ABSTRACT
H2 influenza viruses have not circulated in humans since 1968, and therefore a significant portion of the population would be susceptible to infection should H2 influenza viruses reemerge. H2 influenza viruses continue to circulate in avian reservoirs worldwide, and these reservoirs are a potential source from which these viruses could emerge. Three reassortant cold-adapted (ca) H2 pandemic influenza vaccine candidates with hemagglutinin (HA) and neuraminidase (NA) genes derived from the wild-type A/Japan/305/1957 (H2N2) (Jap/57), A/mallard/6750/1978 (H2N2) (mal/78), or A/swine/MO/4296424/2006 (H2N3) (sw/06) viruses and the internal protein gene segments from the A/Ann Arbor/6/60 ca virus were generated by plasmid-based reverse genetics (Jap/57 ca, mal/78 ca, and sw/06 ca, respectively). The vaccine candidates exhibited the in vitro phenotypes of temperature sensitivity and cold adaptation and were restricted in replication in the respiratory tract of ferrets. In mice and ferrets, the vaccines elicited neutralizing antibodies and conferred protection against homologous wild-type virus challenge. Of the three candidates, the sw/06 ca vaccine elicited cross-reactive antibodies and provided significant protection against the greatest number of heterologous viruses. These observations suggest that the sw/06 ca vaccine should be further evaluated in a clinical trial as an H2 pandemic influenza vaccine candidate.
IMPORTANCE Influenza pandemics arise when novel influenza viruses are introduced into a population with little prior immunity to the new virus and often result in higher rates of illness and death than annual seasonal influenza epidemics. An influenza H2 subtype virus caused a pandemic in 1957, and H2 viruses circulated in humans till 1968. H2 influenza viruses continue to circulate in birds, and the development of an H2 influenza vaccine candidate is therefore considered a priority in preparing for future pandemics. However, we cannot predict whether a human H2 virus will reemerge or a novel avian H2 virus will emerge. We identified three viruses as suitable candidates for further evaluation as vaccines to protect against H2 influenza viruses and evaluated the immune responses and protection that these three vaccines provided in mice and ferrets.
INTRODUCTION
Influenza pandemics arise from an antigenic shift during which a new hemagglutinin (HA) is introduced into a population with little preexisting immunity to the new subtype (1) and often results in substantially higher morbidity and mortality than with annual seasonal influenza epidemics. Pandemic influenza preparedness planning has focused on highly pathogenic H5 and H7 avian influenza viruses. The emergence of the novel swine-origin H1N1 influenza virus in 2009, however, underscores the need to include other influenza subtypes in pandemic preparedness planning.
Of the 18 HA influenza A virus subtypes that have been identified to date, only H1, H2, and H3 have been known to cause influenza pandemics (2, 3), suggesting that these subtypes are capable of sustained transmission in humans. Although H1 and H3 viruses have cocirculated in humans since 1977, H2 influenza viruses have not circulated in humans since 1968 (1). A large segment of the population would thus likely be susceptible to infection should an H2 influenza virus reemerge (4). In addition, as H2 subtype viruses continue to circulate in avian reservoirs worldwide (5–9), they remain a potential pandemic threat. The 1957 H2 pandemic virus was a reassortant that derived the HA, neuraminidase (NA), and PB1 genes from an avian influenza virus and the remaining gene segments from a previously circulating human H1N1 influenza virus (10–12). The development of an H2 influenza vaccine candidate is therefore considered a priority in pandemic preparedness planning.
As it is unlikely that a previously selected vaccine virus will exactly match the pandemic virus, the ability to elicit a broadly cross-reactive antibody response to antigenically distinct viruses within a subtype is an important consideration in the selection of a pandemic influenza vaccine candidate. Earlier studies have examined the ability of inactivated avian H2 influenza viruses to provide cross-protection against mouse-adapted variants of reassortant human influenza viruses and a mouse-adapted avian H2 influenza virus, A/black duck/NJ/1580/1978 (13). As live attenuated influenza vaccines (LAIV) have been observed to confer a greater breadth of heterologous cross-protection in naive hosts (14–17), we evaluated the cold-adapted (ca) A/Ann Arbor/6/1960 (AA ca) virus, an H2N2 influenza virus used as the backbone of the seasonal live attenuated influenza A vaccine currently licensed in the United States. We demonstrated that the AA ca vaccine was efficacious against heterologous virus challenge in mice and ferrets (18). In a subsequent phase I clinical trial, however, the AA ca vaccine was noted to be highly restricted in replication and minimally immunogenic in adults (19). We proceeded to conduct a more extensive evaluation of H2 influenza viruses to identify additional H2 vaccine candidates and selected a group of geographically and temporally diverse H2 influenza viruses from both humans and animal species (20). We observed that the wild-type (wt) A/Japan/305/1957 (H2N2) (Jap/57), A/mallard/6750/1978 (H2N2) (mal/78), and A/swine/MO/4296424/2006 (H2N3) (sw/06) viruses induced the most broadly cross-reactive antibody responses against the panel of H2 viruses (20) and concluded that these three viruses were suitable candidates for further evaluation as live attenuated vaccines to protect against H2 influenza viruses.
The goals of this study were to generate live attenuated cold-adapted H2 vaccines from the wt Jap/57, mal/78, and sw/06 viruses and to compare the immunogenicity of these cold-adapted vaccines and the protection they confer against heterologous challenge in mice and ferrets.
MATERIALS AND METHODS
Viruses.
The A/Swine/MO/4296424/2006 wt (H2N3) influenza virus used in this study was generously provided by Adolfo Garcia-Sastre, Mt. Sinai School of Medicine, New York, NY, and Juergen Richt, ARS-USDA, Ames, IA. Wild-type virus stocks were propagated in the allantoic cavity of 9- to 11-day-old specific-pathogen-free (SPF) embryonated hen's eggs (Charles River Laboratories, Franklin, CT) at 37°C. The 50% tissue culture infectious dose (TCID50) titers were determined in Madin-Darby canine kidney (MDCK) cells (ATCC, Manassas, VA). Viral titers were calculated using the Reed and Muench method (21).
The AA ca virus was obtained from MedImmune. The HA and NA genes derived from either the Jap/57 wt, mal/78 wt, or sw/06 wt viruses and the internal protein gene segments from the AA ca virus were combined by plasmid-based reverse genetics as previously described (22) to generate reassortant ca vaccines (Jap/57 ca, mal/78 ca, and sw/06 ca, respectively). We also generated two 7:1 ca reassortant vaccines containing the HA or NA gene of the sw/06 virus [sw/06 7:1(HA) ca and sw/06 7:1(NA) ca], with the remaining gene segments from the AA ca virus. Each of the ca vaccines were generated by MedImmune and completely sequenced to confirm that they possessed the loci in the PB2, PB1, and NP gene segments that confer the phenotypic properties of the AA ca donor virus. All experiments involving wt sw/06 were conducted in biosafety level 3 (BSL3) containment laboratories at the National Institutes of Health, approved for use by the Centers for Disease Control and Prevention.
Animals.
Four- to 6-week-old female BALB/c mice (Taconic Farms, Inc., Germantown, NY) were used in all mouse experiments. Six- to 10-week-old ferrets (Triple F Farms, Sayre, PA) were used in the ferret studies. All animal experiments were approved by the National Institutes of Health Animal Care and Use Committee or MedImmune's IACUC Committee.
Evaluation of the replication of ca vaccine viruses.
We compared the replication of each of the ca vaccine viruses (Jap/57 ca, mal/78 ca, and sw/06 ca) with the homologous wt viruses (Jap/57 wt, mal/78 wt, and sw/06 wt) and to the AA ca and AA wt viruses in the respiratory tract of ferrets to assess virus attenuation. We administered a single dose of 200 μl of 107 TCID50 of each ca and wt virus to groups of three 8-week-old ferrets. On day 3 following infection, lungs and nasal turbinates were harvested, weighed, and homogenized in L-15 medium (Invitrogen-GIBCO) containing antibiotic-antimycotic (penicillin, streptomycin, and amphotericin B) (Invitrogen-GIBCO) to make 5% (wt/vol) (nasal turbinates [NT]) or 10% (wt/vol) (lungs) tissue homogenates. Tissue homogenates were clarified by centrifugation and titrated in 24- and 96-well tissue culture plates containing MDCK cell monolayers. An additional, more detailed analysis of the replication and associated histopathologic changes following infection with sw/06 wt and ca viruses was undertaken in ferrets, with the AA wt and ca viruses as controls.
Evaluation of immunogenicity and protection against wt virus challenge. (i) Mice.
To determine the immunogenicity of each of the H2 vaccines and the protection they confer against challenge with the homologous and heterologous H2 wt viruses in mice, we administered 50 μl of 1 or 2 doses of 106 TCID50 AA ca, Jap/57 ca, mal/78 ca, or sw/06 ca vaccine virus intranasally to groups of 4 mice that were lightly anesthetized with isoflurane and challenged them with 105 TCID50 AA wt, Jap 57 wt, mal/78 wt, or sw/06 wt 1 month following the final dose of vaccine. Control groups were immunized with L-15 medium. On day 3 following challenge, lungs and nasal turbinates were harvested, weighed, and homogenized in L-15 medium containing antibiotic-antimycotic (penicillin, streptomycin, and amphotericin B) to make 10% (wt/vol) tissue homogenates. Tissue homogenates were clarified by centrifugation and titrated in 24- and 96-well tissue culture plates containing MDCK cell monolayers.
We collected serum from mice prior to immunization, 1 month following dose 1 and 1 month following dose 2, prior to challenge. Neutralizing antibody titers in pre- and postimmunization sera were determined in a microneutralization (MN) assay. Serial 2-fold dilutions of heat-inactivated serum were prepared starting from a 1:10 or 1:20 dilution. Equal volumes of serum and virus were mixed and incubated for 60 min at room temperature. The residual infectivity of the virus-serum mixture was determined in MDCK cells in four replicates for each dilution. Neutralizing antibody titers were defined as the reciprocal of the highest dilution of serum that completely neutralized the infectivity of 100 TCID50 of the virus as determined by the absence of cytopathic effect (CPE) at day 4.
(ii) Ferrets.
To evaluate the immunogenicity of each of the H2 vaccines and the protection they confer against challenge with the homologous and heterologous H2 wt viruses in ferrets, a single dose of 200 μl of 107 TCID50 AA ca, Jap/57 ca, or sw/06 ca virus was administered intranasally to groups of three 6-week-old ferrets lightly anesthetized with isoflurane. Control groups were mock immunized with 1× sucrose-phosphate-glutamate buffer (SPG; 218 mM sucrose-11 mM potassium phosphate buffer-5 mM monosodium glutamate, pH 7.0). All ferrets were bled before administration of virus, and serum was collected between 24 and 31 days postimmunization. Neutralizing antibody titers in pre- and postimmunization ferret sera were determined in an MN assay as described above. The ferrets were challenged with 106 TCID50 of the AA wt, Jap 57 wt, mal/78 wt, or sw/06 wt virus 1 month following vaccination. On day 3 following challenge, nasal turbinates as well as the left and right lower lobes of the lung were harvested from all ferrets. Virus titers in 10% (wt/vol) tissue homogenates of the nasal turbinates and lungs were determined in MDCK cells as described above.
Evaluation of the contribution of the HA and NA genes to replication of sw/06 ca reassortant virus in mice and ferrets.
Following the demonstration of the immunogenicity of the vaccine viruses and the protection they conferred, we evaluated the contribution of the HA and NA genes to the replication of the sw/06 ca virus in mice and ferrets by comparing the level of replication and histopathologic changes following infection with sw/06 ca reassortant viruses bearing the sw06 HA and/or NA gene segments in an AA ca virus background in the respiratory tract of mice and ferrets.
In mice, we administered a single dose of 50 μl of 106 TCID50 of sw/06 wt, sw/06 ca, the single gene reassortant viruses bearing the HA or NA of the sw/06 virus in an AA ca virus called sw/06 7:1(HA) ca or sw/06 7:1(NA) ca, respectively, and the AA ca and AA wt viruses to groups of six 6-week-old BALB/c mice and harvested organs from 4 mice each at days 2, 4, and 7 postinoculation. Virus titers were determined in MDCK cells as described above.
In addition, we also compared the level of viral replication of the 7:1 sw/06 ca reassortants to the sw/06 wt virus and the sw/06 ca vaccine in the respiratory tract of ferrets. We administered a single dose of 200 μl of 107 TCID50 of the sw/06 ca, sw/06 7:1(HA) ca, sw/06 7:1(NA) ca, or sw/06 wt to groups of three 8-week-old ferrets and harvested organs at day 3 postinoculation. In a separate study, we administered a single dose of 200 μl of 107 TCID50 of AA ca or AA wt viruses to groups of three 7-week-old ferrets and harvested organs at day 3 postinoculation. Virus titers in the nasal turbinates and lungs were determined in MDCK cells as described above.
Histopathology.
Tissue sections (4 to 7 μm) from formalin-fixed paraffin-embedded lungs were stained with H&E for histopathological evaluation. Histopathology was evaluated at the airway and interstitium/alveoli levels and also based on the presence of peribronchovascular cuffing. In mice, airway histopathology scores were based on the percentage of necrotic bronchioles found in 5 lung sections from each animal; interstitial inflammation and infiltration of alveolar spaces were scored from 1 to 5, with 0 being within normal limits and 5 defined as the presence of diffuse inflammation and extensive necrosis along with the presence of suppurative exudate in alveolar spaces and extensive areas of consolidation. Peribronchovascular cuffing was estimated subjectively, and grading ranged from none to numerous bronchioles and blood vessels cuffed by mononuclear cells. Immunohistochemistry (IHC) was performed to determine the presence and distribution of viral antigen in selected cases using a commercially available polyclonal goat antibody (Serotec OBT1551) against influenza A virus, strain USSR (H1N1), that recognizes H2N2 antigens; the bound antibody was detected using an alkaline phosphatase polymer system and Vulcan fast red as the chromogen.
RESULTS AND DISCUSSION
Several factors underscore the need for the development of an H2 pandemic influenza vaccine candidate, including the proven capability of the H2 subtype for sustained transmission in humans, the persistence of this subtype in avian reservoirs worldwide, and the waning immunity of the human population. However, we cannot predict whether a human H2 virus will reemerge or a novel avian H2 virus will emerge. Based on genetic analysis and antigenic cross-reactivity of postinfection sera of mice and ferrets infected with 15 H2 viruses (20), we selected three viruses, Jap/57, mal/78, and sw/06, for vaccine development. We evaluated their immunogenicity and protection compared with the AA ca virus as live attenuated pandemic influenza vaccine candidates in mice and ferrets.
Generation and evaluation of reassortant ca viruses in ferrets.
The 6:2 ca reassortant viruses containing the HA and NA gene segments from an H2 wt virus and the six internal protein gene segments from A/Ann Arbor/6/60 ca virus were produced and characterized. An alignment of the predicted amino acid sequences of the HA1 domains of the selected viruses is presented in Fig. 1. There are a number of amino acid differences in the HA sequences among these H2 viruses; AA is more related to Jap/57 and mal/78 is closer to sw/06. All three H2 ca viruses displayed the ca and temperature-sensitive (ts) phenotypes that are conferred by the mutations in the AA ca virus backbone (data not shown).
FIG 1.
HA1 amino acid sequence alignment of the indicated H2N2 viruses. The nonconserved residues are highlighted.
The replication of each ca vaccine virus in the respiratory tract of ferrets on day 3 postinfection is summarized in Fig. 2. In the upper respiratory tract of ferrets, the levels of replication of the AA and mal/78 ca and homologous wt viruses were similar. In contrast, the replication of the Jap/57 and sw/06 ca viruses was significantly lower than that of the corresponding wt viruses. Virus was not detected in the lower respiratory tract of ferrets inoculated with any ca vaccine virus, demonstrating that the ca viruses were restricted in replication and therefore attenuated for ferrets.
FIG 2.
Level of replication of each ca vaccine virus compared with the corresponding wt virus and compared with AA ca and AA wt viruses in the upper (NT, circles) and lower (lungs, square symbols) respiratory tracts of ferrets. Lightly anesthetized ferrets were inoculated intranasally with 107 TCID50 of each virus in a 0.2-ml volume, and tissues were harvested on day 3 postinfection. The dashed horizontal line represents the lower limit of detection. The P values from a t test are included above when significant differences were present.
Replication of the 6:2 sw/06 ca and 7:1 sw/06 ca reassortant viruses in the respiratory tract of ferrets and mice.
Because the sw/06 wt virus was virulent for pigs and mice, we proceeded to more comprehensively assess the level of replication of the sw/06 ca virus in the respiratory tract of ferrets and mice. In the upper respiratory tract of ferrets, the peak of replication for the sw/06 wt virus occurred on day 1 postinfection (mean virus titer = 108.3 TCID50/g), whereas the peak of replication for the sw/06 ca vaccine virus was on day 5 postinfection (mean virus titer = 106.6 TCID50/g) (Table 1). In this study, sw/06 ca and AA ca viruses replicated efficiently in the upper respiratory tract of ferrets, to titers that were higher than the data shown in Fig. 2, likely a reflection of the variability in outbred species. In the lower respiratory tract of ferrets, the sw/06 wt virus replicated efficiently, achieving a mean peak titer of 105.9 TCID50/g on day 3 postinfection, whereas the sw/06 ca vaccine virus was not detectable in the lungs of ferrets at any of the time points examined. The AA wt virus that was included as a control did not replicate efficiently in the lungs of ferrets (peak titer of 102.4 TCID50 on day 1 postinfection) and was not detected beyond day 3 postinfection. The AA ca virus was not detectable in the lungs of ferrets at any time point postinfection. These data show that these H2 viruses had different replication kinetics and that the sw/06 ca vaccine was restricted in replication in the lower respiratory tract of ferrets.
TABLE 1.
Level of replication and airway pathology following infection with sw/06 and AA wt and ca viruses in the respiratory tracts of ferrets
| Virus | Mean virus titera (±SE) in nasal turbinates on day postinfection: |
Mean virus titera (±SE) and pathology scoreb in lungs on day postinfection: |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
3 |
5 |
7 |
|||||||||
| 1 | 3 | 5 | 7 | Mean virus titer (±SE) | Median (range) pathology severity score | Mean virus titer (±SE) | Median (range) pathology severity score | Mean virus titer (±SE) | Median (range) pathology severity score | Mean virus titer (±SE) | Median (range) pathology severity score | |
| sw/06 wt | 8.3 ± 0.3 | 7.4 ± 0.4 | 7.2 ± 0.2 | 3.4 ± 0.3 | 5.1 ± 0.2 | 3 (3–5) | 5.9 ± 0.1 | 4 (4–5) | 5.7 ± 1.0 | 4 (3–5) | 2.2 ± 0.4 | 5 (4–5) |
| sw/06 ca | 5.3 ± 0.2 | 5.0 ± 0.3 | 6.6 ± 0.6 | 4.4 ± 1.0 | ≤1.5 | 1 (1–2) | ≤1.5 | 0 (0) | ≤1.5 | 0 (0) | ≤1.5 | 0 (0) |
| AA wt | 5.5 ± 0.2 | 5.7 ± 0.2 | 4.8 ± 1.0 | ≤1.8 | 2.4 ± 0.2 | 4 (2–4) | 1.8 ± 0.3 | 1 (0–4) | ≤1.5 | 0 (0–2) | ≤1.5 | 1 (1) |
| AA ca | 4.4 ± 0.8 | 5.3 ± 0.2 | 5.5 ± 0.2 | 2.5 ± 0.4 | ≤1.5 | 0 (0–1) | ≤1.5 | 0 (0–1) | ≤1.5 | 0 (0) | ≤1.5 | 0 (0) |
Mean virus titers (expressed in log10 TCID50/g) from 3 ferrets in each group on each of the indicated days postinfection. Titers of 1.8 log10 TCID50/g and 1.5 log10 TCID50/g represent the lower limits of detection in the nasal turbinates and lungs, respectively.
Severity score of airway pathology was defined as follows: 0, within normal limits; 1, minimal inflammation; 2, mild to moderate inflammation; 3, moderate to severe inflammation; 4, severe multifocal to coalescing inflammation; 5, severe diffuse inflammation.
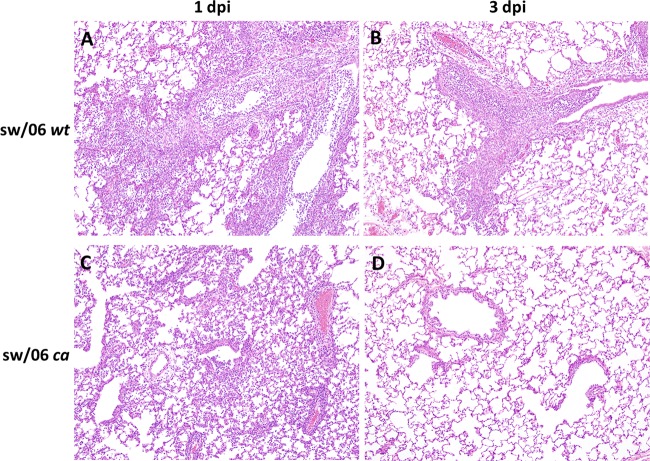
Virus-infected lung tissues were further examined for histopathological changes, and we found that the changes correlated with the level of replication for each of the viruses (Table 1). Severe inflammatory changes were seen in the ferrets that were inoculated with the sw/06 wt virus on days 1 and 3 postinfection (Fig. 3A and B). Ferrets inoculated with this virus had extensive airway involvement consisting of moderate to severe multifocal inflammation. Moderate inflammatory changes were characterized by infiltrates of macrophages, lymphocytes, and neutrophils in the alveolar walls and lumina, with scattered necrosis of airway and interstitial epithelial cells. Severe changes consisted of an interstitial pneumonia that was characterized by the presence of mononuclear cells and neutrophils, extensive parenchymal obliteration by necrosis, with occasional hyperplasia of type II pneumocytes. In contrast, the lungs of ferrets that received the sw/06 ca vaccine virus had minimal to mild/moderate inflammatory changes in the interstitium only on day 1 postinoculation (Fig. 3C) and were within normal limits on day 3 postinoculation (Fig. 3D), in the absence of any detectable replication of virus (data not shown). The mild inflammation observed in the lungs of ferrets infected with sw/06 ca on day 1 could be due to the inoculum (23).
FIG 3.
H&E-stained sections of lung from ferrets infected with sw/06 wt and sw/06 ca viruses and sacrificed at days 1 and 3 postinfection. Moderate to severe inflammatory changes were seen in the lungs of ferrets inoculated with the sw/06 wt virus on both 1 (A) and 3 (A) days postinfection. In contrast, minimal to mild inflammation is present only in the lungs of ferrets infected the sw/06 ca vaccine virus on day 1 postinfection (C) and not on day 3 postinfection (D). Original magnification, ×100.
We next investigated whether the HA or NA genes were important determinants of vaccine virus replication. Reassortant 7:1 ca viruses containing sw/06 HA or NA segments were produced and evaluated in mice. In the upper and lower respiratory tracts of mice (Table 2), the level of replication of the sw/06 ca reassortant viruses was significantly lower than that of the sw/06 wt virus. In the upper respiratory tract, the replication kinetics of the sw/06 ca vaccine and both of the sw/06 ca 7:1 reassortant viruses were similar; peak replication occurred on day 4 postinfection and declined by day 7 (Table 2). In the lower respiratory tract of mice, the sw/06 7:1 ca reassortants replicated to higher titers on day 4 than the sw/06 ca vaccine virus that includes both the HA and NA genes of the sw/06 virus. By day 7, virus was still detected in the lungs of mice immunized with either of the sw/06 ca 7:1 reassortants; in contrast, virus was not detected in any mouse at day 7 following sw/06 ca inoculation (Table 2). These data indicated that the presence of the sw/06 HA or NA alone enhanced viral replication in the respiratory tract of mice.
TABLE 2.
Level of replication of sw/06 ca reassortant and sw/06 wt viruses in the respiratory tract of mice
| Virus | Source of: |
Day postinfection | Virus titera (SE) in nasal turbinates | Lungs |
Lung pathology scoresb |
|||
|---|---|---|---|---|---|---|---|---|
| HA gene | NA gene | Virus titera (SE) | P value vs sw wt | Airway pathology | Airway interstitial | |||
| sw/06 wt | sw/06 wt | sw/06 wt | 2 | 6.4 (0.2) | 8.6 (0.3) | 5, 5 | 3, 5 | |
| 4 | 7.0 (0.2) | 7.6 (0.2) | 5, 4 | 5, 3 | ||||
| 7 | 6.3 (0.3) | 6.6 (0.1) | 5, 5 | 3, 5 | ||||
| sw/06 7:1(HA) ca | sw/06 wt | AA ca | 2 | 4.0c (0.2) | 6.6 (0.1) | <0.0001 | 3, 4 | 3, 5 |
| 4 | 4.7c (0.2) | 5.4 (0.6) | <0.0001 | 4, 3 | 5, 5 | |||
| 7 | 1.9c (0.1) | 2.0 (0.3) | 0.0004 | 2, 2 | 4, 2 | |||
| sw/06 7:1(NA) ca | AA ca | sw/06 wt | 2 | 3.3c (0.3) | 3.6 (0.4) | <0.0001 | 1, 1 | 0, 1 |
| 4 | 4.5c (0.3) | 4.8 (0.3) | <0.0001 | 1, 2 | 0, 0 | |||
| 7 | 2.3c (0.3) | 2.0 (0.3) | <0.0001 | 1, 1 | 0, 2 | |||
| sw/06 ca | sw/06 wt | sw/06 wt | 2 | 3.8c (0.4) | 5.1 (0.3) | <0.0001 | 1, 2 | 5, 5 |
| 4 | 4.3c (0.3) | 3.1 (0.2) | <0.0001 | 1, 2 | 5, 5 | |||
| 7 | 2.2c (0.2) | 1.5 (0) | <0.0001 | 1, 1 | 5, 4 | |||
Mean virus titers (expressed in log10 TCID50/g) from four mice in each group on each of the indicated days postinfection. Titers of 1.8 log10 TCID50/g and 1.5 log10 TCID50/g represent the lower limits of detection in the nasal turbinates and lungs, respectively.
Lung sections were examined from 2 mice in each group on days 2, 4, and 7 postinfection. Pathology scores are listed for each individual mouse.
P < 0.0001 versus sw/06 wt by 2-way analysis of variance (ANOVA).
Lung sections of virus-infected mice were stained by IHC to detect viral antigen, and, in general, the presence of viral antigen correlated well with the virus titer results. Viral antigen was present in the bronchioles and alveoli of mice inoculated with the sw/06 wt virus. In contrast, antigen was present at days 2 and 4 but not at day 7 in mice that were infected with the sw/06 7:1(HA) ca virus and was present at day 2 but not at days 4 and 7 in mice infected with the sw/06 ca virus (Table 2). Airway pathology was most severe in the group inoculated with the sw/06 wt and the sw/06 7:1(HA) ca viruses (Fig. 4A and C) but was not seen with the sw/06 ca virus bearing the same sw/06 HA and NA or sw/06 7:1(NA) ca (Fig. 4B and D). The sw/06 (HA) ca virus was associated with interstitial inflammation; this finding was observed on days 2, 4, and 7 postinfection, with all three viruses bearing the sw/06 HA: the sw/06 wt, sw/06 7:1(HA) ca, and sw/06 ca viruses (Fig. 4A, B, and C). These observations suggest that the presence of the sw/06 HA enhanced viral replication in mice.
FIG 4.
H&E-stained sections of lungs from mice infected with sw/06 wt, sw/06 ca, sw/06 7:1(HA) ca, and sw/06 7:1(NA) ca viruses and sacrificed at 4 days postinfection. Lung pathology was most severe in animals infected with the sw/06 wt and sw/06 7:1(HA) ca viruses (A and C), whereas the lungs of mice infected with the sw/06 7:1(NA) ca virus (D) appeared relatively normal. Original magnification, ×200.
In ferrets (Table 3), the sw/06 7:1(NA) ca reassortant replicated to higher titers than the sw/06 7:1(HA) ca reassortant in the upper respiratory tract but not in the lungs. The difference in level of replication of the sw/06 ca virus in the lower respiratory tract of mice and ferrets likely results from differences in core body temperature of the two hosts in relation to the shutoff temperature of the ca virus. In summary, the HA gene appeared to confer some replicative advantage in the lower respiratory tract of mice that was associated with interstitial inflammation; however, the 7:1 and 6:2 reassortants were completely restricted in the lower respiratory tract of ferrets and were attenuated. Thus, the contribution of the HA and NA to vaccine virus replication in other hosts remains to be determined.
TABLE 3.
Level of replication of sw/06 ca reassortant and sw/06 wt viruses in the respiratory tract of ferretsa
| Virus | Source of: |
Nasal turbinates |
Lungs |
|||
|---|---|---|---|---|---|---|
| HA gene | NA gene | Virus titer (SEM) | P value vs wtb | Virus titer (SEM) | P value vs wtb | |
| sw/06 wt | sw/06 wt | sw/06 wt | 7.5 (0.1) | 5.9 (1.7) | ||
| sw/06 7:1(HA) ca | sw/06 wt | AA ca | 3.1 (0.8) | 0.0009 | 1.5 (0) | 0.0009 |
| sw/06 7:1(NA) ca | AA ca | sw/06 wt | 5.5 (0.3) | NS | 1.5 (0) | 0.0009 |
| sw/06 ca | sw/06 wt | sw/06 wt | 2.0 (0.2) | 0.0001 | 1.5 (0) | 0.0009 |
Viral titers represent the mean titer (expressed in log10 TCID50/g) from 3 ferrets at day 3 postinfection. A titer of 1.5 log10 TCID50/g represents the lower limit of detection in the nasal turbinates and lungs.
Two-way ANOVA. NS, not significant.
Immunogenicity and protection against wt virus challenge. (i) Mice.
The immune responses in mice that received one or two doses of vaccine are summarized in Fig. 5. Following one dose of vaccine, mice in each group developed a modest neutralizing antibody (nAb) response to the homologous wt virus; 50 to 100% of the mal/78 ca-, Jap/57 ca-, and sw/06 ca-vaccinated mice developed low levels of neutralizing antibody against heterologous wt viruses except the AA wt virus (Fig. 5). Following two doses, all of the ca vaccines induced a robust neutralizing antibody response to the homologous wt virus (Fig. 5); sera following two doses of the vaccines were not tested against mal/78 wt virus. Mice vaccinated with Jap/57 ca and sw/06 ca viruses developed cross-reactive antibodies against one of the two heterologous viruses, while the AA ca virus did not elicit robust cross-reactive antibody titers against any of the heterologous H2 viruses.
FIG 5.
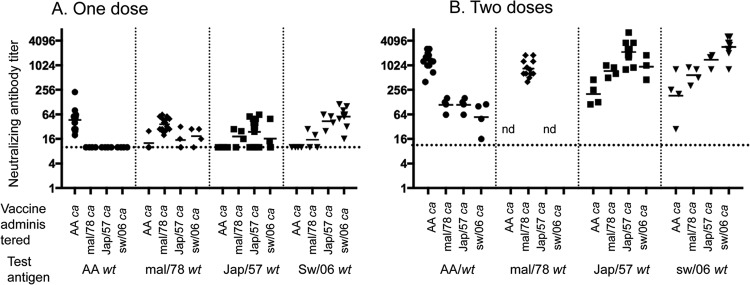
Serum-neutralizing antibody titers in mice following one or two doses of each of the H2 ca vaccines. Mice were intranasally inoculated with 1 or 2 doses of 106 TCID50 of AA ca, Jap/57 ca, mal/78 ca, or sw/06 ca vaccine and bled 1 month following the last vaccine administration. The vaccine administered and the test antigens are listed on the x axis. The neutralizing antibody titer is listed on the y axis. The symbols represent the titers in individual animals, and the bars indicate the geometric mean. The dotted line indicates the lower limit of detection. nd, not done. Antibody was not detectable (<10) on day 0 prior to vaccination. AA, A/Ann Arbor/6/60; Jap 57, A/Japan/305/1957; Mal 78, A/mallard/NY/6750/1978; Sw 06, A/Swine/MO/4296424/2006.
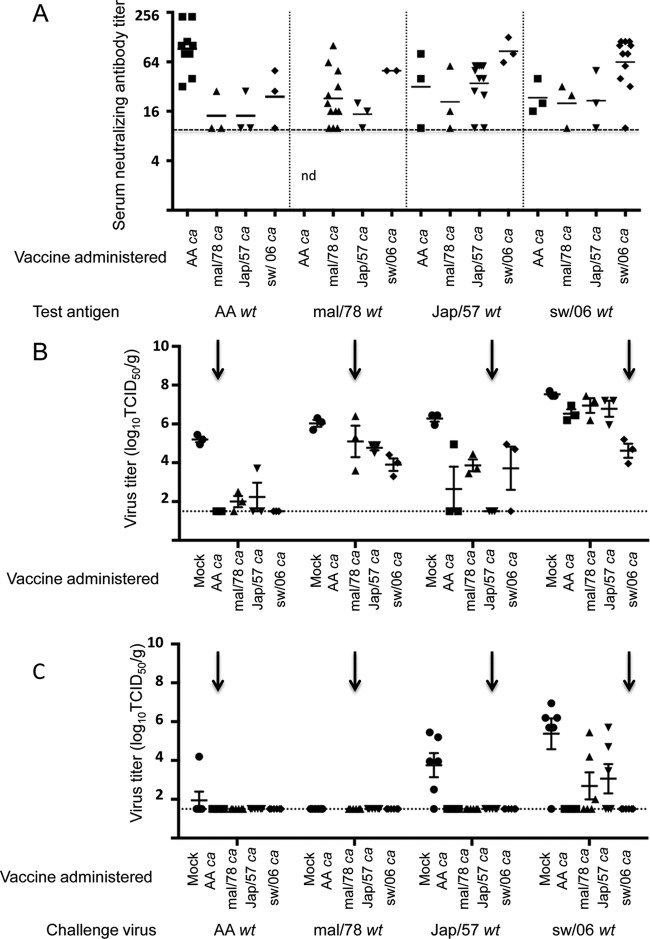
Data on protection from wt virus challenge infection following one (A, B) or two (C, D) doses of vaccine are presented in Fig. 6. In the upper respiratory tract (Fig. 6A), the AA ca vaccine conferred complete protection against the AA wt virus and statistically significant protection (P < 0.05 by Kruskal-Wallis) against Jap/57 wt virus challenge; virus was not detected in three out of four mice. The Jap/57 ca vaccine conferred complete protection only against homologous virus challenge. Notably, the mal/78 wt virus did not replicate well in the upper respiratory tract of mice, and thus the protection conferred by the vaccines against challenge with the mal/78 wt virus could not be fully evaluated. In contrast, the sw/06 ca vaccine conferred complete protection against challenge with the heterologous challenge viruses, AA wt, Jap/57 wt, and sw/06 wt viruses. In the lower respiratory tract of mice (Fig. 6B), the sw/06 ca vaccine also conferred protection against the greatest number of heterologous H2 viruses. The sw/06 ca vaccine conferred statistically significant protection (P < 0.05 by Kruskal-Wallis) against the Jap/57 wt and sw/06 wt viruses, while the AA ca vaccine conferred statistically significant protection (P < 0.05 by Kruskal-Wallis) against the AA wt and Jap/57 wt viruses. The Jap/57 ca vaccine did not confer significant protection against heterologous H2 viruses.
FIG 6.
Protection conferred by the ca vaccines against homologous and heterologous challenge in mice. Mice were intranasally inoculated with either L-15 (mock) or 1 or 2 doses of 106 TCID50/mouse of AA ca, Jap/57 ca, mal/78 ca, or sw/06 ca vaccine and challenged 1 month following the last vaccine administration with 105 TCID50/mouse of the indicated challenge virus. Virus titers were determined on day 3 postchallenge. Levels of replication of the indicated challenge viruses in the upper (A) or lower (B) respiratory tracts of mice that were challenged following 1 dose of the indicated ca vaccine. Levels of replication of the indicated challenge viruses in the upper (C) or lower (D) respiratory tracts of mice that were challenged following 2 doses of the indicated ca vaccine. Homologous challenge is indicated by a arrows. The dashed horizontal line represents the lower limit of detection.
In the upper respiratory tract, two doses of all of the ca vaccines conferred complete protection against homologous and heterologous wt virus challenge (Fig. 6C). The mal/78 wt virus was not included in this study because the virus did not replicate sufficiently well in mice to assess protective efficacy. In the lower respiratory tract, all of the vaccines conferred complete protection against homologous challenge and nearly complete protection against heterologous virus challenge; viral titers were just above the limit of detection in one and two mice, respectively, among sw/06 ca- and AA ca-vaccinated mice, following AA wt and sw/06 wt challenge, respectively (Fig. 6D).
(ii) Ferrets.
The results of the immunogenicity and protection from wt virus challenge infection in ferrets are summarized in Fig. 7. A single dose of each of the ca vaccines elicited a low to modest neutralizing antibody response in a majority of animals against the homologous wt virus. Low levels of cross-reactive neutralizing antibody titers were detected against each of the heterologous wt viruses in a proportion of the ferrets that were immunized with AA ca, Jap/57 ca, mal/78 ca, and sw/06 ca vaccines (Fig. 7A).
FIG 7.
Serum-neutralizing antibody titers and protection against homologous and heterologous challenge infection in ferrets following one dose of each of the H2 ca vaccines. Ferrets were intranasally inoculated with a dose of 107 TCID50 of AA ca, Jap/57 ca, mal/78 ca, or sw/06 ca vaccine (or SPG for the mock-immunized group) and bled and challenged 1 month later with 106 TCID50/ferret of each challenge virus. (A) Immunogenicity of the vaccines: the vaccine administered and the test antigens are listed on the x axis. The neutralizing antibody titer is listed on the y axis. The symbols represent the titers in individual animals, and the bars indicate the geometric mean. The dotted line indicates the lower limit of detection. nd, not done. Antibody was not detectable (<10) on day 0 prior to vaccination. Level of replication of each challenge virus in the upper respiratory tract (B) and lower respiratory tract (C) following 1 dose of each vaccine. Virus titers were determined on day 3 postchallenge. The dashed horizontal line represents the lower limit of detection. Homologous challenge is indicated by arrows.
In the upper respiratory tract, the AA ca, Jap/57 ca, and sw/06 ca vaccines conferred complete or statistically significant (P < 0.05 by Kruskal-Wallis) protection against homologous virus challenge infection, as indicated with an arrow (Fig. 7B); only the sw/06 ca vaccine conferred complete protection against a heterologous challenge virus (AA wt). In the lower respiratory tract, the ca vaccines conferred complete protection against homologous and heterologous challenge with all viruses except the sw/06 wt virus (Fig. 7C). Only the AA ca and sw/06 ca vaccines conferred complete protection in the lower respiratory tract from sw/06 wt challenge. Notably, the mal/78 wt virus did not replicate in the lower respiratory tract of ferrets, and thus the protection conferred by the vaccines against mal/78 wt virus challenge could not be assessed. In a subsequent study, we found that the restricted replication of the mal/78 wt virus could be attributed to HA receptor binding preference because substitutions of Q226L and G228S enabled the virus to replicate to a titer of ∼105 median egg infectious doses/g (24).
Thus, in both mice and ferrets, the sw/06 ca vaccine elicited cross-reactive neutralizing antibodies and conferred protection against a range of antigenically distinct heterologous H2 challenge viruses. This is not surprising, because generally, evolutionarily later strains cross-react well with earlier strains. Although the HA sequence of mal/78 is more closely related to sw/06, it did not protect against replication of challenge virus. None of the three H2 ca viruses offered protection against sw/06 wt virus challenge infection in the NT, but they did offer different degrees of protection against replication of the sw/06 wt virus in the lungs of ferrets.
Live attenuated influenza vaccines may have great potential for use during an influenza pandemic by virtue of the yield of vaccine doses produced in eggs and their ability to rapidly induce immunity and to provide protection against antigenically drifted viruses. The goal of our pandemic influenza vaccine program is to generate pandemic LAIV on the backbone of the licensed seasonal LAIV and determine whether these vaccine viruses are safe, infectious, and immunogenic in preclinical and carefully conducted clinical studies. A theoretical concern associated with the use of LAIV-bearing genes derived from an avian or noncontemporary H2N2 influenza virus is the risk of spread of the vaccine virus or reassortment of the vaccine virus with a circulating influenza virus, resulting in a novel subtype of influenza that could spread in the human population. In order to mitigate these risks, preclinical studies with pandemic LAIV are conducted in appropriate levels of biocontainment, and the phase I clinical trials are conducted with regulatory approval in isolation facilities, at a time when seasonal influenza viruses are not circulating in the community. The risks would be carefully considered by public health authorities before a decision is made to introduce a live attenuated vaccine in a threatened pandemic.
In summary, our studies demonstrate that the sw/06 ca vaccine is attenuated in the lower respiratory tract of ferrets and replicates comparably to the AA ca virus in the respiratory tract of both mice and ferrets. Furthermore, the sw/06 ca vaccine is immunogenic and efficacious against homologous and heterologous challenge in both mice and ferrets. Notably, the recent isolation of the sw/06 virus and its capacity for transmission and to cause disease in mammals without prior adaptation (25) also suggest that this virus would be a logical H2 pandemic vaccine candidate for further evaluation in a phase I clinical trial.
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program of the National Institutes of Health (NIH) and the National Institute of Allergy and Infectious Diseases (NIAID). This research was performed as part of a Cooperative Research and Development Agreement between the Laboratory of Infectious Diseases, NIAID, and MedImmune. X.C., H.J., and G.K. are employees of MedImmune, the manufacturer of seasonal live attenuated influenza virus vaccines.
We thank Jadon Jackson for technical support. We thank Qi Xu, James Zengel, Scott Jacobson, Stephanie Gee, Dan Ye, and Chin-Fen Yang of MedImmune for their excellent technical assistance. We also thank Adolfo Garcia-Sastre and Juergen Richt for providing the sw/06 wild-type virus.
Footnotes
Published ahead of print 26 December 2013
REFERENCES
- 1.Wright PF, Neumann G, Kawaoka Y. 2007. Orthomyxoviruses, p 1691–1740 In Knipe DM, Howley PM, Griffin DE, Martin MA, Lamb RA, Roizman B, Straus SE. (ed), Fields virology, 5th ed, vol 1 Lippincott Williams and Wilkins, Philadelphia, PA [Google Scholar]
- 2.Potter CW. 1998. Chronicle of influenza pandemics, p 3–18 In Nicholson KG, Webster RG, Hay AJ. (ed), Textbook of influenza. Blackwell Science Ltd., Oxford, United Kingdom [Google Scholar]
- 3.Dowdle WR. 1999. Influenza A virus recycling revisited. Bull. World Health Organ. 77:820–828 [PMC free article] [PubMed] [Google Scholar]
- 4.Nabel GJ, Wei CJ, Ledgerwood JE. 2011. Vaccinate for the next H2N2 pandemic now. Nature 471:157–158. 10.1038/471157a [DOI] [PubMed] [Google Scholar]
- 5.Makarova NA, Kaverin NV, Krauss S, Senne D, Webster RG. 1999. Transmission of Eurasian avian H2 influenza virus to shorebirds in North America. J. Gen. Virol. 80:3167–3171 [DOI] [PubMed] [Google Scholar]
- 6.Suss J, Schafer J, Sinnecker H, Webster RG. 1994. Influenza virus subtypes in aquatic birds of eastern Germany. Arch. Virol. 135:101–114. 10.1007/BF01309768 [DOI] [PubMed] [Google Scholar]
- 7.Jonassen CM, Handeland K. 2007. Avian influenza virus screening in wild waterfowl in Norway, 2005. Avian Dis. 51:425–428. 10.1637/7555-033106R1.1 [DOI] [PubMed] [Google Scholar]
- 8.Kishida N, Sakoda Y, Shiromoto M, Bai GR, Isoda N, Takada A, Laver G, Kida H. 2008. H2N5 influenza virus isolates from terns in Australia: genetic reassortants between those of the Eurasian and American lineages. Virus Genes 37:16–21. 10.1007/s11262-008-0235-z [DOI] [PubMed] [Google Scholar]
- 9.Krauss S, Obert CA, Franks J, Walker D, Jones K, Seiler P, Niles L, Pryor SP, Obenauer JC, Naeve CW, Widjaja L, Webby RJ, Webster RG. 2007. Influenza in migratory birds and evidence of limited intercontinental virus exchange. PLoS Pathog. 3:e167. 10.1371/journal.ppat.0030167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawaoka Y, Krauss S, Webster RG. 1989. Avian-to-human transmission of the PB1 gene of influenza A viruses in the 1957 and 1968 pandemics. J. Virol. 63:4603–4608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scholtissek C, Rohde W, Von Hoyningen V, Rott R. 1978. On the origin of the human influenza virus subtypes H2N2 and H3N2. Virology 87:13–20. 10.1016/0042-6822(78)90153-8 [DOI] [PubMed] [Google Scholar]
- 12.Schafer JR, Kawaoka Y, Bean WJ, Suss J, Senne D, Webster RG. 1993. Origin of the pandemic 1957 H2 influenza A virus and the persistence of its possible progenitors in the avian reservoir. Virology 194:781–788. 10.1006/viro.1993.1319 [DOI] [PubMed] [Google Scholar]
- 13.Kaverin NV, Smirnov YA, Govorkova EA, Rudneva IA, Gitelman AK, Lipatov AS, Varich NL, Yamnikova SS, Makarova NV, Webster RG, Lvov DK. 2000. Cross-protection and reassortment studies with avian H2 influenza viruses. Arch. Virol. 145:1059–1066. 10.1007/s007050070109 [DOI] [PubMed] [Google Scholar]
- 14.Armerding D, Rossiter H, Ghazzouli I, Liehl E. 1982. Evaluation of live and inactivated influenza A virus vaccines in a mouse model. J. Infect. Dis. 145:320–330. 10.1093/infdis/145.3.320 [DOI] [PubMed] [Google Scholar]
- 15.Belshe RB, Gruber WC, Mendelman PM, Cho I, Reisinger K, Block SL, Wittes J, Iacuzio D, Piedra P, Treanor J, King J, Kotloff K, Bernstein DI, Hayden FG, Zangwill K, Yan L, Wolff M. 2000. Efficacy of vaccination with live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine against a variant (A/Sydney) not contained in the vaccine. J. Pediatr. 136:168–175. 10.1016/S0022-3476(00)70097-7 [DOI] [PubMed] [Google Scholar]
- 16.Delem A. 1977. Protective efficacy of RIT 4025, a live attenuated influenza vaccine strain, and evaluation of heterotypic immunity to influenza A viruses in ferrets. J. Hyg. 79:203–208. 10.1017/S0022172400053006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Treanor JJ, Kotloff K, Betts RF, Belshe R, Newman F, Iacuzio D, Wittes J, Bryant M. 1999. Evaluation of trivalent, live, cold-adapted (CAIV-T) and inactivated (TIV) influenza vaccines in prevention of virus infection and illness following challenge of adults with wild-type influenza A (H1N1), A (H3N2), and B viruses. Vaccine 18:899–906. 10.1016/S0264-410X(99)00334-5 [DOI] [PubMed] [Google Scholar]
- 18.Chen GL, Lamirande EW, Jin H, Kemble G, Subbarao K. 2010. Safety, immunogencity, and efficacy of a cold-adapted A/Ann Arbor/6/60 (H2N2) vaccine in mice and ferrets. Virology 398:109–114. 10.1016/j.virol.2009.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talaat KR, Karron RA, Liang PH, McMahon BA, Luke CJ, Thumar B, Chen GL, Min JY, Lamirande EW, Jin H, Coelingh KL, Kemble GW, Subbarao K. 2013. An open-label phase I trial of a live attenuated H2N2 influenza virus vaccine in healthy adults. Influenza Other Respir. Viruses 7:66–73. 10.1111/j.1750-2659.2012.00350.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen GL, Lamirande EW, Yang CF, Jin H, Kemble G, Subbarao K. 2010. Evaluation of replication and cross-reactive antibody responses of H2 subtype influenza viruses in mice and ferrets. J. Virol. 84:7695–7702. 10.1128/JVI.00511-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reed LJ, Muench H. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. (Lond.) 27:493–497 [Google Scholar]
- 22.Joseph T, McAuliffe J, Lu B, Vogel L, Swayne D, Jin H, Kemble G, Subbarao K. 2008. A live attenuated cold-adapted influenza A H7N3 virus vaccine provides protection against homologous and heterologous H7 viruses in mice and ferrets. Virology 378:123–132. 10.1016/j.virol.2008.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin H, Manetz S, Leininger J, Luke C, Subbarao K, Murphy B, Kemble G, Coelingh KL. 2007. Toxicological evaluation of live attenuated, cold-adapted H5N1 vaccines in ferrets. Vaccine 25:8664–8672. 10.1016/j.vaccine.2007.10.032 [DOI] [PubMed] [Google Scholar]
- 24.Chen Z, Zhou H, Kim L, Jin H. 2012. The receptor binding specificity of the live attenuated influenza H2 and H6 vaccine viruses contributes to vaccine immunogenicity and protection in ferrets. J. Virol. 86:2780–2786. 10.1128/JVI.06219-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma W, Vincent AL, Gramer MR, Brockwell CB, Lager KM, Janke BH, Gauger PC, Patnayak DP, Webby RJ, Richt JA. 2007. Identification of H2N3 influenza A viruses from swine in the United States. Proc. Natl. Acad. Sci. U. S. A. 104:20949–20954. 10.1073/pnas.0710286104 [DOI] [PMC free article] [PubMed] [Google Scholar]