially, compensate for the two significant deficiencies, reduced sporozoite gliding locomotion and cell traversal. Hence, it can be envisaged that nonreplicating hemocoel sporozoites can substitute salivary gland sporozoites as experimental malaria vaccines in future trials.
Although protection was substantial and, in some cases, 100% effective, we observed a significant difference compared to salivary gland sporozoites. Previous work showed that mosquito saliva modulates local and systemic immune responses toward a protective T-helper 1 (Th1) phenotype (62). Of note, saliva does not affect sporozoite infectivity (63), a finding that is fully supported by our data showing that hemocoel sporozoites establish liver and blood infections. In contrast, murine infections with the mosquito-transmitted West Nile virus demonstrated that saliva substantially enhances virus transmission (64). It is plausible that during transmission of complex eukaryotic pathogens, such as Plasmodium, mosquito saliva affects immunogenicity but not infectivity, whereas during viral transmission the immune modulatory effects are broader. The recent identification of two major immunogenic salivary gland proteins provides a molecular framework to gain a better understanding of immune modulation by mosquito saliva (65). Alternatively, differences in the antigenic repertoire or in shedding of antigenic surface proteins during transmigration between hemocoel and salivary gland sporozoites might contribute to dissimilar immunogenicity. Systematic studies with natural or synthetic adjuvants and differential molecular and immune profiling are warranted to explore whether immunogenicity of hemocoel and, ultimately, axenically cultured sporozoites can be enhanced to reach complete and sustained protection against reinfection.
In conclusion, our study shows that salivary gland invasion is not an absolute prerequisite for infectivity of and immunogenicity in the vertebrate host. This result also strengthens efforts to engineer whole-organism malaria vaccines by mosquito-free culturing of sporozoites.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jan Burgold and Elyzana Putrianti for expert assistance with the cytokine measurements.
This study was supported by the Max Planck Society and, in part, by the EviMalaR Network of Excellence (participant 34). Y.S. is supported by the ZIBI Graduate School, Berlin, Germany (“Research in Infection Biology and Immunology”).
We declare that we have no conflict of interest.
Footnotes
Published ahead of print 30 December 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00758-13.
REFERENCES
- 1.Hafalla JCR, Silvie O, Matuschewski K. 2011. Cell biology and immunology of malaria. Immun. Rev. 240:297–316. 10.1111/j.1600-065X.2010.00988.x [DOI] [PubMed] [Google Scholar]
- 2.Sinden RE, Matuschewski K. 2005. The sporozoite, p 169–190 In Sherman IW. (ed), Molecular approaches to malaria. American Society for Microbiology, Washington, DC [Google Scholar]
- 3.Pimenta PF, Touray M, Miller LH. 1994. The journey of malaria sporozoites in the mosquito salivary gland. J. Eukaryot. Microbiol. 41:608–624. 10.1111/j.1550-7408.1994.tb01523.x [DOI] [PubMed] [Google Scholar]
- 4.Matuschewski K. 2006. Getting infectious: formation and maturation of Plasmodium sporozoites in the Anopheles vectror. Cell. Microbiol. 8:1547–1556. 10.1111/j.1462-5822.2006.00778.x [DOI] [PubMed] [Google Scholar]
- 5.Vanderberg JP. 1975. Development of infectivity by the Plasmodium berghei sporozoite. J. Parasitol. 61:43–50. 10.2307/3279102 [DOI] [PubMed] [Google Scholar]
- 6.Matuschewski K, Ross J, Brown S, Kaiser K, Nussenzweig V, Kappe SHI. 2002. Infectivity-associated changes in the transcriptional repertoire of the malaria sporozoite stage. J. Biol. Chem. 277:41948–41953. 10.1074/jbc.M207315200 [DOI] [PubMed] [Google Scholar]
- 7.Mikolajczak SA, Silva-Rivera H, Peng X, Tarun AS, Camargo N, Jacobs-Lorena V, Daly TM, Bergman LW, de la Vega P, Williams J, Aly AS, Kappe SH. 2008. Distinct malaria parasite sporozoites reveal transcriptional changes that cause differential tissue infection competence in the mosquito vector and mammalian host. Mol. Cell. Biol. 28:6196–6207. 10.1128/MCB.00553-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller AK, Labaied M, Kappe SHI, Matuschewski K. 2005. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature 433:164–167. 10.1038/nature03188 [DOI] [PubMed] [Google Scholar]
- 9.Mueller AK, Camargo N, Kaiser K, Andorfer C, Frevert U, Matuschewski K, Kappe SHI. 2005. Plasmodium liver stage developmental arrest by depletion of a protein at the parasite-host interface. Proc. Natl. Acad. Sci. U. S. A. 102:3022–3027. 10.1073/pnas.0408442102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Touray MG, Warburg A, Laughinghouse A, Krettli A, Miller LH. 1992. Developmentally regulated infectivity of malaria sporozoites for mosquito salivary glands and the vertebrate host. J. Exp. Med. 175:1607–1612. 10.1084/jem.175.6.1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hegge S, Kudryashev M, Smith A, Frischknecht F. 2009. Automated classification of Plasmodium sporozoite movement patterns reveals a shift toward productive motility during salivary gland infection. Biotechnol. J. 4:903–913. 10.1002/biot.200900007 [DOI] [PubMed] [Google Scholar]
- 12.Kudryashev M, Münter S, Lemgruber L, Montagna G, Stahlberg H, Matuschewski K, Meissner M, Cyrklaff M, Frischknecht F. 2012. Structural basis for chirality and directional motility of Plasmodium sporozoites. Cell. Microbiol. 14:1757–1768. 10.1111/j.1462-5822.2012.01836.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kappe S, Bruderer T, Gantt S, Fujioka H, Nussenzweig V, Ménard R. 1999. Conservation of a gliding motility and cell invasion machinery in apicomplexan parasites. J. Cell Biol. 147:937–944. 10.1083/jcb.147.5.937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matuschewski K, Nunes AC, Nussenzweig V, Ménard R. 2002. Plasmodium sporozoite invasion of insect and mammalian cells is directed by the same dual binding system. EMBO J. 21:1597–1606. 10.1093/emboj/21.7.1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinbuechel M, Matuschewski K. 2009. Role for the Plasmodium sporozoite-specific transmembrane protein S6 in parasite motility and efficient malaria transmission. Cell. Microbiol. 11:279–288. 10.1111/j.1462-5822.2008.01252.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engelmann S, Silvie O, Matuschewski K. 2009. Disruption of Plasmodium sporozoite transmission by depletion of sporozoite invasion-associated protein 1. Eukaryot. Cell 8:640–648. 10.1128/EC.00347-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kariu T, Yuda M, Yano K, Chinzei Y. 2002. MAEBL is essential for malarial sporozoite infection of the mosquito salivary gland. J. Exp. Med. 195:1317–1323. 10.1084/jem.20011876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saenz FE, Balu B, Smith J, Mendonca SR, Adams JH. 2008. The transmembrane isoform of Plasmodium falciparum MAEBL is essential for the invasion of Anopheles salivary glands. PLoS One 3:e2287. 10.1371/journal.pone.0002287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson J, Fernandez-Reyes D, Sharling L, Moore SG, Eling WM, Kyes SA, Newbold CI, Kafatos FC, Janse CJ, Waters AP. 2007. Plasmodium cysteine repeat modular proteins 1–4: complex proteins with roles throughout the malaria parasite life cycle. Cell. Microbiol. 9:1466–1480. 10.1111/j.1462-5822.2006.00885.x [DOI] [PubMed] [Google Scholar]
- 20.Frischknecht F, Martin B, Thiery I, Bourgouin C, Ménard R. 2002. Using green fluorescent malaria parasites to screen for permissive vector mosquitoes. Malaria J. 5:23. 10.1186/1475-2875-5-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janse CJ, Franke-Fayard B, Mair GR, Ramesar J, Thiel C, Engelmann S, Matuschewski K, van Gemert GJ, Sauerwein RW, Waters AP. 2006. High-efficiency transfection of Plasmodium berghei facilitates novel selection procedures. Mol. Biochem. Parasitol. 145:60–70. 10.1016/j.molbiopara.2005.09.007 [DOI] [PubMed] [Google Scholar]
- 22.Hegge S, Münter S, Steinbüchel M, Heiss K, Engel U, Matuschewski K, Frischknecht 2010. Multistep adhesion of Plasmodium sporozoites. FASEB J. 24:2222–2234. 10.1096/fj.09-148700 [DOI] [PubMed] [Google Scholar]
- 23.Potocnjak P, Yoshida N, Nussenzweig RS, Nussenzweig V. 1980. Monovalent fragments (Fab) of monoclonal antibodies to a sporozoite surface antigen (Pb44) protect mice against malarial infection. J. Exp. Med. 151:1504–1513. 10.1084/jem.151.6.1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rénia L, Miltgen F, Charoenvit Y, Ponnudurai T, Verhave JP, Collins WE, Mazier D. 1988. Malaria sporozoite penetration: a new approach by double staining. J. Immunol. Methods 112:201–205 [DOI] [PubMed] [Google Scholar]
- 25.Tsuji M, Mattei D, Nussenzweig RS, Eichinger D, Zavala F. 1994. Demonstration of heat-shock protein 70 in the sporozoite stage of malaria parasites. Parasitol. Res. 80:16–21. 10.1007/BF00932618 [DOI] [PubMed] [Google Scholar]
- 26.Lackner P, Beer R, Heussler V, Goebel G, Rudzki D, Helbock R, Tannich E, Schmutzhardt E. 2006. Behavioural and histopathological alterations in mice with cerebral malaria. Neuropathol. Appl. Neurobiol. 32:177–188. 10.1111/j.1365-2990.2006.00706.x [DOI] [PubMed] [Google Scholar]
- 27.Bruña-Romero O, Hafalla JC, González-Aesguinolaza G, Sano G, Tsuji M, Zavala F. 2001. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int. J. Parasitol. 31:1499–1502. 10.1016/S0020-7519(01)00265-X [DOI] [PubMed] [Google Scholar]
- 28.Friesen J, Silvie O, Putrianti ED, Hafalla JCR, Matuschewski K, Borrmann S. 2010. Natural immunization against malaria: causal prophylaxis with antibiotics. Sci. Transl. Med. 2:ra49. 10.1126/scitranslmed.3001058 [DOI] [PubMed] [Google Scholar]
- 29.Kordes M, Matuschewski K, Hafalla JCR. 2011. Caspase-1 activation of IL-1β and IL-18 is dispensable for the induction of experimental cerebral malaria. Infect. Immun. 79:3633–3641. 10.1128/IAI.05459-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart MJ, Vanderberg J. 1988. Malaria sporozoites leave behind trails of circumsporozoite protein during gliding motility. J. Protozool. 35:389–393. 10.1111/j.1550-7408.1988.tb04115.x [DOI] [PubMed] [Google Scholar]
- 31.Frevert U, Engelmann S, Zougbede S, Stange J, Ng B, Matuschewski K, Liebes L, Yee H. 2005. Intravital observation of Plasmodium berghei sporozoite infection of the liver. PLoS Biol. 3:e192. 10.1371/journal.pbio.0030192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montagna GN, Buscaglia CA, Münter S, Goosmann C, Frischknecht F, Brinkmann V, Matuschewski K. 2012. Critical role for heat shock protein 20 (HSP20) in migration of malarial sporozoites. J. Biol. Chem. 287:2410–2422. 10.1074/jbc.M111.302109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van der Heyde HC, Nolan J, Combes V, Granaglia I, Grau GE. 2006. A unified hypothesis for the genesis of cerebral malaria: sequestration, inflammation and hemostasis leading to microcirculatory dysfunction. Trends Parasitol. 22:503–508. 10.1016/j.pt.2006.09.002 [DOI] [PubMed] [Google Scholar]
- 34.de Souza JB, Hafalla JC, Riley EM, Couper KN. 2010. Cerebral malaria: why experimental murine models are required to understand the pathogenesis of disease. Parasitology 137:755–772. 10.1017/S0031182009991715 [DOI] [PubMed] [Google Scholar]
- 35.Grau GE, Fajardo LF, Piguet PF, Allet B, Lambert PH, Vassalli P. 1987. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science 237:1210–1212. 10.1126/science.3306918 [DOI] [PubMed] [Google Scholar]
- 36.Grau GE, Heremans H, Piguet PF, Pointaire P, Lambert PH, Billiau A, Vassalli P. 1989. Monoclonal antibody against interferon gamma can prevent experimental cerebral malaria and its associated overproduction of tumor necrosis factor. Proc. Natl. Acad. Sci. U. S. A. 86:5572–5574. 10.1073/pnas.86.14.5572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engwerda CR, Mynott TL, Sawhney S, de Souza JB, Bickle QD, Kaye PM. 2002. Locally upregulated lymphotoxin alpha, not systemic tumor necrosis factor alpha, is the principle mediator of murine cerebral malaria. J. Exp. Med. 195:1371–1377. 10.1084/jem.20020128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunt NH, Grau GE. 2003. Cytokines: accelerators and brakes in the pathogenesis of cerebral malaria. Trends Immunol. 24:491–499. 10.1016/S1471-4906(03)00229-1 [DOI] [PubMed] [Google Scholar]
- 39.Schofield L, Grau GE. 2005. Immunological processes in malaria pathogenesis. Nat. Rev. Immunol. 5:722–735. 10.1038/nri1686 [DOI] [PubMed] [Google Scholar]
- 40.Hafalla JCR, Burgold J, Dorhoi A, Gross O, Ruland J, Kaufmann SHE, Matuschewski K. 2012. Experimental cerebral malaria develops independently of Card9 signaling. Infect. Immun. 80:1274–1279. 10.1128/IAI.06033-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warburg A, Miller LH. 1992. Sporogonic development of a malaria parasite in vitro. Science 255:448–450. 10.1126/science.1734521 [DOI] [PubMed] [Google Scholar]
- 42.Warburg A, Schneider I. 1993. In vitro culture of the mosquito stages of Plasmodium falciparum. Exp. Parasitol. 76:121–126. 10.1006/expr.1993.1014 [DOI] [PubMed] [Google Scholar]
- 43.Al Olayan AM, Beetsma AL, Butcher GA, Sinden RE, Hurd H. 2002. Complete development of mosquito phases of malaria parasite in vitro. Science 295:677–679. 10.1126/science.1067159 [DOI] [PubMed] [Google Scholar]
- 44.Porter-Kelley JM, Dinglasan RR, Alam U, Ndeta GA, Sacci JB, Jr, Azad AF. 2006. Plasmodium yoelii: axenic development of the parasite mosquito stages. Exp. Parasitol. 112:99–108. 10.1016/j.exppara.2005.09.011 [DOI] [PubMed] [Google Scholar]
- 45.Schneider D, Shahabuddin M. 2000. Malaria parasite development in a Drosophila model. Science 288:2376–2379. 10.1126/science.288.5475.2376 [DOI] [PubMed] [Google Scholar]
- 46.Bounkeua V, Li F, Vinetz JM. 2010. In vitro generation of Plasmodium falciparum ookinetes. Am. J. Trop. Med. Hyg. 83:1187–1194. 10.4269/ajtmh.2010.10-0433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carter V, Nacer ADL, Underhill A, Sinden RE, Hurd H. 2007. Minimum requirements for ookinete to oocyst transformation in Plasmodium. Int. J. Parasitol. 37:1221–1232. 10.1016/j.ijpara.2007.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Atella GC, Bittencourt-Cunha Nunes PR RD, Shahabuddin M, Silva-Neto MAC. 2009. The major insect lipoprotein is a lipid source to mosquito stages of malaria parasite. Acta Trop. 109:159–162. 10.1016/j.actatropica.2008.10.004 [DOI] [PubMed] [Google Scholar]
- 49.Rono MK, Whitten MMA, Oulad-Abdelghani M, Levashina EA, Marois E. 2010. The major yolk protein vitellogenin interferes with the anti-Plasmodium response in the malaria mosquito Anopheles gambiae. PLoS Biol. 8:e1000434. 10.1371/journal.pbio.1000434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Montagna GM, Matuschewski K, Buscaglia CA. 2012. Small heat shock proteins in cellular adhesion and migration: evidence from Plasmodium genetics. Cell Adh. Migr. 6:78–84. 10.4161/cam.20101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spence PJ, Jarra W, Levy P, Reid AJ, Chappell L, Brugat T, Sanders M, Berriman M, Langhorne J. 2013. Vector transmission regulates immune control of Plasmodium virulence. Nature 498:228–231. 10.1038/nature12231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mackinnon MJ, Vell A, Read AF. 2005. The effects of mosquito transmission and population bottlenecking on virulence, multiplication rate and rosetting in rodent malaria. Int. J. Parasitol. 35:145–153. 10.1016/j.ijpara.2004.11.011 [DOI] [PubMed] [Google Scholar]
- 53.Covell G, Nicol WD. 1951. Clinical, chemotherapeutic and immunological studies on induced malaria. Br. Med. Bull. 8:51–55 [DOI] [PubMed] [Google Scholar]
- 54.Nussenzweig RS, Vanderberg J, Most H, Orton C. 1967. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. Nature 216:160–162. 10.1038/216160a0 [DOI] [PubMed] [Google Scholar]
- 55.Epstein JE, Rao S, Williams F, Freilich D, Luke T, Sedegah M, de la Vega P, Sacci J, Richie TL, Hoffman SL. 2007. Safety and clinical outcome of experimental challenge of human volunteers with Plasmodium falciparum-infected mosquitoes: an update. J. Infect. Dis. 196:145–154. 10.1086/518510 [DOI] [PubMed] [Google Scholar]
- 56.Belnoue E, Costa FT, Frankenberg T, Vigario AM, Voza T, Leroy N, Rodrigues MM, Landau I, Snounou G, Rénia L. 2004. Protective T cell immunity against malaria liver stage after vaccination with live sporozoites under chloroquine treatment. J. Immunol. 172:2487–2495 [DOI] [PubMed] [Google Scholar]
- 57.Roesteneberg M, Teirlinck AC, McCall MB, Teelen K, Makandop KN, Wiersma J, Arens T, Beckers P, van Gemert G, van de Vegte-Bolmer M, van der Ven AJ, Luty AJ, Hermsen CC, Sauerwein RW. 2011. Long-term protection against malaria after experimental sporozoite inoculation: an open-label follow-up study. Lancet 377:1770–1776. 10.1016/S0140-6736(11)60360-7 [DOI] [PubMed] [Google Scholar]
- 58.Hoffman SI, Billingsley PF, James E, Richman A, Loyevsky M, Li T, Chakravarty S, Gunasekera A, Chattopadhyay R, Li M, Stafford R, Ahumada A, Epstein JE, Sedegah M, Reyes S, Richie TL, Lyke KE, Edelman R, Laurens MB, Plowe CV, Sim BK. 2010. Development of a metabolically active, non-replicating sporozoite vaccine to prevent Plasmodium falciparum malaria. Hum. Vaccines 6:97–106. 10.4161/hv.6.1.10396 [DOI] [PubMed] [Google Scholar]
- 59.Matuschewski K. 2013. Murine infection models for vaccine development: the malaria example. Hum. Vaccines Immunother. 9:450–456. 10.4161/hv.23218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Epstein JE, Tewari K, Lyke KE, Sim BK, Billingsley PF, Laurens MB, Gunasekera A, Chakravarty S, James ER, Sedegah M, Richman A, Velmurugan S, Reyes S, Li M, Tucker K, Ahumada A, Ruben AJ, Li T, Stafford R, Eappen AG, Tamminga C, Bennett JW, Ockenhouse CF, Murphy JR, Komisar J, Thomas N, Loyevsky M, Birkett A, Plowe CV, Loucq C, Edelman R, Richie TL, Seder RA, Hoffman SL. 2011. Liver attenuated malaria vaccine designed to protect through hepatic CD8+ T cell immunity. Science 334:475–480. 10.1126/science.1211548 [DOI] [PubMed] [Google Scholar]
- 61.Seder RA, Chang LJ, Enema ME, Zephir KL, Sarwar UN, Gordon IJ, Holman LA, James ER, Billingsley PF, Gunasekera A, Richman A, Chakravarty S, Manoj A, Velmurugan S, Li M, Ruben AJ, Li T, Eappen AG, Stafford RE, Plummer SH, Hendel CS, Novik L, Costner PJM, Mendoza FH, Saunders JG, Nason MC, Richardson JH, Murphy J, Davidson SA, Richie TL, Sedegah M, Sutamihardja A, Fahle GA, Lyke KE, Laurens MB, Roederer M, Tewari K, Epstein JE, Sim BKL, Ledgerwood JE, Graham BS, Hoffman SL, The VRC 312 Study Team 2013. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 341:1359–1365. 10.1126/science.1241800 [DOI] [PubMed] [Google Scholar]
- 62.Donovan MJ, Messmore AS, Scrafford DA, Sacks DL, Kamhawi S, McDowell MA. 2007. Uninfected mosquito bites confer protection against infection with malaria parasites. Infect. Immun. 75:2523–2530. 10.1128/IAI.01928-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kebaier C, Voza T, Vanderberg J. 2010. Neither mosquito saliva nor immunity to saliva has a detectable effect on the infectivity of Plasmodium sporozoites injected into mice. Infect. Immun. 78:545–551. 10.1128/IAI.00807-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Styer LM, Lim PY, Louie KL, Albright RG, Kramer LD, Bernard KA. 2011. Mosquito saliva causes enhancement of west nile virus infection in mice. J. Virol. 85:1517–1527. 10.1128/JVI.01112-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.King JG, Vernick KD, Hillyer JF. 2011. Members of the salivary gland surface protein (SGS) family are major immunogenic components of mosquito saliva. J. Biol. Chem. 286:40824–40834. 10.1074/jbc.M111.280552 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.