Abstract
Antibodies m66.6 and 2F5 are the only effective human HIV-1-neutralizing antibodies reported thus far to recognize the N-terminal region of the membrane-proximal external region (MPER) of the gp41 subunit of the HIV-1 envelope glycoprotein. Although 2F5 has been extensively characterized, much less is known about antibody m66.6 or antibody m66, a closely related light-chain variant. Here, we report the crystal structure of m66 in complex with its gp41 epitope, along with unbound structures of m66 and m66.6. We used mutational and binding analyses to decipher antibody elements critical for their recognition of gp41 and determined the molecular basis that underlies their neutralization of HIV-1. When bound by m66, the N-terminal region of the gp41 MPER adopts a conformation comprising a helix, followed by an extended loop. Comparison of gp41-bound m66 to unbound m66.6 identified three light-chain residues of m66.6 that were confirmed through mutagenesis to underlie the greater breadth of m66.6-mediated virus neutralization. Recognition of gp41 by m66 also revealed similarities to antibody 2F5 both in the conformation of crucial epitope residues as well as in the angle of antibody approach. Aromatic residues at the tip of the m66.6 heavy-chain third complementarity-determining region, as in the case of 2F5, were determined to be critical for virus neutralization in a manner that correlated with antibody recognition of the MPER in a lipid context. Antibodies m66, m66.6, and 2F5 thus utilize similar mechanistic elements to recognize a common gp41-MPER epitope and to neutralize HIV-1.
INTRODUCTION
The membrane-proximal external region (MPER) of the gp41 subunit of the HIV-1 transmembrane glycoprotein is one of four primary sites of vulnerability to neutralizing antibodies on the HIV-1 envelope spike (reviewed in reference 1). Comprised of a stretch of ∼25 highly conserved residues immediately upstream of the gp41 transmembrane domain, the MPER is rich in hydrophobic amino acids and plays a critical role in viral infectivity (2–5). Analyses of sera from different cohorts of HIV-infected individuals suggest that the prevalence of patients with MPER-specific neutralizing antibodies can reach up to 30%, although these levels may be unique to the cohorts analyzed (6, 7). To date, only seven human monoclonal antibodies that neutralize HIV-1 through the gp41 MPER have been reported: 2F5, m66, m66.6, z13e1, 4E10, CH12, and most recently 10E8 (1, 6, 8–12). Of this relatively small group of antibodies, 2F5 and the closely related antibodies m66 and m66.6 target the N-terminal region of the MPER (spanning residues 656 to 670 of gp41, HxB2 numbering), while z13e1, 4E10, CH12, and 10E8 target its C-terminal region (spanning residues 668 to 683). Several features have come to characterize neutralizing antibodies that target the gp41 MPER, including recognition of fusion-intermediate states of envelope and the capacity to recognize and extract epitopes from the viral membrane (13–19). In the case of antibody 2F5, hydrophobic residues within its heavy-chain third complementarity-determining region (CDR H3) have been shown to be critical for 2F5-mediated viral neutralization, predominantly through interactions with the viral membrane (20, 21).
While the MPER is highly conserved in sequence across known HIV-1 strains, structures of MPER in unliganded or antibody-bound states reveal that it can adopt a variety of conformations, ranging from extended loops to helices, with the latter being the predominant form it assumes when unbound (6, 15, 22–25). Whether due to inherent functional conformational plasticity of gp41 or to induced antibody constraints, the differences observed between the limited available antibody-bound structures of the MPER have made definition of conserved structural determinants within this region difficult. Nevertheless, the recent finding that the 10E8 antibody targets a conserved α-helix in the C-terminal region of the MPER—one also recognized by antibody 4E10—suggests that the MPER C-terminal region might indeed possess a structurally conserved neutralizing determinant (6, 23). Identification of a similar determinant within the N-terminal region of the MPER has been more difficult since available antibody-bound structural information has been limited to that bound by 2F5 (15, 25).
The isolation of the m66 antibody and of the closely related m66.6 antibody from a phage display antibody library of a donor with 2F5-like neutralizing serum activity provided the first additional examples of neutralizing antibodies that target the N-terminal region of the gp41 MPER, although with approximately half the neutralization breadth of 2F5 (24% for m66.6 versus 54% for 2F5) (8, 26). Antibodies m66 and m66.6 share identical heavy chains, based on VH precursor IGHV5-51*01, but differ by 7.8% in their IGKV1-39*01-based light chains. Both exhibit similar binding affinities to gp41 and show the same sensitivity to alanine mutations within the gp41 MPER, requiring the same five gp41 residues for recognition: Leu660gp41, Leu663gp41, Asp664gp41, Lys665gp41, and Trp666gp41 (HxB2 numbering). Three of these residues—Asp664gp41, Lys665gp41, and Trp666gp41—are also required by 2F5 for binding, consistent with the similarities observed in the virus neutralization fingerprints of m66.6 and 2F5 (9, 27). Although the disparate light chains of m66 and m66.6 do not have a great effect on their respective recognition of gp41, the two antibody variants do differ in a number of respects. m66 displays very low reactivity with autoantigens, whereas m66.6 is highly autoreactive (8). Likewise, m66.6 exhibits greater breadth of virus neutralization, neutralizing ∼10-fold as many HIV-1 strains as m66 (8). The molecular bases by which the distinct light chains of m66 and m66.6 confer these functional differences remain to be determined.
An understanding of antibodies that effectively neutralize HIV-1 has been sought with the goal of shedding light on regions of the virus that are susceptible to the humoral immune system. Comparative structural analyses of different antibodies that target the same region on the HIV-1 spike have the added potential to enable common critical features between the antibodies and their targets to be discerned. We applied this rationale to investigate the N-terminal region of the gp41 MPER. We determined the crystal structure of the antigen-binding fragment (Fab) of antibody m66 in complex with its gp41 MPER epitope, along with the unbound structures of m66 and m66.6. The structures led us to identify critical light-chain residues that were confirmed through mutagenesis to be the molecular basis for the superior neutralization properties of m66.6. Comparison of m66 recognition of gp41 with that of 2F5 revealed structural and mechanistic similarities critical for recognition of MPER in a lipid context and for HIV-1 neutralization. These results provide insight into the N-terminal region of the MPER—one of the few regions on the HIV-1 virus susceptible to antibody—and also shed light on how antibodies are able to access this site and effectively neutralize the virus.
MATERIALS AND METHODS
Antibody expression and purification.
Codon-optimized genes corresponding to the m66 heavy chain and the m66 and m66.6 light chains were synthesized with a 5′ secretion leader sequence (Life Technologies) and subcloned into the mammalian expression vector pVRC-8400. m66 heavy- and light-chain complementarity-determining region (CDR) variants were produced through mutagenesis of template wild-type heavy and light chains (Geneimmune, Inc.). Transient expression of the antibodies and variants was undertaken in 293F cells or 293 Expi cells in accordance with the manufacturer's instruction (Life Technologies) by cotransfection of equal amounts of the respective heavy- and light-chain plasmids. Antibody IgGs were purified from expression supernatants by capture with protein A-Sepharose (Pierce), followed by elution with low-pH elution buffer (Pierce) and adjustment of the eluate pH using Tris-Cl (pH 8.0).
Antibody antigen binding fragments (Fabs).
The antigen binding fragments of m66 and m66.6 were prepared using endoproteinase Lys-C (Roche) digestion as previously described (15, 28). In general, the protease was added at concentrations of 1:1,000 to 1:10,000, and digestion was undertaken at 37°C for 4 to 6 h. Protease digestion reactions were stopped with protease inhibitor tablets (Roche) and cleaved products run over a protein A column to remove the Fc fragment. Antibody Fabs were then subjected to cation exchange (Mono-S) and size exclusion chromatography (Superdex 200) and then dialyzed into 150 mM NaCl–2.5 mM Tris-Cl (pH 7.5)–0.02% NaN3 and concentrated to A280 optical densities of >10 for crystallization.
Crystallization, structure determination, and structural analysis.
Purified m66 and m66.6 Fabs were set up in crystallization screens either in the presence or absence of excess gp41 MPER peptides. 20°C vapor diffusion sitting drop crystallizations of 576 initial conditions adapted from the commercially available Hampton (Hampton Research), Precipitant Synergy (Emerald Biosystems), and Wizard (Emerald Biosystems) crystallization screens were set up with use of a Honeybee 963 robot. Crystal trays were imaged with a Rockimager (Formulatrix), and crystal hits were optimized manually. Crystals of the m66 Fab in complex with a peptide corresponding to gp41 residues 651 to 669 (HxB2 numbering; NQQKRNEQELLELDKWASL; American Peptide, CA) were obtained in a condition comprised of 25% isopropanol, 15 to 20% polyethylene glycol 3350, and 0.2 M ammonium citrate (pH 7.5). Diffraction to a 2.21-Å resolution was obtained in a cryoprotectant composed of mother liquor supplemented with 15% 2R-3R-butanediol and excess peptide. Crystals of free m66 Fab were obtained in 52.5% polyethylene glycol 400, 0.2 M calcium acetate, 0.1 M acetate (pH 4.5). Diffraction to 3.14 Å was obtained in cryoprotectant composed of 59.5% polyethylene glycol 400, 0.17 M calcium acetate, 0.04 M acetate (pH 4.5) supplemented with 7.5% 2R-3R-butanediol and 15% ethylene glycol. Crystals of free m66.6 Fab were obtained in 11% polyethylene glycol 8000, 0.05 M zinc acetate, and 0.05 M sodium cacodylate (pH 6.5). When cryoprotected in 15% polyethylene glycol 8000, 0.075 M zinc acetate, and 0.1 M sodium cacodylate (pH 6.5), supplemented with 15% 2R-3R-butanediol, diffraction to 2.43 Å was obtained. Diffraction data were collected at SER CAT ID-22 or BM-22 beamlines of the Advanced Photon Source (Argonne, IL) and processed using HKL-2000 software (29). All structures were solved with molecular replacement using Phaser (30). The free structure of m66 was solved with search model PDB ID 7FAB, as well as with a threaded Swiss-model of m66 (http://www.expasy.org/spdbv/) (31). All subsequent structures utilized the free structure of m66 as a search model. Refinement of all structures was undertaken with Phenix (32), with iterative model building in Coot (33). Structures were validated with MolProbity (34) and then analyzed using APBS for electrostatics (35), Ligplot for direct contacts (36), PISA for buried surface areas and residues (37), and lsqkab for RMSD alignments (ccp4 Package) (38). All graphics were prepared using PyMOL (PyMOL Molecular Graphics System).
Neutralization assays.
Neutralization of the monoclonal antibodies was measured by using single-round HIV-1 Env-pseudovirus infection of TZM-bl cells (39). HIV-1 Env pseudoviruses were generated by cotransfection of 293T cells with pSG3 delta Env backbone and a second plasmid that expressed HIV-1 Env. At 72 h after transfection, supernatants containing pseudovirus were harvested and frozen at −80°C until further use. In the neutralization assay, 10 μl of 5-fold serially diluted patient serum or monoclonal antibody was incubated with 40 μl of pseudovirus in a 96-well plate at 37°C for 30 min before the addition of TZM-bl cells. After 2 days of incubation, the cells were lysed, and the viral infectivity was quantified by measuring the luciferase activity with a Victor Light luminometer (Perkin-Elmer). The 50% inhibitory concentration (IC50) was calculated as the antibody concentration that reduced infection by 50%.
Liposomes.
Large multilamellar vesicles were synthesized using lipid ratios similar to that of the HIV membrane (40). Dried lipids from Avanti Polar Lipids, Inc. (Alabaster, AL), were resuspended in chloroform or chloroform-methanol to yield a final 9:9:18:20:45 molar ratio for 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC), 1,2-dioleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] (DOPG), egg sphingomyelin (SM), 1,2-dioleoyl-sn-glycero-3-phosphatidylethanolamine (DOPE), and cholesterol (CHOL), respectively. For liposomes containing the MPER epitope, the MPER659-683 peptide (ELLELDKWASLWNWFDITNWLWYIK) in chloroform was added. The lipid mixture was then dried under a nitrogen gas stream and stored under negative pressure overnight. Lipids were resuspended to yield a final MPER659-683 peptide concentration of 16 μM in 50 mM phospholipid.
Liposomal and soluble MPER competition ELISA.
The competition enzyme-linked immunosorbent assay (ELISA) was optimized for each individual antibody. In all experiments, samples were run in duplicate using 5% fetal bovine serum plus 2% dried milk in phosphate-buffered saline (PBS) ± 0.05% Tween 20 as a blocking buffer reagent and PBS ± 0.05% Tween 20 for washes. Mouse IgG antibody 1D4 was directly conjugated to a Nunc Maxisorp 96-well ELISA plate at a concentration of 2 ng/μl at a volume of 100 μl/well. Plates were incubated overnight at 4°C and washed the next day. The plate was then blocked with blocking buffer for 1 h at 25°C. 100 μl of an MPER657-670 peptide linked to a C9 tag (EQELLELDKWASLWGGTETSQVAPA) was added in 5× molar access excess in blocking buffer and incubated for 1 h at 25°C. Simultaneously, 100 μl of the antibody at a concentration of 0.05 μg/ml was added to each well of a 96-well nonadsorbing plate. In the MPER-liposome competition assay, MPER659-683 liposomes were added at 5-fold serial dilutions, starting at concentrations of 3.2 μM peptide (corresponding to 10 mM phospholipid). In the soluble peptide competition assay, a soluble MPER657-670 peptide (EQELLELDKWASLW) linked to Biotin was added at 5-fold serial dilutions starting at a concentration of 16 μM. The antibody-competitor mixture was then added to the washed ELISA plate that was captured with C9-tagged MPER peptide and incubated for 1 h at 25°C. After washing, 100 μl of mouse horseradish peroxidase (HRP)-conjugated anti-human Fc IgG was added at 1:10,000 dilution in blocking buffer to all wells. After 1 h of incubation at 25°C, the plate was washed, and a Bio-Rad HRP substrate detection kit was used to detect bound secondary antibody. The reaction was stopped by adding 0.5 N sulfuric acid, and optical densities were measured at 450 nm. In each case, experiments were run in duplicate.
Hydrophobicity analysis.
Calculations of the free energies of partitioning the m66/m66.6 antibody CDR H3 loops into a lipid bilayer interface were performed using MPEex 3.1 software (http://blanco.biomol.uci.edu/mpex/) (41, 42). Calculations were made using the Totalizer function with no end groups added and the ΔCONH value set at 0. Analysis included residues 98 to 100h of the m66/m66.6 CDR H3 loops.
Prediction of m66.6 neutralization resistance residues.
A neutralization-based epitope prediction method (43) was applied to search for possible additional gp41 residues that may be part of the m66.6 epitope outside the peptide region visualized in the crystal structure. Briefly, residue positions were scored by calculating the mutual information between the amino acid types at each residue position and the neutralization potency on a neutralization panel of 173 viral strains (8). Higher mutual information scores were deemed to indicate possible association between mutations at a given residue position and changes in neutralization susceptibility. For analysis of residue 651, which had the highest mutual information score within the range from residues 649 to 657 directly upstream of MPER, a K651N mutation was introduced into two m66.6-resistant strains (UG037.8 and 3168_V4_C10), and an N651K mutation was introduced into two m66.6-sensitive strains (CNE59 and H086.8) (44), and the resulting viruses then tested for neutralization by m66.6, m66, and variants thereof.
Data deposition and accession codes.
Atomic coordinate and structure factors for m66 in complex with HIV-1 gp41 MPER and for unbound structures of m66 and m66.6 have been deposited in the Protein Data Bank under accession numbers 4NRX, 4NRY, and 4NRZ.
RESULTS
Crystallizations and structure determination.
We undertook crystallizations of the m66 Fab in complex with a panel of gp41 MPER peptides. Cocrystals of m66 Fab in complex with a peptide encompassing gp41 MPER residues 651 to 669 (HxB2 numbering; NQQKRNEQELLELDKWASL), previously optimized for binding to both germ line and mature m66 (Z. Zhu and D. S. Dimitrov, unpublished data), were obtained in space group C2 and diffracted to a 2.21-Å resolution (Table 1). The complex structure was refined to Rcryst = 18.78% and Rfree = 21.52%, revealing three Fabs in the asymmetric unit—only two of which were visibly complexed to peptide. Crystals of unbound m66 Fab were also obtained, yielding diffraction to 3.14 Å in space group P2221. Two unbound m66 Fabs were observed in the asymmetric unit, and the structure was refined to Rcryst = 22.76% and Rfree = 25.57% (Table 1) (45).
TABLE 1.
Crystallographic data collection and refinement statistics
| Parameter | m66+gp41 MPER 651-669 | m66 | m66.6 |
|---|---|---|---|
| PDB ID | 4NRX | 4NRY | 4NRZ |
| Data collection statistics | |||
| Space group | C2 | P2221 | P21 |
| Cell constants | |||
| a, b, c (Å) | 197, 93.5, 89.8 | 98.5, 101, 167 | 59. 9, 118, 79.6 |
| α, β, γ (°) | 90.0, 112, 90.0 | 90.0, 90.0, 90.0 | 90.0 96.8 90.0 |
| Wavelength (Å) | 1.0000 | 1.0000 | 1.0000 |
| Resolution (Å) | 50–2.21 (2.25–2.21) | 50–3.14 (3.24–3.14) | 50–2.43 (2.47–2.43) |
| Rmerge | 0.125 (0.491) | 0.136 (0.521) | 0.156 (0.342) |
| Avg I/σ〈I〉 | 7.92 (2.03) | 10.6 (1.85) | 7.76 (2.55) |
| Completeness (%) | 97.1 (92.2) | 91.3 (50.3) | 85.7 (50.7) |
| Multiplicity | 3.4 (2.9) | 6.5 (4.3) | 4.0 (2.3) |
| Refinement statistics | |||
| Resolution range (Å) | 35.0–2.02 (2.04–2.02) | 44.8–3.11 (3.23–3.11) | 30.2–2.42 (2.49–2.42) |
| No. of unique reflections | 93,609 | 27,003 | 35,840 |
| Rwork/Rfree (%) | 18.78/21.52 | 22.76/25.57 | 17.33/21.70 |
| No. of atoms | 20,854 | 12,732 | 13,421 |
| Protein | 10,250 | 6,439 | 6,719 |
| Ligand/ion | 7 | ||
| Water | 600 | 112 | |
| B-factors (Å2) | 44.7 | 76.0 | 62.5 |
| Protein | 44.5 | 76.0 | 62.6 |
| Ligand/ion | 88.9 | ||
| Water | 47.6 | 53.7 | |
| RMSDs | |||
| Bond length (Å) | 0.009 | 0.003 | 0.008 |
| Bond angle (°) | 1.18 | 0.746 | 1.17 |
| Ramachandran plot (%) | |||
| Most favored regions | 97.74 | 96.84 | 97.57 |
| Additional allowed regions | 2.11 | 3.16 | 2.31 |
| Disallowed regions | 0.15 | 0 | 0.12 |
In parallel, crystallization trials of m66.6 Fab (which shares the same heavy chain as m66) (8) were also undertaken, both with or without gp41 peptide. Invariably, crystals obtained were of unbound m66.6 Fab, despite in most cases the presence of excess gp41 peptide. The lattices of the obtained crystals placed the combining region of m66.6 at lattice contacts that were incompatible with peptide binding. The crystal structure of m66.6 Fab grown in the absence of gp41 peptide was therefore chosen for the current analysis, obtained from crystals that diffracted to 2.43-Å resolution in space group P2 (Table 1). Two m66.6 Fabs were observed in the asymmetric unit, and the structure was refined to Rcryst = 17.33% and Rfree = 21.70%.
Overall structure of the m66-gp41 complex.
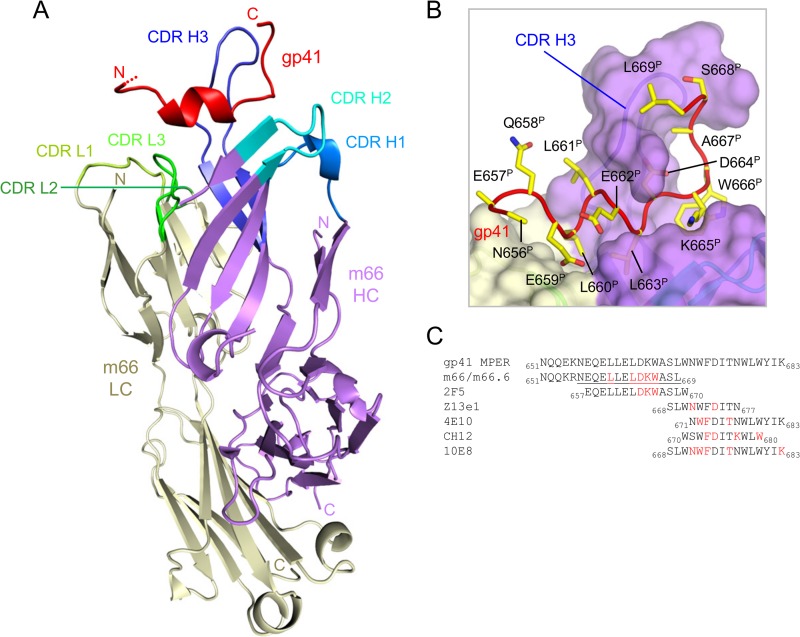
When bound by antibody m66 the N-terminal region of the gp41 MPER adopts a hybrid conformation composed of 310-helix followed by an extended loop (Fig. 1 and see Table S1 in the supplemental material). A high degree of structural homology in the conformations of the gp41 peptides in the two complexes of the asymmetric unit was observed (see Fig. S1A in the supplemental material), yielding Cα- and all-atom root mean square deviations (RMSDs) of 0.27 and 0.90 Å, respectively. Likewise, the m66 antibody variable fragments (Fv) were also highly similar in the two complexes, yielding Cα- and all-atom RMSDs of 0.22 and 0.59 Å, respectively, with their respective third heavy-chain complementarity-determining region (CDR H3, Kabat definition) loops, yielding Cα- and all-atom RMSDs of 0.26 and 0.83 Å, respectively (see Fig. S2A and Table S2 in the supplemental material). Since the electron density for the gp41 peptide in complex 1 (chain P) extended to residue Leu669gp41, compared to Ser668gp41 for complex 2 (chain C), complex 1 was chosen as the focus of the current analysis (see Fig. S1B and C in the supplemental material).
FIG 1.
Structure of m66 Fab in complex with a gp41 MPER peptide. (A) Overall view of the m66 antibody (heavy chain, HC, purple; light chain, LC, wheat) in complex with a peptide of the N-terminal region of the gp41 MPER (red) corresponding to the sequence listed in panel C. Disordered peptide residues are depicted as dot extension at N terminus. m66 heavy- and light-chain complementarity-determining region loops (CDRs) are colored as labeled in the figure. (B) Close-up view of ordered residues (656 to 669) of the gp41 peptide (red) with side chains shown in yellow stick representation (with exception of residues N656P and E657P for which side-chain electron density was disordered; see Fig. S1 in the supplemental material). The m66 combining region is shown in a semitransparent surface representation with the heavy-chain surface in purple and the light-chain surface in wheat. (C) Sequence alignment of epitopes of gp41 MPER-specific neutralizing antibodies. Representative sequences of HIV-1 covering the epitopes of seven gp41 MPER-specific neutralizing antibodies. Antibodies m66, m66.6, and 2F5 bind the MPER N-terminal region while antibodies z13e1, CH12, 4E10, and 10E8 bind its C-terminal region. Ordered peptide residues of the m66 epitope are underlined in the listed sequence. Sequence residues colored red represent positions at which mutation to alanine abrogates antibody binding (6, 8, 10–12, 28).
Overall, main-chain electron density for gp41 peptide chain P was observed from residue Asn656gp41 through to Leu669gp41, with well-ordered side-chain density observed from residue Gln658gp41 to Ser668gp41 (Fig. S1B). No electron density was observed for gp41 residues 651 to 655, suggesting this portion of the peptide was disordered. The helix of the peptide, spanning residues Glu659gp41-Leu663gp41, was observed to contact both the light and the heavy chains of m66, while the extended loop region of the peptide, spanning residues Asp664gp41 to Leu669gp41, interacted predominantly with the heavy chain (Fig. 1A and B). Notably, one face of the m66 CDR H3 loop cradled the extended loop portion of the peptide, forming extensive interactions with gp41 that spanned from the base of the CDR H3 loop through to its tip (Fig. 1B).
m66-gp41 interface.
A total interactive surface area of 856.8 Å2 was observed on the antibody, and a corresponding interactive surface area of 736.6 Å2 was observed on the gp41 peptide, a finding consistent with previously observed interfacial surface areas for antibody-MPER peptide complexes (see Tables S3 to S6 in the supplemental material) (6). The majority of contacts between m66 and gp41 were mediated by the m66 heavy chain, with 19 heavy-chain residues buried by the interaction with gp41 (Fig. 2A), corresponding to 81% of the total interactive surface area on the antibody (Fig. 2A and B and see Tables S3 and S5 in the supplemental material). The m66 heavy-chain first and second complementarity-determining regions (CDR H1 and CDR H2) accounted for 7.2 and 18.3% of the interface, respectively, while the CDR H3 accounted for 55.5% of the interface (Fig. 2A and B and see Tables S3 and S5 in the supplemental material). No interactions were observed between framework regions of the m66 heavy chain and gp41. Of the eight residues of the m66 VH region buried by the interaction with gp41, only two were somatically mutated from the IGHV5-51*01 germ line gene (Fig. 2A).
FIG 2.
m66-gp41 Interface. (A) Sequence alignment of the heavy chains of antibodies m66 and m66.6 against the predicted IGHV5-51*01/IGHD3-10*01/J6*02 germ line precursor, upper panel. Sequence alignment of the m66 light chain against the light chains of antibody m66.6 and the predicted IGKV1-39*01/IGKJ3*01 germ line precursor, lower panel. Symbols represent residues of the m66 heavy and light chains that contact gp41, as described in the figure, with residues buried by gp41 colored red. (B) Footprint of gp41 peptide on the combining region of antibody m66. Shown is a surface representation of the antibody heavy and light chains (colored as in Fig. 1B) with the gp41 footprint on the heavy chain in light blue and on the light chain in pale green. The gp41 peptide is shown in tube representation, red, with side chains of gp41 residues required for binding shown in yellow stick representation. Disordered peptide residues are depicted as dot extension, red, at the N terminus. (C to E) Direct contacts between m66 residues (stick representation, colored according to CDR loop) and gp41 residues required for binding (stick representation, yellow), with hydrogen bonds and salt bridges (C), hydrophobic interactions (D), and interactions between m66 residues and main-chain atoms of gp41 (E). Residue superscripts represent crystal structure chain IDs.
A contact interface between the m66 light chain and gp41 was also observed, with six light-chain residues buried by the interaction, corresponding to 18.9% of the total antibody interface (Fig. 2A and B and see Tables S3 and S6 in the supplemental material). The first and third light-chain complementarity-determining regions (CDR L1 and CDR L3, respectively) accounted for 2.3 and 16.6% of the interface, respectively. As in the case of the heavy chain, no interactions were observed between light-chain framework regions and gp41. Of the six light-chain residues buried by the interaction with gp41, none were somatically mutated from the light-chain precursor IGKV1-39*01 gene (Fig. 2A).
Previous alanine scan mutagenesis analyses revealed that five residues within the N-terminal region of the gp41 MPER were essential for m66 binding: Leu660gp41, Leu663gp41, Asp664gp41, Lys665gp41, and Trp666gp41 (Fig. 2B to D) (8). The structure confirmed the presence of these residues at the m66-gp41 interface. Direct hydrogen bond or salt bridge contacts were observed between the antibody and four of these gp41 residues—Leu663gp41, Asp664gp41, Lys665gp41, and Trp666gp41—while hydrophobic contacts were observed with all five of them (Fig. 2C and D and see Tables S7 and S8 in the supplemental material). Heavy-chain residues (HC) of m66 mediated the majority of these direct contacts, including hydrogen bonds and salt bridges mediated by residues Trp33HC, Thr100gHC, Asp54HC, Asp56HC, and Ser31HC (Fig. 2C and see Table S7 in the supplemental material) and hydrophobic contacts mediated by residues Trp33HC, Tyr52HC, His97HC, Tyr100iHC, and Tyr100kHC (Fig. 2D and see Table S8 in the supplemental material). Three light-chain residues (LC) of m66—Tyr32LC, Thr94LC, and Phe96LC—formed direct contacts with gp41, with two of them—Tyr32LC and Phe96LC—contacting gp41 residues critical for binding, Leu660gp41 and Leu663gp41 (Fig. 2D and see Table S8 in the supplemental material). These contacts were hydrophobic in nature, between side-chain atoms of Tyr32LC and Leu660gp41 and between the side-chain atoms of Phe96LC and Leu660gp41 and Leu663gp41.
Although previous alanine scan binding analyses helped define gp41 residues essential for m66 recognition, as confirmed in the current structure, they mainly revealed interactions between m66 and side-chain atoms of gp41. They largely failed to reveal potential interactions between m66 and main-chain atoms of gp41, such as might occur at sequence variable positions within the epitope. Examination of the m66-gp41 interface did indeed show that m66 interacts with gp41 residues that are insensitive to alanine mutation (see Tables S5 to S8 in the supplemental material) (8). While the majority of these contacts were between the antibody and side-chain atoms of these residues, arguing against their overall importance in recognition, in two cases—Arg100fHC and Tyr100cHC—interactions with main-chain gp41 atoms were observed (Fig. 2E). Arg100fHC formed salt bridges between its guanidinium group and the carbonyl oxygens of Leu661gp41 and Leu669gp41, while Tyr100cHC, through its main-chain carbonyl, formed a hydrogen bond with the main-chain amine of Ser668gp41, as well as hydrophobic contacts with the Cα and C atoms of Ser668gp41 and Ala667gp41, respectively (Fig. 2E; see also Tables S7 and S8 in the supplemental material). Examination of the structure suggests these two CDR H3 residues are situated at key positions within the m66-gp41 MPER interface, forming what appears to be a clamp that holds the extended loop portion of the peptide in place (Fig. 2E). Additional interactions were observed to form between the guanidinium group of Arg100fHC and the backbone carbonyl of Leu669gp41, leading the peptide C terminus to wrap back around the side chain of Arg100fHC (Fig. 2E).
Comparison of gp41 MPER-bound m66 with unbound m66.6 and m66.
The m66 variant m66.6, which shares the same heavy chain as m66 but differs in its light chain, has a greater capacity for HIV-1 neutralization than its counterpart (8). We sought to determine whether m66.6's greater neutralization capacity could be inferred from comparison of its unbound structure with that of m66, both bound to gp41 MPER and unbound. Specifically, we sought to determine whether contacts observed between the m66 light chain and gp41 were likely maintained by m66.6, and if they were maintained could additional determinants of neutralization in the m66.6 light chain be identified. Toward that end, the variable fragment (Fv) of unbound m66.6 was superimposed onto the Fv of gp41-bound m66 (Fig. 3A). With the exception of their CDR H3 loops, which exhibited a Cα-atom RMSD of 2.5 Å (see Fig. S3 and Table S2 in the supplemental material), the two Fv regions displayed remarkable structural similarity in both their heavy and light chains, with a Cα-atom RMSD of 0.48 Å (1.3 Å if the CDR H3s are included in the comparison). Of the three residues of the m66 light chain that contacted gp41 in the co-crystal structure, Tyr32LC, Thr94LC, and Phe96LC, all three were highly conserved in the m66.6 structure. Comparison of their spatial positions yielded a combined all-atom RMSD of 0.15 Å (Fig. 3B). While the possibility that the m66.6 light chain undergoes conformational rearrangement upon gp41 binding cannot be excluded, these three light-chain residues are at the very least prepositioned in unbound m66.6 to form contacts with gp41 similar to those formed by m66.
FIG 3.
Comparison of gp41 MPER-bound m66 with unbound m66.6 and m66. (A) Upper panel, superposition of the Fv region of the unbound structure of antibody m66.6 (heavy chain, HC, cyan; light chain, LC, orange) onto the Fv region of antibody m66 (heavy chain, purple; light chain, wheat) bound to gp41 (red). Residues of the m66.6 light chain that differ from those of the m66 light chain are shown in orange sticks with a semitransparent surface around them. Lower panel, image rotated by 70°. (B) Close-up view of panel A, lower panel. Three residues of the m66 light chain (green sticks) form direct contacts with gp41, including with gp41 residues required for antibody recognition (yellow sticks). The positions of all three of these light-chain residues are conserved in the m66.6 light chain (magenta sticks). (C) Upper panel, superposition of the Fv region of the unbound structure of antibody m66 (heavy chain, marine; light chain, charcoal) onto the Fv of m66 (heavy chain, purple; light chain, wheat) bound to gp41 (red). Lower panel, image rotated by 70°. Although part of the CDR H3 loop of unbound m66 is disordered (depicted as dot extensions, marine), little conformational movement of the m66 heavy and light chains is observed upon gp41 binding. (D) Close-up view of panel C, lower panel. The three residues of the m66 light chain that interact with gp41 (gp41-bound, green sticks; unbound, charcoal sticks) are prepositioned for gp41 binding and do not undergo large conformational changes upon epitope binding. In all panels, disordered residues are depicted as dot extensions and residue superscripts represent crystal structure chain IDs.
We also compared the structure of unbound m66 with that of gp41 MPER-bound m66 (Fig. 3C). While the CDR H3 loop of unbound m66 was disordered in the structure and could not be compared, the remaining components of the Fv regions of the two antibodies displayed a high degree of structural homology, with a Cα-atom RMSD of 0.45 Å, suggesting little conformational rearrangement upon gp41 binding. Comparison of the spatial positions of the three gp41-contacting light-chain residues in the bound and unbound structures of m66 also revealed very little conformational movement of these residues upon gp41 binding, with a combined all-atom RMSD of 0.24 Å (Fig. 3D). Thus, as in the case of unbound m66.6, the light-chain residues in unbound m66 also appear to be prepositioned for gp41 binding.
Molecular basis for m66.6 neutralization of HIV-1.
Given the high degree of structural similarity observed for critical light-chain residues of gp41 MPER-bound m66 and unbound m66.6, we next sought to identify light-chain residues unique to m66.6 that were positioned in proximity to gp41, under the hypothesis that such residues could underlie the greater breadth of m66.6-mediated virus neutralization. In total, the m66.6 light chain differs from the m66 light chain at 11 residue positions (Fig. 2A, 3A, and 4A). Examination of the Fv superposition of unbound m66.6 and gp41 MPER-bound m66 revealed that of these 11 light-chain residues, those within its CDR L1 loop fell in proximity to gp41 in the alignment (Fig. 4A). m66.6 CDR L1 residue Lys30LC was positioned closest to gp41, though at a distance greater than 7.5 Å away from the nearest (non-hydrogen) gp41 atom—the carbonyl oxygen of residue Glu657gp41. While additional contacts between the m66.6 light chain and gp41 could therefore not be directly inferred, the predicted proximity of the m66.6 CDR L1 to gp41 suggested it might nonetheless be critical for m66.6-mediated virus neutralization.
FIG 4.
Molecular basis for m66.6 neutralization of HIV-1. (A) The upper panel shows an alignment of Fv regions of gp41-bound m66 (heavy chain, purple; light chain, wheat; gp41, red) and unbound antibody m66.6 (heavy chain, cyan; light chain, orange). Of the residues unique to the m66.6 light chain (shown in semitransparent orange surface representation), those that lie closest to the gp41 peptide in the alignment are found in the m66.6 CDR L1 loop (shown as magenta sticks). Corresponding CDR L1 residues of m66 are shown as green sticks. Residues of the m66-bound gp41 peptide that lie closest to the m66.6 CDR L1 loop in the superposition are shown in yellow stick representation (the side chain of E657P is only partially ordered). Lower panel, close-up view of residues of the m66.6 CDR L1 that differ from those of m66, colored as in upper panel but without surrounding surface. In both panels, disordered peptide residues are depicted as dot extensions. Residue superscripts represent crystal structure chain IDs. (B) Residues of the m66.6 CDR L1 were swapped into the CDR L1 of m66 as listed, and the resulting variants tested for HIV-1 neutralization. Shown are breadth-potency curves against a 41-isolate panel (Tables 2 and 3).
To directly test whether residues of the m66.6 CDR L1 were the basis for its greater neutralization capacity (over that of m66), we substituted m66.6 CDR L1 residues into the CDR L1 of m66 and then tested the resulting m66 variants for HIV-1 neutralization. Specifically, three residues of the m66.6 CDR L1—His28LC, Lys30LC, and Lys31LC—were introduced into the CDR L1 of m66, replacing residues Ser28LC, Gly30LC, and Ser31LC, respectively. Substitutions were made either individually, in combination, or by swapping the CDR L1 as a whole. The capacity of the m66 CDR L1 variants to neutralize HIV-1 was then tested in a TZM-bl-based luciferase reporter assay against a panel of 41 HIV-1 Envelope pseudotyped strains previously determined to be largely sensitive to m66.6 but resistant to m66 (Table 2) (8). As shown in Fig. 4B and Tables 2 and 3, while wild-type (WT) m66.6 was able to neutralize 93% of strains tested (38 out of 41) at an inhibitory concentration (IC50) of <50 mg/ml, WT m66 was only able to neutralize 2% of these strains (1 out of 41). As residues of the m66.6 CDR L1 were introduced into the m66 CDR L1, m66 neutralization of HIV-1 began to improve. The single substitutions S28LCH and S31LCK increased the percentage of strains neutralized to 7 and 17% of total, respectively (Fig. 4B and Tables 2 and 3). Remarkably, a single lysine substitution at m66 residue Gly30LC (G30LCK) led to m66-mediated neutralization of over 65% of the strains tested, representing a >30-fold increase in breadth over WT m66 (Fig. 4B and Tables 2 and 3). Although the double substitution S28LCH-G30LCK had a slightly detrimental effect on neutralization breadth compared to G30LCK alone, neutralization potency for this double mutant did increase against neutralized strains (Fig. 4B and Tables 2 and 3). Lastly, when all three unique residues of the m66.6 CDR L1 were introduced into the m66 CDR L1—S28LCH, G30LCK, S31LCK—in effect swapping the CDR L1 as a whole, 83% of the tested strains were neutralized (34 of 41 viruses neutralized) representing a >40-fold increase in breadth over WT m66 (Fig. 4B and Tables 2 and 3). These experiments confirm that residues of the m66.6 CDR L1 are critical for HIV-1 neutralization, with Lys30LC playing a central role.
TABLE 2.
m66 variant neutralization IC50s against 41-isolate panela

Color shading in the table represents neutralization IC50s: orange, 0.10 to 1.0 μg/ml; yellow, 1.00 to 10.0 μg/ml; green, 10.0 to 50.0 μg/ml.
TABLE 3.
Summary statistics for 41-isolate neutralization panela

LC, light chain; HC, heavy chain; WT, wild type. Residues shown in red represent m66.6 CDR L1 residues that were introduced into the m66 CDR L1 (top) or a point mutation introduced into the m66 CDR H3 (bottom).
Recognition of gp41 MPER N-terminal region by neutralizing and non-neutralizing antibodies.
To establish whether or not conserved structural elements characterize recognition of the N-terminal region of the gp41 MPER by neutralizing antibodies, we compared m66 recognition of the MPER N-terminal region with recognition by the neutralizing antibody 2F5, and with recognition by two non-neutralizing antibodies, 11F10 and 13H11 (22, 28). Antibody 11F10 is a mouse antibody that was elicited by structural mimics of the 2F5 epitope and recognizes a similar conformation of gp41 (28), while antibody 13H11, also a mouse antibody, is specific for a helical conformation of the MPER thought to represent the gp41 postfusion six-helix bundle (22). Two structural features were examined in these comparisons: the recognized antibody-bound conformations of gp41 and the angles at which the antibodies approached gp41. For 2F5 and 11F10, the comparison with m66 was restricted to residues within the extended loop portions of the m66 epitope, focusing on residues required for gp41 recognition: Asp664gp41, Lys665gp41, and Trp666gp41 (Fig. 5A). For 13H11, the comparison was limited to residues within the helical portion of the m66 epitope, covering residues 659 to 663.
FIG 5.
Recognition of the gp41 MPER N-terminal region by neutralizing and non-neutralizing antibodies. (A) Structures of gp41 MPER bound by neutralizing antibodies m66 and 2F5 and by non-neutralizing antibodies 11F10 and 13H11. Orientations of the loop forms of gp41 MPER (red) are based on alignment with m66 epitope residues 664 to 666 (shown as yellow sticks) which are required for m66, 2F5, and 11F10 recognition. Orientation of the helical form of gp41 MPER bound by antibody 13H11 (blue) is based on alignment with helical residues of the m66 epitope, 659 to 663 (shown as yellow sticks). (B) The upper row shows orientations of the 2F5, 11F10, and 13H11 Fv regions (gray) relative to the Fv region of m66 (heavy chain, purple; light chain, wheat) based on the alignments of their epitopes shown in panel A. In each case the m66-bound gp41 peptide is shown for reference. The lower row shows images rotated by 90°. A dashed line is shown for reference and represents a hypothetical steric planar boundary near the tips of the m66 and 2F5 CDR H3 loops. (C) Relative angles of antibody approach to the gp41 MPER N-terminal region. Directions of approach (based on the Fv orientations shown in lower row of panel B) were defined by a line drawn from the midpoint between the heavy- and light-chain disulfide bonds to the Cα atom of gp41 residue Lys665P (m66, black line; 2F5, blue line; 11F10, magenta line; 13H11, green line). Angles listed are relative to the direction of approach of m66. Circles shown are centered around the Cα atom of Lys665P.
Starting with 2F5, superposition of its core epitope residues with those of m66 yielded RMSDs of 0.03 and 2.5 Å for Cα- and all-atom alignments, respectively (corresponding to 0.01 and 0.83 Å per residue, respectively). Extension of the range of the alignment in the C-terminal direction to individually include residues Ala667gp41, Ser668gp41, and Leu669gp41 yielded Cα-atom RMSDs of 0.74, 0.85, and 2.4 Å, respectively (or 0.19, 0.17, and 0.40 Å per residue, respectively), suggesting that the highest degree of structural similarity predominantly spanned their three shared core epitope residues Asp664gp41, Lys665gp41, and Trp666gp41. Comparison of the same core epitope residues of the non-neutralizing antibody 11F10 revealed even higher structural homology to the m66 epitope, with Cα- and all-atom alignments yielding RMSDs of 0.014 and 2.5 Å, respectively (corresponding to 0.005 and 0.83 Å per residue, respectively) (Fig. 5A). Comparison of the helical portion of the m66 epitope (residues 659 to 663) to corresponding epitope residues of antibody 13H11 yielded Cα- and all-atom RMSDs of 0.76 Å and 1.8 Å, respectively (corresponding to 0.15 and 0.36 Å per residue, respectively) (Fig. 5A). Taken as a whole, although a high degree of structural homology was observed for backbone atoms of the m66 and 2F5 epitopes, across their three core residues, since similarity was also observed with the 11F10 and 13H11 epitope structures, structural homology of bound epitopes alone is likely not a sufficient parameter for distinguishing neutralizing from non-neutralizing antibodies.
We next examined the relative angles at which the antibodies approached conserved structural elements within the N-terminal region of the gp41 MPER. Utilizing alignments of structurally homologous epitope residues (residues 664 to 666 for 2F5 and 11F10 and residues 659 to 663 for 13H11, as shown in Fig. 5A) to orient the antibody Fv regions (Fig. 5B), the direction of approach of each respective Fv was defined as the line drawn from the midpoint between its heavy- and light-chain Fv disulfide bonds to the Cα atom of gp41 residue Lys665gp41 (Fig. 5C). Starting with antibody 2F5, when its core epitope residues Asp664gp41 to Trp666gp41 were aligned against the corresponding residues of m66-bound gp41, its direction of approach was found to differ from that of m66 by 30.2° (Fig. 5B and C). A similar comparison with non-neutralizing antibody 11F10 revealed that its direction of approach differed from that of m66 by an angle of 50.3° (Fig. 5B and C). In stark contrast, the direction of approach of the non-neutralizing antibody 13H11 (based on alignment with m66-bound gp41 helical residues, 659 to 663) was almost diametrically opposed to that of m66, approaching at an angle 167.2° away from the direction of m66 (Fig. 5B and C). This highly pronounced difference between 13H11 and m66 could be a result of their targeting distinct conformational states of HIV-1 Env, with m66 likely targeting an intermediate conformation and 13H11 targeting a postfusion conformation (see Fig. S5 in the supplemental material) (22). Thus, as a whole, the angle of approach of neutralizing antibody 2F5 was the most similar to that of m66 and the angle of approach of the non-neutralizing antibody 13H11 was the most divergent. In the case of non-neutralizing antibody 11F10, its angle of approach relative to that of m66 was much closer than that of 13H11, only an additional 20° away from that observed for 2F5, suggesting either little margin for error in approaching this region on the viral spike or that additional parameters could be involved in defining recognition that leads to effective neutralization.
Comparison of m66 and 2F5 CDR H3 loops.
One feature that has previously been shown to characterize neutralizing antibodies that target the gp41 MPER is that they are able to bind their epitopes in the context of the viral membrane, while those that are non-neutralizing cannot (13). In the case of antibody 2F5, while the base of its CDR H3 loop is involved in extensive interactions with the gp41 MPER, hydrophobic residues at the tip of its CDR H3 loop, which do not appear to directly interact with gp41, have been shown to be critical for virus neutralization—likely through interactions with the viral membrane (20, 21). While the m66 CDR H3 loop differs from the 2F5 CDR H3 in that it forms interactions with gp41 from its base through to its tip (albeit along one face of the loop), it resembles the 2F5 CDR H3 loop in that it also possesses a stretch of aromatic residues at its apex: Y100cHC, F100dHC, and Y100eHC (Fig. 6). Remarkably, when m66 and 2F5 are aligned based on superposition of their core epitope residues Asp664gp41 to Trp666gp41, the tips of their CDR H3 loops fall along a similar plane (Fig. 5B and 6). The positioning of the tips of the m66 and 2F5 CDR H3 loops along this plane—one that could potentially represent a steric boundary defined by the viral membrane—places apical residues of the m66 CDR H3 loop in position to form membrane interactions similar to those mediated by apical residues of the 2F5 CDR H3 loop (Fig. 5B) (20, 21). In contrast, when the non-neutralizing antibody 11F10 is aligned to m66 using the same core epitope residues, it crosses this putative steric boundary (Fig. 5B), a finding consistent with its inability to recognize the gp41 MPER in the context of a lipid membrane and its inability to neutralize HIV-1 (28).
FIG 6.
Comparison of CDR H3 loops of antibodies m66 and 2F5. (A) Close-up view of the m66 CDR H3 loop shown in cartoon representation (upper panel) and semitransparent electrostatic surface representation (lower panel). Residues at the tip of the m66 CDR H3 loop are displayed as green sticks (also colored green in the accompanying CDR H3 sequence below). The m66 heavy chain is colored purple, the light chain wheat, and gp41 red. Acidic surfaces are depicted in red, basic surfaces in blue, and nonpolar surfaces in white. (B) Close-up view of 2F5 CDR H3 loop in cartoon representation (upper panel) and semitransparent electrostatic surface representation (lower panel), based on alignment with m66 epitope residues 664 to 666. Hydrophobic residues at the tip of the 2F5 CDR H3 loop previously shown to be required for HIV-1 neutralization are displayed as green sticks (also colored green in the accompanying CDR H3 sequence below). The 2F5 heavy chain is colored light blue, the light chain gray, and gp41 red. Acidic surfaces are depicted in red, basic surfaces in blue, and nonpolar surfaces in white. (C) Close-up view of the superposition of the m66 and 2F5 CDR H3 loops based on alignment of their core epitope residues 664 to 666. The tips of the CDR H3 loops of both antibodies fall along a similar plane, positioning apical residues (green sticks) for interaction with the viral membrane. Antibodies and gp41 colored as in panels A and B.
Role of the m66.6/m66 CDR H3 loop in lipid-context gp41 MPER recognition and HIV-1 neutralization.
Due to the similarity in the positioning of the CDR H3 loops of m66 and 2F5, based on alignment of their epitopes, we sought to assess the functional role of residues at the apex of the m66 and m66.6 CDR H3 loops in virus neutralization and MPER recognition in a lipid environment. Previous studies have shown that residues at the tip of the 2F5 CDR H3 loop are required for HIV-1 neutralization in a manner that correlates with MPER recognition in a membrane context (20, 21). Since our ultimate goal was to determine the role of the CDR H3 loop in virus neutralization, we focused our analysis on variant m66.6, which shares an identical CDR H3 as m66, but has a wider breadth of virus neutralization. Mutations of four residues at the distal tip of the m66.6 CDR H3 loop—Ser100bHC, Tyr100cHC, Phe100dHC, and Tyr100eHC—were introduced to alter hydrophobicity of the loop based on Wimley-White scales of hydrophobicity defined by free energies of partitioning whole residues from water to a lipid bilayer interface, ΔGwif (Fig. 6A; see also Fig. S6 in the supplemental material) (42). Single or double substitutions to serine were used to degrade hydrophobicity of the loop, while single or double substitutions to tryptophan were used to enhance hydrophobicity of the loop (see Fig. S6 in the supplemental material).
An ELISA-based competition assay was then used to assess the capacity of the m66.6 CDR H3 variants to recognize the MPER both in soluble and lipid environments (20) (see Fig. S7 in the supplemental material). As a first step, the competition assay was validated by assessing the capacities of wild-type antibodies m66.6, 2F5, and 11F10 to recognize the MPER in these environments (Fig. 7A). For lipid-context recognition, liposome competitors containing an MPER peptide corresponding to gp41 residues 659 to 683 were formulated using lipid ratios similar to those found in HIV-1 viral membranes (40). For soluble-context recognition, an MPER peptide corresponding to gp41 residues 657 to 670 was utilized. Each antibody was incubated with increasing amounts of the liposomal or soluble MPER competitor, and then added to an ELISA plate on which gp41 MPER was captured (see Fig. S7 in the supplemental material). Within this assay, the capacity of the antibody to be competed by the liposomal or soluble MPER competitors represented the capacity to bind MPER in membrane or soluble contexts, respectively. As shown in Fig. 7A, while all three of the MPER-specific antibodies were successfully competed by a soluble MPER competitor, only antibodies m66.6 and 2F5 were competed by a lipid-embedded MPER epitope. The finding that the non-neutralizing antibody 11F10 does not have the capacity to recognize a lipid-embedded MPER epitope is consistent with earlier data (13) and the postulation that recognition of the MPER in a lipid environment represents a correlate of neutralization for antibodies that target this region.
FIG 7.
Role of CDR H3 loop in recognition of MPER in lipid environment and in HIV-1 neutralization. (A) Binding profiles of antibodies m66.6 (black), 2F5 (brown), and 11F10 (gray) to gp41 MPER, competed with either liposomal (left panel) or soluble (right panel) MPER. Although all three antibodies were competed by the soluble competitor (right panel), only antibodies m66.6 and 2F5 were competed by the liposomal MPER competitor (left panel). (B) Binding profiles of m66.6 CDR H3 hydrophobicity variants to gp41 MPER. The curves represent the percent binding to the respective liposomal (left panel) or soluble (right panel) MPER competitors. Altered hydrophobicity of the m66.6 CDR H3 had a minimal effect on binding to soluble MPER. Mutations that degraded m66 CDR H3 hydrophobicity reduced antibody recognition of liposomal MPER, while those that augmented hydrophobicity enhanced recognition of liposomal MPER. (C) Role of CDR H3 loop hydrophobicity in the context of the less broad antibody m66. Mutation Y100eHCW was introduced into the CDR H3 loop and virus neutralization tested against a 41-isolate panel. The breadth-potency curve of m66-Y100eHCW (black) is shown in comparison to that of WT m66 (blue) (Tables 2 and 3).
We next examined the capacity of m66.6 variants with altered CDR H3 loop hydrophobicity (see Fig. S6 in the supplemental material) to recognize the MPER in both soluble and lipid environments. Although changes in CDR H3 hydrophobicity had little effect on the degree of MPER recognition in a soluble context, the degree to which an m66.6 variant could recognize MPER in a lipid context was highly dependent on hydrophobicity of the CDR H3 loop (Fig. 7B and Tables 4 and 5). Single serine mutations that degraded hydrophobicity of the loop reduced recognition of the MPER epitope in a lipid environment by a >50-fold increase in the half-maximal inhibitory concentration (IC50), whereas the double serine mutation Y100cHCS-F100dHCS knocked out binding in lipid completely (Fig. 7B and Table 4). In contrast, mutations that enhanced hydrophobicity of the loop led to enhanced recognition of MPER in a lipid environment, though the improvements observed did not exceed 3-fold reductions in IC50s, with the mutation Y100eHCW leading to the most pronounced effect (Fig. 7B and Table 4). Assessment of the statistical relationships between m66.6 CDR H3 loop hydrophobicity, ΔGwif, and the IC50s of MPER recognition revealed a statistically significant relationship between CDR H3 hydrophobicity and lipid-context MPER recognition (P = 0.007) but not between hydrophobicity and MPER recognition in a soluble context (P = 0.955) (Table 5). Although we cannot exclude the possibility that truncation of the soluble peptide at residue 670 could be a factor in the observed results, we note that previous studies have found no evidence for interactions of m66 or m66.6 with gp41 residues downstream of residue 670 (8). In addition, it is possible that these results were also influenced by the lack of a transmembrane domain in the liposomal peptides used, possibly affecting the orientation of MPER within the lipid bilayer or its extraction from the bilayer, though we note that even in the absence of a transmembrane domain the liposomal peptide was not extracted by antibody 11F10 (Fig. 7A).
TABLE 4.
Binding and HIV-1 neutralization IC50s for m66.6 CDR H3 variantsa

*, in cases where 50% inhibition was not achieved, the upper limit of the competitor concentration is listed. Color shading in the table represents neutralization IC50s: orange, 0.10 to 1.0 μg/ml; yellow, 1.00 to 10.0 μg/ml; green, 10.0 to 50.0 μg/ml. HC, heavy chain; WT, wild type.
TABLE 5.
Correlates of HIV-1 neutralization for m66.6 CDR H3 variants
| Parameter |
P valuea |
||
|---|---|---|---|
| ΔGwif | Binding IC50 |
||
| Soluble MPER | Lipid MPER | ||
| Neutralization IC50 HIV-1 strain | |||
| MN.3 | 0.0506 (NS) | 0.4338 (NS) | 0.0066 (**) |
| HxB2.DG | 0.0044 (**) | 0.6674 (NS) | 0.0001 (***) |
| H086.8 | 0.0162 (*) | 0.8152 (NS) | 0.0002 (***) |
| CNE59 | 0.0133 (*) | 0.9394 (NS) | 0.0013 (**) |
| ADA.DG | 0.0039 (**) | 0.8078 (NS) | 0.0004 (***) |
| 5768.04 | 0.0698 (NS) | 0.7336 (NS) | 0.0031 (**) |
| HT593.1 | 0.0224 (*) | 0.6890 (NS) | 0.0011 (**) |
| CDR H3 hydrophobicity | |||
| ΔGwif | NA | 0.9554 (NS) | 0.0070 (**) |
As determined by Spearman nonparametric two-tailed correlation. Comparison data for neutralization IC50s of various strains or CDR H3 ΔGwif versus the indicated parameters are shown. NS, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001. NA, not applicable.
We next sought to assess the effect of m66.6 CDR H3 hydrophobicity on HIV-1 neutralization. m66.6 CDR H3 variants were tested for neutralization of HIV-1 using a single-round infectivity assay (Tzm-bl target cells) against a panel of seven envelope-pseudotyped strains previously shown to be sensitive to m66.6 (Table 4). Single or double serine mutations in the m66.6 CDR H3 loop that reduced overall hydrophobicity led to reduced breadth and potency of HIV-1 neutralization, with the single mutation F100dHCS and the double mutations Y100cHCS-F100dHCS and Y100cHCS-Y100eHCS almost completely ablating HIV-1 neutralization (Table 4). In contrast, single or double mutations to tryptophan which increased overall hydrophobicity of the CDR H3 loop were found to largely maintain, though not exceed, the neutralization capacity of WT m66.6 (Table 4). Assessment of nonparametric Spearman correlations between virus neutralization IC50s and soluble- and lipid-context MPER binding IC50s of the m66.6 CDR H3 variants revealed a statistically significant relationship between virus neutralization and lipid-context MPER recognition, across all strains tested, but not between virus neutralization and soluble-context MPER recognition (Table 5). The correlation between HIV-1 neutralization and m66.6 CDR H3 hydrophobicity (ΔGwif) was also robust, with a statistically significant P value observed for five of the seven strains tested (Table 5). Thus, as a whole, the data confirm the relevance of the m66.6 CDR H3 in recognition of the MPER in lipid context and in HIV-1 neutralization, as previously observed for 2F5.
CDR H3 mutation Y100eHCW potentiates m66 neutralization of HIV-1.
Although mutations that increased hydrophobicity of the m66.6 CDR H3 loop correlated with improved lipid-context MPER recognition, they largely left m66.6-mediated virus neutralization unchanged (Table 4). One possible explanation for this observation is that in the context of m66.6, where determinants within its light chain strongly contribute to neutralization, apical residues of the CDR H3 loop may already be functionally optimal. We postulated that an improvement in lipid-context recognition might nevertheless translate into improved HIV-1 neutralization if tested in the context of the less broad and potent antibody m66. Mutation Y100eHCW, which displays the most pronounced improvement in m66.6 lipid-context recognition, was therefore introduced into m66 and tested against the panel of 41 envelope-pseudotyped isolates described above. Remarkably, as shown in Fig. 7C and Tables 2 and 3, increased hydrophobicity of the m66 CDR H3 at this residue position led to a dramatic increase in the breadth of m66 neutralization, leading to neutralization of >30% of the strains tested. This finding confirms the relevance of the CDR H3 loop in the context of antibody m66 and suggests that increasing m66 CDR H3 loop hydrophobicity may compensate for m66's less optimal light chain (relative to that of its counterpart m66.6), possibly by improving its binding capacity to the native virion or by helping it extract its epitope from the viral membrane.
DISCUSSION
We have determined the crystal structure of the neutralizing antibody m66 in complex with a gp41 peptide corresponding to the N-terminal region of the gp41 MPER, along with the unbound structures of m66 and its light-chain variant m66.6. Although m66 and m66.6 are less potent and broad than the previously characterized MPER-specific antibody 2F5, the structures reported here represent the first structures after that of 2F5 of human neutralizing antibodies that target this specific subregion of the gp41 MPER. Through a comparative structural and biochemical analysis, we uncovered determinants of m66- and m66.6-mediated HIV-1 neutralization, and through comparison with antibody 2F5, we defined common structural and functional elements of antibody-mediated virus neutralization through the N-terminal region of the gp41 MPER.
While antibodies m66 and m66.6 share an identical heavy chain, the distinct light chain of antibody m66.6 confers a significantly greater capacity for virus neutralization. Our structural comparison of gp41-bound m66 with unbound m66.6 allowed us to pinpoint, in conjunction with mutagenesis, unique residues within the m66.6 CDR L1 loop that underlie its neutralization. We showed that the introduction of m66.6 CDR L1 residue Lys30LC into the m66 CDR L1 increased m66 neutralization breadth >30-fold, while swapping the entire m66.6 CDR L1 into the m66 light chain led to a 40-fold improvement in neutralization. Since the overall backbone structures of the CDR L1s in m66.6 and m66 are similar, as are their general interactions with other domains of the antibody (Fig. 3 and see Fig. S3 and Tables S9 and S10 in the supplemental material), the precise molecular mechanism by which residues of the CDR L1 confer superior virus neutralization remains to be fully determined.
One hypothesis, due to the proximity of the CDR L1 to gp41 in the m66 complex structure, is that in the context of m66.6, additional interactions with gp41 are formed—contacts not present in the context of m66. However, when antibodies m66.6 and m66 and its CDR L1 variants were tested for binding to gp41 MPER peptides with extended N termini—spanning residues 647 to 669, 651 to 669, and 656 to 669—no significant differences in binding were observed, regardless of peptide residue range or antibody CDR L1 (see Fig. S4 in the supplemental material), providing little evidence for additional contacts between the antibody and gp41 in this context. Likewise, a computational analysis of the virus neutralization fingerprint of antibody m66.6 aimed at identifying gp41 residues upstream of MPER (within residues 649 to 657) that could confer resistance to neutralization also did not provide evidence for additional interactions with gp41 when followed up with mutagenesis (see Fig. S8 and Table S11 in the supplemental material) (43, 44). Other hypotheses will likely need to be investigated to fully define the mechanism by which residues of the m66.6 CDR L1 confer virus neutralization. It is possible that their role may only be detectable in the context of the native virion spike. Given the requirement for recognition of the epitope in a lipid context, the basic nature of m66.6 residues Lys30LC and Lys31LC could potentially enable such recognition through interactions with negatively charged phospholipid headgroups. Our finding that mutation Y100eHCW in m66 can recover neutralization of ∼30% of strains tested, in the absence of any changes to its CDR L1, suggests that functional elements within the CDR L1 and CDR H3 loops possibly compensate for one another in this manner. We note that m66 and m66.6 also differ considerably in their reactivity with autoantigens (8), and it remains to be determined whether residues of the CDR L1 play a role in these differences and whether these differences relate to membrane-context MPER recognition.
The structures reported in the present study enabled us to undertake a comparative analysis with antibody 2F5—the only other neutralizing antibody known to target the N-terminal region of the MPER. Antibodies m66 and 2F5 were found to recognize similar backbone conformations of core epitope residues 664 to 666 and to approach these residues from similar directions, although epitope similarities outside these residues were more limited. Additional parallels between m66 and 2F5 were observed in the planar positioning of the hydrophobic tips of their CDR H3 loops, as well as in the functional dependence on these loops for membrane-context MPER recognition and virus neutralization. The structural and functional similarities observed between 2F5 and antibodies m66 and m66.6 could suggest that both lineages evolved under similar constraints for recognition and neutralization of HIV-1. As more antibodies against the N-terminal region of the MPER are isolated and characterized, these and other conserved determinants of their recognition and neutralization of the virus will be further deciphered and confirmed, providing a means to identify requisite structural elements for their elicitation.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the Structural Biology and Structural Bioinformatics Core Sections, Vaccine Research Center, and P. Prabakaran for discussions and comments on the manuscript. We thank J. Stuckey for assistance with graphics. We thank J. Baalwa, D. Ellenberger, D. Gabuzda, F. Gao, B. Hahn, K. Hong, J. Kim, F. McCutchan, D. Montefiori, L. Morris, J. Overbaugh, E. Sanders-Buell, G. Shaw, R. Swanstrom, M. Thomson, S. Tovanabutra, C. Williamson, and L. Zhang for contributing the HIV-1 Envelope plasmids used in our neutralization panels.
Support for this study was provided by the Intramural Research Program of the Vaccine Research Center, National Institute of Allergy and Infectious Diseases, and the Center for Cancer Research, National Cancer Institute. Use of sector 22 (Southeast Region Collaborative Access team) at the Advanced Photon Source was supported by the U.S. Department of Energy, Basic Energy Sciences, Office of Science, under contract W-31-316 109-Eng-38.
Footnotes
Published ahead of print 11 December 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.02837-13.
REFERENCES
- 1.Kwong PD, Mascola JR. 2012. Human antibodies that neutralize HIV-1: identification, structures, and B cell ontogenies. Immunity 37:412–425. 10.1016/j.immuni.2012.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salzwedel K, West JT, Hunter E. 1999. A conserved tryptophan-rich motif in the membrane-proximal region of the human immunodeficiency virus type 1 gp41 ectodomain is important for Env-mediated fusion and virus infectivity. J. Virol. 73:2469–2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munoz-Barroso I, Salzwedel K, Hunter E, Blumenthal R. 1999. Role of the membrane-proximal domain in the initial stages of human immunodeficiency virus type 1 envelope glycoprotein-mediated membrane fusion. J. Virol. 73:6089–6092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shang L, Yue L, Hunter E. 2008. Role of the membrane-spanning domain of human immunodeficiency virus type 1 envelope glycoprotein in cell-cell fusion and virus infection. J. Virol. 82:5417–5428. 10.1128/JVI.02666-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suarez T, Gallaher WR, Agirre A, Goni FM, Nieva JL. 2000. Membrane interface-interacting sequences within the ectodomain of the human immunodeficiency virus type 1 envelope glycoprotein: putative role during viral fusion. J. Virol. 74:8038–8047. 10.1128/JVI.74.17.8038-8047.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, Imamichi H, Bailer RT, Chakrabarti B, Sharma SK, Alam SM, Wang T, Yang Y, Zhang B, Migueles SA, Wyatt R, Haynes BF, Kwong PD, Mascola JR, Connors M. 2012. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature 491:406–412. 10.1038/nature11544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray ES, Taylor N, Wycuff D, Moore PL, Tomaras GD, Wibmer CK, Puren A, DeCamp A, Gilbert PB, Wood B, Montefiori DC, Binley JM, Shaw GM, Haynes BF, Mascola JR, Morris L. 2009. Antibody specificities associated with neutralization breadth in plasma from human immunodeficiency virus type 1 subtype C-infected blood donors. J. Virol. 83:8925–8937. 10.1128/JVI.00758-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu Z, Qin HR, Chen W, Zhao Q, Shen X, Schutte R, Wang Y, Ofek G, Streaker E, Prabakaran P, Fouda GG, Liao HX, Owens J, Louder M, Yang Y, Klaric KA, Moody MA, Mascola JR, Scott JK, Kwong PD, Montefiori D, Haynes BF, Tomaras GD, Dimitrov DS. 2011. Cross-reactive HIV-1-neutralizing human monoclonal antibodies identified from a patient with 2F5-like antibodies. J. Virol. 85:11401–11408. 10.1128/JVI.05312-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muster T, Steindl F, Purtscher M, Trkola A, Klima A, Himmler G, Ruker F, Katinger H. 1993. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J. Virol. 67:6642–6647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zwick MB, Labrijn AF, Wang M, Spenlehauer C, Saphire EO, Binley JM, Moore JP, Stiegler G, Katinger H, Burton DR, Parren PW. 2001. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol. 75:10892–10905. 10.1128/JVI.75.22.10892-10905.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson JD, Brunel FM, Jensen R, Crooks ET, Cardoso RM, Wang M, Hessell A, Wilson IA, Binley JM, Dawson PE, Burton DR, Zwick MB. 2007. An affinity-enhanced neutralizing antibody against the membrane-proximal external region of human immunodeficiency virus type 1 gp41 recognizes an epitope between those of 2F5 and 4E10. J. Virol. 81:4033–4043. 10.1128/JVI.02588-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris L, Chen X, Alam M, Tomaras G, Zhang R, Marshall DJ, Chen B, Parks R, Foulger A, Jaeger F, Donathan M, Bilska M, Gray ES, Abdool Karim SS, Kepler TB, Whitesides J, Montefiori D, Moody MA, Liao HX, Haynes BF. 2011. Isolation of a human anti-HIV gp41 membrane proximal region neutralizing antibody by antigen-specific single B cell sorting. PLoS One 6:e23532. 10.1371/journal.pone.0023532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim M, Sun ZY, Rand KD, Shi X, Song L, Cheng Y, Fahmy AF, Majumdar S, Ofek G, Yang Y, Kwong PD, Wang JH, Engen JR, Wagner G, Reinherz EL. 2011. Antibody mechanics on a membrane-bound HIV segment essential for GP41-targeted viral neutralization. Nat. Struct. Mol. Biol. 18:1235–1243. 10.1038/nsmb.2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun ZY, Oh KJ, Kim M, Yu J, Brusic V, Song L, Qiao Z, Wang JH, Wagner G, Reinherz EL. 2008. HIV-1 broadly neutralizing antibody extracts its epitope from a kinked gp41 ectodomain region on the viral membrane. Immunity 28:52–63. 10.1016/j.immuni.2007.11.018 [DOI] [PubMed] [Google Scholar]
- 15.Ofek G, Tang M, Sambor A, Katinger H, Mascola JR, Wyatt R, Kwong PD. 2004. Structure and mechanistic analysis of the anti-human immunodeficiency virus type 1 antibody 2F5 in complex with its gp41 epitope. J. Virol. 78:10724–10737. 10.1128/JVI.78.19.10724-10737.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, Robinson J, Scearce RM, Plonk K, Staats HF, Ortel TL, Liao HX, Alam SM. 2005. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science 308:1906–1908. 10.1126/science.1111781 [DOI] [PubMed] [Google Scholar]
- 17.Frey G, Peng H, Rits-Volloch S, Morelli M, Cheng Y, Chen B. 2008. A fusion-intermediate state of HIV-1 gp41 targeted by broadly neutralizing antibodies. Proc. Natl. Acad. Sci. U. S. A. 105:3739–3744. 10.1073/pnas.0800255105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golding H, Zaitseva M, de Rosny E, King LR, Manischewitz J, Sidorov I, Gorny MK, Zolla-Pazner S, Dimitrov DS, Weiss CD. 2002. Dissection of human immunodeficiency virus type 1 entry with neutralizing antibodies to gp41 fusion intermediates. J. Virol. 76:6780–6790. 10.1128/JVI.76.13.6780-6790.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Rosny E, Vassell R, Jiang S, Kunert R, Weiss CD. 2004. Binding of the 2F5 monoclonal antibody to native and fusion-intermediate forms of human immunodeficiency virus type 1 gp41: implications for fusion-inducing conformational changes. J. Virol. 78:2627–2631. 10.1128/JVI.78.5.2627-2631.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Julien JP, Huarte N, Maeso R, Taneva SG, Cunningham A, Nieva JL, Pai EF. 2010. Ablation of the complementarity-determining region H3 apex of the anti-HIV-1 broadly neutralizing antibody 2F5 abrogates neutralizing capacity without affecting core epitope binding. J. Virol. 84:4136–4147. 10.1128/JVI.02357-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ofek G, McKee K, Yang Y, Yang ZY, Skinner J, Guenaga FJ, Wyatt R, Zwick MB, Nabel GJ, Mascola JR, Kwong PD. 2010. Relationship between antibody 2F5 neutralization of HIV-1 and hydrophobicity of its heavy chain third complementarity-determining region. J. Virol. 84:2955–2962. 10.1128/JVI.02257-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicely NI, Dennison SM, Spicer L, Scearce RM, Kelsoe G, Ueda Y, Chen H, Liao HX, Alam SM, Haynes BF. 2010. Crystal structure of a non-neutralizing antibody to the HIV-1 gp41 membrane-proximal external region. Nat. Struct. Mol. Biol. 17:1492–1494. 10.1038/nsmb.1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cardoso RM, Zwick MB, Stanfield RL, Kunert R, Binley JM, Katinger H, Burton DR, Wilson IA. 2005. Broadly neutralizing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusion-associated motif in gp41. Immunity 22:163–173. 10.1016/j.immuni.2004.12.011 [DOI] [PubMed] [Google Scholar]
- 24.Pejchal R, Gach JS, Brunel FM, Cardoso RM, Stanfield RL, Dawson PE, Burton DR, Zwick MB, Wilson IA. 2009. A conformational switch in human immunodeficiency virus gp41 revealed by the structures of overlapping epitopes recognized by neutralizing antibodies. J. Virol. 83:8451–8462. 10.1128/JVI.00685-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Julien JP, Bryson S, Nieva JL, Pai EF. 2008. Structural details of HIV-1 recognition by the broadly neutralizing monoclonal antibody 2F5: epitope conformation, antigen-recognition loop mobility, and anion-binding site. J. Mol. Biol. 384:377–392. 10.1016/j.jmb.2008.09.024 [DOI] [PubMed] [Google Scholar]
- 26.Shen X, Parks RJ, Montefiori DC, Kirchherr JL, Keele BF, Decker JM, Blattner WA, Gao F, Weinhold KJ, Hicks CB, Greenberg ML, Hahn BH, Shaw GM, Haynes BF, Tomaras GD. 2009. In vivo gp41 antibodies targeting the 2F5 monoclonal antibody epitope mediate human immunodeficiency virus type 1 neutralization breadth. J. Virol. 83:3617–3625. 10.1128/JVI.02631-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Georgiev IS, Doria-Rose NA, Zhou T, Kwon YD, Staupe RP, Moquin S, Chuang GY, Louder MK, Schmidt SD, Altae-Tran HR, Bailer RT, McKee K, Nason M, O'Dell S, Ofek G, Pancera M, Srivatsan S, Shapiro L, Connors M, Migueles SA, Morris L, Nishimura Y, Martin MA, Mascola JR, Kwong PD. 2013. Delineating antibody recognition in polyclonal sera from patterns of HIV-1 isolate neutralization. Science 340:751–756. 10.1126/science.1233989 [DOI] [PubMed] [Google Scholar]
- 28.Ofek G, Guenaga FJ, Schief WR, Skinner J, Baker D, Wyatt R, Kwong PD. 2010. Elicitation of structure-specific antibodies by epitope scaffolds. Proc. Natl. Acad. Sci. U. S. A. 107:17880–17887. 10.1073/pnas.1004728107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Otwinowski Z, Minor W. 1997. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276:307–326. 10.1016/S0076-6879(97)76066-X [DOI] [PubMed] [Google Scholar]
- 30.McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. 2007. Phaser crystallographic software. J. Appl. Crystallogr. 40:658–674. 10.1107/S0021889807021206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guex N, Peitsch MC. 1997. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18:2714–2723. 10.1002/elps.1150181505 [DOI] [PubMed] [Google Scholar]
- 32.Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC. 2002. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. Sect. D Biol. Crystallogr. 58:1948–1954. 10.1107/S0907444902016657 [DOI] [PubMed] [Google Scholar]
- 33.Emsley P, Cowtan K. 2004. Coot: model-building tools for molecular graphics. Acta Crystallogr. Sect. D Biol. Crystallogr. 60:2126–2132. 10.1107/S0907444904019158 [DOI] [PubMed] [Google Scholar]
- 34.Davis IW, Leaver-Fay A, Chen VB, Block JN, Kapral GJ, Wang X, Murray LW, Arendall WB, Snoeyink J, Richardson JS, Richardson DC. 2007. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic acids research 35:W375–W383. 10.1093/nar/gkm216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. 2001. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc. Natl. Acad. Sci. U. S. A. 98:10037–10041. 10.1073/pnas.181342398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDonald IK, Thornton JM. 1994. Satisfying hydrogen-bonding potential in proteins. J. Mol. Biol. 238:777–793. 10.1006/jmbi.1994.1334 [DOI] [PubMed] [Google Scholar]
- 37.Krissinel E, Henrick K. 2007. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372:774–797. 10.1016/j.jmb.2007.05.022 [DOI] [PubMed] [Google Scholar]
- 38.Winn MD, Ballard CC, Cowtan KD, Dodson EJ, Emsley P, Evans PR, Keegan RM, Krissinel EB, Leslie AG, McCoy A, McNicholas SJ, Murshudov GN, Pannu NS, Potterton EA, Powell HR, Read RJ, Vagin A, Wilson KS. 2011. Overview of the CCP4 suite and current developments. Acta Crystallogr. Sect. D Biol. Crystallogr. 67:235–242. 10.1107/S0907444910045749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, Bilska M, Kothe DL, Salazar-Gonzalez JF, Wei X, Decker JM, Hahn BH, Montefiori DC. 2005. Human immunodeficiency virus type 1 Env clones from acute and early subtype b infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 79:10108–10125. 10.1128/JVI.79.16.10108-10125.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brugger B, Glass B, Haberkant P, Leibrecht I, Wieland FT, Krausslich HG. 2006. The HIV lipidome: a raft with an unusual composition. Proc. Natl. Acad. Sci. U. S. A. 103:2641–2646. 10.1073/pnas.0511136103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snider C, Jayasinghe S, Hristova K, White SH. 2009. MPEx: a tool for exploring membrane proteins. Protein Sci. 18:2624–2628. 10.1002/pro.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wimley WC, White SH. 1996. Experimentally determined hydrophobicity scale for proteins at membrane interfaces. Nat. Struct. Biol. 3:842–848. 10.1038/nsb1096-842 [DOI] [PubMed] [Google Scholar]
- 43.Chuang GY, Acharya P, Schmidt SD, Yang Y, Louder MK, Zhou T, Kwon YD, Pancera M, Bailer RT, Doria-Rose NA, Nussenzweig MC, Mascola JR, Kwong PD, Georgiev IS. 2013. Residue-level prediction of HIV-1 antibody epitopes based on neutralization of diverse viral strains. J. Virol. 87:10047–10058. 10.1128/JVI.00984-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doria-Rose NA, Georgiev I, O'Dell S, Chuang GY, Staupe RP, McLellan JS, Gorman J, Pancera M, Bonsignori M, Haynes BF, Burton DR, Koff WC, Kwong PD, Mascola JR. 2012. A short segment of the HIV-1 gp120 V1/V2 region is a major determinant of resistance to V1/V2 neutralizing antibodies. J. Virol. 86:8319–8323. 10.1128/JVI.00696-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. 2010. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66:213–221. 10.1107/S0907444909052925 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.