Abstract
Human astroviruses (HAstV) are a frequent cause of gastroenteritis in young children and immunocompromised patients. To understand the early steps of HAstV infection in the highly permissive Caco-2 cell line, the binding and entry processes of the virus were characterized. The half-time of virus binding to the cell surface was about 10 min, while virus decapsidation took around 130 min. Drugs affecting clathrin-mediated endocytosis, endosome acidification, and actin filament polymerization, as well as those that reduce the presence of cholesterol in the cell membrane, decreased the infectivity of the virus. The infection was also reduced by silencing the expression of the clathrin heavy chain (CHC) by RNA interference or by overexpression of dominant-negative mutants of dynamin 2 and Eps15. Furthermore, the entry of HAstV apparently depends on the maturation of endosomes, since the infection was reduced by silencing the expression of Rab7, a small GTPase involved in the early- to late-endosome maturation. Altogether, our results suggest that HAstV enters Caco-2 cells using a clathrin-dependent pathway and reaches late endosomes to enter cells. Here, we have characterized the mechanism used by human astroviruses, important agents of gastroenteritis in children, to gain entry into their host cells. Using a combination of biochemical and genetic tools, we found that these viruses enter Caco-2 cells using a clathrin-dependent endocytic pathway, where they most likely need to travel to late endosomes to reach the cytoplasm and begin their replication cycle.
INTRODUCTION
Human astroviruses (HAstV) are an important cause of gastroenteritis among young children (1), although a recent isolate was associated with encephalitis in an immunodeficient patient (2). These viruses belong to the genus Mammastrovirus in the family Astroviridae. Virions are formed by a nonenveloped protein capsid and a positive-strand RNA genome of approximately 6.8 kb (3). The RNA genome has three open reading frames (ORF1a, -1b, and -2) encoding three polyproteins (4). The structural polyprotein, with approximately 780 amino acid residues (4), is produced from ORF2 after the synthesis of a polyadenylated subgenomic RNA (sgRNA), which is 3′ colinear with the genomic RNA (5, 6). In the case of a HAstV serotype 8 (HAstV-8) strain, this polyprotein is 90 kDa (VP90) (7). VP90 is intracellularly cleaved at residue Asp-657 to yield a smaller protein, VP70, which is present in the extracellular particles (8, 9). VP70 has at least two domains distinguished by sequence comparison of virus strains belonging to the eight currently recognized HAstV serotypes (10–12). Most of the amino-terminal domain, from amino acid residues 1 to 415, is highly conserved, whereas the carboxy-terminal region downstream of residue 415 is hypervariable. The crystal structure of the HAstV-8 carboxy-terminal domain indicates that it forms the spikes of the viral particles (13); therefore, it is likely involved the initial interactions with the host cell, probably through binding carbohydrate molecules (13). The conserved N-terminal domain is predicted to form a jelly roll structure and represents the capsid core of the particles, interacting with the viral RNA genome (14). Astrovirus particles formed by VP70 require trypsin treatment to become infectious; during this process, three final polypeptides are produced: VP34, derived from the N-terminal domain, and VP27 and VP25, which partially overlap and are derived from the hypervariable region (7). Neutralizing epitopes are located in this region (15, 16), but they have not been precisely mapped.
HAstV infection seems to be mainly restricted to epithelial enteric cells (17), although the recent identification of HAstV in the central nervous system (CNS) suggests that atypical HAstV might infect different cell types in vivo (2). In vitro, a limited number of cell lines have been shown to be permissive to all HAstV serotypes (18), with Caco-2 cells being the most efficient in replicating the virus (18, 19). Little is known about the mechanism of cell entry of astroviruses. By electron microscopy, HAstV-1 was suggested to enter HEK-293 cells by adsorptive endocytosis (20).
To enter a host cell, most viruses take advantage of the endocytosis pathways of the cell. While there is much information about the entry of several enveloped viruses (21), less is known about the mechanisms used by nonenveloped viruses to enter cells. In general, the viral fusion proteins present at the surfaces of enveloped viruses mediate the fusion of viral and cellular membranes, allowing the viral particle to enter the cell. The cell entry mechanism of nonenveloped viruses adds the complication that a large hydrophilic virus particle needs to traverse a lipid membrane without the resource of fusing two lipid membranes. The endocytic pathways used by different viruses include clathrin-mediated endocytosis, uptake via caveolae, macropinocytosis, and novel nonclathrin, noncaveola pathways still poorly characterized (21). The study of a particular virus entry pathway is initially based on the use of inhibitory drugs, which often have more than one target in the cell and provide limited information. Currently, more specific methods are available, such as the use of dominant-negative (DN) mutants or inhibition of the expression of a specific protein by RNA interference. These techniques have become important tools to characterize the routes used by viruses to enter cells (22, 23). In this work, we used a combination of these methodologies to explore the mechanism by which astroviruses gain access into their hosts. Here, we report that HAstV-8 enters Caco-2 cells using a clathrin-dependent pathway and it most likely needs to travel to late endosomes to reach the cytoplasm.
MATERIALS AND METHODS
Cells and virus.
The colon adenocarcinoma Caco-2 cell line used in this study was obtained from the American Type Culture Collection. For infection, cells were cultured for 3 to 4 days at 37°C with Advanced D-MEM (Adv-DMEM [Dulbecco's modified Eagle's medium]) (Invitrogen, Carlsbad, CA) supplemented with 2 mM glutamine and 3% fetal bovine serum (FBS) in a 10% CO2 atmosphere. Stocks of HAstV-8 (strain Yuc8) (12) were prepared and titrated in Caco-2 cells grown in 96-well plates by a focus-forming unit (FFU) assay, as previously described (9). The virus was activated with 200 μg/ml of trypsin for 1 h at 37°C, and soybean trypsin inhibitor (Sigma, St. Louis, MO) at 200 μg/ml was added immediately before inoculating the cells. HAstV stocks were prepared using a low multiplicity of infection (MOI) (<0.1) unless otherwise indicated. Stocks of rotavirus strain RRV (kindly donated by H. B. Greenberg, Stanford University) and reovirus type 1 (kindly donated by C. Ramos, National Institute of Health, Cuernavaca, Mexico) were used as controls; they were propagated and the titers were determined in Caco-2 cells by an immunoperoxidase assay with the appropriate primary antibodies, as previously described (24). Rotavirus infectivity was activated by treatment with trypsin (10 μg/ml for 30 min at 37°C).
Sera and reagents.
Rabbit polyclonal antibodies to the HAstV-8 strain, to V-Pro, and to VP90666-780 have been described previously (7, 9, 25). Mouse monoclonal antibody against the hemagglutinin (HA) tag was purchased from Santa Cruz Biotechnology (Santa Cruz, CA), mouse anti-tubulin antibody was from Zymed (San Francisco, CA), and mouse anti-Rab7 antibody was from Abcam (Cambridge, United Kingdom). Mouse monoclonal antibody to clathrin heavy chain (CHC) was purchased from ABR Antibodies (Golden, CO). Alexa 488- and 568-conjugated secondary antibodies were obtained from Molecular Probes (Eugene, OR). Horseradish peroxidase-conjugated goat anti-rabbit polyclonal antibody was from PerkinElmer Life Sciences (Boston, MA). Chlorpromazine, dansylcadaverine, bafilomycin-A1, cytochalasin D, jasplakinolide, nocodazole, inhibitor for cathepsin B (CA-074), brefeldin A, and methyl-β-cyclodextrin (MBC) were purchased from Sigma (St. Louis, MO), and inhibitor I for cathepsin L was from Calbiochem (Merck, Darmstadt, Germany), whereas Aspergillus giganteus alpha-sarcin was from Oncogene-Science (Cambridge, MA).
Binding assay.
The binding assay was carried out as previously described (26). Briefly, ice-cold trypsin-activated virus was added to Caco-2 cells at an MOI of 0.1. The cells were maintained in an ice-cold water bath to prevent virus entry. At different time points, the unbound particles were diluted and washed out from the cells. After two additional washes with ice-cold minimal essential medium (MEM) to completely remove unbound virus, warm MEM was added and the cells were incubated at 37°C for 16 h. Infected cells were detected by an immunoperoxidase assay as described previously (7). Thus, infected cells represent the number of infectious particles that were able to bind at a given time at 4°C and subsequently entered cells at 37°C. Data were processed using the following equation: ln(V0/Vt) = (KC)t, where V0 is the total number of input infectious particles (normalized to the number detected after 1 h of adsorption at 37°C), Vt is the unattached virus at the indicated time (calculated as the total virus added minus the number of infected cells), C is the cell concentration, and K is the attachment rate constant (26). Given that HAstV cell receptors were considered to be present in large excess and the virus in limited amounts, it was expected that the virus-cell interaction was a first-order reaction. This assumption was confirmed by the linear graph obtained (Fig. 1A); therefore, the half-time of attachment was calculated by the following equation: t1/2 = 0.693/k, where k is the slope of the curve.
FIG 1.
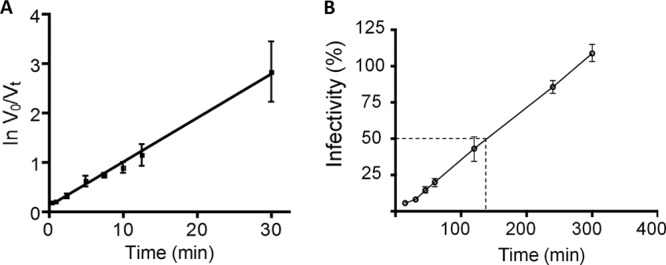
Astrovirus binding and entry into Caco-2 cells. (A) Trypsin-treated HAstV-8 (at an MOI of 0.1) was added to Caco-2 cells and incubated at 4°C for the indicated times to allow virus attachment, but not entry. The unbound virus was removed, the cells were washed twice, and the infection was allowed to proceed for 16 h at 37°C. Finally, the cells were fixed and immunostained as described in Materials and Methods. The input virus (V0) was considered to be that determined by infecting the cells for 1 h at 37°C, while the unbound virus (Vt, calculated as the total virus added minus the number of infected cells) was considered the difference of the input and the bound virus detected at a given time. (B) Two-fold dilutions of NR-labeled HAstV-8 were adsorbed to Caco-2 cells for 1 h at 4°C in the dark and then shifted to 37°C and exposed to white light at the indicated time points. The titer obtained in each case is expressed as a percentage of the infectivity obtained with the NR-HAstV kept in darkness. The arithmetic means ± standard deviations of three independent experiments performed in duplicate are shown. The dashed line indicates the time at which 50% of HAstV infectivity was no longer affected by white light.
RNA release assay.
To prepare neutral-red (NR)-labeled virus (NR-HAstV), HAstV-8 was grown in Caco-2 cells in the presence of neutral red (10 μg/ml), which was added to the medium after the adsorption period, as previously described for poliovirus and norovirus (27, 28). The cells were incubated at 37°C protected from light, and NR-HAstV was harvested 18 h p.i. by freeze-thawing twice. To confirm their photosensitivity, the titers of virus stocks were determined either in the dark or under white light. Virus infectivity was 100 times higher when the virus was kept in the dark during the infection than under white light. To evaluate the kinetics of RNA release, 2-fold dilutions of NR-HAstV were adsorbed to cells for 1 h at 4°C in the dark, and then the cultures were shifted to 37°C and exposed to white light at the indicated times postadsorption; the cells were kept for 16 h at 37°C, at which time the cells were fixed and the infectivity was determined by an immunoperoxidase assay (9).
Alpha-sarcin coentry assay.
A mixture of activated virus and 10 μM alpha-sarcin was added to Caco-2 cells and incubated for 1 h at 37°C. The cells were washed and incubated with 10 μCi/ml of Easy Tag Express-[35S]-Protein-labeling mix (NEG-772; Dupont) for 30 min at 37°C. Finally, the cells were washed, lysed, and analyzed by radioactive incorporation in trichloroacetic acid (TCA)-precipitable material and by SDS-10% PAGE and autoradiography, as described previously (29).
Cell infection in the presence of drugs.
Caco-2 cells were cultured in 96-well plates for 3 to 4 days until they reached 100% confluence. The cells were treated at 37°C with different concentrations of the drugs for 1 h before infection and during the adsorption period unless otherwise indicated. Approximately 2,000 FFU (MOI = 0.04) of activated virus was inoculated per well, and the inoculum was removed after 1 h at 37°C. Sixteen hours p.i., the cells were fixed and stained with an anti-HAstV-8 antiserum as described previously (9) to detect infected cells. The FFU were counted with the help of a Visiolab 1000 station (Biocom, France), as previously reported (24). The effects of drugs on viral infectivity are reported as the percentages of infected cells observed in the absence of any treatment. None of these treatments caused cell death, as determined by a lactate dehydrogenase (LDH) release assay. For LDH determinations, an LDH release kit (TOX-7; Sigma) was used. The medium was collected from each well, and the cells attached to the plate were lysed with the lysis buffer included in the kit. The LDH activity was determined following the manufacturer's instructions.
Infectivity of HAstV in cells transfected with dominant-negative mutants.
Plasmids carrying Eps15 mutant genes (pD3Δ2 and pEΔ95/295) (30) fused to green fluorescent protein (GFP) were kindly donated by A. Benmerah, INSERM, France. The pD3Δ2 construct expresses the carboxy-terminal truncated fragment of Eps15, and it was used as a control, since it has a wild-type (wt) phenotype. Construct pEΔ95/295 encodes an Eps15 deletion mutant lacking the second and third EH domains, and it has a DN phenotype (30). Plasmids carrying the wt and DN mutant (K44A) genes of dynamin 2 (Dyn2) containing the HA epitope fused at the amino terminus (31) were donated by S. L. Schmidt (Scripps Institute, La Jolla, CA). Caco-2 cells grown on coverslips were transfected with 1.5 to 2 μg of plasmid per well in 12-well plates using TransFast transfection reagent (Promega, Madison, WI), following the manufacturer's instructions. Expression of these proteins was monitored by direct fluorescence observation (for Eps15 mutants) or by indirect immunofluorescence (IF) using an anti-HA tag antibody (for dyn2 constructs) and antibodies labeled with Alexa Fluor-488 as secondary antibodies. Plasmid-transfected cells were infected with HAstV (at an MOI of 3) and were detected by IF using a rabbit polyclonal antibody to HAstV-8 and a goat anti-rabbit antibody coupled to Alexa-Fluor 568 after fixing with 2% formaldehyde and permeabilizing with 0.05% Triton X-100 in phosphate-buffered saline (PBS). Bovine serum albumin (BSA) (1% [wt/vol]) in PBS was used as a blocking buffer. The slides were analyzed with a fluorescence microscope (Zeiss Axioskop 2 mot plus) coupled to a digital camera (Photometrics Cool Snap HQ). Astrovirus infection was determined in at least 300 transfected cells in three independent experiments.
siRNA transfections.
Clathrin heavy chain small interfering RNA (siRNA-CHC) and an siRNA to firefly luciferase that was used as an irrelevant control (siRNA-Irr) were obtained from Dharmacon Research (Lafayette, CO); the sequences of these siRNAs were previously reported (32, 33). To silence Rab7, an siGenome smart-pool siRNA (Dharmacon) to Rab7 (siRNA-Rab7) was used. In these assays Caco-2 cells were electroporated with the appropriate siRNA using Solution-T and the Nucleofector device, according to the instructions of the manufacturer (Amaxa, Germany), and then seeded on 48-well tissue culture plates. After 3 days at 37°C, the cells were infected at an MOI of 3, as described above, and processed for IF or harvested for protein analysis by Western blotting as previously described (8, 9).
Statistical analysis.
Statistical significance was evaluated by using a one-way analysis of variance (ANOVA) test followed by Dunnett's multiple-comparison test using GraphPad Prism 5.0 (GraphPad Software, Inc.), in which a comparative analysis against a control is made. P values of less than 0.05 were considered significant.
RESULTS
Astrovirus binding to Caco-2 cells.
One of the main problems encountered when virus binding to cells is determined by physical methods is that it has been observed that viral preparations usually contain a high ratio of physical to infectious particles. Even though this ratio has not been established for astroviruses, to overcome this potential problem, we determined the binding kinetics of astrovirus using an assay that measures the binding of infectious rather than physical particles. In this assay, virus at an MOI of 0.1 was added to cells and incubated in an ice water bath to prevent virus entry; at different time points, unbound virus was removed, and the cells were washed and then shifted to 37°C to allow virus entry; 16 h p.i., the cells were fixed and immunostained with a polyclonal antibody to HAstV, and the virus-infected cells were counted. Considering the total amount of virus added (V0) and the unbound virus at a given time (Vt), the data were processed [ln(V0/Vt)] and plotted against time (Fig. 1A). The linearity of the plot indicates that the virus-cell interaction is a first-order reaction, because it seemed to depend only on the inoculated infectious particles and not on the concentration of the cell receptor(s), which was expected to be in large excess. Thus, under the conditions of the assay, HAstV-8 had a half-time of attachment of 9.24 min.
RNA release.
To determine the time at which the viral particle uncoats after entering the cell, the release of viral RNA from the particles was evaluated using HAstV particles grown in the presence of neutral red (NR-HAstV). This method has been used to evaluate the decapsidation of genomic RNA from different single-stranded positive-RNA (ssRNA+) viruses, such as poliovirus (27) and calicivirus (28). Neutral red is a photoactive dye that is incorporated into the virion and makes the genomic RNA, and therefore virus infectivity, sensitive to white-light irradiation; however, once viral RNA is released from the virion, the dye diffuses, and viral infectivity is no longer affected (27). To evaluate whether this assay could be useful to detect RNA decapsidation, cells infected with NR-HAstV were exposed to white light immediately after the attachment period. Similarly to other ssRNA+ viruses, the infectivity of NR-HAstV was reduced 2 log units by white-light irradiation compared to conditions where the virus was kept in the dark (not shown). To determine the time at which the infectivity was no longer affected by white light, and therefore the time at which viral RNA release had occurred, cells infected with NR-HAstV were exposed to white light at different time points after attachment. Virus infectivity was dependent on the time at which the monolayers were exposed to white-light irradiation (Fig. 1B). Based on these results, the half-time for RNA release was estimated to be about 130 min. Thus, although virus binding to the cell surface seems to be relatively fast (Fig. 1A), virus entry and/or viral RNA decapsidation takes longer.
Astrovirus induces the coentry of alpha-sarcin.
Cell infection with a variety of nonenveloped viruses induces transient membrane permeability that causes the coentry of small molecules, such as antibiotics and toxins (34). Alpha-sarcin is a small toxin with RNase activity to which the cell membrane is not permeable and has been used as an indicator for cell entry of a variety of viruses (29, 35, 36). Under conditions where the membrane integrity is affected, the toxin enters the cell and degrades cellular RNA, blocking protein translation (34). To determine whether astrovirus was able to induce membrane permeability during entry, and therefore the coentry of alpha-sarcin, cells were infected for 1 h at 37°C in the presence of the toxin, and protein synthesis was then followed by adding 35S protein-labeling mix for 30 min at 37°C. The protein pattern observed by autoradiography of SDS-polyacrylamide gels showed that protein synthesis was reduced in cells infected with HAstV (Fig. 2A). Accordingly, decreased incorporation of [35S]Met was dependent on the trypsin treatment of the virus and on the MOI used (Fig. 2B). Alpha-sarcin coentry was also promoted by simian rotavirus RRV treated with trypsin, which was used as a positive control (29, 35). These data indicated that only infectious astrovirus particles were able to induce membrane permeabilization and that translation inhibition caused by alpha-sarcin could be used as an indicator of the ability of astrovirus to enter Caco-2 cells.
FIG 2.
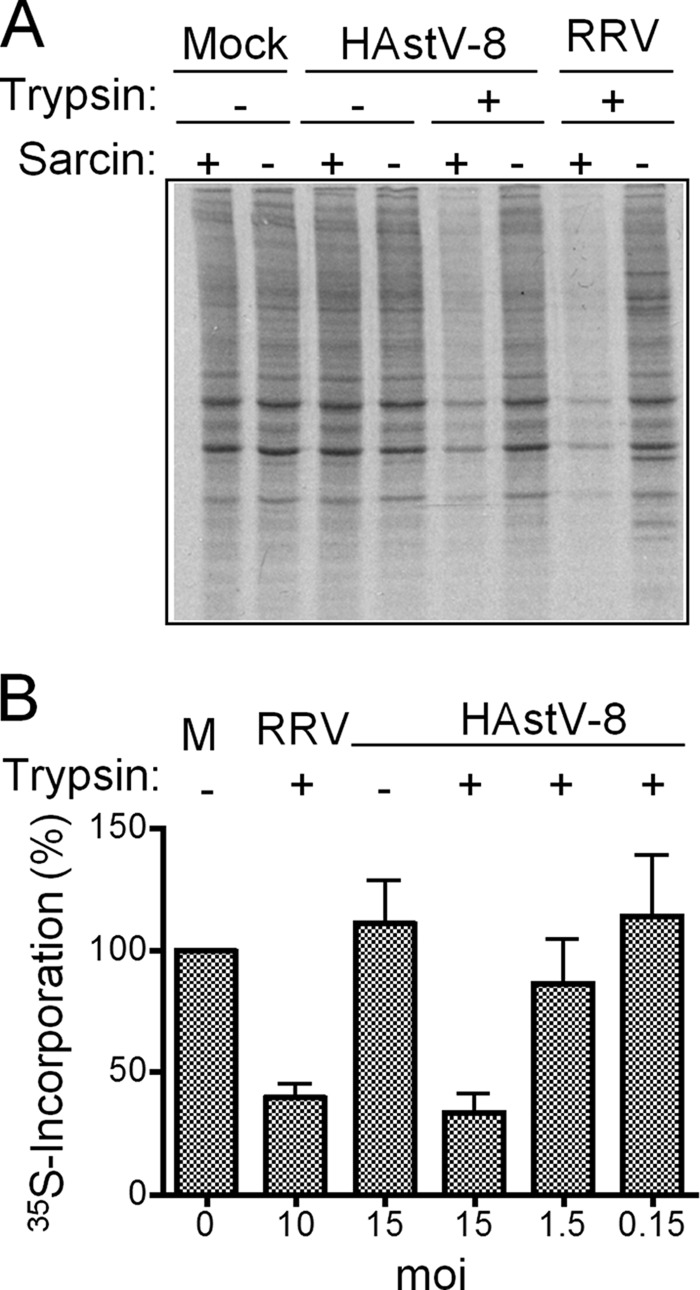
Astrovirus induces the coentry of alpha-sarcin into Caco-2 cells. (A) Monolayers of Caco-2 cells were infected or not (Mock) with trypsin-treated (+) or untreated (−) HAstV-8 or rotavirus (RRV) at an MOI of 10 in the presence (+) or absence (−) of 10 μM alpha-sarcin for 1 h at 37°C. The inoculum was then removed, and the cells were labeled and processed as described in Materials and Methods. The labeled proteins were resolved by SDS-10% PAGE and detected by autoradiography. (B) Cells were infected and incubated with alpha-sarcin as for panel A using the indicated MOIs of HAstV-8 or RRV, which were treated (+) or not (−), with trypsin. 35S incorporation was determined as TCA-precipitable material and expressed as a percentage of the 35S incorporation of mock-infected (M) cells treated under the same conditions. The error bars indicate standard deviations obtained from two experiments performed in duplicate.
Effects of drug treatments on HAstV infection.
To better understand the pathway used by astroviruses to enter Caco-2 cells, virus infection was evaluated on cells treated with different drugs that have been previously used as initial criteria to determine the endocytosis pathways employed by viruses (23). The drugs included those that block clathrin assembly, cytoskeleton integrity, and endosome acidification. For these experiments, reovirus type 1, whose entry depends on an acid- and clathrin-dependent mechanism, was used as a control (37, 38).
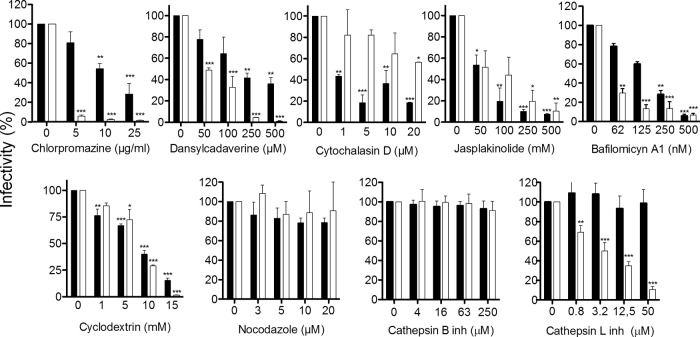
Drugs that were able to block astrovirus infection in a dose-dependent manner (Fig. 3, black bars) included chlorpromazine and dansylcadaverine, which affect the assembly of coated pits formed by clathrin (39); cytochalasin D and jasplakinolide, which impair actin filament function by depolymerizing actin and promoting disordered actin polymerization, respectively (40); and bafilomycin A1 (Baf-A1), which specifically inhibits the vacuolar ATPase (v-ATPase), preventing the acidification of endocytic vesicles and the maturation of endosomes and lysosomes (41). As observed, these drugs also blocked reovirus infection (Fig. 3, white bars). HAstV infectivity was also impaired in cells treated with MBC, which removes cholesterol from cell membranes (42). In contrast, nocodazole, which disassembles microtubules (43), and the inhibitors of the endosomal proteases cathepsin B and cathepsin L (44) did not alter astrovirus infection. None of these treatments caused cell death, as determined by an LDH release assay (results not shown).
FIG 3.
Sensitivity of HAstV-8 infection to treatments with different drugs. Caco-2 cells grown in 96-well plates were pretreated with the indicated concentrations of drugs for 1 h at 37°C. The cells were then infected with HastV-8 (black bars), or reovirus (white bars) (2,000 FFU per well), maintaining the drugs during the adsorption period of 1 h at 37°C. The virus inoculum was then removed, and the infection was left to proceed for 16 h at 37°C. Infected cells were fixed and stained as indicated in Materials and Methods. The data are expressed as the percentages of virus infectivity observed in control untreated cells, which were taken as 100%. The arithmetic means and standard deviations of three independent experiments performed in duplicate are shown. *, P < 0.05; **, P < 0.01; ***, P < 0.001. inh, inhibitor.
In all these assays, the infectivity of astrovirus was determined by staining infected cells with an anti-capsid antibody. To discard the possibility that some of the drugs tested affected the synthesis of sgRNA, which encodes the capsid protein, drug-treated cells were infected and immunostained with antibodies to the viral protease, V-Pro, whose synthesis is directed by the incoming genomic ssRNA. No difference was found between the two antibodies (not shown), confirming that the drug treatments affect virus entry and not the synthesis of the sgRNA. Taken together, these results suggest that HAstV enters cells by clathrin-mediated endocytosis and depends on the presence of cholesterol in the cell membrane, the actin cytoskeleton, and the acidification of endosomes.
Silencing the expression of clathrin reduces astrovirus infectivity.
To confirm the involvement of clathrin in HAstV entry, virus infection was evaluated in cells transfected with siRNA-CHC. Immunoblot analysis showed that transfection of Caco-2 cells with siRNA-CHC significantly reduced the levels of both CHC and astrovirus VP90 compared to the expression of these proteins in HAstV-infected cells transfected with siRNA-Irr (Fig. 4A). Furthermore, when the expression of CHC was silenced, the infectivity of HAstV was reduced by about 50% (Fig. 4B), although this effect could be more pronounced, since the transfection efficiency of Caco-2 cells, although not determined in this work, was low, as judged by the number of cells with reduced expression of clathrin by IF. In addition, the cells with apparently normal levels of CHC were clearly infected, while cells with reduced levels of CHC were poorly stained with anti-HAstV antibodies (Fig. 4C). These results suggest that the assembly of the clathrin lattice is necessary for HAstV infection.
FIG 4.
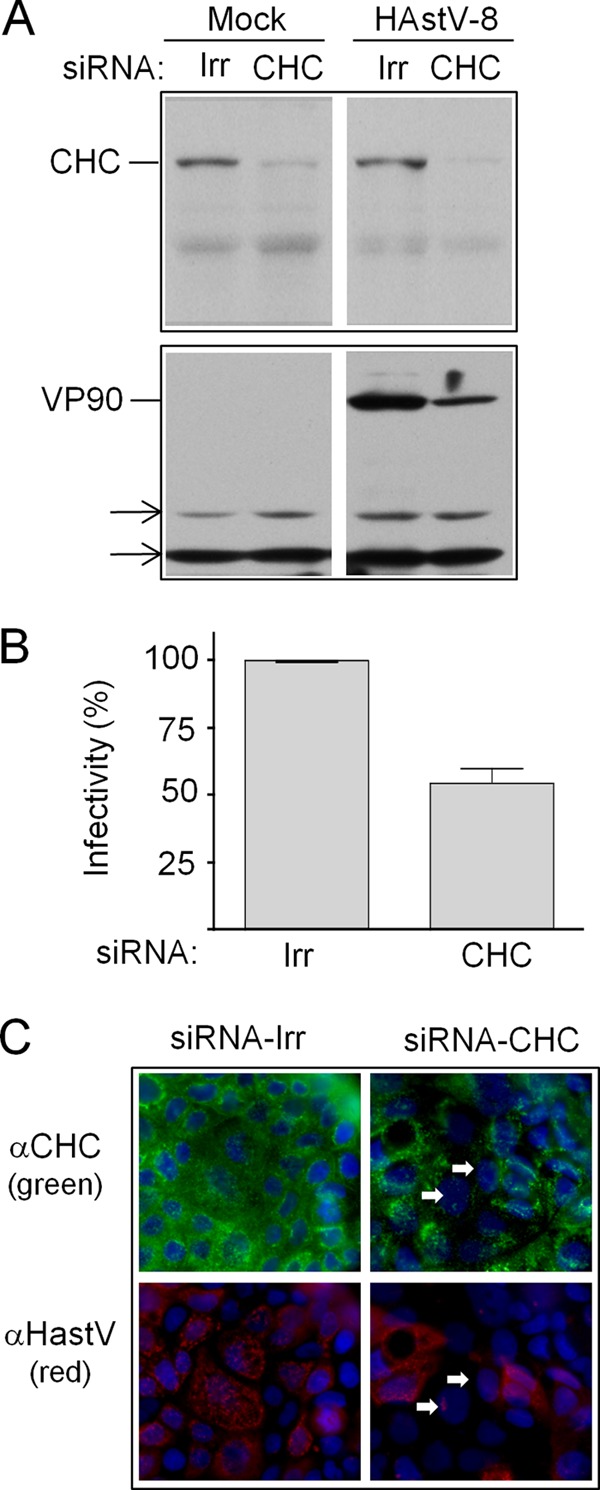
Effect of silencing the expression of CHC on HAstV-8 infectivity. (A) Caco-2 cells were transfected with the indicated siRNAs and, 72 h posttransfection, were infected with HAstV-8. Ten hours postinfection, the expression of CHC and VP90 was tested by Western blotting, using anti-HAstV-8 and anti-clathrin antibodies, as indicated. The arrows indicate cellular proteins that were nonspecifically recognized by the primary antibody and that served as loading controls. (B) Caco-2 cells were transfected with the indicated siRNAs and, 72 h posttransfection, were infected with HAstV-8. At 10 h p.i., the cells were processed for IF. Infectivity was determined by counting infected cells in cultures transfected with the indicated siRNAs. The data are expressed as percentages of the virus infectivity observed in cells treated with the irrelevant siRNA (Irr). The arithmetic means and standard deviations of three independent experiments performed in duplicate are shown. (C) Immunofluorescence assay showing the silencing of CHC. Caco-2 cells grown in coverslips were transfected with the indicated siRNAs, and 72 h posttransfection, the cells were infected with HAstV-8; at 10 h p.i., the cells were fixed and processed for IF using using anti-HAstV-8 (green) and anti-clathrin (red) antibodies, as indicated. Nuclei were stained with DAPI (blue). The arrows indicate cells transfected with the siRNA to CHC and infected with HAstV.
Dominant-negative mutants of dynamin and Eps15 decrease astrovirus infectivity.
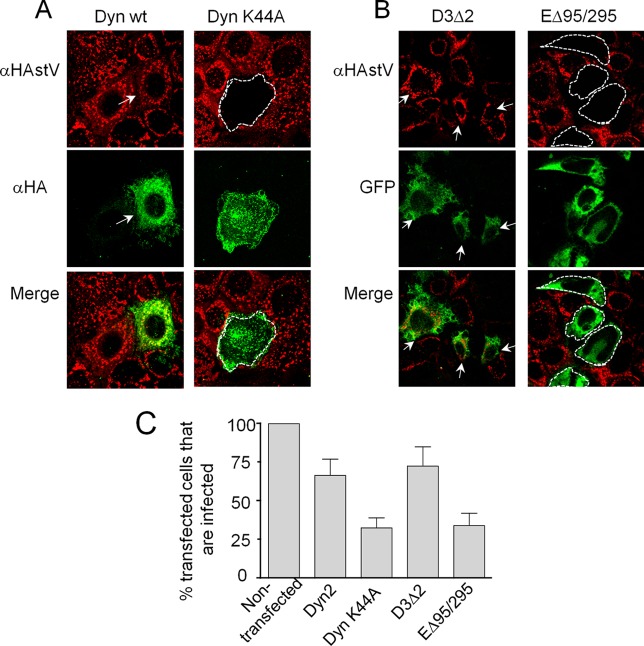
Dyn2, a GTPase that participates in the excision of endosomes from the plasma membrane (45), and Eps15, one of the adaptor proteins that interact with clathrin during endocytosis, have been involved in the clathrin-mediated endocytosis of some molecules and viruses (21). To determine whether these proteins participate in the entry of astroviruses into Caco-2 cells, the cells were transfected with plasmids encoding DN mutants of Eps15 (EΔ95/295) and Dyn2 (Dyn-K44A) or with their wt counterparts, D3Δ2 and Dyn2, respectively (30, 31). Since plasmid transfection efficiency on Caco-2 cells was very low (less than 5%), the effects of these proteins on astrovirus infection was estimated by counting the transfected cells that became infected. Even though the expression level of the transgenes was heterogeneous in the cell monolayer, as suggested by differences in the fluorescence intensity detected (Fig. 5A and B), a significant reduction in the number of infected cells was observed after transfection with the DN mutants (Fig. 5C). In the case of Dyn2, virus infectivity was reduced from 67 to 33% when cells were transfected with the Dyn-K44A DN mutant, whereas infectivity dropped from 73 to 34% in cells transfected with the EΔ95/295 construct, compared with cells transfected with the wt plasmids. Thus, a clathrin-, Dyn2-, and Eps15-dependent endocytosis pathway seems to be important for HAstV entry into Caco-2 cells.
FIG 5.
Infectivity of HAstV-8 in cells transfected with Dyn2 and Eps15 dominant-negative mutants. (A) Caco-2 cells were transfected with the indicated constructs as described under Materials and Methods and incubated for 48 h, at which time they were infected with trypsin-treated HAstV-8. Fourteen hours p.i., the cells were fixed and immunostained with anti-HA (green; to detect the expression of Dyn2) and with anti-HAstV-8 (red) antibodies. (B) Cells expressing Eps15 mutants were detected by the fluorescence of the fused GFP. Cells transfected with the wild-type proteins and also infected are marked with arrows, whereas cells transfected with the DN mutants that are not infected are marked with dotted lines. (C) At least 300 cells transfected with the indicated plasmids were screened for HAstV-8 infection, and the numbers are expressed as percentages of the infected cells observed in nontransfected cultures. The arithmetic means and standard deviations of three independent experiments performed in duplicate are shown. The images were obtained by confocal microscopy with slices of 0.2 μm.
Role of Rab7 in astrovirus infection.
The negative effect of Baf-A1 on HAstV infection and the time needed for virus penetration/RNA decapsidation (see above) suggest that astrovirus might need to reach late endosomes for cell entry (46). To explore this possibility, the effect of silencing the expression of Rab7, a small GTPase involved in a number of trafficking processes (47), including the maturation early of into late endosomes and lysosomes, was tested. For this, the expression of Rab7 was knocked down by RNA interference (RNAi), and the infectivity and viral protein expression of HAstV were evaluated under these conditions. Rab7 expression was reduced upon transfection with siRNA-Rab7, as was the expression of the viral nonstructural protein V-Pro (Fig. 6A), which proved to be a good marker for early virus infection, since it is produced immediately after viral RNA decapsidation. Under these conditions, virus infectivity was also reduced to about 50% by staining the cells with antibodies specific for the capsid protein VP90 (Fig. 6B) compared to cells transfected with an irrelevant siRNA.
FIG 6.
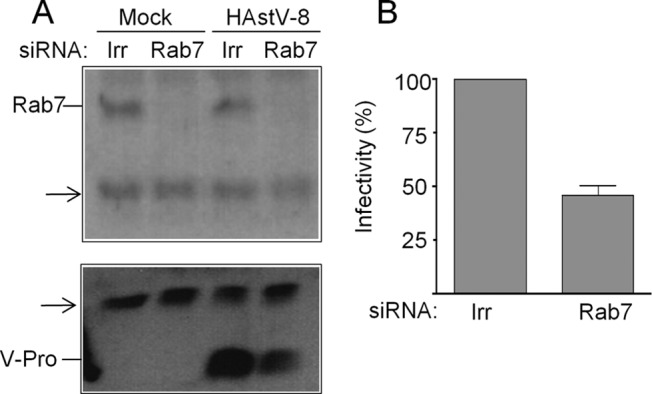
HAstV-8 infectivity is reduced in Rab7 knockdown cells. Caco-2 cells were transfected with the indicated siRNAs and infected with HAstV-8, as indicated in Fig. 4. (A) Inmunoblot analysis of cell lysates from cells transfected with the indicated siRNAs. Expression of Rab7 and the viral protease (V-Pro) was monitored with the indicated antibodies; the arrows indicate cellular proteins recognized nonspecifically by the primary antibodies. (B) Caco-2 cells were transfected with the indicated siRNAs for 72 h and then infected with HAstV-8. At 10 h p.i., the cells were fixed and stained by an immunoperoxidase assay using anti-HAstV-8 antibody as described in Materials and Methods. Infectivity was determined by counting infected cells in cultures transfected with the indicated siRNAs. The data are expressed as percentages of the virus infectivity observed in cells treated with the irrelevant siRNA. The arithmetic means and standard deviations of three independent experiments performed in duplicate are shown.
DISCUSSION
Studies on the initial steps of human astrovirus infection have been limited to human embryo kidney cells (HEK-293) (20), which have shown to be modestly permissive for some HAstV strains (18); however, this process has not been characterized in colon adenocarcinoma Caco-2 cells, which are currently considered the best model for the isolation and study of the biology of HAstV (18). This work aimed to study aspects of the early interactions between HAstV-8 and Caco-2 cells, including the mechanism the virus uses to enter and initiate a productive infection.
The binding kinetics indicated that HAstV has an attachment half-time of about 10 min at 4°C under the conditions employed, which is close to that reported for other naked viruses, such as rotaviruses, under similar conditions (26), suggesting either an abundance of cell attachment factors or high affinity for cell receptors.
On the other hand, the results obtained with the RNA release assay indicated that virus internalization and/or genome release from particles took a longer time, about 130 min. It is not clear in what cellular compartment the viral particles disassemble; however, given the time taken for the virus genome to reach the cytoplasm and the fact that Rab7 seems necessary for cell infection, it is very likely that the viral particles need to reach late endosomes to release their genomes (21), behaving like late-penetrating viruses (46). The infectivity assays performed in the presence of inhibitors that affect endosome maturation, as well as different types of endocytosis and vesicular transport, such as Baf-A1, support this idea.
It seems that HAstV uses a clathrin-dependent pathway to enter the cell, since its infectivity was reduced by silencing the expression of CHC and by overexpression of DN mutants of Eps15 and Dyn2. It was intriguing to note that none of these treatments completely abolished the infectivity of the virus. However, these results could be explained by the fact that since Caco-2 cells are poorly transfectable, CHC silencing was incomplete, and the expression of the DN mutants varied between transfected cells, as judged by the fluorescence intensities observed in the IF assays. Similar reductions of infectivity have been reported for other viruses when using Eps15 DN mutants, like dengue virus (48), arenavirus (49), and bovine viral diarrhea virus (BVDV) (50), which also use a clathrin-dependent pathway for cell entry. In particular, the reduction of BVDV infectivity was dependent on the level of expression of the Eps15 DN mutant (50).
The decreased infectivity observed after cell treatment with cytochalasin D and jasplakinolide, which affect the dynamics of actin filaments, either by depolymerizing them or by inducing their disordered polymerization, respectively (40), is consistent with the observation that astroviruses enter cells via a clathrin-mediated pathway, In addition, the presence of cholesterol in the cell membrane is important for astrovirus infection, since treatment with MBC was able to block infection. Although cholesterol has been considered more important for caveola- than for clathrin-dependent endocytosis (23, 51), different reports have demonstrated that the lipid is important for viruses that use different mechanisms for cell entry, including clathrin-, caveola-, and macropinocytosis-dependent pathways (52, 53), as well as unconventional entry routes, such as those described for rotavirus and simian virus 40 (SV40) (24, 54). Astrovirus infection was not blocked by cell treatment with drugs that affect microtubules, such as nocodazole, or with inhibitors for the lysosomal proteases cathepsins B and L, which block the infection of other viruses that enter by a clathrin-dependent mechanism, such as Ebola virus, reovirus, and coronavirus (37, 55, 56), suggesting that the activities of these proteases are not necessary for astrovirus infection.
In general, the entry of nonenveloped viruses depends on conformational changes of their capsid proteins that can be triggered by different factors, including proteolysis, exposure to a specific environments, and/or interactions with cell molecules (57). In the case of astrovirions, trypsin treatment causes virus changes that notably enhance their infectivity (7, 58); however, it is not known whether additional changes are necessary to complete viral entry and, if so, the factors that could trigger them.
Similarly to other naked viruses, such as poliovirus (59), rotavirus (29, 35), and feline calicivirus (60), astrovirus was able to promote the coentry of the toxin alpha-sarcin, suggesting that astrovirus entry induces transient changes in cell membrane permeability that depend on the infectious status of the virus, since viruses that were not treated with trypsin did not promote the coentry of alpha-sarcin. It would be expected that membrane permeabilization would be induced at the endosomal membrane, where virus entry occurs. In agreement with this possibility, both membrane permeabilization to the toxin and release of the viral RNA from the particles took place after at least 90 min, suggesting they are closely related events, as has been observed during poliovirus entry (59). The precise subcellular compartment where the virion or viral RNA and alpha-sarcin are translocated to the cytoplasm remains to be determined.
The activation of the extracellular signal-regulated mitogen-active kinase (ERK1/2) in the first 15 min after virus attachment is apparently necessary for HAstV-1 infection (61). Since no virus replication is needed for ERK1/2 activation (61) and virus binding takes place during the first 10 min (this work), the attachment of the virus to the cell surface might be the event that triggers the activation of the kinase, which might contribute to the formation of clathrin-coated endosomes. ERK1/2 activation and clathrin-dependent endocytosis could be related events during HAstV entry into Caco-2 cells, as has been shown for the internalization of the lactoferrin receptor in Caco-2 cells (62).
In summary, in this work, we have characterized the entry pathway of HAstV-8 in cultured cells, an important step for understanding the biology of these viruses. This is particularly relevant in light of the recent discovery of novel astroviruses isolated from humans that show genetic similarities to animal strains, some of which have been associated with diseases other than gastroenteritis (63). It remains to be tested if these other astrovirus strains follow the same route of entry as HAstV and, even more importantly, to determine the cell entry mechanism used by astroviruses to infect target cells in a natural infection.
ACKNOWLEDGMENTS
We thank A. Benmerah and S. L. Schmidt for donating to us the plasmid constructs used in this work.
This work was partially supported by grants 44884-Q and CB07-79574 from CONACyT-Mexico and IN226106 and IN219910 from DGAPA-UNAM.
Footnotes
Published ahead of print 11 December 2013
REFERENCES
- 1.Walter JE, Mitchell DK. 2003. Astrovirus infection in children. Curr. Opin. Infect. Dis. 16:247–253. 10.1097/00001432-200306000-00011 [DOI] [PubMed] [Google Scholar]
- 2.Quan PL, Wagner TA, Briese T, Torgerson TR, Hornig M, Tashmukhamedova A, Firth C, Palacios G, Baisre-De-Leon A, Paddock CD, Hutchison SK, Egholm M, Zaki SR, Goldman JE, Ochs HD, Lipkin WI. 2010. Astrovirus encephalitis in boy with X-linked agammaglobulinemia. Emerg. Infect. Dis. 16:918–925. 10.3201/eid1606.091536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang B, Monroe SS, Koonin EV, Stine SE, Glass RI. 1993. RNA sequence of astrovirus: distinctive genomic organization and a putative retrovirus-like ribosomal frameshifting signal that directs the viral replicase synthesis. Proc. Natl. Acad. Sci. U. S. A. 90:10539–10543. 10.1073/pnas.90.22.10539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendez E, Arias CF. 2013. Astroviruses, p 609–628 In Knipe DM, Howley PM. (ed), Fields virology, vol I Lippincott, Williams and Wilkins, Philadelphia, PA [Google Scholar]
- 5.Monroe SS, Jiang B, Stine SE, Koopmans M, Glass RI. 1993. Subgenomic RNA sequence of human astrovirus supports classification of Astroviridae as a new family of RNA viruses. J. Virol. 67:3611–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willcocks MM, Carter MJ. 1993. Identification and sequence determination of the capsid protein gene of human astrovirus serotype 1. FEMS Microbiol. Lett. 114:1–7. 10.1111/j.1574-6968.1993.tb06542.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendez E, Fernandez-Luna T, Lopez S, Mendez-Toss M, Arias CF. 2002. Proteolytic processing of a serotype 8 human astrovirus ORF2 polyprotein. J. Virol. 76:7996–8002. 10.1128/JVI.76.16.7996-8002.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banos-Lara MDR, Mendez E. 2010. Role of individual caspases induced by astrovirus on the processing of its structural protein and its release from the cell through a non-lytic mechanism. Virology 401:322–332. 10.1016/j.virol.2010.02.028 [DOI] [PubMed] [Google Scholar]
- 9.Mendez E, Salas-Ocampo E, Arias CF. 2004. Caspases mediate processing of the capsid precursor and cell release of human astroviruses. J. Virol. 78:8601–8608. 10.1128/JVI.78.16.8601-8608.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang QH, Kakizawa J, Wen LY, Shimizu M, Nishio O, Fang ZY, Ushijima H. 2001. Genetic analysis of the capsid region of astroviruses. J. Med. Virol. 64:245–255. 10.1002/jmv.1043 [DOI] [PubMed] [Google Scholar]
- 11.Jonassen CM, Jonassen TO, Saif YM, Snodgrass DR, Ushijima H, Shimizu M, Grinde B. 2001. Comparison of capsid sequences from human and animal astroviruses. J. Gen. Virol. 82:1061–1067 [DOI] [PubMed] [Google Scholar]
- 12.Mendez-Toss M, Romero-Guido P, Munguia ME, Mendez E, Arias CF. 2000. Molecular analysis of a serotype 8 human astrovirus genome. J. Gen. Virol. 81:2891–2897 [DOI] [PubMed] [Google Scholar]
- 13.Dong J, Dong L, Mendez E, Tao Y. 2011. Crystal structure of the human astrovirus capsid spike. Proc. Natl. Acad. Sci. U. S. A. 108:12681–12686. 10.1073/pnas.1104834108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krishna NK. 2005. Identification of structural domains involved in astrovirus capsid biology. Viral Immunol. 18:17–26. 10.1089/vim.2005.18.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bass DM, Upadhyayula U. 1997. Characterization of human serotype 1 astrovirus-neutralizing epitopes. J. Virol. 71:8666–8671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanchez-Fauquier A, Carrascosa AL, Carrascosa JL, Otero A, Glass RI, Lopez JA, San Martin C, Melero JA. 1994. Characterization of a human astrovirus serotype 2 structural protein (VP26) that contains an epitope involved in virus neutralization. Virology 201:312–320. 10.1006/viro.1994.1296 [DOI] [PubMed] [Google Scholar]
- 17.Sebire NJ, Malone M, Shah N, Anderson G, Gaspar HB, Cubitt WD. 2004. Pathology of astrovirus associated diarrhoea in a paediatric bone marrow transplant recipient. J. Clin. Pathol. 57:1001–1003. 10.1136/jcp.2004.017178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brinker JP, Blacklow NR, Herrmann JE. 2000. Human astrovirus isolation and propagation in multiple cell lines. Arch. Virol. 145:1847–1856. 10.1007/s007050070060 [DOI] [PubMed] [Google Scholar]
- 19.Willcocks MM, Carter MJ, Laidler FR, Madeley CR. 1990. Growth and characterisation of human faecal astrovirus in a continuous cell line. Arch. Virol. 113:73–81. 10.1007/BF01318354 [DOI] [PubMed] [Google Scholar]
- 20.Donelli G, Superti F, Tinari A, Marziano ML. 1992. Mechanism of astrovirus entry into Graham 293 cells. J. Med. Virol. 38:271–277. 10.1002/jmv.1890380408 [DOI] [PubMed] [Google Scholar]
- 21.Mercer J, Schelhaas M, Helenius A. 2010. Virus entry by endocytosis. Annu. Rev. Biochem. 79:803–833. 10.1146/annurev-biochem-060208-104626 [DOI] [PubMed] [Google Scholar]
- 22.Mayor S, Pagano RE. 2007. Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol. 8:603–612. 10.1038/nrm2216 [DOI] [PubMed] [Google Scholar]
- 23.Sieczkarski SB, Whittaker GR. 2002. Dissecting virus entry via endocytosis. J. Gen. Virol. 83:1535–1545 [DOI] [PubMed] [Google Scholar]
- 24.Guerrero CA, Zarate S, Corkidi G, Lopez S, Arias CF. 2000. Biochemical characterization of rotavirus receptors in MA104 cells. J. Virol. 74:9362–9371. 10.1128/JVI.74.20.9362-9371.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendez E, Salas-Ocampo MP, Munguia ME, Arias CF. 2003. Protein products of the open reading frames encoding nonstructural proteins of human astrovirus serotype 8. J. Virol. 77:11378–11384. 10.1128/JVI.77.21.11378-11384.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendez E, Lopez S, Cuadras MA, Romero P, Arias CF. 1999. Entry of rotaviruses is a multistep process. Virology 263:450–459. 10.1006/viro.1999.9976 [DOI] [PubMed] [Google Scholar]
- 27.Kirkegaard K. 1990. Mutations in VP1 of poliovirus specifically affect both encapsidation and release of viral RNA. J. Virol. 64:195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perry JW, Wobus CE. 2010. Endocytosis of murine norovirus 1 into murine macrophages is dependent on dynamin II and cholesterol. J. Virol. 84:6163–6176. 10.1128/JVI.00331-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cuadras MA, Arias CF, Lopez S. 1997. Rotaviruses induce an early membrane permeabilization of MA104 cells and do not require a low intracellular Ca2+ concentration to initiate their replication cycle. J. Virol. 71:9065–9074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benmerah A, Bayrou M, Cerf-Bensussan N, Dautry-Varsat A. 1999. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112:1303–1311 [DOI] [PubMed] [Google Scholar]
- 31.Damke H, Gossen M, Freundlieb S, Bujard H, Schmid SL. 1995. Tightly regulated and inducible expression of dominant interfering dynamin mutant in stably transformed HeLa cells. Methods Enzymol. 257:209–220 [DOI] [PubMed] [Google Scholar]
- 32.Blanchard E, Belouzard S, Goueslain L, Wakita T, Dubuisson J, Wychowski C, Rouille Y. 2006. Hepatitis C virus entry depends on clathrin-mediated endocytosis. J. Virol. 80:6964–6972. 10.1128/JVI.00024-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Motley A, Bright NA, Seaman MN, Robinson MS. 2003. Clathrin-mediated endocytosis in AP-2-depleted cells. J. Cell Biol. 162:909–918. 10.1083/jcb.200305145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otero MJ, Carrasco L. 1987. Proteins are cointernalized with virion particles during early infection. Virology 160:75–80. 10.1016/0042-6822(87)90046-8 [DOI] [PubMed] [Google Scholar]
- 35.Liprandi F, Moros Z, Gerder M, Ludert JE, Pujol FH, Ruiz MC, Michelangeli F, Charpilienne A, Cohen J. 1997. Productive penetration of rotavirus in cultured cells induces coentry of the translation inhibitor alpha-sarcin. Virology 237:430–438. 10.1006/viro.1997.8803 [DOI] [PubMed] [Google Scholar]
- 36.Madan V, Sanz MA, Carrasco L. 2005. Requirement of the vesicular system for membrane permeabilization by Sindbis virus. Virology 332:307–315. 10.1016/j.virol.2004.11.001 [DOI] [PubMed] [Google Scholar]
- 37.Ebert DH, Deussing J, Peters C, Dermody TS. 2002. Cathepsin L and cathepsin B mediate reovirus disassembly in murine fibroblast cells. J. Biol. Chem. 277:24609–24617. 10.1074/jbc.M201107200 [DOI] [PubMed] [Google Scholar]
- 38.Ehrlich M, Boll Van Oijen WA, Hariharan R, Chandran K, Nibert ML, Kirchhausen T. 2004. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 118:591–605. 10.1016/j.cell.2004.08.017 [DOI] [PubMed] [Google Scholar]
- 39.Wang LH, Rothberg KG, Anderson RG. 1993. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123:1107–1117. 10.1083/jcb.123.5.1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bubb MR, Spector I, Beyer BB, Fosen KM. 2000. Effects of jasplakinolide on the kinetics of actin polymerization. An explanation for certain in vivo observations. J. Biol. Chem. 275:5163–5170. 10.1074/jbc.275.7.5163 [DOI] [PubMed] [Google Scholar]
- 41.Bowman EJ, Siebers A, Altendorf K. 1988. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc. Natl. Acad. Sci. U. S. A. 85:7972–7976. 10.1073/pnas.85.21.7972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ilangumaran S, Hoessli DC. 1998. Effects of cholesterol depletion by cyclodextrin on the sphingolipid microdomains of the plasma membrane. Biochem. J. 335:433–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vasquez RJ, Howell B, Yvon AM, Wadsworth P, Cassimeris L. 1997. Nanomolar concentrations of nocodazole alter microtubule dynamic instability in vivo and in vitro. Mol. Biol. Cell 8:973–985. 10.1091/mbc.8.6.973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chapman HA. 2006. Endosomal proteases in antigen presentation. Curr. Opin. Immunol. 18:78–84. 10.1016/j.coi.2005.11.011 [DOI] [PubMed] [Google Scholar]
- 45.Sever S, Damke H, Schmid SL. 2000. Garrotes, springs, ratchets, and whips: putting dynamin models to the test. Traffic 1:385–392. 10.1034/j.1600-0854.2000.010503.x [DOI] [PubMed] [Google Scholar]
- 46.Lozach PY, Huotari J, Helenius A. 2011. Late-penetrating viruses. Curr. Opin. Virol. 1:35–43. 10.1016/j.coviro.2011.05.004 [DOI] [PubMed] [Google Scholar]
- 47.Wang T, Ming Z, Xiaochun W, Hong W. 2011. Rab7: role of its protein interaction cascades in endo-lysosomal traffic. Cell Signal. 23:516–521. 10.1016/j.cellsig.2010.09.012 [DOI] [PubMed] [Google Scholar]
- 48.Mosso C, Galvan-Mendoza IJ, Ludert JE, del Angel RM. 2008. Endocytic pathway followed by dengue virus to infect the mosquito cell line C6/36 HT. Virology 378:193–199. 10.1016/j.virol.2008.05.012 [DOI] [PubMed] [Google Scholar]
- 49.Vela EM, Zhang L, Colpitts TM, Davey RA, Aronson JF. 2007. Arenavirus entry occurs through a cholesterol-dependent, non-caveolar, clathrin-mediated endocytic mechanism. Virology 369:1–11. 10.1016/j.virol.2007.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lecot S, Belouzard S, Dubuisson J, Rouille Y. 2005. Bovine viral diarrhea virus entry is dependent on clathrin-mediated endocytosis. J. Virol. 79:10826–10829. 10.1128/JVI.79.16.10826-10829.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ivanov AI. 2008. Pharmacological inhibition of endocytic pathways: is it specific enough to be useful? Methods Mol. Biol. 440:15–33. 10.1007/978-1-59745-178-9_2 [DOI] [PubMed] [Google Scholar]
- 52.Martin-Acebes MA, Gonzalez-Magaldi M, Sandvig K, Sobrino F, Armas-Portela R. 2007. Productive entry of type C foot-and-mouth disease virus into susceptible cultured cells requires clathrin and is dependent on the presence of plasma membrane cholesterol. Virology 369:105–118. 10.1016/j.virol.2007.07.021 [DOI] [PubMed] [Google Scholar]
- 53.Nitschke M, Korte T, Tielesch C, Ter-Avetisyan G, Tunnemann G, Cardoso MC, Veit M, Herrmann A. 2008. Equine arteritis virus is delivered to an acidic compartment of host cells via clathrin-dependent endocytosis. Virology 377:248–254. 10.1016/j.virol.2008.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Damm EM, Pelkmans L, Kartenbeck J, Mezzacasa A, Kurzchalia T, Helenius A. 2005. Clathrin- and caveolin-1-independent endocytosis: entry of simian virus 40 into cells devoid of caveolae. J. Cell Biol. 168:477–488. 10.1083/jcb.200407113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chandran K, Sullivan NJ, Felbor U, Whelan SP, Cunningham JM. 2005. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science 308:1643–1645. 10.1126/science.1110656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simmons G, Gosalia DN, Rennekamp AJ, Reeves JD, Diamond SL, Bates P. 2005. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. U. S. A. 102:11876–11881. 10.1073/pnas.0505577102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Banerjee M, Johnson JE. 2008. Activation, exposure and penetration of virally encoded, membrane-active polypeptides during non-enveloped virus entry. Curr. Protein Pept. Sci. 9:16–27. 10.2174/138920308783565732 [DOI] [PubMed] [Google Scholar]
- 58.Bass DM, Qiu S. 2000. Proteolytic processing of the astrovirus capsid. J. Virol. 74:1810–1814. 10.1128/JVI.74.4.1810-1814.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perez L, Carrasco L. 1993. Entry of poliovirus into cells does not require a low-pH step. J. Virol. 67:4543–4548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stuart AD, Brown TD. 2006. Entry of feline calicivirus is dependent on clathrin-mediated endocytosis and acidification in endosomes. J. Virol. 80:7500–7509. 10.1128/JVI.02452-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moser LA, Schultz-Cherry S. 2008. Suppression of astrovirus replication by an ERK1/2 inhibitor. J. Virol. 82:7475–7482. 10.1128/JVI.02193-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang R, Lopez V, Kelleher SL, Lonnerdal B. 2011. Apo- and holo-lactoferrin are both internalized by lactoferrin receptor via clathrin-mediated endocytosis but differentially affect ERK-signaling and cell proliferation in Caco-2 cells. J. Cell. Physiol. 226:3022–3031. 10.1002/jcp.22650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Finkbeiner SR, Holtz LR. 2013. New human astroviruses, p 119–133 In Schultz-Cherry S. (ed), Astrovirus research: essential ideas, everyday impacts, future directions, vol 1 Springer Science and Business, New York, NY [Google Scholar]