Abstract
The obligate intracellular bacterial pathogen Chlamydia trachomatis is the causative agent of a variety of infectious diseases such as trachoma and sexually transmitted diseases. In infected target cells, C. trachomatis replicates within parasitophorous vacuoles and expresses the protease-like activity factor CPAF. Previous studies have suggested that CPAF degrades the host transcription factors RFX5 and NF-κB p65, which are involved in the regulation of constitutive and inducible expression of major histocompatibility complex class I (MHC I). It was speculated that Chlamydia suppresses the surface presentation of MHC I in order to evade an effective immune response. Nevertheless, a recent study suggested that RFX5 and NF-κB p65 may not serve as target substrates for CPAF-mediated degradation, raising concerns about the proposed MHC I subversion by Chlamydia. Hence, we investigated the direct influence of Chlamydia on MHC I expression and surface presentation in infected host cells. By using nine different human cells and cell lines infected with C. trachomatis (serovar D or LGV2), we demonstrate that chlamydial infection does not interfere with expression, maturation, transport, and surface presentation of MHC I, suggesting functional antigen processing in bacterium-infected cells. Our findings provide novel insights into the interaction of chlamydiae with their host cells and should be taken into consideration for the design of future therapies and vaccines.
INTRODUCTION
The intracellular Gram-negative bacterium Chlamydia trachomatis causes more cases of sexually transmitted diseases than any other bacterial pathogen, making C. trachomatis infections an enormous public health problem (1). Infection with Chlamydia can result in acute salpingitis and pelvic inflammatory disease, whose long-term consequences include chronic pain, ectopic pregnancy, and infertility (2). Different studies have also described an association between C. trachomatis and the risk of cervical cancer (3, 4). Moreover, ocular infections can lead to trachoma, the leading cause of infectious blindness worldwide (5). Members of the genus Chlamydia share a life cycle of 48 to 72 h with a distinct biphasic stage. Chlamydiae initiate their intracellular life cycle by invading cells in the form of elementary bodies (EBs) (1). EBs rapidly differentiate into reticulate bodies (RBs) that are metabolically active and proliferate inside cytoplasmic parasitophorous vacuoles termed inclusions (1). Finally, RBs differentiate back into EBs before they exit infected cells and spread to new cells.
The primary targets of C. trachomatis are epithelial cells of the urogenital tract and conjunctiva (6), which are able to present pathogenic antigens via major histocompatibility complex class I (MHC I) molecules (7). In the classical antigen presentation pathway, MHC I heavy chains associate with β2-microglobulin in the endoplasmic reticulum (ER) and enter the peptide loading complex (7). Peptides are generated from antigens following processing by the proteasome, transported into the ER through the transporter associated with antigen processing (TAP), and then loaded onto MHC I molecules. Finally, MHC I/peptide complexes are transported through the Golgi compartment to the cell surface, where they present their bound antigens to CD8+ cytotoxic T cells (7). The MHC I antigen presentation pathway enables the immune system to detect infected cells displaying peptides from foreign proteins. Studies using mouse models have underscored the role of the CD8+ T cell response in the recognition of Chlamydia-infected cells (8). Thus, tight control of MHC I expression and surface presentation is essential for the outcome of a successful and efficient immune response to the pathogens.
The NF-κB signaling pathway participates in the control of basal MHC I gene expression, as well as in its transcriptional upregulation following treatment with cytokines like tumor necrosis factor alpha (9). Other transcription factors, which compose the RFX protein complex, such as RFX5, cooperate with gamma interferon (IFN-γ)-regulated transactivator proteins (NLRC5/CITA) for basal, as well as IFN-γ-inducible, MHC I expression (10). Studies by Zhong and coworkers (11) have suggested that MHC I is downregulated in C. trachomatis-infected epithelial cells such as HeLa cells, and the authors hypothesized that chlamydial interference with antigen presentation provides an effective immune evasion strategy that contributes to the growth and propagation of the bacterial pathogen (11). The protease-like activity factor CPAF is thought to be the main virulence factor of C. trachomatis (12). It was proposed that CPAF-mediated degradation of the transcription factor RFX5 is directly responsible for MHC I suppression in infected epithelial cells (11, 13). Furthermore, Christian and colleagues (14) suggested that CPAF is responsible for the degradation of NF-κB subunit p65 during infection and thereby reduces the sensitivity of host cells to proinflammatory stimuli, which are required for efficient antigen presentation. However, recent findings by Chen et al. (15) have raised doubts that RFX5 and NF-κB p65 are real substrates for CPAF in infected host cells. The authors found that the reported proteolysis of the putative CPAF substrates RFX5 (11) and NF-κB (14), as well as several others, is due to enzymatic activity in cell lysates rather than in intact cells. Therefore, the study of Chen et al. (15) highlights the need to reevaluate the Chlamydia literature on CPAF and demands new investigations of the proposed CPAF functions in infected host cells and reinterpretation of models involving the role of this bacterial enzyme in infection. The authors of that study (15) suggested that maybe other mechanisms could be responsible for the previously observed Chlamydia-host interactions that have been attributed to CPAF-dependent proteolysis of host polypeptides (such as Golgi compartment reorganization, apoptosis resistance, and host cytoskeletal remodeling) and the suggested roles of CPAF in chlamydial pathogenesis (15).
The aim of our study was to explore whether and to what extent C. trachomatis infection directly affects the expression and surface presentation of MHC I in Chlamydia-infected host cells. By analyzing nine different human cells and cell lines (epithelial cells and fibroblasts) infected with C. trachomatis (serovar D or LGV2), we found that Chlamydia does not interfere with the transcription and protein synthesis of MHC I. Furthermore, we did not observe any detectable change in intracellular localization, transport, surface stability, or presentation of MHC I. Thus, our data demonstrate for the first time that Chlamydia-infected cells retain their full ability to perform MHC I-mediated antigen presentation in the presence or absence of IFN-γ.
MATERIALS AND METHODS
Cell culture and C. trachomatis (serovars D and LGV2) infection.
HeLa cells (human cervical epithelium line, ATCC CCL-2), HeLa 229 cells (human cervical epithelium line, ATCC CCL-2.1), WISH cells (human epithelial line, ATCC CCL-25), Hep-2 cells (human epithelial line, ATCC CCL-23), HL cells (human airway epithelium line, kindly provided by Andreas Essig, Uniklinik Ulm, Ulm, Germany), MRC-5 cells (fibroblast line, ATCC CCL-171), MCF-7 cells (mammary epithelium line, ATCC HTB-22), WSI cells (fibroblast line, kindly provided by Peter J. van den Elsen, Leiden University Medical Center, Leiden, The Netherlands) and Daudi cells (lymphoblast line, ATCC CCL-213, used as a control for IL-10 and IL-10 receptor production [16]), were grown in Iscove's modified Dulbecco's medium (IMDM; Invitrogen) with 10% heat-inactivated fetal calf serum (FCS; Biochrom). Human dermal fibroblasts (juvenile foreskin, C-12300; PromoCell) were grown in minimal essential medium (Opti-MEM; Gibco, Invitrogen) with 10% heat-inactivated FCS (PromoCell).
TAP-deficient T2 cells (ATCC CRL-1992) are derivatives of the human TAP-proficient lymphoblastoid cell line T1 (ATCC CRL-1991) expressing HLA-A2 and HLA-B51 (17). Transfectants of T2 cells expressing wild-type TAP (18) were cultured in IMDM (Invitrogen) supplemented with 1 mg/ml G418 (PAA Laboratories, GE Healthcare Life Sciences, Piscataway, NJ). C. trachomatis serovar D strain IC Cal 8 (obtained from the Institute of Ophthalmology, London, United Kingdom) and serovar LGV2 strain 434/BU (kindly provided by Thomas Rudel, University of Würzburg, Würzburg, Germany) were propagated in buffalo green monkey (19) and HeLa cells as described previously (8). Cell monolayers were inoculated with C. trachomatis at a multiplicity of infection (MOI) of 5 and centrifuged at 1,600 × g for 60 min at 37°C. Noninfected and infected cells were further incubated in IMDM with 5% FCS. For some experiments (see Fig. 1 to 5 and 7), cells were pretreated with IFN-γ (distributed by the National Institute of Allergy and Infectious Diseases, Bethesda, MD) at 100 IU/ml for 48 h and also during the following 48 h of chlamydial infection. The proper IFN-γ response of infected and noninfected cells was controlled via cytokine-mediated TAP1 induction (20) in Western blot assays. For cell culture experiments (see Fig. 7A), we used bioactive recombinant human IL-10 obtained from Life Technologies.
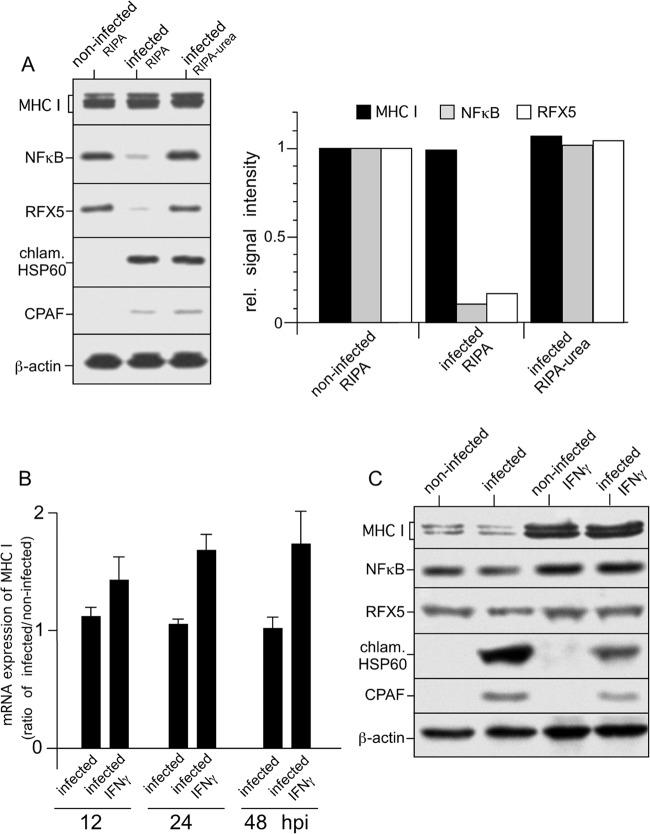
FIG 1.
Expression of MHC I in C. trachomatis serovar D-infected HeLa cells. (A) Proteolysis of NF-κB and RFX5 is dependent on cell processing and does not affect steady-state levels of MHC I heavy chains. Cells were infected at an MOI of 5, and samples were prepared at 48 hpi. Lysates of infected HeLa cells were prepared in RIPA buffer or by direct lysis in RIPA buffer containing 4 M urea, separated by SDS-PAGE, and probed with antibodies to MHC I, NF-κB, RFX5, chlamydial Hsp60 (chlam. HSP60), CPAF, and β-actin (loading control). Fluorographs were analyzed by densitometric scanning. rel., relative. (B) Levels of MHC I mRNA in infected cells. Samples were analyzed by real-time RT-PCR at 12, 24, and 48 hpi. Relative gene expression represents the ratio of mRNA levels in Chlamydia-infected and noninfected cells. Data were calculated as described in Materials and Methods. Values represent the mean of three independent experiments (with standard deviations). (C) Neither constitutive nor IFN-γ-inducible MHC I expression is affected in Chlamydia-infected cells. HeLa cells infected or not infected with Chlamydia were cultured with or without IFN-γ. Therefore, HeLa cells were pretreated with 100 IU of IFN-γ/ml for 48 h and also during the following 48 h of chlamydial infection. Lysates were prepared in RIPA buffer containing 4 M urea. MHC I, NF-κB, RFX5, chlamydial Hsp60, and CPAF from lysates of infected and noninfected HeLa cells were detected by Western blot assays with specific antibodies. β-Actin staining was used as a loading control and for standardization.
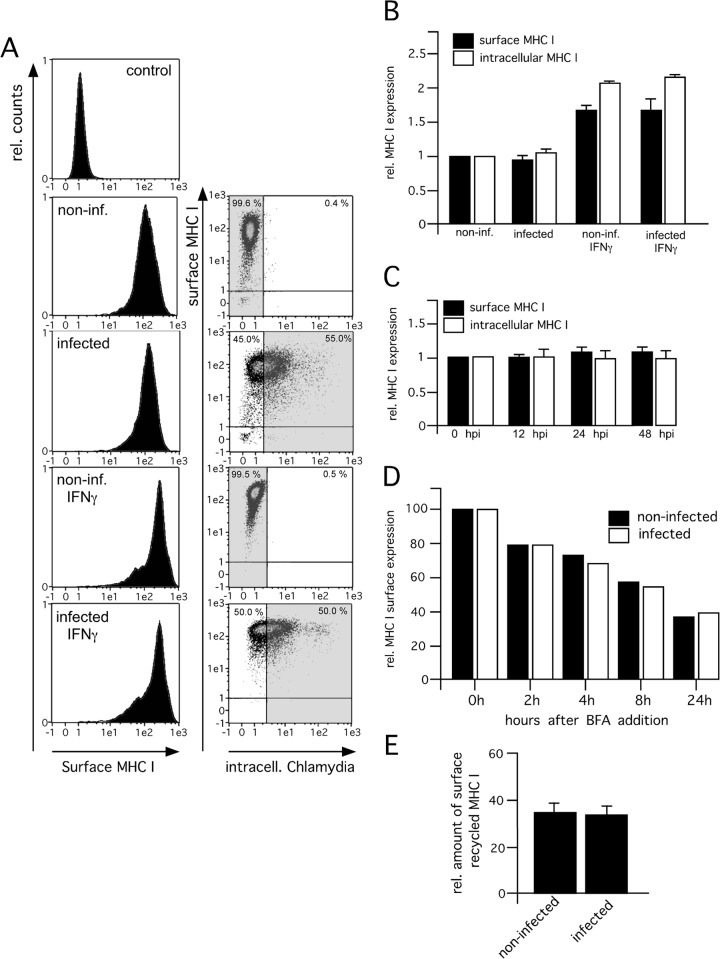
FIG 5.
MHC I surface presentation properties of infected (inf.) and noninfected HeLa cells in the absence or presence of IFN-γ. (A) Analysis of surface-expressed MHC I. Infected (48 hpi) and noninfected HeLa cells were costained for surface MHC I (red fluorescence) and intracellular Chlamydia (green fluorescence). HeLa cells were treated with IFN-γ for 48 h prior to and for 48 h during infection with Chlamydia. The proper IFN-γ response of infected and noninfected cells was controlled via cytokine-mediated TAP1 induction analyzed by corresponding Western blot assays (not shown). Infected cells were identified by their green fluorescent intensity (right panel, dot plot analysis of MHC I fluorescence versus Chlamydia fluorescence). The histogram plots representing MHC I surface expression (left panel) were gated on the populations of Chlamydia-negative and -positive cells, respectively (indicated by gray areas in the respective dot plots, right panel). The topmost histogram in Fig. 5A represents an isotype control staining with a suitable mouse IgG2a κ MAb (BioLegend). The percentage of cells present in the upper and lower left quadrants is indicated at the top left of each plot, whereas the value at the top right represents the percentage of cells located within the upper and lower right quadrants. One representative result of three independent experiments is shown. Data from MHC I staining experiments (intracellular and surface-expressed MHC I) are summarized in panel B. Results are plotted as the relative (rel.) increase in mean fluorescence intensity compared with the mean fluorescence intensity in the control experiments. (C) Time course analysis of MHC I expression during chlamydial infection. HeLa cells infected for 0, 12, 24, and 48 h were stained for intracellular and surface-expressed MHC I. Results are plotted as the relative change in mean fluorescence intensity compared with the mean fluorescence intensity in the control experiments. (D) Survival of MHC I molecules on the cell surface. Transfectants were treated with BFA, which inhibits protein export from the ER. After the cells were cultured for different times, MHC I surface staining with W6/32 was performed. Flow cytometry results are presented as the percent reductions of the mean fluorescence intensities at 2, 4, 8, and 24 h compared with the mean fluorescence intensity at 0 h. (E) Endosomal recycling of surface MHC I in infected and noninfected HeLa cells. Cells were infected (48 hpi), and expression of surface MHC I in the presence or absence of primaquine was analyzed by flow cytometry. The reduction in the mean fluorescence intensity compared with the mean fluorescence intensity in the control experiments is plotted as a percentage of surface recycled MHC I.
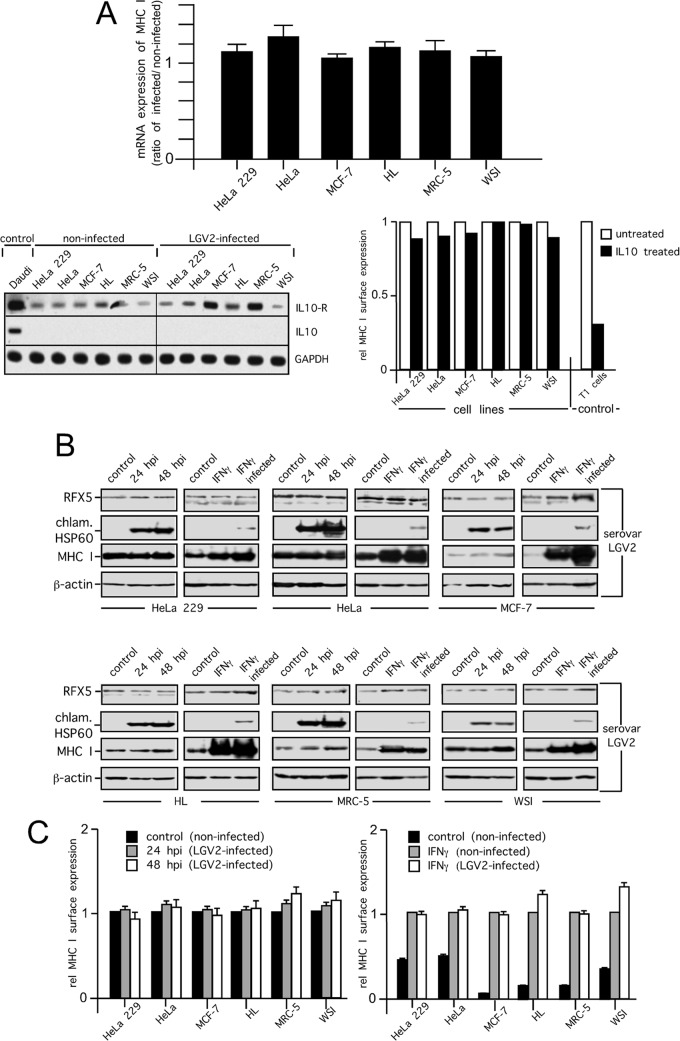
FIG 7.
MHC I expression and surface presentation in C. trachomatis serovar LGV2-infected cells. (A) Levels of MHC I mRNA in C. trachomatis serovar LGV2-infected cells (upper panel). Samples of HeLa 229, HeLa, MCF-7, HL, MRC-5, and WSI cells were analyzed by quantitative PCR at 30 hpi. Relative (rel.) gene expression represents the ratio of the mRNA level in Chlamydia-infected cells to that in noninfected cells. Data were calculated as described in Materials and Methods. IL-10 and IL-10 receptor mRNA expression of noninfected and infected cells (30 hpi) was assayed by RT-PCR (lower panel, left). Daudi cells were used as a positive control for IL-10 (16) and IL-10 receptor mRNA expression. Inverted images of ethidium bromide-stained gels are shown. Chlamydial infections were controlled by parallel microscopic analysis. For the analysis of MHC I surface expression in the presence or absence of exogenously applied IL-10 (100 IU/ml), cells were cytokine treated for 48 h and analyzed by flow cytometry. T1 cells were used as a positive control (64). Results of surface MHC I staining are plotted as the relative change in mean fluorescence intensity compared with the mean fluorescence intensity in the control experiments, which was set to 1 (lower panel, right). Representative results of three independent experiments are shown. (B) Analysis of MHC I expression in cells infected with C. trachomatis serovar LGV2. Cells were infected with C. trachomatis serovar LGV2 at an MOI of 5, and samples were prepared at 24 and 48 h after infection. Lysates of infected HeLa 229, HeLa, MCF-7, HL, MRC-5, and WSI cells were prepared by direct lysis in RIPA buffer containing 4 M urea, separated by SDS-PAGE, and probed with antibodies to MHC I, RFX5, chlamydial Hsp60 (chlam. HSP60), and β-actin (loading control). (C) Analysis of surface-expressed MHC I. C. trachomatis serovar LGV2-infected and noninfected human cell lines (24 and 48 hpi) were costained for surface MHC I and intracellular Chlamydia. Flow cytometry results of surface MHC I staining of infected cells are plotted as the relative change in mean fluorescence intensity compared with the mean fluorescence intensity in noninfected control experiments, which was set to 1 (left panel). In further flow cytometry studies, cells were pretreated with IFN-γ for 48 h and also during the following 48 h of chlamydial infection (right panel). As a control, noninfected cells were pretreated and treated or not treated with IFN-γ for the same incubation times. Results of surface MHC I staining are plotted as the relative change in mean fluorescence intensity compared with the mean fluorescence intensity of IFN-γ-treated noninfected cells, which was set to 1 (right panel). The proper IFN-γ response of infected and noninfected cells was additionally controlled via cytokine-mediated TAP1 induction analyzed by corresponding Western blot assays (not shown).
Antibodies.
Rabbit polyclonal sera against LAMP1, EEA1, trans-Golgi compartment network 46 (TGN46), Giantin, GM130, RFX5, and β-actin were purchased from Abcam. Rabbit polyclonal serum against β-actin was purchased from Sigma-Aldrich. W6/32 is a conformation-dependent mouse monoclonal antibody (MAb) reacting with assembled HLA-A, -B, and -C (21). 3B10.7 is a rat MAb recognizing human MHC I heavy chains (22). The polyclonal rabbit NF-κBp65 antiserum was purchased from eBioscience. The mouse polyclonal antiserum against CPAF was generously supplied by Andreas Essig (Uniklinik Ulm, Ulm, Germany). Rat monoclonal anti-KDEL (MAC 256) antibody was obtained from Geoff Butcher (Babraham Institute, Cambridge, United Kingdom). Mouse anti-chlamydial Hsp60 MAb A57-B9 was purchased from Acris. Fluorescein isothiocyanate (FITC)-conjugated mouse anti-Chlamydia MAb was taken from the IMAGEN Chlamydia kit purchased from Oxoid.
Western blotting.
Cells were lysed in radioimmunoprecipitation assay (RIPA) buffer (150 mM NaCl, 50 mM Tris-HCl, 1% NP-40, 0.25% Na-deoxycholate, Complete protease inhibitor [Roche], 50 mM Na-F) with or without 4 M urea. After incubation on ice for 60 min, cell lysates were centrifuged for 30 min at 20,000 × g at 4°C. For the analysis of intracellular MHC I maturation, we performed endoglycosidase H (Endo H) cleavage experiments with detergent extracts of infected and noninfected cells. Therefore, 10-μl volumes of the lysates were mixed with 20 μl Endo H buffer (50 mM C6H5Na3O7 [pH 5.5] containing 0.1 M β-mercaptoethanol, 0.01% SDS). Endo H cleaves asparagine-linked high-mannose-type oligosaccharide chains from glycoproteins. These carbohydrate residues are usually attached to MHC I molecules in the ER, thereby rendering the proteins sensitive to Endo H cleavage. Later, in the Golgi compartment, the residues are modified, resulting in Endo H resistance. For SDS-polyacrylamide gel electrophoresis and Western blot analysis, lysate supernatants were mixed with an equal volume of 2× Laemmli sample buffer (0.5 M Tris, 20% glycerol, 7% β-mercaptoethanol, 2% SDS, 0.1% bromophenol blue). Blots were incubated with appropriate antibodies, and bands were visualized by enhanced chemiluminescence. Fluorographs were quantified with GelEval 1.32 software (FrogDance Software).
Flow cytometry.
Analysis of cell lines was performed on a MACSQuant analyzer (Miltenyi Biotec) with MACSQuantify software version 2.4. The viability of infected cells was first checked by trypan blue exclusion. Dead cells and cell debris were removed from the cultures by extensive washing. Cells were detached from the culture wells with 0.05% trypsin–0.02% EDTA, washed in cold dichloride-FCS-NaN3 buffer (DFN; phosphate-buffered saline containing 0.9 mM CaCl2, 0.5 mM MgCl2, 10% FCS [vol/vol], and 0.1% sodium azide [wt/vol]), and incubated with the primary antibody for 60 min at 4°C. Cells were washed twice with cold DFN and incubated with the fluorochrome-conjugated secondary antibody for 30 min at 4°C. Cells were gated for live cells, and 10,000 cells were measured for each sample. To exclude background fluorescence, cells incubated with suitable isotype control IgGs (BioLegend) were used as controls. For intracellular staining of chlamydiae (8), cells were fixed with 2% paraformaldehyde permeabilized with phosphate-buffered saline containing 0.5% saponin and 0.5% bovine serum albumin (BSA) at room temperature for 30 min, and intracellularly immunostained with the IMAGEN kit (Oxoid).
Immunofluorescence microscopy.
For fluorescence microscopy, cells were grown on coverslips and fixed for 20 min in 2% paraformaldehyde, quenched with 3% BSA, permeabilized with 0.1% saponin (Sigma-Aldrich), and incubated serially with the indicated primary and corresponding secondary antibodies. For the staining of surface MHC I, HeLa cells were stained without prior permeabilization. Images were taken with an Axiovert 200M/ApoTome microscope and a confocal Exciter 5 laser scanning microscope (Zeiss). Colocalization was measured by AxioVision colocalization and Zen 2009 software (Zeiss). Pearson coefficients were measured by CoLocalizer Express software (CoLocalization Research Software).
RT-PCR and quantitative PCR.
Total RNA was isolated from human cell lines infected or not infected with C. trachomatis (serovars LGV2 and D) with the peqGOLD Total RNA kit (Peqlab). A DNase digestion step was included. For each sample, cDNA was reverse transcribed from 1 μg of RNA in a reaction volume of 20 μl with the Promega reverse transcription (RT) system and oligo(dT) primers (Promega). The following PCR primer pairs for pan-MHC I (HLA-ABC) were generated with the NCBI primer design tool (http://www.ncbi.nlm.nih.gov/tools/primer-blast/): pan-MHC I HLA-ABC, 5′-CTGAGGTGCTGGGCCCTG-3′ and 5′-CCCACTTCTGGAAGGTTC-3′; glyceraldehyde 3-phosphate dehydrogenase (GAPDH), 5′-TCAAGTGGGGCGATGCTGGC-3′ and 5′-TGGGGGCATCAGCAGAGGGG-3′; interleukin-10 (IL-10), 5′-CTGTGAAAACAAGAGCAAGGC-3′ and 5′-GAAGCTTCTGTTGGCTCCC-3′; IL-10 receptor, 5′-CCATCTTGCTGACAACTTCC-3′ and 5′-GTGTCTGATACTGTCTTGGC-3′. Validation of the primers (specificity and annealing temperature) was performed by melting curve analysis and conventional RT-PCR with agarose gel electrophoresis. Conventional semiquantitative RT-PCR amplification of MHC I, GAPDH, IL-10, and the IL-10 receptor included a denaturation step of 94°C for 5 min; 30 to 35 cycles of ≤1 min of denaturation at 94°C, ≤1 min of annealing, and ≤1 min of elongation at 72°C; and finally 10 min of extension at 72°C. The PCR products were analyzed in a 1% agarose gel and quantified with GelEval 1.32 software (FrogDance Software). After normalization to GAPDH, the MHC mRNA ratio of infected to noninfected cells was determined. The standard SYBR green-based real-time PCR was carried out with a Smart cycler II (Cepheid, BD), and cycle threshold (CT) values were determined with Dx software (Cepheid, BD). Relative gene expression was defined as the ratio of MHC I gene mRNA levels relative to the level of GAPDH mRNA as a reference. Primer efficiency (E) was determined by analyzing serial dilutions of cDNA. The CT values were plotted against log cDNA concentrations, and the slope was calculated by linear regression. In accordance with Pfaffl (23), the ratio was calculated with the formula (Etarget)ΔCTtarget(noninfected − Chlamydia infected)/(Ereference)ΔCTreference(noninfected − Chlamydia infected). Gene expression values were calculated from three independent experiments. For the PCR experiments with fibroblasts, IFN-α/β receptor chain 2 neutralizing antibody (MAb 1155, clone MMHAR-2; Millipore) was added to the culture medium immediately after infection to neutralize endogenously produced IFN-β. Production of IFN-β by infected cells was examined by enzyme-linked immunosorbent assay (Fujirebio Inc., Life Technologies).
RESULTS
To investigate the influence of chlamydial infection on MHC I expression and surface presentation in epithelial cells, we first assessed the steady-state protein levels of MHC I, NF-κB p65, and RFX5 in C. trachomatis serovar D-infected HeLa cells (the established model system for chlamydial cell infection studies) lysed with or without urea as a denaturing agent to block postlytic enzymatic activity in the cell lysates (including CPAF activity [15]). Comparison of noninfected and infected cells lysed in the absence of urea showed that in infected cells, the appearance of CPAF coincides with the degradation of NF-κB p65 and RFX5 (Fig. 1A). However, when Chlamydia-infected cells were lysed in the presence of urea, no such proteolytic breakdown of NF-κB p65 and RFX5 was observed (Fig. 1A). The Western blot signals for both transcription factors are comparable to those obtained with noninfected cells lysed in the absence of urea (Fig. 1A). These results strongly suggest that the reported CPAF-dependent degradation of NF-κB p65 and RFX5 does not occur in intact cells. Rather, it seems that proteolysis of both host polypeptides occurs during or after cell lysis and is due to CPAF activity in the lysates of infected cells (15). In agreement with this notion, the results in Fig. 1A demonstrate that the lysis condition-dependent degradation of NF-κB p65 and RFX5 is not associated with reduced steady-state levels of MHC I, suggesting that in Chlamydia-infected cells, MHC I expression is not downregulated by the bacteria. Since chlamydial degradation of RFX5 had been correlated with effects on constitutive, as well as IFN-γ-induced, MHC I expression in a previous study (13), we next evaluated levels of MHC I mRNA in infected and noninfected HeLa cells by real-time RT-PCR. The MHC I mRNA ratios of infected versus noninfected cells obtained (12, 24, and 48 h postinfection [hpi]), depicted in Fig. 1B, show that MHC I gene expression is not reduced in Chlamydia-infected cells. After infection, IFN-γ-treated HeLa cells even show a slight increase in the MHC I mRNA level, suggesting that Chlamydia also does not inhibit induced MHC I transcription. Chlamydial infection has been reported to be accompanied by a decline in host cell ATP levels with a concomitant increase in ADP levels (24). Because the ATP required for the protein translation machinery in cells is significant (25), nucleotide parasitism of Chlamydia might also interfere with the energy requirements of inducible protein synthesis in infected host cells. Therefore, we additionally performed immunoblot experiments (Fig. 1C) in which we compared the amounts of constitutive and IFN-γ-induced MHC I heavy chains in infected and noninfected HeLa cells. Our results in Fig. 1C show that infected HeLa cells display constitutive and IFN-γ-induced MHC I protein levels that are comparable to those observed in corresponding noninfected cells. Thus, our findings suggest that Chlamydia-infected epithelial cells retain their full capability to express IFN-γ-inducible MHC I. No detectable influence on MHC I gene transcription or protein synthesis was observed. It should also be noted that IFN-γ-treated cells show a detectable reduction in the expression of chlamydial Hsp60 and CPAF (Fig. 1C).
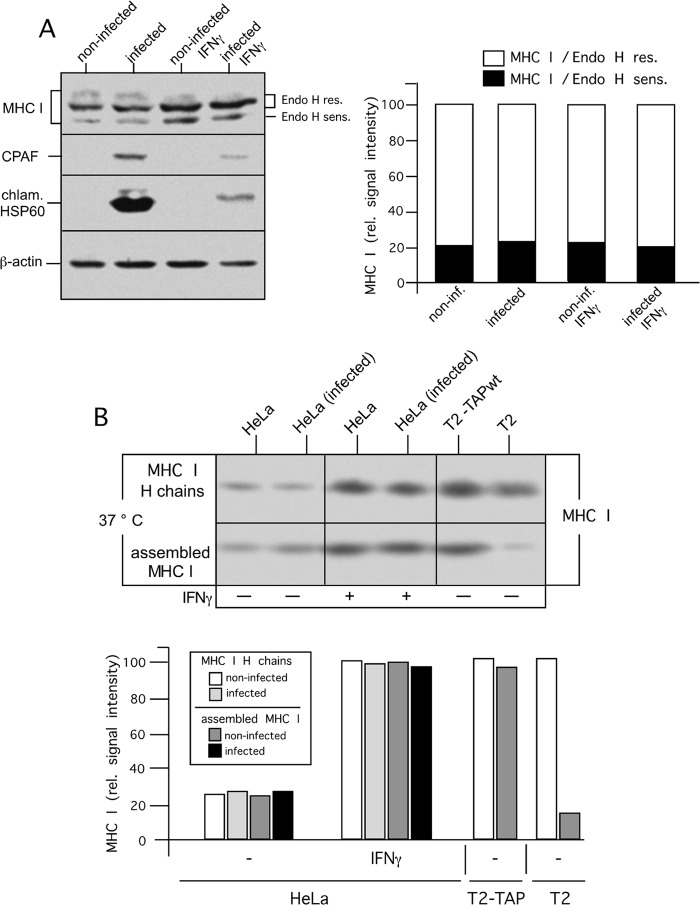
To assess whether and to what extent chlamydial infection affects posttranslational maturation and transport processes, which are involved in intracellular MHC I translocation from the ER to the plasma membrane, we first analyzed whether intracellular maturation of MHC I along the secretory route might be affected by chlamydial infection. To this end, Endo H cleavage experiments were performed with detergent extracts of infected and noninfected HeLa cells. As shown in the SDS-PAGE analysis in Fig. 2A (left panel), 48 h after chlamydial infection, intracellular MHC I maturation is comparable in infected and noninfected cells. In both situations, we observed a 4:1 signal ratio of Endo H-resistant to -sensitive MHC I molecules (Fig. 2A, right panel). To address whether Chlamydia might affect the quality of peptide loading of MHC I, we performed thermostability experiments that made use of the established correlation between the heat resistance of MHC I molecules and the conformational integrity of MHC I peptide complexes (26). Therefore, lysates of infected and noninfected HeLa cells treated or not treated with IFN-γ were incubated for 1 h at 37°C before MHC I heavy chains (control for MHC I expression levels) and intact assembled MHC I molecules were immunoisolated with conformation-independent (3B10.7) (22) and conformation-dependent (W6/32) (21) antibodies, respectively. The immunoisolation studies depicted in Fig. 2B show that in infected and noninfected HeLa cells, MHC I molecules are largely heat stable, suggesting comparable amounts of stable MHC I peptide complexes. Corresponding control experiments with human T2 cells expressing or lacking the antigenic transporter TAP (26) (Fig. 2B, last two lanes from the left) verified that MHC I complexes resist heat treatment only when suitable peptide ligands are present.
FIG 2.
MHC I maturation in C. trachomatis serovar D-infected HeLa cells. (A) HeLa cells were treated or not treated with IFN-γ for 48 h prior to infection with Chlamydia and for 48 h during infection with Chlamydia. After lysis, extracts of infected and noninfected HeLa cells were digested with Endo H before separation by SDS-PAGE and analysis by Western blotting (left panel). Fluorographs were analyzed with GelEval 1.32 software (FrogDance Software), and peak integrals were plotted as bar graphs in arbitrary units (right panel). Endo H-sensitive (sens.) and -resistant (res.) MHC I forms are indicated. Immunoblot assays were probed with anti-MHC I, anti-chlamydial Hsp60 (chlam. HSP60), and anti-β-actin antibodies. rel. relative; inf., infected. (B) Thermostability of MHC I molecules in infected and noninfected HeLa cells. Cell lysates were incubated for 1 h at 37°C. The conformational integrity of MHC I peptide complexes was analyzed by immunoprecipitation with the conformation-independent and -dependent MHC I antibodies 3B10.7 and W6/32, respectively. Immunoisolates were analyzed in Western blot assays probed for MHC I (upper panel). Fluorographs were analyzed as described above, and peak integrals were plotted as bar graphs in arbitrary units (lower panel). wt, wild type.
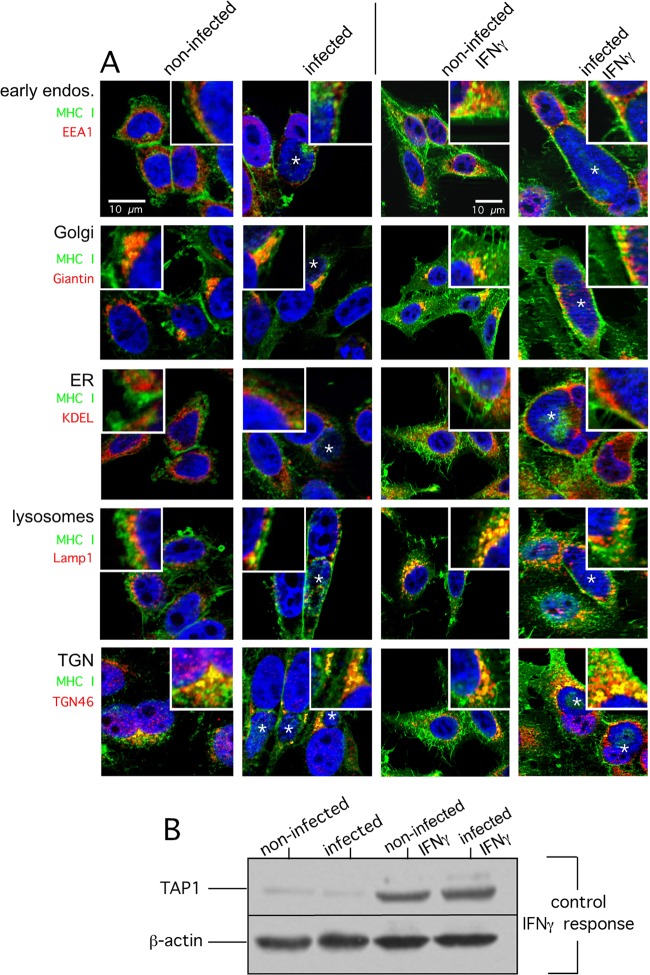
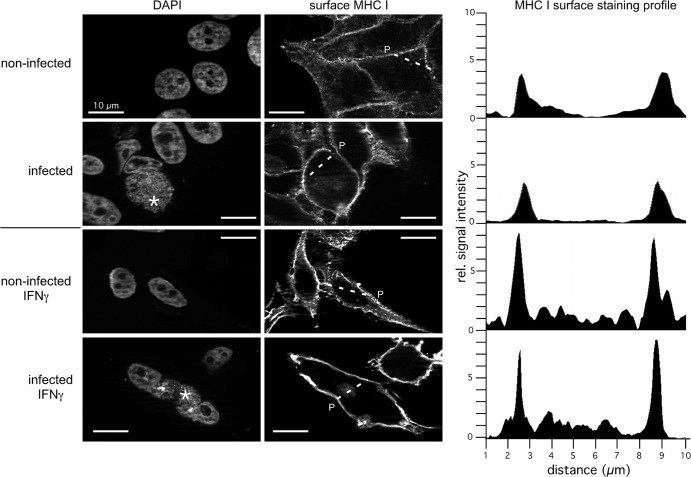
Next, we investigated and compared the intracellular localization of MHC I in Chlamydia-infected and noninfected cells treated or not treated with IFN-γ. To this end, we costained HeLa cells with antibodies specific for MHC I and a set of different organelle markers. The immunofluorescence experiments shown in Fig. 3A revealed that in noninfected, as well as infected, cells (treated or not treated with IFN-γ), MHC I partially colocalizes with late secretory, as well as endocytic, compartments (Golgi compartment, TGN, early endosomes, and late endosomes/lysosomes). In line with the Endo H experiments (Fig. 2A), we observed much less pronounced colocalization between MHC I and the ER in infected and noninfected HeLa cells (with or without IFN-γ). Thus, although chlamydial infection is accompanied by fragmentation of post-ER compartments like the Golgi compartment (27), it seems that Chlamydia-infected and noninfected epithelial cells do not differ in the intracellular localization of MHC I along the secretory route. The proper IFN-γ response of infected and noninfected HeLa cells was controlled via cytokine-mediated TAP1 induction (20) in corresponding Western blot assays (Fig. 3B). Immunofluorescence staining of nonpermeabilized infected and noninfected HeLa cells (with or without IFN-γ) suggests that in addition to post-ER compartments, a large quantity of MHC I molecules is expressed on the cell surface (Fig. 4, left and middle panels). Fluorescence intensity profiles (Fig. 4, right panels) measured across nonpermeabilized cells revealed that constitutive and IFN-γ-induced surface expression of MHC I is comparable in infected and noninfected HeLa cells. To investigate the characteristics of surface MHC I in more detail, we performed flow cytometry experiments (Fig. 5A and B) in which we measured the IFN-γ-inducible MHC I expression levels of surface-presented (Fig. 5A) and intracellular populations (Fig. 5B) of infected and noninfected cells. Chlamydia-positive and -negative cells were distinguished by intracellular staining (Fig. 5A, dot plots, right panel) with the IMAGEN detection kit for Chlamydia. The histogram plots representing MHC I expression (surface MHC I, Fig. 5A, left panel) were gated on the population of Chlamydia-positive and negative HeLa cells, respectively. The results in Fig. 5A and B (comparison of intracellular and surface MHC I) demonstrate that infected and noninfected cells show comparable levels of intracellular and surface-expressed MHC I. This is true for constitutive, as well as IFN-γ-induced, MHC I expression (Fig. 5A and B). Furthermore, an analysis over 0, 12, 24, and 48 hpi confirmed that intracellular and surface expression of MHC I was unaffected (Fig. 5C). Accordingly, we observed that in the presence of brefeldin A (BFA), which blocks transport from the ER to the Golgi compartment (28), the time course of the survival of surface MHC I (half-life of about 8 h) is almost the same in Chlamydia-infected and noninfected HeLa cells (Fig. 5D). This suggests that Chlamydia does not modulate the turnover of preexisting surface-expressed MHC I in infected epithelial cells.
FIG 3.
MHC I subcellular localization in C. trachomatis serovar D-infected HeLa cells. (A) HeLa cells were infected for 48 h or not infected (columns 1 and 2) and costained for MHC I (green) and EEA1 (early endosomes), Giantin (Golgi compartment), KDEL proteins (ER), LAMP1 (lysosomes), and TGN46 (trans-Golgi compartment network) (red). Cells were infected with Chlamydia or not infected as in columns 1 and 2 and additionally treated with IFN-γ (columns 3 and 4). In particular, cells were treated with IFN-γ for 48 h prior to infection with Chlamydia and for 48 h during infection with Chlamydia. (B) The proper IFN-γ response of infected and noninfected cells was controlled via the cytokine-mediated induction of TAP1 analyzed by corresponding Western blot assays. The corresponding overlays of the immunostainings are depicted. The insets show magnifications of representative cell areas where MHC I and organelle markers colocalized. For direct comparison, all cells were photographed with the same exposure time. DNA of the host nucleus and chlamydial inclusions were labeled with 4′,6-diamidino-2-phenylindole (blue). Parasitophorous vacuoles are indicated by asterisks.
FIG 4.
MHC I localization on the plasma membrane of infected and noninfected HeLa cells in the absence or presence of IFN-γ. HeLa cells were pretreated with IFN-γ for 48 h and also during the following 48 h of chlamydial infection. The proper IFN-γ response of infected and noninfected cells was controlled via cytokine-mediated TAP1 induction in Western blot assays (not shown). Nonpermeabilized cells were immunostained for surface MHC I (right panel). DNA of the host nucleus and inclusions of the same cells were labeled with 4′,6-diamidino-2-phenylindole (left panel). Parasitophorous vacuoles are indicated by asterisks. Noninfected and infected cells were photographed with the same exposure time. To compare MHC I surface expression in infected and noninfected HeLa cells, we measured the intensity of transmitted fluorescent light along a line segment drawn across the cellular area of interest (indicated by a broken line and P). The representative fluorescence intensity profiles measured across the cells along the dashed lines are shown on the right. rel., relative.
Chlamydial inclusions have characteristics in common with recycling endosomes of the host (29), have been shown to interact with vesicles of the endocytic pathway (30), and might affect the endosomal recycling of surface receptors (29). To assess the rate of endosomal MHC I surface recycling in infected and noninfected HeLa cells, we analyzed MHC I surface expression in the presence of primaquine, which selectively inhibits endosomal recycling of cell surface molecules (31). By using this approach, we observed no detectable differences in the recycling of surface MHC I (Fig. 5E).
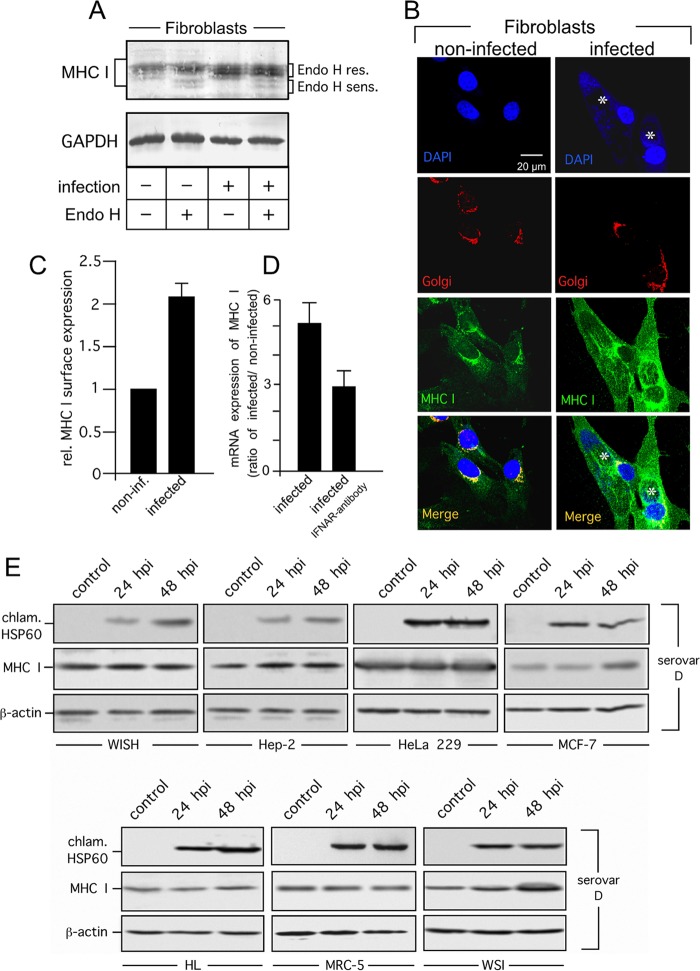
Fibroblasts do not belong to the main target cells of C. trachomatis. However, it has been shown that chlamydiae infect fibroblasts in the articular synovial membrane of patients developing reactive arthritis following genital infection (32) Thus, we were also interested in investigating MHC I expression and surface presentation in Chlamydia-infected human primary fibroblasts. In fibroblasts, Endo H cleavage patterns do not differ between noninfected and infected cells (Fig. 6A). The majority of the MHC I heavy chains detected were identified as Endo H resistant, suggesting that they are located in post-ER compartments. Indeed, further immunofluorescence studies demonstrated that in infected and noninfected fibroblasts, MHC I colocalizes with the Golgi compartment (Fig. 6B). In line with the observation that Chlamydia-infected HeLa cells produce cytokines (IL-6 and IL-8) (33) that are not involved in the upregulation of antigen presentation, we did not observe any elevation of surface MHC I expression in infected HeLa cells. However, in Chlamydia-infected primary fibroblasts, steady-state levels of MHC I heavy chains and mRNA were elevated (Fig. 6A and D), suggesting that MHC I expression is induced during chlamydial infection. In support of this, flow cytometry studies revealed that C. trachomatis infection resulted in a 2-fold increase in surface-expressed MHC I (Fig. 6C). In agreement with our previous findings (34, 35), we found that C. trachomatis induces the release of IFN-β from human primary fibroblasts (3.5 ± 3.1 IU/ml at 48 h after infection, n = 3), which is known to play a critical role in the induction of MHC I expression (36). The addition of neutralizing antibodies directed against the IFN type I receptor reduced the upregulation of MHC I mRNA (Fig. 6D), indicating that endogenously produced IFN-β contributes significantly to Chlamydia-mediated MHC I induction in human primary fibroblasts. In addition to HeLa and human primary fibroblasts, MHC I expression was also analyzed in C. trachomatis serovar D-infected human WISH, Hep-2, HeLa 229, MRC-5, MCF-7, HL, and WSI cells (MOI of 5, 24 and 48 hpi) via immunoblot analysis (Fig. 6E). In all of the human cell lines tested, MHC I was clearly not downregulated by chlamydial infection (Fig. 6E), demonstrating that the ability to retain MHC I expression in the presence of C. trachomatis serovar D infection is also true for other human cell lines.
FIG 6.
MHC I expression and surface presentation in C. trachomatis serovar D-infected primary fibroblasts and human cell lines. (A) MHC I expression in infected fibroblasts. Cell lysates were treated with or without Endo H to distinguish between Endo H-resistant (res.) post-ER and Endo H-sensitive (sens.) ER forms of MHC I. Western blot assays were probed with antibodies to MHC I and GAPDH (loading control). (B) Colocalization of MHC I with the Golgi compartment in C. trachomatis serovar D-infected primary fibroblasts. Cell monolayers were costained with 4′,6-diamidino-2-phenylindole for DNA (nuclei and chlamydial inclusions, indicated by asterisks, blue), GM130 antibody (Golgi compartment, red), and MHC I antibody (green). (C) MHC I surface expression of fibroblasts determined by flow cytometry. Samples were prepared at 48 h after infection. Surface MHC I was stained with a fluorophore-conjugated antibody. C. trachomatis serovar D-positive cells in infected cultures were identified following intracellular staining with a FITC-conjugated antibody against C. trachomatis (see Fig. 5). Mean fluorescence intensities are depicted as histograms. Values represent the mean of three independent experiments (with standard deviations). (D) Expression of MHC I mRNA in infected and noninfected fibroblasts. Samples were analyzed by real-time RT-PCR. Relative (rel.) gene expression represents the ratio of the mRNA level in Chlamydia-infected cells to that in noninfected cells. Data were calculated as described in Materials and Methods. To neutralize IFN-β activity in fibroblast cultures, an antibody against type I IFN receptor (IFNAR) was added immediately after infection. (E) MHC I expression in C. trachomatis serovar D-infected WISH, Hep-2, HeLa 229, MCF-7, HL, MRC-5, and WSI cells. Lysates of infected (24 and 48 hpi) and noninfected (control) cells were separated by SDS-PAGE and analyzed by Western blotting with antibodies against MHC I, chlamydial Hsp60 (chlam. HSP60), and β-actin.
Finally, to confirm that maintenance of MHC I synthesis and surface presentation is not a phenomenon unique to C. trachomatis serovar D-infected human cells, we also examined MHC I expression in the C. trachomatis serovar LGV2-infected cell lines that were originally used by Zhong and coworkers (11, 13). To this end, we first evaluated the levels of MHC I mRNA in infected and noninfected HeLa, HeLa 229, MRC-5, MCF-7, HL, and WSI cells by RT-PCR by following the infection protocol (MOI of 5, 30 hpi) of Zhong et al. (11) (Fig. 7A, upper panel). The MHC I mRNA ratios of infected versus noninfected cells obtained revealed that MHC I gene transcription remained unchanged in C. trachomatis serovar LGV2-infected cells (Fig. 7A, upper panel). The effects proposed by Zhong and coworkers (11, 13) might also be based on immunosuppressive reactions of the infected host cell lines. Thus, in addition, we also analyzed IL-10 receptor and IL-10 mRNA expression (Fig. 7A, lower left panel). IL-10 is thought to downregulate MHC I surface expression in Chlamydia-infected monocytes (37) via self-production and -stimulation. All of the cell lines tested (noninfected or LGV2 infected) expressed IL-10 receptor mRNA (Fig. 7A, lower left panel). However, they did not produce any detectable IL-10 mRNA (Fig. 7A, upper panel) and displayed no or only negligible modulation of surface MHC I in the presence of exogenous IL-10 (Fig. 7A, lower right panel), suggesting that IL-10-mediated effects on MHC I can be excluded for the cell lines analyzed. In agreement with the results shown in Fig. 7A, all of the cell lines treated or not treated with IFN-γ, displayed detectable steady-state levels of RFX5 and MHC I that were not reduced after chlamydial infection. This shows that C. trachomatis serovar LGV2 does not degrade RFX5 and/or suppresses MHC I protein expression (Fig. 7B). Further, we also examined MHC I surface expression in the different cell lines infected or not infected with C. trachomatis serovar LGV2 (MOI of 5, 24 and 48 hpi) in the absence or presence of IFN-γ (Fig. 7C, left and right panel). In support of our flow cytometry experiments with C. trachomatis serovar D-infected cells (Fig. 5), none of the cell lines analyzed showed any detectable decrease in MHC I surface expression after C. trachomatis serovar LGV2 infection (Fig. 7C, left and right panel).
In summary, our findings obtained with nine different cell lines and two different C. trachomatis strains provide strong evidence of proper MHC I-mediated antigen processing in C. trachomatis-infected human epithelial cells and fibroblasts. Our results suggest that neither the expression (transcription and translation) nor the posttranslational (localization, transport, and surface expression/stability) processes are disturbed or impaired in Chlamydia-infected cells.
DISCUSSION
C. trachomatis is an obligate intracellular bacterial pathogen and a leading cause of sexually transmitted bacterial diseases (38). It has adapted an intravacuolar replication life cycle and can persist in hosts for a long time (38). The expansion of the inclusion in the cytoplasm and the alteration of host cell pathways during parasite acquisition of nutrients are inevitably destructive to the infected cells (39). However, at the same time, the bacteria have to maintain the integrity and viability of the infected host cells (40) before completing their own intracellular replication. Chlamydiae have evolved different strategies for manipulating infected cells (41). It was proposed by Zhong et al. that Chlamydia suppresses constitutive and IFN-γ-inducible MHC I transcription via CPAF-mediated degradation of RFX5, a transcription factor that regulates MHC gene expression (11, 13). Degradation of the p65 subunit of NF-κB, which is relevant for MHC I expression, was also thought to be mediated by CPAF (14). However, the observed activation of specific CD8+ T cells in chlamydial infections and the involvement of cytotoxic T lymphocytes in the immune response to C. trachomatis (42–44) provide an argument against the hypothesis of MHC I subversion in Chlamydia-infected cells. Besides the ability to lyse infected cells, production of IFN-γ seems to be another important mechanism by which CD8+ T cells limit the spread of chlamydial infection (45). Moreover, a recent study provided evidence that the reported chlamydial proteolysis of transcription factors RFX5 and NF-κB (11, 13, 14) is due to enzymatic activity in cell lysates but not in intact cells (15). In support of this, our experiments demonstrate for the first time that Chlamydia-infected epithelial cells and fibroblasts retain the ability to perform MHC I-mediated antigen presentation. MHC I expression, intracellular transport, cell surface expression, and MHC I surface stability are not affected in Chlamydia-infected cells treated or not treated with IFN-γ compared to those in respective noninfected cells. Thus, in contrast to the studies of Zhong et al. (11), no detectable differences in protein levels (and mRNA levels) between noninfected and infected cells were observed. The same was also true for posttranslational processes of intracellular MHC I maturation, such as intracellular trafficking, subcellular localization, and surface presentation/stability. Moreover, in fibroblasts, which represent another host cell type, we also detected no chlamydial subversion of MHC I presentation. A possible explanation for the contradictory findings on MHC I expression in Chlamydia-infected cells is the improvement of experimental methods and approaches between the two different studies. For instance, Zhong et al. (11) neither performed confocal and/or ApoTome immunofluorescence studies nor combined flow cytometry experiments to directly compare MHC I expression and surface presentation in infected and noninfected cells. Moreover, they did not use cell preparation and lysis conditions preventing postlytic protein degradation via CPAF. Thus, it is tempting to speculate that previously observed effects on RFX5 and MHC I (11) are associated with experimental limitations and processes that occur accidently during and/or after cell harvesting and extraction (15).
Recent studies provide evidence that Chlamydia-infected dendritic cells (46) are not impaired in MHC II antigen presentation, suggesting that neither the MHC I nor the MHC II pathway serves as a direct target for Chlamydia-specific evasion strategies. Our findings from MHC I thermostability assays with infected and noninfected cells (see Fig. 2B) exclude major changes in the quality control of MHC I peptide loading. However, we cannot formally exclude the possibility that chlamydiae influence MHC I-restricted presentation to cytotoxic T cells by more subtle mechanisms analogous to what has been described for viruses (47), which directly disturb the generation of immunorelevant and suitable peptide antigens. Thus, it will be of interest so see whether and to what extent Chlamydia manipulates the MHC I-bound peptide reservoir presented by infected epithelial cells.
It should be noted that our results do not contradict the observation by Caspar-Bauguil et al. (37) and Ibana et al. (48) describing cell-specific paracrine/autocrine modulation of surface MHC I presentation in Chlamydia-infected monocytic and endocervical cell lines. In the case of U293 monocytes, the authors (37) identified monocyte-characteristic IL-10 secretion (49) as a specific autocrine mechanism for MHC I downregulation, whereas for the endocervical cell line A2EN (48), a potential involvement of CXCL12/CXCR4 in combination with other soluble factors is discussed. Thus, it is conceivable that under certain cellular conditions or activities, Chlamydia might contribute rather indirectly to local MHC I suppression by stimulating the secretion of inhibitory factors that decrease the ability to eliminate infected cells.
Positive correlations between chlamydial infection and cervical cancer in human papillomavirus (HPV)-positive women suggest that C. trachomatis infection might act as a cofactor in cervical cancer development (3, 50, 51). One of the postulated mechanisms by which C. trachomatis was suggested to support the development of cervical cancer had been related to the ability of the bacteria to degrade the RFX5 transcription factor in order to subvert surface MHC I antigen recognition of both Chlamydia and HPV by CD8+ T cells (52, 53). However, the findings of our study strongly suggest that the impact of Chlamydia on cancer development is not based on immune evasion mechanisms impairing MHC I presentation in host cells. As chlamydial infection causes host cell DNA fragmentation and aberrant chromosomal segregation in dividing cells (39, 54–56), it is more likely that the contribution to carcinogenesis is based on DNA damage mechanisms rather than on MHC I subversion.
The development of a C. trachomatis T cell vaccine is the current focus of many research groups (57–59). Although effective antimicrobial therapies exist, vaccination is considered to be the best approach by which to reduce the prevalence of chlamydial infections (57, 60). However, currently, no vaccines against C. trachomatis infection are available, despite the many efforts that have been made throughout the years. Vaccines based on humoral immunity alone are unlikely to efficiently protect against infections caused by intracellular pathogens (61–63). Antigen-presenting cells (APCs) and their MHC I- and II-mediated antigen presentation are at the center of the initiation of immune responses by T cells and appear to be particularly important for the development of antichlamydial immunity (57). Hence, T cell vaccines that induce cellular immune responses (including activation of APCs and the generation of long-lived T-cell memory) are thought to be essential to elicit protective immunity against Chlamydia (57). Our finding that Chlamydia-infected epithelial cells retain the ability to express and present MHC I molecules provides important novel insights for the development of such future strategies designed to support and maintain T cell-mediated immune responses that help to control and eradicate chlamydial infections.
ACKNOWLEDGMENTS
This work was supported by the Friedrich Loeffler Institute and the Federal Ministry of Education and Research of the Federal Republic of Germany (grant 01 KI 0720). C.G. was supported by a fellowship grant from the Jena School for Microbial Communication.
We thank Mareen Sens, Katharina Wolf, and Martin Förster for technical assistance and Ralf M. Leonhardt and Eberhard Straube for helpful discussions.
Footnotes
Published ahead of print 16 December 2013
REFERENCES
- 1.Manavi K. 2006. A review on infection with Chlamydia trachomatis. Best Pract. Res. Clin. Obstet. Gynecol. 20:941–951. 10.1016/j.bpobgyn.2006.06.003 [DOI] [PubMed] [Google Scholar]
- 2.Dalaker K, Gjonnaess H, Kvile G, Urnes A, Anestad G, Bergan T. 1981. Chlamydia trachomatis as a cause of acute perihepatitis associated with pelvic inflammatory disease. Br. J. Vener. Dis. 57:41–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madeleine MM, Anttila T, Schwartz SM, Saikku P, Leinonen M, Carter JJ, Wurscher M, Johnson LG, Galloway DA, Daling JR. 2007. Risk of cervical cancer associated with Chlamydia trachomatis antibodies by histology, HPV type and HPV cofactors. Int. J. Cancer 120:650–655. 10.1002/ijc.22325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.da Silva Barros NK, Costa MC, Alves RR, Villa LL, Derchain SF, Zeferino LC, Dos Santos Carneiro MA, Rabelo-Santos SH. 2012. Association of HPV infection and Chlamydia trachomatis seropositivity in cases of cervical neoplasia in midwest Brazil. J. Med. Virol. 84:1143–1150. 10.1002/jmv.23312 [DOI] [PubMed] [Google Scholar]
- 5.Wright HR, Turner A, Taylor HR. 2008. Trachoma. Lancet 371:1945–1954. 10.1016/S0140-6736(08)60836-3 [DOI] [PubMed] [Google Scholar]
- 6.Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP, Mariotti SP. 2004. Global data on visual impairment in the year 2002. Bull. World Health Organ. 82:844–851 http://www.scielosp.org/scielo.php?script=sci_arttext&pid=S0042-96862004001100009&lng=en&nrm=iso&tlng=en [PMC free article] [PubMed] [Google Scholar]
- 7.Cresswell P, Ackerman AL, Giodini A, Peaper DR, Wearsch PA. 2005. Mechanisms of MHC class I-restricted antigen processing and cross-presentation. Immunol. Rev. 207:145–157. 10.1111/j.0105-2896.2005.00316.x [DOI] [PubMed] [Google Scholar]
- 8.Fiegl D, Kagebein D, Liebler-Tenorio EM, Weisser T, Sens M, Gutjahr M, Knittler MR. 2013. Amphisomal route of MHC class I cross-presentation in bacteria-infected dendritic cells. J. Immunol. 190:2791–2806. 10.4049/jimmunol.1202741 [DOI] [PubMed] [Google Scholar]
- 9.Dejardin E, Deregowski V, Greimers R, Cai Z, Chouaib S, Merville MP, Bours V. 1998. Regulation of major histocompatibility complex class I expression by NF-kappaB-related proteins in breast cancer cells. Oncogene 16:3299–3307. 10.1038/sj.onc.1201879 [DOI] [PubMed] [Google Scholar]
- 10.Meissner TB, Liu YJ, Lee KH, Li A, Biswas A, van Eggermond MC, van den Elsen PJ, Kobayashi KS. 2012. NLRC5 cooperates with the RFX transcription factor complex to induce MHC class I gene expression. J. Immunol. 188:4951–4958. 10.4049/jimmunol.1103160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong G, Liu L, Fan T, Fan P, Ji H. 2000. Degradation of transcription factor RFX5 during the inhibition of both constitutive and interferon gamma-inducible major histocompatibility complex class I expression in chlamydia-infected cells. J. Exp. Med. 191:1525–1534. 10.1084/jem.191.9.1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw AC, Vandahl BB, Larsen MR, Roepstorff P, Gevaert K, Vandekerckhove J, Christiansen G, Birkelund S. 2002. Characterization of a secreted Chlamydia protease. Cell. Microbiol. 4:411–424. 10.1046/j.1462-5822.2002.00200.x [DOI] [PubMed] [Google Scholar]
- 13.Zhong G, Fan P, Ji H, Dong F, Huang Y. 2001. Identification of a chlamydial protease-like activity factor responsible for the degradation of host transcription factors. J. Exp. Med. 193:935–942. 10.1084/jem.193.8.935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christian J, Vier J, Paschen SA, Hacker G. 2010. Cleavage of the NFκB family protein p65/RelA by the chlamydial protease-like activity factor (CPAF) impairs proinflammatory signaling in cells infected with chlamydiae. J. Biol. Chem. 285:41320–41327. 10.1074/jbc.M110.152280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen AL, Johnson KA, Lee JK, Sutterlin C, Tan M. 2012. CPAF: a chlamydial protease in search of an authentic substrate. PLoS Pathog. 8:e1002842. 10.1371/journal.ppat.1002842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gary-Gouy H, Harriague J, Bismuth G, Platzer C, Schmitt C, Dalloul AH. 2002. Human CD5 promotes B-cell survival through stimulation of autocrine IL-10 production. Blood 100:4537–4543. 10.1182/blood-2002-05-1525 [DOI] [PubMed] [Google Scholar]
- 17.DeMars R, Chang CC, Shaw S, Reitnauer PJ, Sondel PM. 1984. Homozygous deletions that simultaneously eliminate expressions of class I and class II antigens of EBV-transformed B-lymphoblastoid cells. I. Reduced proliferance responses of autologous and allogeneic T cells that have decreased expression of class II antigens. Hum. Immunol. 11:77–97 [DOI] [PubMed] [Google Scholar]
- 18.Knittler MR, Alberts P, Deverson EV, Howard JC. 1999. Nucleotide binding by TAP mediates association with peptide and release of assembled MHC class I molecules. Curr. Biol. 9:999–1008. 10.1016/S0960-9822(99)80448-5 [DOI] [PubMed] [Google Scholar]
- 19.Barron AL, Olshevsky C, Cohen MM. 1970. Characteristics of the BGM line of cells from African green monkey kidney. Brief report. Arch. Gesamte Virusforsch. 32:389–392. 10.1007/BF01250067 [DOI] [PubMed] [Google Scholar]
- 20.Min W, Pober JS, Johnson DR. 1998. Interferon induction of TAP1: the phosphatase SHP-1 regulates crossover between the IFN-alpha/beta and the IFN-gamma signal-transduction pathways. Circ. Res. 83:815–823. 10.1161/01.RES.83.8.815 [DOI] [PubMed] [Google Scholar]
- 21.Brodsky FM, Lem L, Solache A, Bennett EM. 1999. Human pathogen subversion of antigen presentation. Immunol. Rev. 168:199–215. 10.1111/j.1600-065X.1999.tb01294.x [DOI] [PubMed] [Google Scholar]
- 22.Lutz PM, Cresswell P. 1987. An epitope common to HLA class I and class II antigens, Ig light chains, and beta 2-microglogulin. Immunogenetics 25:228–233. 10.1007/BF00404692 [DOI] [PubMed] [Google Scholar]
- 23.Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. 10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang G, Burczynski F, Hasinoff B, Zhong G. 2002. Infection of myocytes with chlamydiae. Microbiology 148:3955–3959 http://mic.sgmjournals.org/content/148/12/3955.long [DOI] [PubMed] [Google Scholar]
- 25.Otero MJ, Carrasco L. 1984. Action of oligomycin on cultured mammalian cells. Permeabilization to translation inhibitors. Mol. Cell. Biochem. 61:183–191 [DOI] [PubMed] [Google Scholar]
- 26.Leonhardt RM, Keusekotten K, Bekpen C, Knittler MR. 2005. Critical role for the tapasin-docking site of TAP2 in the functional integrity of the MHC class I-peptide-loading complex. J. Immunol. 174:5104–5114 http://www.jimmunol.org/content/175/8/5104.long [DOI] [PubMed] [Google Scholar]
- 27.Heuer D, Rejman Lipinski A, Machuy N, Karlas A, Wehrens A, Siedler F, Brinkmann V, Meyer TF. 2009. Chlamydia causes fragmentation of the Golgi compartment to ensure reproduction. Nature 457:731–735. 10.1038/nature07578 [DOI] [PubMed] [Google Scholar]
- 28.Nuchtern JG, Bonifacino JS, Biddison WE, Klausner RD. 1989. Brefeldin A implicates egress from endoplasmic reticulum in class I restricted antigen presentation. Nature 339:223–226. 10.1038/339223a0 [DOI] [PubMed] [Google Scholar]
- 29.van Ooij C, Apodaca G, Engel J. 1997. Characterization of the Chlamydia trachomatis vacuole and its interaction with the host endocytic pathway in HeLa cells. Infect. Immun. 65:758–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scidmore MA, Fischer ER, Hackstadt T. 2003. Restricted fusion of Chlamydia trachomatis vesicles with endocytic compartments during the initial stages of infection. Infect. Immun. 71:973–984. 10.1128/IAI.71.2.973-984.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Weert AW, Geuze HJ, Groothuis B, Stoorvogel W. 2000. Primaquine interferes with membrane recycling from endosomes to the plasma membrane through a direct interaction with endosomes which does not involve neutralisation of endosomal pH nor osmotic swelling of endosomes. Eur. J. Cell Biol. 79:394–399. 10.1078/0171-9335-00062 [DOI] [PubMed] [Google Scholar]
- 32.Nanagara R, Li F, Beutler A, Hudson A, Schumacher HR., Jr 1995. Alteration of Chlamydia trachomatis biologic behavior in synovial membranes. Suppression of surface antigen production in reactive arthritis and Reiter's syndrome. Arthritis Rheum. 38:1410–1417 [DOI] [PubMed] [Google Scholar]
- 33.Al-Mously N, Eley A. 2007. Interaction of Chlamydia trachomatis serovar E with male genital tract epithelium results in secretion of proinflammatory cytokines. J. Med. Microbiol. 56:1025–1032. 10.1099/jmm.0.47241-0 [DOI] [PubMed] [Google Scholar]
- 34.Rödel J, Groh A, Vogelsang H, Lehmann M, Hartmann M, Straube E. 1998. Beta interferon is produced by Chlamydia trachomatis-infected fibroblast-like synoviocytes and inhibits gamma interferon-induced HLA-DR expression. Infect. Immun. 66:4491–4495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rödel J, Vogelsang H, Prager K, Hartmann M, Schmidt KH, Straube E. 2002. Role of interferon-stimulated gene factor 3gamma and beta interferon in HLA class I enhancement in synovial fibroblasts upon infection with Chlamydia trachomatis. Infect. Immun. 70:6140–6146. 10.1128/IAI.70.11.6140-6146.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Offermann MK, Faller DV. 1989. Autocrine induction of major histocompatibility complex class I antigen expression results from induction of beta interferon in oncogene-transformed BALB/c-3T3 cells. Mol. Cell. Biol. 9:1969–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caspar-Bauguil S, Puissant B, Nazzal D, Lefevre JC, Thomsen M, Salvayre R, Benoist H. 2000. Chlamydia pneumoniae induces interleukin-10 production that down-regulates major histocompatibility complex class I expression. J. Infect. Dis. 182:1394–1401. 10.1086/315856 [DOI] [PubMed] [Google Scholar]
- 38.Belland R, Ojcius DM, Byrne GI. 2004. Chlamydia. Nat. Rev. Microbiol. 2:530–531. 10.1038/nrmicro931 [DOI] [PubMed] [Google Scholar]
- 39.Grieshaber SS, Grieshaber NA, Miller N, Hackstadt T. 2006. Chlamydia trachomatis causes centrosomal defects resulting in chromosomal segregation abnormalities. Traffic 7:940–949. 10.1111/j.1600-0854.2006.00439.x [DOI] [PubMed] [Google Scholar]
- 40.Greene W, Xiao Y, Huang Y, McClarty G, Zhong G. 2004. Chlamydia-infected cells continue to undergo mitosis and resist induction of apoptosis. Infect. Immun. 72:451–460. 10.1128/IAI.72.1.451-460.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abdelrahman YM, Belland RJ. 2005. The chlamydial developmental cycle. FEMS Microbiol. Rev. 29:949–959. 10.1016/j.femsre.2005.03.002 [DOI] [PubMed] [Google Scholar]
- 42.Igietseme JU, Magee DM, Williams DM, Rank RG. 1994. Role for CD8+ T cells in antichlamydial immunity defined by Chlamydia-specific T-lymphocyte clones. Infect. Immun. 62:5195–5197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Magee DM, Williams DM, Smith JG, Bleicker CA, Grubbs BG, Schachter J, Rank RG. 1995. Role of CD8 T cells in primary Chlamydia infection. Infect. Immun. 63:516–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim SK, Angevine M, Demick K, Ortiz L, Rudersdorf R, Watkins D, DeMars R. 1999. Induction of HLA class I-restricted CD8+ CTLs specific for the major outer membrane protein of Chlamydia trachomatis in human genital tract infections. J. Immunol. 162:6855–6866 [PubMed] [Google Scholar]
- 45.Lampe MF, Wilson CB, Bevan MJ, Starnbach MN. 1998. Gamma interferon production by cytotoxic T lymphocytes is required for resolution of Chlamydia trachomatis infection. Infect. Immun. 66:5457–5461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang X, Shen C, Rey-Ladino J, Yu H, Brunham RC. 2008. Characterization of murine dendritic cell line JAWS II and primary bone marrow-derived dendritic cells in Chlamydia muridarum antigen presentation and induction of protective immunity. Infect. Immun. 76:2392–2401. 10.1128/IAI.01584-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hansen TH, Bouvier M. 2009. MHC class I antigen presentation: learning from viral evasion strategies. Nat. Rev. Immunol. 9:503–513. 10.1038/nri2575 [DOI] [PubMed] [Google Scholar]
- 48.Ibana JA, Schust DJ, Sugimoto J, Nagamatsu T, Greene SJ, Quayle AJ. 2011. Chlamydia trachomatis immune evasion via downregulation of MHC class I surface expression involves direct and indirect mechanisms. Infect. Dis. Obstet. Gynecol. 2011:420905. 10.1155/2011/420905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mandrekar P, Catalano D, Girouard L, Szabo G. 1996. Human monocyte IL-10 production is increased by acute ethanol treatment. Cytokine 8:567–577. 10.1006/cyto.1996.0076 [DOI] [PubMed] [Google Scholar]
- 50.Wallin KL, Wiklund F, Luostarinen T, Angstrom T, Anttila T, Bergman F, Hallmans G, Ikaheimo I, Koskela P, Lehtinen M, Stendahl U, Paavonen J, Dillner J. 2002. A population-based prospective study of Chlamydia trachomatis infection and cervical carcinoma. Int. J. Cancer 101:371–374. 10.1002/ijc.10639 [DOI] [PubMed] [Google Scholar]
- 51.Samoff E, Koumans EH, Markowitz LE, Sternberg M, Sawyer MK, Swan D, Papp JR, Black CM, Unger ER. 2005. Association of Chlamydia trachomatis with persistence of high-risk types of human papillomavirus in a cohort of female adolescents. Am. J. Epidemiol. 162:668–675. 10.1093/aje/kwi262 [DOI] [PubMed] [Google Scholar]
- 52.Alibek K, Karatayeva N, Bekniyazov I. 2012. The role of infectious agents in urogenital cancers. Infect. Agents Cancer 7:35–42. 10.1186/1750-9378-7-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khosla S. 2012. Is Chlamydia trachomatis a cofactor for cervical cancer? Aust. Med. Stud. J. 3:55–57 http://www.amsj.org/archives/2050 [Google Scholar]
- 54.Satta A, Stivala A, Garozzo A, Morello A, Perdichizzi A, Vicari E, Salmeri M, Calogero AE. 2006. Experimental Chlamydia trachomatis infection causes apoptosis in human sperm. Hum. Reprod. 21:134–137. 10.1093/humrep/dei269 [DOI] [PubMed] [Google Scholar]
- 55.Gallegos G, Ramos B, Santiso R, Goyanes V, Gosalvez J, Fernandez JL. 2008. Sperm DNA fragmentation in infertile men with genitourinary infection by Chlamydia trachomatis and Mycoplasma. Fertil. Steril. 90:328–334. 10.1016/j.fertnstert.2007.06.035 [DOI] [PubMed] [Google Scholar]
- 56.Chumduri C, Gurumurthy RK, Zadora PK, Mi Y, Meyer TF. 2013. Chlamydia infection promotes host DNA damage and proliferation but impairs the DNA damage response. Cell Host Microbe 13:746–758. 10.1016/j.chom.2013.05.010 [DOI] [PubMed] [Google Scholar]
- 57.Karunakaran KP, Yu H, Foster LJ, Brunham RC. 2010. Development of a Chlamydia trachomatis T cell vaccine. Hum. Vaccin. 6:676–680. 10.4161/hv.6.8.12299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Howie SE, Horner PJ, Horne AW, Entrican G. 2011. Immunity and vaccines against sexually transmitted Chlamydia trachomatis infection. Curr. Opin. Infect. Dis. 24:56–61. 10.1097/QCO.0b013e3283421081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Picard MD, Cohane KP, Gierahn TM, Higgins DE, Flechtner JB. 2012. High-throughput proteomic screening identifies Chlamydia trachomatis antigens that are capable of eliciting T cell and antibody responses that provide protection against vaginal challenge. Vaccine 30:4387–4393. 10.1016/j.vaccine.2012.01.017 [DOI] [PubMed] [Google Scholar]
- 60.Schautteet K, De Clercq E, Vanrompay D. 2011. Chlamydia trachomatis vaccine research through the years. Infect. Dis. Obstet. Gynecol. 2011:963513. 10.1155/2011/963513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seder RA, Hill AV. 2000. Vaccines against intracellular infections requiring cellular immunity. Nature 406:793–798. 10.1038/35021239 [DOI] [PubMed] [Google Scholar]
- 62.Rodrigues MM, Boscardin SB, Vasconcelos JR, Hiyane MI, Salay G, Soares IS. 2003. Importance of CD8 T cell-mediated immune response during intracellular parasitic infections and its implications for the development of effective vaccines. An. Acad. Bras. Cienc. 75:443–468. 10.1590/S0001-37652003000400005 [DOI] [PubMed] [Google Scholar]
- 63.Robinson HL, Amara RR. 2005. T cell vaccines for microbial infections. Nat. Med. 11:S25–S32. 10.1038/nm1212 [DOI] [PubMed] [Google Scholar]
- 64.Terrazzano G, Romano MF, Turco MC, Salzano S, Ottaiano A, Venuta S, Fontana S, Manzo C, Zappacosta S, Carbone E. 2000. HLA class I antigen downregulation by interleukin (IL)-10 is predominantly governed by NK-kappaB in the short term and by TAP1+2 in the long term. Tissue Antigens 55:326–332. 10.1034/j.1399-0039.2000.550406.x [DOI] [PubMed] [Google Scholar]