Abstract
Coxsackieviruses require phosphatidylinositol-4-kinase IIIβ (PI4KIIIβ) for replication but can bypass this need by an H57Y mutation in protein 3A (3A-H57Y). We show that mutant coxsackievirus is not outcompeted by wild-type virus during 10 passages in vitro. In mice, the mutant virus proved as virulent as wild-type virus, even when mice were treated with a PI4KIIIβ inhibitor. Our data suggest that upon emergence, the 3A-H57Y mutant has the fitness to establish a resistant population with a virulence similar to that of wild-type virus.
TEXT
Enteroviruses, belonging to the family of the Picornaviridae, are small, positive-stranded RNA viruses and include many important human pathogens, including poliovirus, rhinovirus, coxsackievirus, and enterovirus 71. Since the genus Enterovirus comprises more than 250 (sero)types, the development of a panenterovirus vaccine will be unfeasible. Hence, the development of broad-spectrum antienteroviral drugs will be crucial for disease control. Like most RNA viruses, enteroviruses have a high mutation rate, resulting in a rapid emergence of drug resistance which eventually could lead to therapy failure. Drugs that target host factors that are crucial for virus replication are believed to impede this process. In recent years, several studies identified the essential membrane-modifying host cell factor phosphatidylinositol-4-kinase IIIβ (PI4KIIIβ) as the target of broad-spectrum and highly potent inhibitors of enterovirus replication (1–8). However, following lengthy selection processes, coxsackievirus B3 (CVB3) mutants that are resistant to these inhibitors emerged and were shown to harbor single point mutations in the nonstructural protein 3A (4, 7, 8). The H57Y mutation (3A-H57Y) was identified most frequently and provided the strongest resistance against PI4KIIIβ inhibitors, as well as to depletion of this factor. This indicates that the mutant coxsackievirus can bypass this host factor and/or the need for its product, phosphatidylinositol-4-phosphate (PI4P), in cell culture (8).
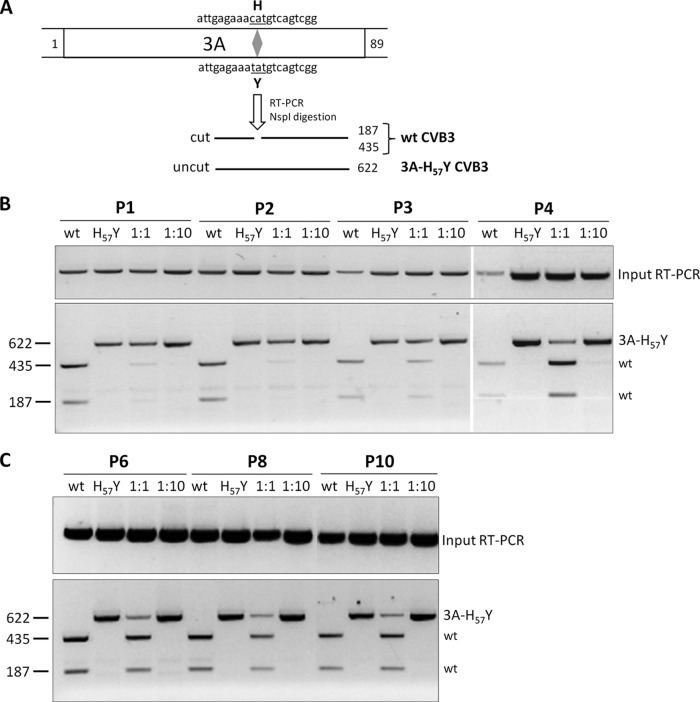
The aim of this study was to explore the fitness of this mutant virus that can replicate independently of a host factor that is essential for the replication of the wild-type (wt) virus. We therefore studied whether the PI4KIIIβ-independent CVB3 mutant would be outgrown by wt virus within a mixed population. Previously, we performed a side-by-side comparison of the replication kinetics of both viruses in a single replication cycle in vitro. CVB3 3A-H57Y was shown to grow with kinetics similar to those of the wt virus in the absence of a PI4KIIIβ inhibitor, suggesting that neither virus is able to outcompete the other (8). However, single-replication-cycle assays do not allow the detection of slight differences in the growth kinetics between two virus populations. Therefore, a multicycle growth-competition experiment was carried out, in which BGM cells were infected at a multiplicity of infection of 0.001 with either wt CVB3 (strain Nancy) or CVB3 3A-H57Y or were coinfected with a mixture of the viruses at a ratio of 1:1 or 1:10 (wt/mutant). When cytopathic effect became apparent, the virus cultures were harvested by freeze-thawing and were titrated for infectious virus content. This process was repeated for 10 consecutive passages, and the ratio of mutant to wt virus was analyzed in every passage. To this end, viral RNA was isolated (Qiagen), reverse transcription (RT)-PCR was carried out using random hexamer primers (Roche), and the resulting cDNA (Fig. 1B and C, RT-PCR input) was then used as a template in a PCR. This reaction was performed with primers designed around the H57Y mutation (forward primer, 5′-TAA TAC GAC TCA CTA TAG GAT AGC GTG GGG ACC ACG-3′, and reverse primer, 5′-ATT AAC CTC CAC TTC CTC CTT GGC-3′) to yield a 622-nucleotide (nt) DNA product. Next, a genotype-specific digestion was performed using restriction enzyme NspI, which only cleaves cDNA amplified from the wt CVB3, resulting in two fragments of 435 nt and 187 nt (Fig. 1A). Consequently, RNA isolated from CVB3 3A-H57Y is represented by a single 622-nt product (Fig. 1A).
FIG 1.
(A) Schematic representation of the basis of RT-PCR for analysis of a coinfection growth-competition experiment between wt and 3A-H57Y CVB3. BGM cells were infected either with wt or mutant CVB3 or with a mixture of both viruses (ratios of 1:1 and 1:10) for 10 consecutive passages. Viral RNAs were extracted after every passage, and RT-PCR products were digested with NspI and visualized on a 1% agarose gel using ethidium bromide. Cleavage products are indicated with lines. (B) Gels for passages 1 to 4 (P1 to P4). (C) Gels for passages 6, 8, and 10. (Top) Input cDNA for restriction analysis following RT-PCR.
When wt virus was mixed with an equal amount of CVB3 3A-H57Y, products from both viruses could be readily detected during 10 consecutive passages. We observed that after 3 passages, the viral RNA ratio shifted from favoring CVB3 3A-H57Y toward favoring wt CVB3 (Fig. 1B and C). However, it remains unclear why this shift occurred. Nevertheless, CVB3 3A-H57Y was not outcompeted by the wt virus throughout the course of the experiment. This finding was further substantiated by using a 10-fold excess of mutant over wt CVB3 (1:10 ratio, wt to mutant), which revealed the sole presence of the CVB3 3A-H57Y product. Altogether, these observations suggest that upon emergence, CVB3 3A-H57Y may have the fitness to establish a resistant virus population, at least in vitro.
A comparable fitness of two virus variants in vitro does not necessarily imply a comparable virulence in the infected host. Importantly, these growth competition experiments were performed in the absence of a PI4KIIIβ inhibitor. Since we previously demonstrated that the presence of such inhibitor induced a 1- to 2-h delay in a single replication cycle of the mutant virus in vitro, we wondered whether this delay in vitro might be translated to an impaired virulence in vivo (8). To this end, we studied the replication of both viruses in a CVB-induced pancreatitis mouse model that has been described previously (9). In this model, virus-induced pancreatic tissue damage is the most prominent symptom and, hence, it served as the basis to study viral fitness. Employing this model, we compared the virulence and pathogenicity of wt CVB3 and CVB3 3A-H57Y, either in the absence or presence of compound 2 (a recently published PI4KIIIβ inhibitor of enterovirus replication both in vitro and in vivo [7]). Mice (n = 5 per group) were infected intraperitoneally with equivalent amounts (5 × 105 TCID50/ml) of either wt virus or CVB3 3A-H57Y, which was previously determined to result in a peak of pancreatic symptoms at day 3 postinfection (data not shown). Immediately after infection, mice were treated (via the subcutaneous route) twice daily with 50 mg/kg of body weight/day of compound 2 or were left untreated (Fig. 2). At 3 days postinfection, the mice were sacrificed, and following extensive transcardial perfusion with phosphate-buffered saline, the pancreas was removed. One part of the pancreas was fixed in 4% formaldehyde and embedded in paraffin. Tissue sections were stained with hematoxylin and eosin, and histopathological damage was quantified using a standardized scoring system ranging from 0 (absence of damage) to 3 (diffuse damage throughout the tissue) (10). Histopathological analysis revealed similar scores for interstitial inflammation, necrosis, and edema in mice infected with either wt virus or 3A-H57Y CVB3. Compound 2 provided complete protection against all parameters of pancreatitis in mice infected with wt CVB3, whereas no protective effects were observed in mice infected with CVB3 3A-H57Y (Fig. 2A). Interestingly, the signs of inflammation in these mice were comparable to those of untreated infected mice, suggesting a similar virulence even in the presence of the compound.
FIG 2.
Effects of compound 2 in CVB3-induced pancreatitis. Mice were infected with wt or 3A-H57Y CVB3 and treated subcutaneously twice a day with 50 mg/kg/day of compound 2 or were left untreated. Effects on histopathology scores (A), virus replication (B), and biochemical parameters for pancreatitis (C) are shown. Data are presented as means ± standard deviations (n = 5). Statistical significance was calculated by means of the Mann-Whitney U test.
Another part of the pancreas was homogenized in saline using mechanical disruption (Precellys 24; Bertin) to quantify infectious virus titers by means of endpoint titrations, expressed as the 50% cell culture infectious dose (TCID50) per ml (Fig. 2B). As a marker for pancreatitis, we also quantified the serum amylase and lipase levels, using an enzymatic colorimetric reaction (Cobas/Roche) on the modular P analyzer (Roche Diagnostics). To this end, 500 μl of blood was collected by cardiac puncture before the transcardial perfusion, and following 1 h of incubation at room temperature, blood was centrifuged at 13,000 rpm for 10 min (Fig. 2C). Similar to the histopathological analysis, the virus titers and amylase/lipase levels of untreated infected animals did not differ significantly from those of the treated, CVB3 3A-H57Y-infected animals. On the contrary, mice treated with compound 2 and infected with wt CVB3 exhibited a marked reduction of virus titers (∼4 log10) and normalization of amylase and lipase levels.
Overall, our data demonstrate that, upon acquiring an H57Y mutation in protein 3A, CVB3 exhibited a reduced drug susceptibility to PI4KIIIβ inhibitors not only in vitro but also in the infected host. Although our previous in vitro studies suggested a reduced fitness of this mutant CVB3 in the presence of a PI4KIIIβ inhibitor, this was not translated to an impaired virulence or pathogenicity in vivo (either in in the absence or presence of such an inhibitor). Furthermore, we show that in a 1:1 mixture with wt virus, CVB3 3A-H57Y was not outcompeted by wt virus but remained present in the viral population throughout the course of the experiment. Collectively, these data suggest that upon the emergence of CVB3 3A-H57Y in vivo under the selective pressure of a PI4KIIIβ inhibitor, this mutant virus has sufficient fitness to compete with wt virus and to establish a resistant virus population with a similar virulence. However, given the lengthy selection process in vitro and the fact that, thus far, only one 3A-H57Y isolate has been recovered from a patient (GenBank accession number JQ040513), it remains questionable whether this mutation would emerge during a short-acting treatment of these acute infections in infected patients.
ACKNOWLEDGMENTS
We acknowledge Carolien De Keyzer and Kim Donckers for their excellent assistance in performing the animal experiments and generating the in vivo data.
The research leading to these results has received funding from the European Union Seventh Framework Program (FP7/2007-2013) under SILVER grant agreement number 260644, the KU Leuven Geconcerteerde Onderzoeksactie (GOA/10/014), the Netherlands Organization for Scientific Research (NWO-VENI-863.12.005 to H.M.V.D.S. and NWO-VICI-91812628 to F.J.M.V.K.) and the European Union 7th Framework Program (EUVIRNA Marie Curie Initial Training Network), grant agreement number 264286.
Footnotes
Published ahead of print 26 December 2013
REFERENCES
- 1.Arita M, Kojima H, Nagano T, Okabe T, Wakita T, Shimizu H. 2011. Phosphatidylinositol 4-kinase III beta is a target of enviroxime-like compounds for antipoliovirus activity. J. Virol. 85:2364–2372. 10.1128/JVI.02249-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arita M, Takebe Y, Wakita T, Shimizu H. 2010. A bifunctional anti-enterovirus compound that inhibits replication and the early stage of enterovirus 71 infection. J. Gen. Virol. 91:2734–2744. 10.1099/vir.0.023374-0 [DOI] [PubMed] [Google Scholar]
- 3.Arita M, Wakita T, Shimizu H. 2009. Cellular kinase inhibitors that suppress enterovirus replication have a conserved target in viral protein 3A similar to that of enviroxime. J. Gen. Virol. 90:1869–1879. 10.1099/vir.0.012096-0 [DOI] [PubMed] [Google Scholar]
- 4.De Palma AM, Thibaut HJ, van der Linden L, Lanke K, Heggermont W, Ireland S, Andrews R, Arimilli M, Al-Tel TH, De Clercq E, van Kuppevald F, Neyts J. 2009. Mutations in the nonstructural protein 3A confer resistance to the novel enterovirus replication inhibitor TTP-8307. Antimicrob. Agents Chemother. 53:1850–1857. 10.1128/AAC.00934-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heinz BA, Vance LM. 1995. The antiviral compound enviroxime targets the 3A coding region of rhinovirus and poliovirus. J. Virol. 69:4189–4197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heinz BA, Vance LM. 1996. Sequence determinants of 3A-mediated resistance to enviroxime in rhinoviruses and enteroviruses. J. Virol. 70:4854–4857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Schaar HM, Leyssen P, Thibaut HJ, de Palma A, van der Linden L, Lanke KH, Lacroix C, Verbeken E, Conrath K, Macleod AM, Mitchell DR, Palmer NJ, van de Poel H, Andrews M, Neyts J, van Kuppeveld FJ. 2013. A novel, broad-spectrum inhibitor of enterovirus replication that targets host cell factor phosphatidylinositol 4-kinase IIIβ. Antimicrob. Agents Chemother. 57:4971–4981. 10.1128/AAC.01175-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Schaar HM, van der Linden L, Lanke KH, Strating JR, Purstinger G, de Vries E, de Haan CA, Neyts J, van Kuppeveld FJ. 2012. Coxsackievirus mutants that can bypass host factor PI4KIIIbeta and the need for high levels of PI4P lipids for replication. Cell Res. 22:1576–1592. 10.1038/cr.2012.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Palma AM, Verbeken E, Van Aelst I, Van den Steen PE, Opdenakker G, Neyts J. 2008. Increased gelatinase B/matrix metalloproteinase 9 (MMP-9) activity in a murine model of acute coxsackievirus B4-induced pancreatitis. Virology 382:20–27. 10.1016/j.virol.2008.08.046 [DOI] [PubMed] [Google Scholar]
- 10.De Palma AM, Thibaut HJ, Li S, Van AI, Dillen C, Swinnen M, Verbeken E, Neyts J, Opdenakker G. 2009. Inflammatory rather than infectious insults play a role in exocrine tissue damage in a mouse model for coxsackievirus B4-induced pancreatitis. J. Pathol. 217:633–641. 10.1002/path.2501 [DOI] [PubMed] [Google Scholar]