Abstract
Allergies are mainly characterized as an unrestrained Th2-biased immune response. Epidemiological data associate protection from allergic diseases with the exposure to certain infectious agents during early stages of life. Modulation of the immune response by pathogens has been considered to be a major factor influencing this protection. Recent evidence indicates that immunoregulatory mechanisms induced upon infection ameliorate allergic disorders. A longitudinal study has demonstrated reduced frequency and incidence of asthma in children who reported a prior infection with Salmonella. Experimental studies involving Salmonella enterica serovar Typhimurium-infected murine models have confirmed protection from induced allergic airway inflammation; however, the underlying cause leading to this amelioration remains incompletely defined. In this study, we aimed to delineate the regulatory function of Salmonella Typhimurium infection in the amelioration of allergic airway inflammation in mice. We observed a significant increase in CD11b+ Gr1+ myeloid cell populations in mice after infection with S. Typhimurium. Using in vitro and in vivo studies, we confirmed that these myeloid cells reduce airway inflammation by influencing Th2 cells. Further characterization showed that the CD11b+ Gr1+ myeloid cells exhibited their inhibitory effect by altering GATA-3 expression and interleukin-4 (IL-4) production by Th2 cells. These results indicate that the expansion of myeloid cells upon S. Typhimurium infection could potentially play a significant role in curtailing allergic airway inflammation. These findings signify the contribution of myeloid cells in preventing Th2-mediated diseases and suggest their possible application as therapeutics.
INTRODUCTION
The global surveillance report on the prevalence of chronic respiratory diseases published in 2011 by the Global Initiative for Asthma (GINA) estimated that about 300 million individuals worldwide suffer from asthma, with 250,000 deaths annually attributed to this disease. Asthma is mainly characterized as a Th2-biased inflammatory disease, defined by cellular infiltration, increased mucus production, and structural remodeling of the lungs, resulting in airway obstruction. Epidemiological studies have correlated the rise in allergic diseases over the past few decades, especially in the developed part of the world, to changes in environmental factors and improvement in the field of disease prevention, resulting in reduced exposure to microbial and helminth antigens during early childhood (1–3). As allergic disorders are typically a result of Th2-skewed immune responses, modulation toward a Th1 immune phenotype induced during microbial infection was considered to be critical in preventing acute Th2 diseases (4, 5). Subsequent studies in mice have indicated the vital role of various immunoregulatory mechanisms in the modulation of allergic and autoimmune diseases. Among them, regulatory T (Treg) cells have been demonstrated to be essential in restoring the balance of the immune system in order to prevent and to provide protection from various diseases (6). Induction and expansion of Treg cells during various helminth and bacterial infections have led to the inhibition of allergic airway inflammation in mouse models (7–11). In contrast, certain infection models have also confirmed suppression of allergies in a Treg cell-independent manner, for which the mechanisms are yet undefined (12, 13).
A longitudinal study by Pelosi et al. on Sardinian children demonstrated an inverse correlation between the exposure to pathogen and the susceptibility to allergic reactions (14). In comparison to children suffering from enteritis of a nonbacterial etiology, children infected by Salmonella during their infancy had reduced frequency and delayed occurrence of asthma and allergic rhinoconjunctivitis in later stages of their lives. Consequently, investigation in a murine model of allergic airway inflammation confirmed that infection with Salmonella enterica serovar Typhimurium resulted in reduced airway inflammation; however, the mechanism still remains unclear (15). In the present study, we attempted to identify the mechanism(s) induced upon Salmonella Typhimurium infection in mediating this suppression of airway inflammation.
Following an analogous regimen adopted from Wu et al., we observed a decrease in airway inflammation in auxotrophic S. Typhimurium AroA strain SL 7207-infected mice (15). Contrary to the previous hypothesis, the reduction in immune pathology was mediated by a mechanism independent of a clear shift toward a Th1 response. Additionally, there was no demonstrable change in the frequency of Foxp3+ Treg cells in the infected group of mice.
Subsequently, we detected a considerable increase in a population of cells expressing CD11b and Gr1 in mice infected with S. Typhimurium. This heterogeneous population of cells has been shown to be comprised of macrophages, immature granulocytes, early myeloid progenitors, and dendritic cells (DCs) (16). Using in vitro coculture systems, we attempted to determine whether these myeloid cells influenced the differentiation or the stability of Th2 cells. We demonstrate that these myeloid cells do not influence the in vitro differentiation of naive T cells into a Th2 phenotype but considerably destabilize already differentiated Th2 cells by downmodulating the expression of key regulatory factors. Our results implicate a potential mechanism of protection from asthma mediated by S. Typhimurium infection through the expansion of CD11b+ Gr1+ cells, which negatively influence the stability of Th2 cells.
MATERIALS AND METHODS
Animals and bacteria.
BALB/c and DO11.10 mice were bred at the animal facility of Twincore (Twincore, Hanover, Germany) and at the Helmholtz Centre for Infection Research (HZI; Braunschweig, Germany). Six- to 12-week-old sex- and age-matched mice were used for all experiments. All animals were housed under specific-pathogen-free conditions. Mice were sacrificed by intraperitoneal (i.p.) injection of 6.25 mg of ketamine hydrochloride and 0.75 mg of xylazine hydrochloride as approved by the German animal welfare law. All mouse experiments were conducted in accordance with the described procedures in ethics applications approved by the institutional animal welfare committee and by the local government, namely, the Lower Saxony State Office for Consumer Protection and Food Safety. The auxotrophic S. Typhimurium aroA strain SL 7207 was used for all experiments. Bacteria were grown in Luria-Bertani (LB) medium (Roth, Karlsruhe, Germany) at 37°C and were used at an optical density corresponding to 0.5 × 109 to 1.0 × 109 CFU/ml.
Fluorescence cytometry.
Antibodies against CD4 (GK1.5 and RM4-5), CD3 (17A2 and 145-2C11), CD8 (H35-17.2), Foxp3 (FJK-16S), GATA-3 (TWAJ), gamma interferon (IFN-γ; XMG1.2), Gr-1 (RB6-8C5), CD11b (M1/70), CD25 (PC61), CD19 (1D3), CD49b (DX5), NKp46 (29A1.4), CD11c (N418), and B220 (RA3-6B2) were purchased from eBioscience (Frankfurt, Germany), and anti-Ly6C (HK1.4) was from Biolegend (Fell, Germany). Fluorescence-activated cell sorter (FACS) acquisition was performed on an LSRII (Becton, Dickinson, Heidelberg, Germany) instrument using DIVA software (6.1.2), and data were analyzed using FlowJo software (Tree Star, Inc., OR, USA). FACS analysis was performed at the Cell Sorting Core Facility of the Hanover Medical School on a FACS Aria (Becton, Dickinson, Heidelberg, Germany), XDP, or MoFlo (Beckman Coulter, Krefeld, Germany) cell sorter.
Induction of allergic airway inflammation and measurement of cellular infiltration in BAL fluid.
Induction of allergic airway inflammation with parallel infection with the auxotrophic S. Typhimurium aroA strain SL 7207 was adapted from a regimen described earlier (15). Mice were sensitized with 10 μg of ovalbumin (OVA) i.p. (grade VI; Sigma-Aldrich, Munich, Germany) adsorbed on 1.5 mg of aluminum hydroxide (Sigma-Aldrich, Munich, Germany) on days 7, 8, 9, and 20. One group of OVA-sensitized mice was infected intragastrically with 0.5 × 109 to 1.0 × 109 CFU of S. Typhimurium (SL 7207) (Sal group) in LB medium containing 3% sodium bicarbonate on days 0, 7, 20, and 27. All OVA-sensitized mice were subsequently challenged on days 20, 24, 27, 30, and 34 by intranasal (i.n.) administration of 30 μg of OVA (grade V). Sham-sensitized (phosphate-buffered saline [PBS] plus alum) and sham-challenged mice were used as negative controls (Neg group). Animals were sacrificed 24 h after the last challenge using ketamine and xylazine, and their trachea were cannulated. Airways were flushed three times with 800 μl of ice-cold PBS, and total cell number in the bronchoalveolar lavage (BAL) fluid was calculated using trypan blue exclusion dye. Approximately 5 × 104 to 10 × 104 cells in 100 μl of PBS were used for cytospinning (700 rpm for 5 min) (Cytospin3; Shandon). Slides were stained with a Diff-Quik staining kit (Medion Diagnostics, Deudingen, Switzerland) according to the manufacturer's protocol. Two hundred leukocytes were counted per slide from random fields in a single-blinded manner.
Measurement of OVA-specific Ig in serum.
Quantification of antigen-specific serum immunoglobulins IgE, IgG1, and IgG2a was performed using enzyme-linked immunosorbent assays (ELISAs) as described previously (17). The detection limits were 3.125 ng/ml for IgG1, 1.56 ng/ml for IgE, and 0.78 ng/ml for IgG2a. The absorbance was measured at 405 nm with a 570-nm filter as a reference.
Cytokine profiling of OVA-restimulated meLN cells.
The cytokine secretion profile of cells from lung-draining lymph nodes was determined using an in vitro restimulation assay. Single-cell suspensions from mediastinal lymph nodes (meLN) were obtained by mechanical disruption, and 1 × 106 total cells were seeded in 96-well round-bottom plates in 200 μl of complete RPMI medium (Gibco, Darmstadt, Germany) containing 100 μg/ml OVA (grade VI). Culture supernatants were harvested after 72 h and frozen at −80°C until further use. Interleukin-4 (IL-4), IL-5, IL-13, IL-10, and IFN-γ levels were measured in cell-free supernatants by ELISAs using matched antibody pairs purchased from R&D Systems (Wiesbaden-Nordenstadt, Germany). ELISAs were performed according to the manufacturer's instructions.
Analysis of cellular infiltrates in spleen and lymph nodes and their cytokine profiles.
A total of 1 × 106 cells from the spleen were stimulated with 0.1 μg/ml phorbol myristate acetate (PMA; Sigma-Aldrich, Munich, Germany) and 1 μg/ml ionomycin (Sigma-Aldrich, Munich, Germany). After 4 h, 1 μg/ml brefeldin A (ebiosciences, San Diego, CA, USA) was added and incubated for an additional 2 h. Unstimulated cells incubated with brefeldin A were used as a control. Consequently, cells were washed, and intracellular staining was performed to determine the expression of IFN-γ in CD4+ and CD8+ cells. Unstimulated cells were used to determine the frequencies of Foxp3+ Treg cells and myeloid cell populations expressing CD11b and Gr1.
In order to determine the influence of an oral infection with S. Typhimurium on the cellular composition in the spleen and lung, mice were infected with 0.5 × 109 to 1 × 109 CFU of SL 7207 on days 0, 4, and 9. On day 14 the mice were sacrificed and analyzed for the frequency of CD3+ T cells, CD19+ B cells, CD11c+ dendritic cells (DCs), CD49b+ NK cells, Foxp3+ Treg cells, and CD11b+ Gr1+ myeloid cells in the spleen and lung. Uninfected mice were used as controls.
Quantitative PCR.
The left lobe of the lung was excised and frozen immersed in TRIzol (Invitrogen, Darmstadt, Germany) at −80°C until further use. Total RNA was isolated according to the manufacturer's protocol. RNA was quantified using a NanoDrop-1000 spectrophotometer (Peqlab, Erlangen, Germany). cDNA synthesis was performed using oligo(dT) primers and a Fermentas Revert enzyme kit (Fermentas, Schwerte, Germany) using 1 μg of total RNA as the template. The MUC5AC gene was quantified as described previously (18) using the primers (5′-CTTCAACGGCAGTCCAAAAT-3′) and (5′-CTCAAGGGGTGTCAGCCTAA-3′). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. Primers for GAPDH and SYBR green mix were obtained from SAbiosciences (Hilden, Germany). Quantitative PCRs (qPCRs) and data analysis were carried out using a LightCycler 480 (Roche, Penzberg, Germany).
Lung histology and quantification.
The lungs were fixed by intratracheal instillation of 4% buffered paraformaldehyde, ligated, and stored in fixative until further use. Specimens were trimmed according to standardized tissue trimming RITA (registry of industrial toxicology animal data) industrial guidelines (19). Uniform samples were embedded in paraffin, and 3-μm sections were stained with either hematoxylin and eosin (H&E) or periodic acid-Schiff (PAS) stain to estimate cellular infiltration or mucus production by airway epithelial cells, respectively. The surface area of mucus-containing goblet cells (Sgc) per total surface area of airway epithelial basal membrane (Sep) was determined using a computer-assisted tool (Axio Vision, version 4.8; Carl Zeiss).
Ex vivo isolation of myeloid cells from the spleen.
On day 14, splenocytes from mice previously infected with 0.5 × 109 to 1 × 109 CFU of auxotrophic S. Typhimurium aroA strain SL 7207 on days 0, 4, and 9 were depleted of CD3+ and CD19+ cells using anti-CD3/anti-CD19 phycoerythrin (PE) antibodies in conjunction with anti-PE magnetic cell sorting (MACS) microbeads (Miltenyi, Bergisch Gladbach, Germany). Myeloid cells were further sorted by a FACS instrument as CD11b+ and Gr1+ cells. FACS-sorted CD11b− Gr1− cells from the infected mice were used as negative controls.
Influence of myeloid cells on differentiation of Th2 cells.
To investigate the influence of myeloid cells on Th2 differentiation, sorted CD11b+ Gr1+ cells were cocultured in vitro with naive CD4+ DO11.10+ T cells at a ratio of 1:1 under Th2 differentiation conditions in the presence of IL-4 (1 μg/ml), IL-2 (25 U/ml), anti-IFN-γ (2.5 μg/ml), and granulocyte-macrophage colony-stimulating factor (GM-CSF)-derived bone marrow DCs. After 5 days of culture, the expression of the key transcription factor GATA-3 was measured by FACS on live gated CD4+ DO11.10+ cells to determine the frequency of differentiated Th2 cells. CD11b− Gr1− cells sorted from the same infected mice were used as internal controls for these Th2 differentiation assays.
Influence of myeloid cells on differentiated Th2 cells.
Th2 cells were differentiated in vitro as described previously. The effect of myeloid cells on the stability of differentiated Th2 cells was assessed by coculturing the in vitro-generated Th2 cells with CD11b+ Gr1+ cells, and their GATA-3 expression was analyzed after 2 days by FACS analysis and Western blotting. After coculture, Th2 cells were sorted positively using anti-CD4 PE and anti-PE MACS microbeads. For estimation of GATA-3 expression using Western blotting, sorted cells were washed with PBS and lysed using buffer (Pierce, Rockford, IL, USA) containing Na3VO4 and protease inhibitors. Protein concentration was estimated from the cell extract using a bicinchoninic acid (BCA) assay (Pierce, Rockford, IL, USA), and an equal amount of total protein was used for Western blotting. Blots were probed with anti-GATA-3 (clone sc268; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-IgG-horseradish peroxidase (HRP; (Jackson ImmunoResearch, West Grove, PA, USA). β-Actin (clone A5441; Sigma-Aldrich, Munich, Germany) was used as a loading control. Chemiluminescence was detected using an Intas ChemoCam detection system every 2 min after addition of the HRP substrate (GE Health Care, Buckinghamshire, United Kingdom), and images were sequentially integrated. Quantification was carried out using LabImage 1D software (Intas). To determine the mechanism, 20 μM nitric oxide synthase inhibitor N-(3-aminomethyl) benzylacetamidine (1400W; Merck, Darmstadt, Germany) or 50 μM arginase (Arg) inhibitor (S)-(2-boronoethyl-l-cysteine) hydrochloride (BEC HCl; Merck, Darmstadt, Germany) was added to the cocultures. At 2 days postcoculture the Th2 cells were analyzed for their GATA-3 expression profiles. As controls, the noncocultured Th2 cells were also treated with the respective concentrations of the inhibitors.
Additionally, ELISAs were performed to estimate IL-4, IL-5, and IL-13 production by Th2 cells. To this end, equal numbers of live sorted Th2 cells postcoculture were stimulated using PMA (0.1 μg/ml) and ionomycin (1 μg/ml) for 6 h, and cell-free supernatants were used. ELISAs were performed using matched antibody pairs from R&D Systems.
Induction of allergic airway inflammation by adoptive transfer of Th2 cells cocultured with CD11b+ Gr1+ cells.
In vitro-differentiated Th2 cells were cocultured with ex vivo-isolated CD11b+ cells expressing intermediate and high levels of Gr1 (CD11b+ Gr1int and CD11b+ Gr1hi, respectively) at a ratio of 1:1. After 5 days, CD4+ DO11.10+ cells were sorted by FACS, and 3.5 × 106 to 4 × 106 sorted cells were adoptively transferred intravenously (i.v.) per BALB/c recipient mouse. Mice were challenged intranasally with 30 μg of OVA on two successive days. Positive (Pos)- and negative (Neg)-control mice were adoptively transferred with noncocultured Th2 cells. Positive-control mice were challenged as described above, and the negative-control mice were left unchallenged. At day 3 posttransfer mice were sacrificed, and BAL fluid was collected and analyzed as described earlier.
Statistical analysis.
Data are represented as means plus standard deviations (SD). Comparative groups were tested for statistical significance by analysis of variance (ANOVA) or a Mann-Whitney U test using Prism, version 5, software (GraphPad, San Diego, CA, USA). P values of less than 0.05 were considered statistically significant.
RESULTS
S. Typhimurium infection results in amelioration of cellular airway inflammation in mice.
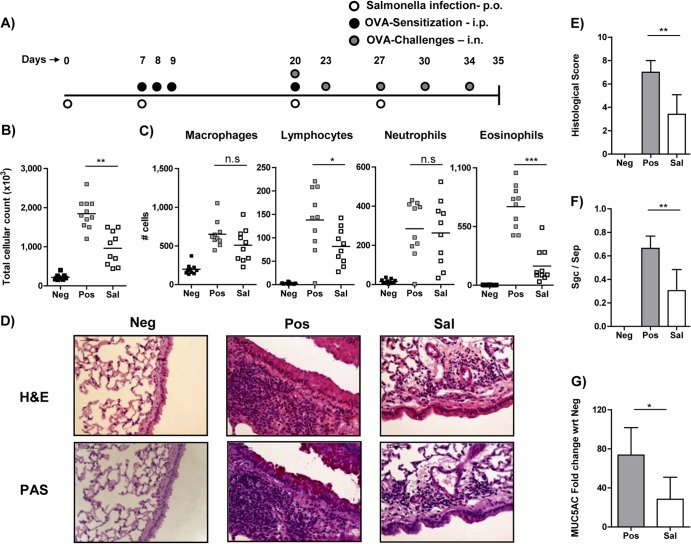
To determine the influence of S. Typhimurium infection on airway inflammation, we used a murine model of systemic sensitization with ovalbumin and alum (Fig. 1A). The positive-control group of mice (Fig. 1, Pos), which were OVA sensitized and challenged, showed an increased inflammatory response, as scored by total cellular infiltration and especially eosinophilic infiltration in BAL fluid in comparison to sham-sensitized and sham-challenged negative-control mice (Fig. 1B and C, Neg). The experimental group of mice (Sal) infected with the attenuated auxotrophic strain of S. Typhimurium aroA (SL 7207) prior to and during the sensitization period showed a significant reduction in total cellular infiltration in BAL fluid (Fig. 1B). In particular, eosinophils, which are critical cellular players in the pathology of allergic airway inflammation, were significantly decreased (Fig. 1C). Additionally, lymphocytic infiltration in the BAL fluid of infected mice was also distinctly reduced; however, there was no alteration in either the macrophage or neutrophil population between the groups. These observations are in accordance with published data (15) and thus confirm that gastric infection of mice with S. Typhimurium results in a reduced cellular infiltration into the lungs.
FIG 1.
Influence of S. Typhimurium infection on allergic airway inflammation. (A) Schematic representation of regimen used for inducing allergic airway inflammation. Mice sensitized against OVA on days 7, 8, 9, and 20 were subsequently challenged with OVA on days 20, 23, 27, 30, and 34. One group of mice (Sal) was infected with S. Typhimurium on days 0, 7, 20, and 27. Negative-control (Neg) mice were sham sensitized and sham challenged with PBS, unlike the positive control (Pos) and experimental (Sal) groups. p.o., orally. (B) Total cellular infiltration in the BAL fluid was determined by counting live cells using trypan blue exclusion dye. (C) Macrophage, lymphocyte, neutrophil and eosinophil numbers were ascertained by differential cellular count of cytospots using standard morphological parameters in a single-blinded manner. (D) Histology of formalin-fixed lung tissues stained with H&E (upper panel) or PAS (lower panel) dyes to determine cellular infiltration and mucus production, respectively, in lung. (E) Overall lung extension, peribronchial and perivascular cellular infiltration, and interstitial edema were the parameters considered for histological scoring in a double-blinded manner. (F) Mucus secretion was assessed by measuring the surface area of mucus-containing goblet cells (Sgc) per total surface of airway epithelial area. (G) Expression of the MUC5AC gene in the lung was determined using quantitative PCR and is represented as fold change with respect to (wrt) the Neg group. In panels B and C, each dot represents data from an individual mouse. Data shown were pooled from three individual experiments using 4 to 6 mice in each group. Data are represented as means plus SD and are representative of three individual experiments with 4 to 6 mice per group. A Mann-Whitney test was used for panel G, and ANOVA was used to determine statistical significance for the other data. n.s, nonsignificant; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Histological analysis reveals reduced pathology and mucus production in the lungs of S. Typhimurium-infected mice.
By histological analysis and quantitative PCR (qPCR), we assessed cellular pathology and mucus secretion in the lungs. Lung sections were stained with hematoxylin-eosin (H&E) to determine lung pathology (Fig. 1D, top panel) or with periodic acid-Schiff (PAS) stain to assess mucus production (Fig. 1D, bottom panel). A double-blinded quantitative analysis revealed a significant reduction in the overall inflammatory score in the lungs of S. Typhimurium-infected mice in comparison to the uninfected group (Fig. 1E, Sal and Pos, respectively). The Sal group mice also showed reduction in mucus production, as assessed by the ratio between the area of surface airway epithelium containing goblet cells (Sgc) and the total area of airway epithelium (Sep) (Fig. 1F). Additionally, qPCR analysis of MUC5AC expression in the lungs from the Sal group of mice showed relatively reduced expression compared to Pos group of mice (Fig. 1G), corroborating the quantitative histological data for mucus production.
Infection with S. Typhimurium does not interfere with the sensitization.
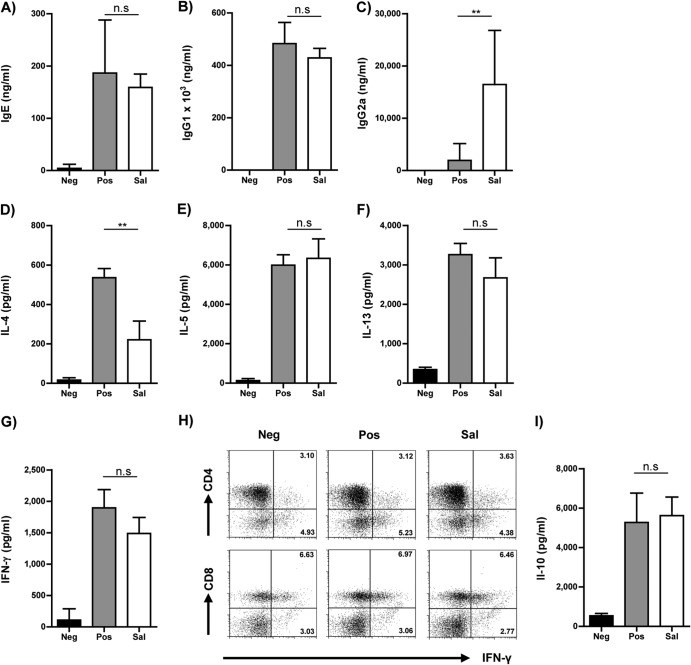
As S. Typhimurium infection was carried out during sensitization, it was essential to validate whether the infection resulted in an impairment of sensitization against the allergen, which may influence overall airway inflammation later. Thus, we collected serum from individual mice and evaluated the allergen-specific antibody titers by ELISA. The group of infected mice showed substantial titers of OVA-specific IgE (Fig. 2A) and IgG1 (Fig. 2B), which were equivalent to those of the uninfected but sensitized group (Pos) of mice, thus confirming comparable sensitization against the allergen. Additionally, the Sal group also exhibited enhanced titers of OVA-specific IgG2a in their sera (Fig. 2C). While the presence of antigen-specific IgE and IgG1 levels signifies the induction of a robust and equivalent Th2 response in both the infected and noninfected groups of mice, the increased titers of IgG2a in the infected mice indicates a tendency toward a Th1-mediated immune response.
FIG 2.
Modulation of T cell responses. OVA-specific serum IgE (A), IgG1 (B), and IgG2a (C) levels were quantified from individual serum samples by ELISA. Histograms represent means, and error bars represent SD of titers from individual mice. Cell-free supernatants of OVA-restimulated mediastinal lymph node cells were analyzed for IL-4 (D), IL-5 (E), IL-13 (F), IFN-γ (G), and IL-10 (I) by ELISA using matched antibody pairs. Histograms represent means, and error bars represent SD of ELISA replicates performed on three individual stimulations from each group. (H) Representative FACS plot of splenocytes analyzed for the frequency of IFN-γ-producing CD4+ and CD8+ T cells on the day of analysis. The plots are gated on live CD3+ lymphocytes. The FACS plots are representative plots from two individual experiments with 4 to 6 mice per group. Data are represented as means plus SD and are representative of three individual experiments with 3 to 6 mice per group. An ANOVA test was used to determine statistical significance. n.s, nonsignificant; **, P < 0.01.
Reduced pathology is not mediated by an apparent diversion toward a Th1 phenotype.
As the increased levels of IgG2a in the sera of S. Typhimurium-infected mice indicate a Th1-biased immune response, we analyzed the production of the Th1 signature cytokine IFN-γ. In parallel we assessed the production of Th2 cytokines such as IL-4, IL-5, and IL-13. For this purpose, single-cell suspensions from meLN were stimulated with OVA in vitro, and the cell-free supernatants were analyzed for cytokine production by ELISA. Among the Th2 cytokines analyzed, we observed a significant reduction in IL-4 (Fig. 2D) but not in IL-5 (Fig. 2E) or IL-13 (Fig. 2F) production by the Sal group of mice in contrast to the Pos group. Interestingly, we did not observe any significant alteration in IFN-γ secretion by the cells isolated from the Sal group of mice in comparison to their uninfected counterparts (Fig. 2G). We also analyzed IFN-γ production at a cellular level in splenocytes and cells from mesenteric and mediastinal lymph nodes (mLN and meLN, respectively). There were no differences in the relative numbers of IFN-γ-producing CD4+ and CD8+ cells between either of the groups in the spleen (Fig. 2H), mLN, and meLN (data not shown). With no visible shift toward a Th1-biased response in spite of reduced IL-4 production, we sought to determine the immunoregulatory mechanisms that might result in curbing airway inflammation. In this regard, we quantified the level of IL-10 in the supernatant of meLN cells stimulated with OVA in vitro but observed no significant differences between the Sal and Pos groups of mice (Fig. 2I). Subsequently, we analyzed CD4+ Foxp3+ Treg cells, which could exhibit an immunoregulatory function in an IL-10-independent manner.
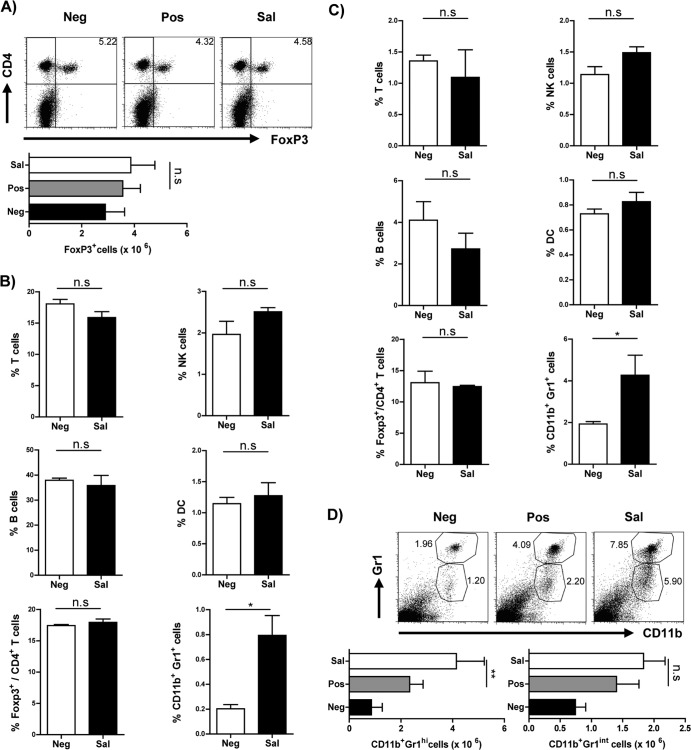
S. Typhimurium infection does not result in expansion of the Foxp3+ Treg cell population.
Treg cells have been implicated in playing a critical role in inhibiting airway inflammation in certain bacterial and helminth infections (7, 11). Consequently, we investigated the probable expansion of Treg cells in an in vivo infection by evaluating Foxp3+ cells in the spleen. No significant alteration was observed in either the frequency or total cell numbers of Foxp3+ Treg cells in the spleen (Fig. 3A).
FIG 3.
Alteration of cellular compartment upon S. Typhimurium infection. (A) Representative FACS plots gated on live CD3+ lymphocytes and histograms indicate the frequency and the total numbers of CD4+ Foxp3+ Treg cells in the spleen of mice from the different groups used in the airway inflammation model. Histograms indicate the frequency of various cell populations, i.e., T cells (CD3+), B cells (CD19+), NK cells (CD49b+), conventional DCs (cDCs) (CD11c+ and major histocompatibility complex class II-positive [MHCII+]), Treg cells (Foxp3+), and myeloid cells (CD11b+ Gr1+), in the spleen (B) and lungs (C) of mice infected with S. Typhimurium (Sal) in contrast to uninfected mice (Neg). The frequencies of all cell types were determined from the total-live-cell gate. Foxp3+ cell frequency was determined from gating live CD3+ CD4+ lymphocytes. (D) Representative FACS plots and histograms indicate the frequencies and total numbers of CD11b+ Gr1hi and CD11b+ Gr1int myeloid cells in the spleen of mice from the different groups used in the airway inflammation model. These cell frequencies were ascertained from the total live-cell gate. In panels A and D, data are representative plots from three individual experiments with 4 to 6 mice per group. ANOVA was used to determine statistical significance. In panels B and C data are represented as means plus SD and are representative of two individual experiments with 3 to 4 mice per group. A Mann-Whitney test was used for determining statistical significance. n.s, nonsignificant; *, P < 0.05; **, P < 0.01.
S. Typhimurium infection results in expansion of CD11b+ Gr1+ myeloid cells.
Since there was no apparent induction of a Th1-biased immune response or IL-10 levels and since the protection could not be attributed to the expansion of Treg cells, we investigated the changes in other predominant immune cell populations upon infection with S. Typhimurium. Mice infected with S. Typhimurium showed no demonstrable change in the frequencies of B cells (CD19+), T cells (CD3+), NK cells (CD49+), dendritic cells (CD11c+), and Treg cells (CD4+ Foxp3+), except for a significant increase in the frequency of CD11b+ Gr1+ cells in the spleens (Fig. 3B) and lungs (Fig. 3C). We further confirmed the expansion of CD11b+ Gr1+ myeloid cells in our experimental regimen for airway inflammation. There was a considerable increase in this myeloid cell population in the Sal group compared to levels in the other two groups (Fig. 3D). Further characterization of this population indicated that there was a significant increase in frequency and total cell numbers of the CD11b+ Gr1hi cell population and a modest increase in the CD11b+ Gr1int population. These myeloid cells were further examined for the surface expression of F4/80, Ly6C, and CD11c. In contrast to the CD11b+ Gr1hi cells which were CD11c− F4/80− Ly6Cint, the CD11b+ Gr1int cells were F4/80+ Ly6Chi but CD11c− (data not shown).
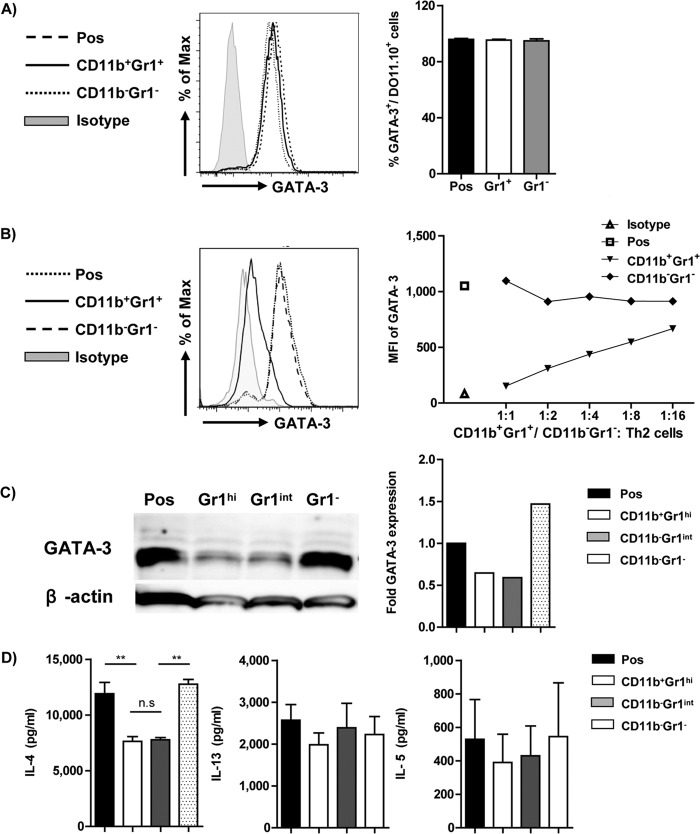
CD11b+ Gr1+ cells do not inhibit Th2 differentiation but influence GATA-3 expression.
Although S. Typhimurium infection moderates airway inflammation, the antibody and cytokine profiles of these infected mice indicate a pronounced Th2-dominated immune response. Earlier studies pertaining to the influence of CD11b+ Gr1+ cells on Th2 responses have demonstrated conflicting results (20, 21). Hence, we next sought to determine whether S. Typhimurium infection-induced CD11b+ Gr1+ myeloid cells inhibited the differentiation of naive T cells into the Th2 lineage or had any influence on the stability of already differentiated Th2 cells. In vitro coculture of naive T cells with sorted CD11b+ Gr1+ myeloid cells under Th2-polarizing conditions did not influence differentiation into Th2 cells (Fig. 4A, right panel). Also, the CD11b+ Gr1+ myeloid cells did not influence the strength of Th2 induction, as estimated by the levels of expression of GATA-3 (Fig. 4A, left panel). However, differentiated Th2 cells, upon coculture with CD11b+ Gr1+ myeloid cells, demonstrated a significant downregulation of GATA-3 expression at 2 days postcoculture in a dose-dependent manner (Fig. 4B). CD11b+ Gr1+ cells have been broadly classified as CD11b+ Gr1hi and CD11b+ Gr1int. Recently, CD11b+ Gr1int cells have been demonstrated to influence the Th2 effector function. As S. Typhimurium infection resulted in a significant increase in the CD11b+ Gr1hi cell population, we subsequently tried to determine the role of this subfraction in modulating GATA-3 expression using CD11b+ Gr1int cells as a positive reference. The CD11b+ Gr1hi cells demonstrated a comparable reduction in GATA-3 expression to CD11b+ Gr1int cells, as quantified by Western blot analysis (Fig. 4C). CD11b− Gr1− cells sorted from infected mice were used as a negative control, and they did not have any influence on GATA-3 expression.
FIG 4.
Myeloid cells alter Th2 stability but not differentiation. (A) A representative FACS plot and bar graph indicating the frequency of GATA-3+ cells of total live CD4+ DO11.10+ cells as an indicator of Th2 differentiation when T cells are cocultured with CD11b+ Gr1+ cells at ratio of 1:1. CD11b− Gr1− cells isolated from the same infected mice were used as an internal control. Noncocultured differentiated Th2 cells (Pos) were used as controls. Data are representative of three individual experiments. (B) CD11b+ Gr1+ myeloid cells were cocultured with differentiated Th2 cells at various ratios to ascertain their influence on the stability of Th2 cells. The FACS plot and line graph indicate the mean fluorescence intensity (MFI) of GATA-3 expression in live CD4+ DO11.10+ (Th2) cells. Noncocultured Th2 cells (Pos) and Th2 cells cocultured with CD11b− Gr1− cells sorted from the infected mice were used as controls. CD11b+ Gr1+ and CD11b− Gr1− cells were pooled from 3 to 4 infected mice. Data are representative of three individual experiments. (C) A representative Western blot analysis of GATA-3 and a bar graph indicating the quantification of GATA-3 expression from sorted Th2 cells after coculture with CD11b+ Gr1hi, CD11b+ Gr1int, or CD11b− Gr1− cells. Data are representative plots from two individual experiments. Noncocultured Th2 cells (Pos) were also used as negative controls. (D) A representative graph indicating IL-4, IL-13, and IL-5 production by noncocultured Th2 cells (Pos) and Th2 cells cocultured with CD11b+ Gr1hi, CD11b+ Gr1int, or CD11b− Gr1− cells. Data are represented as means plus SD and are representative of three individual experiments. ANOVA was used to determine statistical significance. n.s, nonsignificant; **, P < 0.01.
CD11b+ Gr1+ cells diminish IL-4 production by Th2 cells.
To ascertain whether the down-modulation of GATA-3 expression in Th2 cells altered their functional characteristics, Th2 cells were sorted at 5 days postcoculture, and equal numbers of sorted T cells were restimulated in vitro with PMA and ionomycin. Cell-free supernatants from these cultures were analyzed for IL-4 production by ELISA. Coculture of differentiated Th2 cells with CD11b+ Gr1hi or CD11b+ Gr1int cells exhibited a significant reduction in IL-4 cytokine production in comparison to only Th2 cells (Pos) or Th2 cells cocultured with CD11b− Gr1− cells (Fig. 4D, left panel). However, no significant difference was observed in either IL-13 (Fig. 4D, middle panel) or IL-5 (Fig. 4D, right panel) production levels between the various groups.
Adoptive transfer of Th2 cells cocultured with CD11b+ Gr1+ cells exhibit attenuation in eosinophil recruitment to the airways of experimental mice.
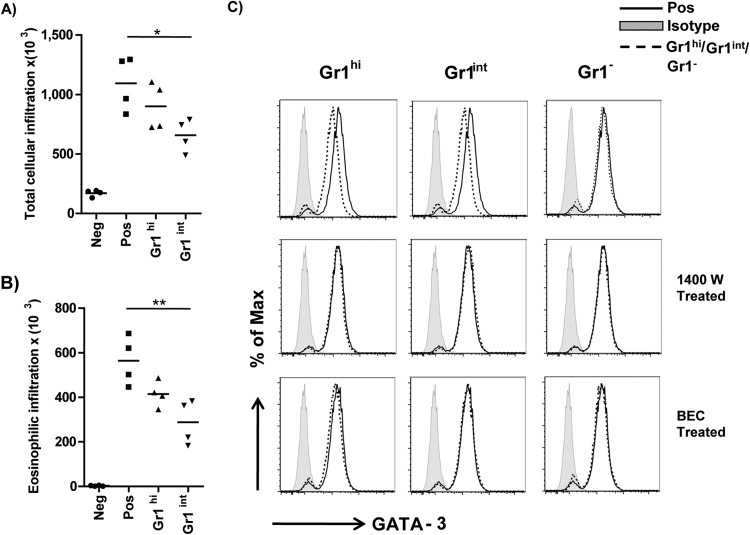
As coculture of Th2 cells with CD11b+ Gr1+ cells demonstrated down-modulation of key Th2 factors (GATA-3 and IL-4), we investigated the potential of these Th2 cells to induce allergic airway inflammation. After 5 days of coculture, equal numbers of FACS-sorted Th2 cells were adoptively transferred into recipient BALB/c mice. Non cocultured, FACS-sorted Th2 cells were transferred in the control mice. Except for the negative-control group, all mice were challenged with 30 μg of OVA (grade V) for two consecutive days to induce airway inflammation. In contrast to the positive-control (Pos) group of mice that received noncocultured Th2 cells, mice that received Th2 cells cocultured with CD11b+ Gr1hi cells demonstrated reduced total cellular (Fig. 5A) and eosinophilic infiltration in BAL fluid (Fig. 5B). Th2 cells cocultured with CD11b+ Gr1int cells were used as positive controls and demonstrated a significant inhibition of cellular infiltration in BAL fluid.
FIG 5.
Downmodulation of GATA-3 expression was mediated in an arginase- and NO-dependent manner, and adoptive transfer of the cocultured Th2 cells resulted in reduced airway eosinophilia. Th2 cells cocultured with CD11b+ Gr1hi and CD11b+ Gr1int myeloid cells were sorted and adoptively transferred into mice to evaluate their ability to induce allergic airway inflammation. The Neg- and Pos-control group mice received only noncocultured Th2 cells. Total cellular (A) and eosinophilic (B) infiltration into the BAL fluid was determined in the various groups. Data are represented as means and are representative of three individual experiments with 3 to 4 mice per group. (C) In order to delineate the mechanism by which these myeloid cells modulate the downregulation of GATA-3 expression, the coculture experiments were carried out in the presence of a nitric oxide synthase inhibitor (1400W) or an arginase inhibitor (BEC). Th2 cells cocultured in the absence of any inhibitors were used as a control (top panel). The FACS histograms represent the data from three independent experiments. An ANOVA test was used to determine statistical significance. Max, maximum. *, P < 0.05; **, P <0.01.
Mechanism mediating the down-modulation of GATA-3 expression in Th2 cells.
Previous studies have indicated the ability of these myeloid cells to induce the suppression of T cells by mechanisms mediated by either nitric oxide (NO), arginase I (ArgI), or reactive oxygen species (22, 23). The study by Arora et al. demonstrated the ability of CD11b+ Gr1int cells to induce down-modulation of GATA-3 expression by Th2 cells in an arginase- and IL-10-dependent manner (20). Hence, by using specific inhibitors for the enzymes nitric oxide synthase (NOS) (N-(3-aminomethyl)benzylacetamidine [1400W]) and arginase [(S)-(2-boronoethyl-l-cysteine), or BEC], we tried to ascertain whether these enzymes influenced the expression of GATA-3 expression in Th2 cells upon coculture with CD11b+ Gr1hi cells. In the absence inhibitors, coculture with myeloid cells demonstrated reduced production of GATA-3 in Th2 cells (Fig. 5C, top row). In contrast, coculture in either the presence of a NOS inhibitor (1400W) (Fig. 5C, middle row) or arginase inhibitor (BEC) (Fig. 5C, bottom row) prevented the down-modulation of GATA-3 expression in Th2 cells, implying a role for both nitric oxide synthase and arginase in the mechanism mediated by myeloid cells in influencing the Th2 cells.
DISCUSSION
A number of investigations involving the administration of pathogens in mouse models have demonstrated moderation of manifestations of atopic asthma, such as lung inflammation, mucus production, and airway eosinophilia. The species, strain, formulation, and route of application of the pathogen are critical in shaping the type of immune response being generated. Studies involving bacteria or helminths suggested that the mechanism ameliorating allergic disorders are mediated by either a diversion toward a Th1 response or by expansion of regulatory T cells. Even subtypes of a pathogen have been shown to differ in their abilities and mechanisms to induce suppression of airway inflammation (24). While Mycobacterium vaccae induces Treg cells to mediate the inhibition of airway inflammation, Mycobacterium bovis treatment results in a similar outcome but by inducing a Th1 response (10, 25). Epidemiological and experimental evidence has established the immunomodulatory properties of S. Typhimurium in attenuating allergic responses, but the immune response mediating the amelioration still remained elusive (15). Depending upon the strain of Salmonella, an infection can either cause enterocolitis, which remains localized to the intestine and produces disease symptoms within a period of 12 to 72 h, or typhoid fever, which is a systemic infection and manifests only after a median incubation period of 5 to 9 days. The inflammatory responses induced vary between the two diseases as typhoid fever is characterized by a slowly developing infiltrate composed predominantly of mononuclear inflammatory cells, while in enterocolitis the immune response involves a rapidly developing infiltrate consisting predominantly of neutrophils (26, 27). In this study, using the typhoid fever model regimen adopted from Wu et al., we demonstrate the potential mechanism mediated by an oral infection with the Salmonella Typhimurium aroA strain (SL 7207) in reducing lung inflammation in an antigen-sensitized and challenged mouse. The reduced virulence of the auxotrophic mutant and its ability to induce an innate immune response similar to that of a wild-type strain (28) were the reasons for employing this mutant strain of Salmonella Typhimurium for our study.
In accordance with previously published results, we also observed elevated titers of allergen-specific serum IgG2a and a reduction in secretion of IL-4, indicating a possible shift toward a Th1 response. The signature Th2 cytokine IL-4 has been implicated in the differentiation of goblet cells, expression of mucin (MUC5AC) by epithelial cells, differentiation of T lymphocytes, and recruitment of eosinophils (29, 30). Concomitantly, in our study the reduction in IL-4 secretion correlates with decreased eosinophil infiltration and mucus production. However, unlike secretion of IL-4, there was no alteration in either IL-5 or IL-13 production in the infected group of mice. Previous clinical evidence (31), along with in vitro (32) and in vivo (33) studies, has suggested a probable differential regulation in the production of Th2 cytokines though the exact cause remains unknown. Furthermore, recently IL-5 production was shown to be predominantly confined to a subset of IL-13-expressing CD4+ cells, suggesting that IL-13 and IL-5 share similar regulatory pathways that may be distinct from the pathway of IL-4 (34). Surprisingly, there was no increase in the levels of the Th1 cytokine IFN-γ or any significant reduction in antigen-specific IgG1 in the S. Typhimurium-infected group of mice. Additionally, intracellular cytokine profiling of T cells also could not affirm an increase in IFN-γ-producing cell numbers, suggesting that this amelioration is not due to an apparent Th1-diverted mechanism.
A number of similar investigations involving the administration of pathogens orally emphasize the importance of complex interactions occurring in the gastrointestinal milieu, resulting in the shaping of a tolerogenic immune response against environmental antigens (24, 35). Constituting a nonredundant immunoregulatory cell population, Treg cells execute a crucial role in maintenance of immune homeostasis (36). Numerous studies have confirmed the decisive contribution of Treg cells in ameliorating allergic disorders (10, 17, 24). Colonization of the intestinal tract by commensal bacteria like Enterobacteriaceae, Clostridium, and Bifidobacterium has been shown to induce Treg cells, resulting in the prevention of inflammatory bowel disease and maintenance of mucosal tolerance (37, 38). In contrast to certain commensal bacteria and helminths and in accordance with other infection models like Acinetobacter lwoffii F78 and Acinetobacter baumannii, S. Typhimurium infection exhibited no demonstrable change in the frequencies and numbers of Foxp3+ Treg cells (12, 13). Subsequently, we detected a considerable increase in a population of cells expressing CD11b and Gr1 in the spleens and lungs of mice infected with S. Typhimurium. There was a significant increase in CD11b+ Gr1hi cells and a moderate increase in the frequencies and absolute numbers of CD11b+ Gr1int cells in the S. Typhimurium-infected mice. These CD11b+ Gr1+ cells are comprised of a heterogeneous population of cells and have been predominantly studied in the context of cancer (39–41), and their induction and function in many other diseases and infection models are being currently investigated. The profiles of these cells have been previously characterized into either a granulocytic or monocytic phenotype based on the expression of Gr1, Ly6G, and Ly6C (42). Similar to studies involving helminths and tumors, infection with S. Typhimurium was also shown to induce nonspecific immunosuppression mediated by macrophage precursors in a nitric oxide-dependent mechanism (43, 44). These cells have been predominantly associated with inhibition of immune responses by using free radicals such as nitric oxide (NO) and by secreting immunoregulatory cytokines (45, 46). Recent investigations have indicated their ability to modulate Th2-mediated responses (47). The study by Arora et al. indicated that lipopolysaccharide (LPS)-induced CD11b+ Gr1int cells in the lungs affected Th2 cell stability and could prevent allergic airway inflammation. These populations of cells that were induced in a MyD88- and TLR4-dependent manner have been shown to inhibit airway inflammation in an IL-10- and arginase I-dependent manner (20). Additionally production of nitric oxide synthase (NOS) (48) and IFN-γ by these cells has also been attributed to their suppressive ability (20, 49). However, administration of LPS can also result in aggravation of asthma, indicating that the dosage of LPS is critical in determining the type of response generated (50). In contrast, Delano et al. demonstrated using a sepsis model that elevated levels of CD11b+ Gr1+ cells in the spleen resulted in enhanced Th2 cell polarization (21). These cells were induced in an MyD88-dependent but TLR4-independent mechanism unlike those in the lung. With contrasting results pertaining to similar cells isolated from different target organs, our objective was to understand the role of CD11b+ Gr1+ cells in the spleen expanded upon S. Typhimurium infection. Using in vitro coculture systems, we demonstrated that under Th2-polarizing conditions, the myeloid cells isolated ex vivo from the spleens of S. Typhimurium-infected mice did not influence the differentiation of naive T cells into a Th2 phenotype. However, they considerably destabilized already differentiated Th2 cells by down-modulating the expression of the key transcription factor GATA-3 and the production of IL-4 in these cells. Subsequently, using a Th2 adoptive transfer model to induce airway inflammation, we demonstrate that Th2 cells cocultured with CD11b+ Gr1+ cells had reduced effector function, as demonstrated by the attenuated cellular infiltration in BAL fluid compared to that in the control group receiving noncocultured Th2 cells. Even though the CD11b+ Gr1int cells demonstrated a relatively stronger influence on cellular infiltration, the significant increase in CD11b+ Gr1hi cell numbers upon Salmonella infection could simulate comparable influences in vivo. Finally, using inhibitors, we determined that both arginase (Arg) and nitric oxide synthase (NOS) contributed to the regulatory mechanism mediated by both CD11b+ Gr1int and CD11b+ Gr1hi cells in down-modulating GATA-3 expression in Th2 cells. Recent findings have indicated the possibility of these subsets using different mechanisms as the granulocytic subset was found to express low levels of NO, whereas the monocytic subset expressed high levels of NO. However, both the subsets also expressed arginase I (51). Though Arg and NOS are competitively regulated by Th1 and Th2 cytokines, LPS, which is commonly referred to as a Th1 cytokine inducer, has been demonstrated to activate the expression of both of the enzymes (52, 53). The myeloid cells therefore induced upon an infection with Salmonella may be a collection of different cells that are capable of expressing either NOS or Arg. Collectively, from available data on CD11b+ Gr1int cells and our data on CD11b+ Gr1hi cells, we can speculate that the expansion of CD11b+ Gr1hi and CD11b+ Gr1int cells upon S. Typhimurium infection could synergistically orchestrate amelioration of allergic airway inflammation by influencing the stability of Th2 cells and suppressing their effector function.
ACKNOWLEDGMENTS
We thank Maxine Swallow and Friederike Kruse for maintaining mouse colonies and technical assistance. We thank Aline Sandouk for proofreading the manuscript.
We declare that we have no financial or commercial conflicts of interest.
This work was supported by Deutsche Forschungsgemeinschaft (DFG) grant SFB587. V.G. and C.H. were supported by Hanover Biomedical Research School Molecular Medicine and GRK 1441 programs, respectively. C.F. was supported by the DFG.
Footnotes
Published ahead of print 16 December 2013
REFERENCES
- 1.Bach JF. 2002. The effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 347:911–920. 10.1056/NEJMra020100 [DOI] [PubMed] [Google Scholar]
- 2.Wills-Karp M, Santeliz J, Karp CL. 2001. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat. Rev. Immunol. 1:69–75. 10.1038/35095579 [DOI] [PubMed] [Google Scholar]
- 3.Yazdanbakhsh M, Kremsner PG, van Ree R. 2002. Allergy, parasites, and the hygiene hypothesis. Science 296:490–494. 10.1126/science.296.5567.490 [DOI] [PubMed] [Google Scholar]
- 4.Kumar M, Behera AK, Matsuse H, Lockey RF, Mohapatra SS. 1999. A recombinant BCG vaccine generates a Th1-like response and inhibits IgE synthesis in BALB/c mice. Immunology 97:515–521. 10.1046/j.1365-2567.1999.00782.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeung VP, Gieni RS, Umetsu DT, DeKruyff RH. 1998. Heat-killed Listeria monocytogenes as an adjuvant converts established murine Th2-dominated immune responses into Th1-dominated responses. J. Immunol. 161:4146–4152 [PubMed] [Google Scholar]
- 6.Berod L, Puttur F, Huehn J, Sparwasser T. 2012. Tregs in infection and vaccinology: heroes or traitors? Microb. Biotechnol. 5:260–269. 10.1111/j.1751-7915.2011.00299.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold IC, Dehzad N, Reuter S, Martin H, Becher B, Taube C, Muller A. 2011. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J. Clin. Invest. 121:3088–3093. 10.1172/JCI45041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grainger JR, Smith KA, Hewitson JP, McSorley HJ, Harcus Y, Filbey KJ, Finney CA, Greenwood EJ, Knox DP, Wilson MS, Belkaid Y, Rudensky AY, Maizels RM. 2010. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-beta pathway. J. Exp. Med. 207:2331–2341. 10.1084/jem.20101074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lagranderie M, Abolhassani M, Vanoirbeek JA, Lima C, Balazuc AM, Vargaftig BB, Marchal G. 2010. Mycobacterium bovis bacillus Calmette-Guerin killed by extended freeze-drying targets plasmacytoid dendritic cells to regulate lung inflammation. J. Immunol. 184:1062–1070. 10.4049/jimmunol.0901822 [DOI] [PubMed] [Google Scholar]
- 10.Zuany-Amorim C, Sawicka E, Manlius C, Le Moine A, Brunet LR, Kemeny DM, Bowen G, Rook G, Walker C. 2002. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nat. Med. 8:625–629. 10.1038/nm0602-625 [DOI] [PubMed] [Google Scholar]
- 11.Wilson MS, Taylor MD, Balic A, Finney CA, Lamb JR, Maizels RM. 2005. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J. Exp. Med. 202:1199–1212. 10.1084/jem.20042572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu H, Kuolee R, Harris G, Zhou H, Miller H, Patel GB, Chen W. 2011. Acinetobacter baumannii infection inhibits airway eosinophilia and lung pathology in a mouse model of allergic asthma. PLoS One 6:e22004. 10.1371/journal.pone.0022004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conrad ML, Ferstl R, Teich R, Brand S, Blumer N, Yildirim AO, Patrascan CC, Hanuszkiewicz A, Akira S, Wagner H, Holst O, von Mutius E, Pfefferle PI, Kirschning CJ, Garn H, Renz H. 2009. Maternal TLR signaling is required for prenatal asthma protection by the nonpathogenic microbe Acinetobacter lwoffii F78. J. Exp. Med. 206:2869–2877. 10.1084/jem.20090845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelosi U, Porcedda G, Tiddia F, Tripodi S, Tozzi AE, Panetta V, Pintor C, Matricardi PM. 2005. The inverse association of salmonellosis in infancy with allergic rhinoconjunctivitis and asthma at school-age: a longitudinal study. Allergy 60:626–630. 10.1111/j.1398-9995.2005.00747.x [DOI] [PubMed] [Google Scholar]
- 15.Wu CJ, Chen LC, Kuo ML. 2006. Attenuated Salmonella typhimurium reduces ovalbumin-induced airway inflammation and T-helper type 2 responses in mice. Clin. Exp. Immunol. 145:116–122. 10.1111/j.1365-2249.2006.03099.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heithoff DM, Enioutina EY, Bareyan D, Daynes RA, Mahan MJ. 2008. Conditions that diminish myeloid-derived suppressor cell activities stimulate cross-protective immunity. Infect. Immun. 76:5191–5199. 10.1128/IAI.00759-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baru AM, Hartl A, Lahl K, Krishnaswamy JK, Fehrenbach H, Yildirim AO, Garn H, Renz H, Behrens GM, Sparwasser T. 2010. Selective depletion of Foxp3+ Treg during sensitization phase aggravates experimental allergic airway inflammation. Eur. J. Immunol. 40:2259–2266. 10.1002/eji.200939972 [DOI] [PubMed] [Google Scholar]
- 18.Baru AM, Ganesh V, Krishnaswamy JK, Hesse C, Untucht C, Glage S, Behrens G, Mayer CT, Puttur F, Sparwasser T. 2012. Absence of Foxp3+ regulatory T cells during allergen provocation does not exacerbate murine allergic airway inflammation. PLoS One 7:e47102. 10.1371/journal.pone.0047102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kittel B, Ruehl-Fehlert C, Morawietz G, Klapwijk J, Elwell MR, Lenz B, O'Sullivan MG, Roth DR, Wadsworth PF, RITA Group, NACAD Group 2004. Revised guides for organ sampling and trimming in rats and mice—part 2. A joint publication of the RITA and NACAD groups. Exp. Toxicol. Pathol. 55:413–431 [DOI] [PubMed] [Google Scholar]
- 20.Arora M, Poe SL, Oriss TB, Krishnamoorthy N, Yarlagadda M, Wenzel SE, Billiar TR, Ray A, Ray P. 2010. TLR4/MyD88-induced CD11b+ Gr-1int F4/80+ non-migratory myeloid cells suppress Th2 effector function in the lung. Mucosal Immunol. 3:578–593. 10.1038/mi.2010.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O'Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, Swan R, Chung CS, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, Laface D, Heyworth PG, Clare-Salzler M, Moldawer LL. 2007. MyD88-dependent expansion of an immature GR-1+ CD11b+ population induces T cell suppression and Th2 polarization in sepsis. J. Exp. Med. 204:1463–1474. 10.1084/jem.20062602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deshane J, Zmijewski JW, Luther R, Gaggar A, Deshane R, Lai JF, Xu X, Spell M, Estell K, Weaver CT, Abraham E, Schwiebert LM, Chaplin DD. 2011. Free radical-producing myeloid-derived regulatory cells: potent activators and suppressors of lung inflammation and airway hyperresponsiveness. Mucosal Immunol. 4:503–518. 10.1038/mi.2011.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, Zanovello P, Segal DM. 2002. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J. Immunol. 168:689–695 [DOI] [PubMed] [Google Scholar]
- 24.Forsythe P, Inman MD, Bienenstock J. 2007. Oral treatment with live Lactobacillus reuteri inhibits the allergic airway response in mice. Am. J. Respir. Crit. Care. Med. 175:561–569. 10.1164/rccm.200606-821OC [DOI] [PubMed] [Google Scholar]
- 25.Hopfenspirger MT, Agrawal DK. 2002. Airway hyperresponsiveness, late allergic response, and eosinophilia are reversed with mycobacterial antigens in ovalbumin-presensitized mice. J. Immunol. 168:2516–2522 [DOI] [PubMed] [Google Scholar]
- 26.Zhang S, Kingsley RA, Santos RL, Andrews-Polymenis H, Raffatellu M, Figueiredo J, Nunes J, Tsolis RM, Adams LG, Baumler AJ. 2003. Molecular pathogenesis of Salmonella enterica serotype Typhimurium-induced diarrhea. Infect. Immun. 71:1–12. 10.1128/IAI.71.1.1-12.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raffatellu M, Wilson RP, Winter SE, Baumler AJ. 2008. Clinical pathogenesis of typhoid fever. J. Infect. Dev. Ctries. 2:260–266 [DOI] [PubMed] [Google Scholar]
- 28.VanCott JL, Chatfield SN, Roberts M, Hone DM, Hohmann EL, Pascual DW, Yamamoto M, Kiyono H, McGhee JR. 1998. Regulation of host immune responses by modification of Salmonella virulence genes. Nat. Med. 4:1247–1252. 10.1038/3227 [DOI] [PubMed] [Google Scholar]
- 29.Dabbagh K, Takeyama K, Lee HM, Ueki IF, Lausier JA, Nadel JA. 1999. IL-4 induces mucin gene expression and goblet cell metaplasia in vitro and in vivo. J. Immunol. 162:6233–6237 [PubMed] [Google Scholar]
- 30.Temann UA, Prasad B, Gallup MW, Basbaum C, Ho SB, Flavell RA, Rankin JA. 1997. A novel role for murine IL-4 in vivo: induction of MUC5AC gene expression and mucin hypersecretion. Am. J. Respir. Cell Mol. Biol. 16:471–478. 10.1165/ajrcmb.16.4.9115759 [DOI] [PubMed] [Google Scholar]
- 31.Kagi MK, Wuthrich B, Montano E, Barandun J, Blaser K, Walker C. 1994. Differential cytokine profiles in peripheral blood lymphocyte supernatants and skin biopsies from patients with different forms of atopic dermatitis, psoriasis and normal individuals. Int. Arch. Allergy Immunol. 103:332–340. 10.1159/000236651 [DOI] [PubMed] [Google Scholar]
- 32.Bohjanen PR, Okajima M, Hodes RJ. 1990. Differential regulation of interleukin 4 and interleukin 5 gene expression: a comparison of T-cell gene induction by anti-CD3 antibody or by exogenous lymphokines. Proc. Natl. Acad. Sci. U. S. A. 87:5283–5287. 10.1073/pnas.87.14.5283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheever AW, Finkelman FD, Caspar P, Heiny S, Macedonia JG, Sher A. 1992. Treatment with anti-IL-2 antibodies reduces hepatic pathology and eosinophilia in Schistosoma mansoni-infected mice while selectively inhibiting T cell IL-5 production. J. Immunol. 148:3244–3248 [PubMed] [Google Scholar]
- 34.Liang HE, Reinhardt RL, Bando JK, Sullivan BM, Ho IC, Locksley RM. 2012. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat. Immunol. 13:58–66. 10.1038/ni.2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navarro S, Cossalter G, Chiavaroli C, Kanda A, Fleury S, Lazzari A, Cazareth J, Sparwasser T, Dombrowicz D, Glaichenhaus N, Julia V. 2011. The oral administration of bacterial extracts prevents asthma via the recruitment of regulatory T cells to the airways. Mucosal Immunol. 4:53–65. 10.1038/mi.2010.51 [DOI] [PubMed] [Google Scholar]
- 36.Mayer CT, Berod L, Sparwasser T. 2012. Layers of dendritic cell-mediated T cell tolerance, their regulation and the prevention of autoimmunity. Front. Immunol. 3:183. 10.3389/fimmu.2012.00183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeon SG, Kayama H, Ueda Y, Takahashi T, Asahara T, Tsuji H, Tsuji NM, Kiyono H, Ma JS, Kusu T, Okumura R, Hara H, Yoshida H, Yamamoto M, Nomoto K, Takeda K. 2012. Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon. PLoS Pathog. 8:e1002714. 10.1371/journal.ppat.1002714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K. 2011. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331:337–341. 10.1126/science.1198469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsui A, Yokoo H, Negishi Y, Endo-Takahashi Y, Chun NA, Kadouchi I, Suzuki R, Maruyama K, Aramaki Y, Semba K, Kobayashi E, Takahashi M, Murakami T. 2012. CXCL17 expression by tumor cells recruits CD11b+ Gr1high F4/80− cells and promotes tumor progression. PLoS One 7:e44080. 10.1371/journal.pone.0044080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao L, Lim SY, Gordon-Weeks AN, Tapmeier TT, Im JH, Cao Y, Beech J, Allen D, Smart S, Muschel RJ. 2013. Recruitment of a myeloid cell subset (CD11b/Gr1mid) via CCL2/CCR2 promotes the development of colorectal cancer liver metastasis. Hepatology 57:829–839. 10.1002/hep.26094 [DOI] [PubMed] [Google Scholar]
- 41.Woller N, Knocke S, Mundt B, Gurlevik E, Struver N, Kloos A, Boozari B, Schache P, Manns MP, Malek NP, Sparwasser T, Zender L, Wirth TC, Kubicka S, Kuhnel F. 2011. Virus-induced tumor inflammation facilitates effective DC cancer immunotherapy in a Treg-dependent manner in mice. J. Clin. Invest. 121:2570–2582. 10.1172/JCI45585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. 2008. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 111:4233–4244. 10.1182/blood-2007-07-099226 [DOI] [PubMed] [Google Scholar]
- 43.al-Ramadi BK, Brodkin MA, Mosser DM, Eisenstein TK. 1991. Immunosuppression induced by attenuated Salmonella. Evidence for mediation by macrophage precursors. J. Immunol. 146:2737–2746 [PubMed] [Google Scholar]
- 44.al-Ramadi BK, Meissler JJ, Jr, Huang D, Eisenstein TK. 1992. Immunosuppression induced by nitric oxide and its inhibition by interleukin-4. Eur. J. Immunol. 22:2249–2254. 10.1002/eji.1830220911 [DOI] [PubMed] [Google Scholar]
- 45.Greifenberg V, Ribechini E, Rossner S, Lutz MB. 2009. Myeloid-derived suppressor cell activation by combined LPS and IFN-gamma treatment impairs DC development. Eur. J. Immunol. 39:2865–2876. 10.1002/eji.200939486 [DOI] [PubMed] [Google Scholar]
- 46.Rossner S, Voigtlander C, Wiethe C, Hanig J, Seifarth C, Lutz MB. 2005. Myeloid dendritic cell precursors generated from bone marrow suppress T cell responses via cell contact and nitric oxide production in vitro. Eur. J. Immunol. 35:3533–3544. 10.1002/eji.200526172 [DOI] [PubMed] [Google Scholar]
- 47.Pesce JT, Ramalingam TR, Mentink-Kane MM, Wilson MS, El Kasmi KC, Smith AM, Thompson RW, Cheever AW, Murray PJ, Wynn TA. 2009. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 5:e1000371. 10.1371/journal.ppat.1000371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodriguez D, Keller AC, Faquim-Mauro EL, de Macedo MS, Cunha FQ, Lefort J, Vargaftig BB, Russo M. 2003. Bacterial lipopolysaccharide signaling through Toll-like receptor 4 suppresses asthma-like responses via nitric oxide synthase 2 activity. J. Immunol. 171:1001–1008 [DOI] [PubMed] [Google Scholar]
- 49.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, Bicciato S, Bronte V. 2006. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J. Clin. Invest. 116:2777–2790. 10.1172/JCI28828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. 2002. Lipopolysaccharide-enhanced, Toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J. Exp. Med. 196:1645–1651. 10.1084/jem.20021340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. 2008. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol. 181:5791–5802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bogdan C. 2001. Nitric oxide and the immune response. Nat. Immunol. 2:907–916. 10.1038/ni1001-907 [DOI] [PubMed] [Google Scholar]
- 53.Bronte V, Serafini P, Mazzoni A, Segal DM, Zanovello P. 2003. L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol. 24:302–306. 10.1016/S1471-4906(03)00132-7 [DOI] [PubMed] [Google Scholar]