Abstract
Carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) is an adhesion molecule expressed in a wide variety of tissues including epithelial cells, leukocytes, and tumors that may establish both homotypic and heterotypic interactions. The aim of this work was to study the protein expression pattern of CEACAM1 in cervical cancer and precursor lesions in the context of human papillomavirus (HPV) infection. We used immunohistochemistry to analyze CEACAM1 expression in formalin-fixed, paraffin-embedded cervical tissues from 15 healthy women, 15 patients with low-grade squamous intraepithelial lesions (SIL), 15 patients with high-grade SIL, and 15 patients with squamous carcinomas. HPV types were identified by PCR. CEACAM1 was either undetectable (13/15) or low (2/15) in normal cervical tissues. By contrast, CEACAM1 expression was increased in high-grade SIL (10 samples staining intermediate/high and 4 samples staining low) as compared with low-grade SIL with undetectable (n=3) or low (n= 12) expression. CEACAM1 expression was undetectable or low in cervical carcinoma. Our results suggest that CEACAM1 may be an interesting progression marker in SIL and cervical cancer, in particular due to reported immunoregulatory properties.
Keywords: carcinoembryonic, antigen-related cell, adhesion molecule 1, cell adhesion, squamous intraepithelial lesions, cervical cancer, human papillomavirus
Cervical cancer is the second most common malignant tumor in women worldwide, with 80% of cases arising in developing countries. Infection with oncogenic human papillomavirus (HPv) types is necessary but not sufficient to cause cervical cancer. Other risk factors include smoking, genetic factors, and immune system dysfunction (Schiffman and Castle 2003; Waggoner 2003). Cervical carcinogenesis encompasses HPV infection, viral persistence, progression to precancer lesions, and invasion (Schiffman and Kjaer 2003). Cell adhesion molecules play an essential role in different physiological and pathological conditions such as tissue architecture, cell migration, cell activation, inflammatory reactions, immune response, and cancer (Dustin 2001; Comoglio et al. 2003; Cavallaro and Christofori 2004). Carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1), also known as C-CAM, BGP, and CD66a, is a member of the immunoglobulin superfamily (Singer et al. 2000). It is widely expressed in a variety of epithelial, endothelial, and hematopoietic cells (Naka-jima et al. 2002; Chen et al. 2004). CEACAM1 mediates cell-cell adhesion via homophilic or heterophilic binding and regulates cell proliferation, apoptosis, tumor growth, and differentiation/polarization of epithelial cells, angiogenesis, NK cell cytotoxicity, and T-cell-mediated immune response (Greicius et al. 2003). Some CEACAM1 isoforms display cytoplasmic “immunoreceptor tyrosine-based inhibition motifs” (ITIM) that, upon phosphorylation, recruit SH2 domain containing tyrosine phosphatases (i.e., SHP1) (Sundberg and Obrink 2002).
Downregulation of CEACAM1 was reported in many malignant tumors such as breast, colon, endometrium, prostate, and hepatocellular carcinomas, suggesting that CEACAM1 may function as a tumor suppressor protein, maintaining the normal phenotype of epithelial cells (Phan et al. 2004). In contrast, de novo expression of CEACAM1 has been associated with poor response and reduced disease-free survival in patients with melanoma and non-small cell lung carcinoma (Kammerer et al. 2004). To date, no information is available on the expression of CEACAM1 in cervical cancer and only sparse data regarding normal cervical tissue have been reported. The aim of this work was to study the protein expression pattern of CEACAM1 in normal cervical tissue, squamous intraepithelial lesions (SIL), and cervical cancer, in the context of HPV infection.
Materials and Methods
Tissue Samples
Tissue samples were obtained from the Pathology Department of the OPD Hospital Civil de Guadalajara, Guadalajara, Mexico. The study included formalin-fixed, paraffin-embedded specimens from 15 invasive squamous cervical carcinomas (large-cell keratinizing and non-keratinizing types), 15 high-grade SIL, 15 low-grade SIL, and 15 normal cervical tissues. Mean age of subjects ranged from 37 to 45 years (low-grade SIL subjects being the youngest and cervical cancer patients the oldest). Difference between groups was not significant. High parity was more frequent in patients with cervical cancer. There was no significant difference between groups with respect to socioeconomic status, smoking habit, or other clinical history. Two different pathologists (FTR and CCF) independently confirmed the diagnosis for all specimens. The protocol was approved by the Biomedicine Sciences Committee according to the guidelines of the World Medical Association Declaration of Helsinki (amended by the 52nd WMA General Assembly, Edinburgh, Scotland, October 2000).
Immunohistochemical (IHC) Staining
Serial sections from the formalin-fixed, paraffin-embedded blocks were used for detection of CEACAM1 by IHC method. All sections (6 × 5 mm) included the transformation zone, ectocervix, endocervix, stroma, glands, and blood vessels. Thin sections (5 μm) were deparaffinized in xylene (J.T. Baker; Xalostoc, Mexico), rehydrated through a graded series of ethanol (Sigma; St Louis, MO), and heated in a steamer for 30 min in citrate buffer (10 mM, pH 6.0) for antigen retrieval. Slides were washed in Tris-buffered saline (TBS: 50 mmol/liter Tris, 150 mmol/liter NaCl, pH 7.4) and treated with Dako Peroxidase Block (Dako; Carpinteria, CA) for 5 min at room temperature to quench endogenous peroxidase activity. Sections were incubated with anti-CEACAM1 (HP-NC8 developed by Miguel Lopez-Botet; Universitat Pompeu Fabra, Barcelona, Spain). The HP-NC8 hybridoma was obtained immunizing Balb/c mice with activated NK cell populations. Specificity of the HP-NC8 MAb for CEACAM-1 was established based on immunofluorescence and immunoprecipitation analysis and was confirmed testing its reactivity by immunofluorescence against a panel of HeLa cells transfected with different family members (CEACAM1, CEACAM-5, CEACAM-6, or CEACAM-8); under these conditions only CEACAM1+ cells were clearly stained by HP-NC8 (Löpez-Botet M et al., unpublished data). The appropriate dilution was determined by serial 2-fold dilution at different times and temperature conditions. We selected 1:10 dilution in TBS for 60 min at room temperature. The two-step EnVision system (Dako) was used for IHC stain. Application of the primary antibody was followed by polymeric conjugate [secondary anti-mouse antibodies bond to a dextran backbone containing horseradish peroxidase (HRP)] incubation for 30 min at room temperature. The highly sensitive 3-amino-9-ethylcarbazol plus (AEC+) chromogen (Dako) was used as substrate for the EnVision HRP enzymes. All sections were counterstained with hematoxylin QS (Vector Laboratories; Burlingame, CA), mounted with gelatin-glycerin-based medium (Glycergel, Dako Faramount; Dako), and examined by light microscopy with a grid eyepiece. Negative controls included prostate cancer sections negative for CEACAM1 and nonspecific mouse IgG at the same protein concentration as the primary anti-CEACAM1 antibody. Normal placental tissues were used as positive controls in each staining run.
Evaluation of IHC Staining CEACAM1 Expression
Histological and IHC evaluation were performed independently by two pathologists. Slides with discrepant evaluations were re-evaluated, and a consensus was reached. For each sample, at least 3000 cells were evaluated for CEACAM1 expression, and the percentage of cells with membranous or membranous and cytoplasmic stain was determined. In addition, CEACAM1 expression was also categorized into “low” (<33% positive cells) and “intermediate/high” (>66% positive cells) (Sienel et al. 2003).
DNA Extraction
Tissue regions of interest were defined by characteristic morphological criteria and included the transformation zone with normal cervical tissue, low-grade SIL, high-grade SIL, or invasive cervical tissue, depending on the histopathological diagnosis. Tissue regions were outlined and excised from the paraffin block using a small scalpel (3 × 3 mm). Fragments were collected in autoclaved plastic microtubes (1.5 ml). Paraffin was dissolved in xylene (1 ml) for 10 min for two changes. Then, 0.5 ml of 100% ethanol was added and mixed for 5 min, followed by centrifugation at 10,000 × g for 3 min with two changes. After ethanol evaporation at 37C, 200 μg/ml of proteinase K (Sigma) was added for 36 hr at 37C. Proteinase K was inactivated at 94C for 10 min. Aqueous supernatant was transferred to another fresh microtube. DNA was precipitated by adding 100% ethanol and 20 μg/ml glycogen (Sigma) for 30 min at −20C. The pellet was washed twice with 70% ethanol, dried, resuspended in 20 ml distilled water, and measured spectrophotometrically.
PCR Assay
HPV detection was detected using specific primers (Table 1). All PCR reactions were performed in a total volume of 50 μl. PCR mixture contained 75 mM Tris-HCl pH 8.8, 20 mM (NH4)2SO4, 0.01% Tween 20,2 mM MgCl2, 0.2 mM dNTPs, 0.6 μM of each primer, 1.25 U Taq DNA recombinant polymerase (Fermentas International Inc; Burlington, ON, Canada), and 100 ng DNA. Genomic DNAs from SiHa (HPV16) and HeLa (HPV18), tissue samples with known HPV infection for HPV6/11, HPV31, and 33, were used as positive controls. Genomic DNA from C33A cervical carcinoma cells was used as negative control. The cycling protocol for CpI/CPII was 94C for 30 sec, 51C for 30 sec, and 72C for 60 sec for 40 cycles; HPV16, 92C for 120 sec, 48C for 90 sec; HPV6/11 and HPV18, 92C for 120 sec, 48C for 90 sec, and 72C for 60 sec for 38 cycles; and HPV31 and HPV33, 94Cfor 60 sec, 45C for 60 sec, and 72C for 60 sec for 45 cycles. Amplification products were electrophoresed on 1.8% agarose gel and visualized after ethidium bromide staining under UV light.
Table 1.
Primer sequences for HPV
| Name | Region of virus genome | Sequences | Amplimer length (base pair) |
| HPV (CpI/CpII) | E1 | ttatcawatgcccaytgtaccat atgttaatwsagccwccaaaatt | 188 |
| HPV 6/11 | E6 | ctctgccggtggtcagtgcat atgcctccacgtctgcaac | 120 |
| HPV16 | URR | gcagctctgtgcataac ctgcacatgggtgtgtgc | 229 |
| HPV18 | E2-Hinge | gaattcactctatgtgcag tagttgttgcctgtaggtg | 221 |
| HPV31 | E6 | ttcaaaaatcctgcagaaaag ctttgacacgttatacacct | 320 |
| HPV33 | E6/E7 | acctttgcaacgatctgagg gaaccgcaaacacagtttac | 108 |
HPV, human papillomavirus.
Statistical Analysis
Statistical analysis was performed using the SPSS software package (version 10.0; SPSS Inc., Chicago, IL). Measures of central tendency and dispersion were determined. For CEACAM1 expression and HPV infection, X 2 test was used to evaluate homogeneity. CEACAM1 expression in normal cervical tissue, low-grade SIL, high-grade SIL, and cervical cancer were compared with ANOVA and Mann-Whitney U test. We also performed a two-factor ANOVA using CEACAM1 expression as dependent variable, considering study groups and high-risk HPV infection as factors. Spearman rank correlation method was used to correlate CEACAM1 expression and HPV infection (low- and high-risk HPV). Results were expressed as r value together with significance level. Differences were considered significant at p<0.05.
Results
IHC analysis showed that CEACAM1 protein was not expressed or scantly expressed in squamous epithelial cells from normal cervical tissues. We only detected CEACAM1 protein in 2/15 normal cervical samples. In both positive samples, CEACAM1 expression was very low (5% and 10%, respectively) and restricted to superficial epithelial layers with a membranous staining pattern. We studied 30 SIL classified into two groups according to the Bethesda System: low-grade (15) and high-grade SIL (15). Of 15 low-grade SIL, three were negative for CEACAM1 expression. Of the remaining 12 low-grade SIL, all were low positive (staining ranged from 3% to 30%). Pattern of CEACAM1 expression was membranous and restricted to superficial layers. In contrast, 10/15 high-grade intraepithelial lesions displayed intermediate- to high-positive CEACAM1 expression (staining ranged from 33% to 76%), and four showed low expression (staining ranged from 5% to 25%). Only one high-grade SIL was negative for CEACAM1. In high-grade intraepithelial lesions, both cytoplasmic and membranous staining were observed through all epithelial layers. Of 15 invasive cervical carcinomas, 2 tissues were negative and 13 were low positive to CEACAM1. The vast majority of CEACAM1-positive tumor samples showed staining from 3% to 10% (11/13 samples), and in all cases positivity was restricted to keratin pearls or well-differentiated tumors (membranous staining). Statistical analysis with ANOVA and Mann-Whitney U test showed that CEACAM1 expression was significantly elevated in high-grade SIL in comparison with normal cervical specimens (p<0.0001 and p=0.000043, respectively), low-grade SIL (p<0.001 and p=0.0037, respectively), and cervical cancer (p<0.0001 and p=0.0004, respectively). CEACAM1 expression did not differ significantly between low-grade SIL and cervical cancer. Representative photographs of CEACAM1 expression in normal cervical tissues, SIL, and invasive cervical cancer are shown in Figure 1. Complete IHC and HPV infection data are summarized in Table 2.
Figure 1.
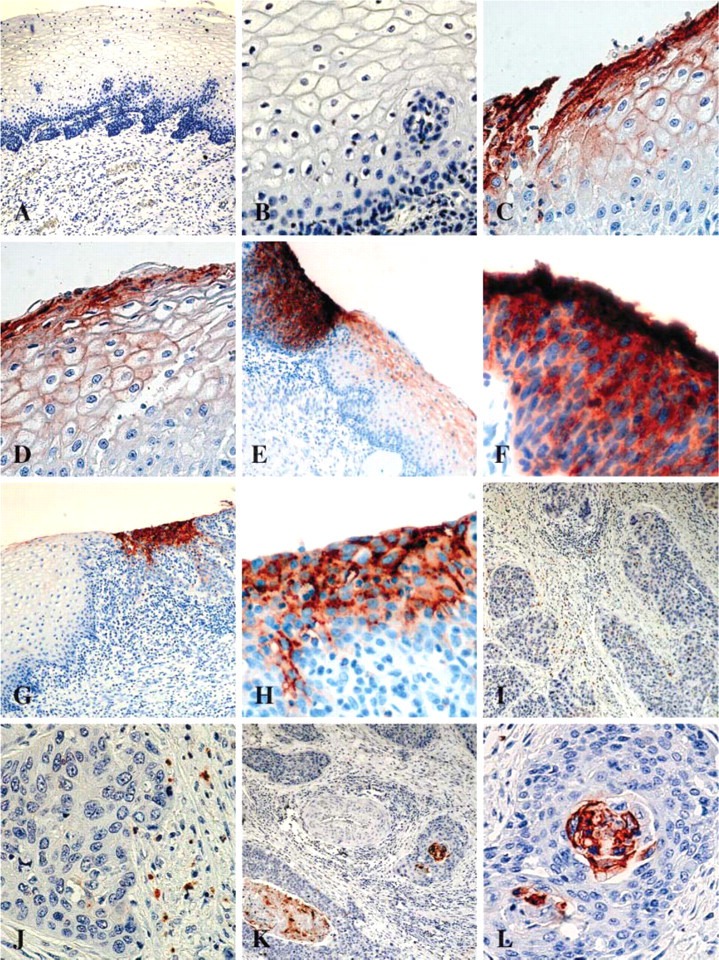
Immunohistochemical detection of carcinoembryonic antigen-related adhesion molecule 1 (CEACAM1) on paraffin-embedded sections. (A,B) Normal tissue: note absence of expression in squamous epithelial cells. (C,D) Low-grade squamous intraepithelial lesion (LGSIL): low expression of CEACAM1 in the superficial epithelial edge with membranous staining pattern. (E) Coexistence of LGSIL: low expression of CEACAM1 with membranous staining pattern and high-grade SIL (HGSIL) with high expression of CEACAM1 with cytoplasmic-membranous pattern. (F) Magnification of previous HGSIL. (G) Coexistence of normal tissue and HGSIL: note absence of expression in normal squamous epithelial cells and intermediate expression of CEACAM1 in HGSIL with cytoplasmic-membranous pattern. (H) Magnification of previous HGSIL: intermediate expression of CEACAM1 in squamous epithelial cells. (I,J) Invasive cancer: note absence of CEACAM1 expression in tumor cells and sparse but CEACAM1-positive neutrophils. (K,L) Invasive cancer: low expression of CEACAM1 in keratin pearls. No staining was observed with normal mouse serum or isotype-matched negative control antibodies.
The vast majority of normal cervical tissues (13/15) were negative for CEACAM1. Ten of these CEACAM1-negative samples were also negative for high-risk HPV, whereas the other three CECAM1-negative samples were positive for low-risk HPV 6/11. The remaining two normal low-positive CEACAM1 samples were negative for both high- and low-risk HPV. Three low-grade SIL were negative for CEACAM1, and one of these samples was positive for high-risk HPV. Of 12 low-grade SIL that were positive for CEACAM1 (low positive), six were also positive for high-risk HPV. Almost all high-grade SIL with CEACAM1 staining 33% to 75% (7/10) were positive for high-risk HPV (HPV 16 > HPV 31 > HPV 33 or HPV 18 and three with coinfections). One high-grade SIL with CEACAM1 staining 33% to 75% was negative for high-risk HPV, another was positive for low-risk HPV, and the third was positive for HPV, but HPV typification was not possible. Three of four high-grade SIL with CEACAM1 staining <33% were positive for high-risk HPV (two with low-risk HPV coinfections). However, the unique CEACAM1-negative high-grade SIL was positive for high-risk HPV. Practically all CECAM1-negative or -positive invasive cervical carcinoma tissues were positive for high-risk HPV (see Table 2). There was a significant correlation between CEACAM1 expression and high-risk HPV infection (HPV 16, 18, 31, or 33) when all groups were analyzed at the same time (Spearman r = 0.552, p<0.001). However, we did not find a significant correlation between CEACAM1 expression and high-risk HPV infection when the four groups were analyzed separately. A two-factor ANOVA analysis showed that CEACAM1 expression differences were explained by study group factor (p<0.0001) and not important by high-risk HPV infection factor (p=0.113).
Table 2.
CEACAM1 expression and HPV infection in normal cervical tissues, LGSIL, HGSIL, and invasive cervical cancera
| CEACAM1 expression | HPV infection (positive samples, %) | |||||
| Clinical status | Categories | Number (%) | Staining pattern | Negative | Low risk (6/11) | High risk (16, 18, 31, 33) |
| Controls | Negative | 13 (86.6) | 10 (66.6) | 3 (20.0) | ||
| <33% | 2 (13.3) | Membranous | 2 (13,3) | |||
| >33% | ||||||
| Median (range) | 0 (0-10) | |||||
| LGSIL | Negative | 3 (20.0) | 2 (13.3) | 1 (6.6) | ||
| <33% | 12 (80.0) | Membranous | 1 (6.6) | 7 (46.6) two coinfections | 6 (40.0) two coinfections | |
| >33% | ||||||
| Median (range) | 10 (0-30) | |||||
| HGSIL | Negative | 1 (6.6) | 1 (6.6) | |||
| <33% | 4 (26.6) | Membranous | 3 (20.0) two coinfections | 3 (20.0) two coinfections | ||
| >33% | 10 (66.6) | Cytoplasmic/membranous (all epithelium) | 1 (6.6) | 4 (26.6) three coinfections | 7 (46.6) three coinfections | |
| Median range | 40 (0-76) | |||||
| INV CA | Negative | 2 (13.3) | 2 (13.3) | |||
| <33% | 13 (86.6) | Keratin pearls and membranous | 5 (33.3) three coinfections | 11 (73.3) three coinfections | ||
| >33% | ||||||
| Median (range) | 5 (0-30) | |||||
Carcinoembryonic antigen-related adhesion molecule 1 (CEACAM1) expression (ANOVA and Mann-Whitney U test): high-grade squamous intraepithelial lesions (HGSIL) vs controls, p<0.0001 and p=0.000043; vs low-grade squamous intraepithelial lesions (LGSIL), p<0.001 and p=0.0037; vs invasive carcinoma (INV CA), p<0.0001 and p=0.0004; LGSIL vs INV CA, not significant. One HGSIL (CEACAM1 >33%) was positive for HPV, but typification was not possible.
Discussion
Cervical cancer implies a complex and not entirely understood interaction between tumor and host factors. SIL progression is tightly linked to HPV persistence and local immune response (Wang and Hildesheim 2003). It is important to distinguish between women at high risk for SIL progression or recurrence after SIL treatment. Efforts to accomplish this goal include evaluation of resection margins (Orbo et al. 2004), HPV detection (Kalof et al. 2005), and the use of biological markers such as p16INK4 (Kalof et al. 2005). It has been reported that local immunity alterations play an important role in progression to cervical cancer such as dendritic cell reduction (Jimenez-Flores et al. 2006) and suppression by proteases (Daneri-Navarro et al. 2005). However, key factors implicated in HPV persistence, SIL progression, and recurrence posttreatment are still unknown (Orbo et al. 2004; Sarian et al. 2004).
In this work we describe for the first time the expression pattern of CEACAM1 in cervical cancer and precursor lesions. We show that CEACAM1 immunostaining is significantly increased in high-grade SIL in comparison with low-grade SIL and normal cervical tissues (virtually absent). Statistical analysis reveals that CEACAM1 is a marker of SIL progression. Our data are important in view of the described inhibitory role of CEACAM1 in NK and T cells (Markel et al. 2002; Phan et al. 2004). It has been reported that CEACAM1 functions as a regulatory coreceptor for both lymphocytes and myeloid cells, in an ITIM-dependent manner (Gray-Owen and Blumberg 2006). We propose two alternative hypotheses regarding CEACAM1 expression in SIL. In the first scenario, high-risk HPV-infected cells expressing CEACAM1 may inhibit T, NK, or dendritic cell function through homophilic interactions during the antiviral immune response. Negative effects mediated by CEACAM1 in infectious diseases include the ability of Neisseria gonorrhoeae to suppress antibody production by killing CEACAM1-expressing B cells (Pantelic et al. 2005) and T-cell suppression by Neisseria gonorrhoeae Opa proteins-CEACAM1 interaction (Boulton and Gray-Owen 2002). Haemophilus influenzae, Neisseria gonorrhoeae, Neisseria meningitides, and SARS coronavirus use CEACAM1 as cellular receptor (Virji et al. 2000; Yang et al. 2004). In the second scenario, CEACAM1 expression in high-risk HPV-infected cervical cells is linked to tumor suppressor activity. In this last case, CEACAM1-positive, high-grade SIL will be associated with proliferation control and regression. CEACAM1 downregulation in invasive cervical cancer supports this last scenario. To resolve this dilemma, it will be necessary to study CEACAM1 expression and SIL progression or recurrence posttreatment in a prospective cohort study.
CEACAM1 is a fascinating adhesion molecule that may have apparently opposite actions (i.e., tumorpromoting vs tumor-suppressive functions) depending on the tumor lineage and differentiation grade (Estrera et al. 2001; Sienel et al. 2003; Oliveira-Ferrer et al. 2004). In this work we observed that CEACAM1 expression in invasive cervical carcinoma was weak or restricted to keratin pearls and well-differentiated tumor cells, supporting the view that CEACAM1 must be downregulated at invasive stages. Similar findings were observed in prostate, colon, and breast cancer (Bamberger et al. 2002; Nittka et al. 2004; Phan et al. 2004). CEACAM1 downregulation is mediated through transcriptional repression by Sp2 in prostate cancer (Phan et al. 2004). The mechanism(s) that mediate CEACAM1 upregulation in SIL or downregulation in invasive cervical carcinoma are unknown. Our data suggest that CEACAM1 expression is linked to high-grade SIL, followed by down-regulation during cervical carcinoma invasion. To better understand the role of high-risk HPV in CEACAM1 expression, it is important to correlate HPV viral load and HPV DNA status: episomal vs integrated HPV DNA with CEACAM1 upregulation in high-grade SIL. HPV in situ hybridization is a useful tool to discriminate between episomal and integrated HPV DNA. Preliminary studies in our laboratory suggest that CEACAM1 upregulation may be related to integrated HPV DNA in high-grade SIL (unpublished data). Together our results suggest that CEACAM1 may be an important biological marker in SIL and cervical cancer progression.
Acknowledgments
This work was supported by a grant from the Terry Fox Foundation (to AD-N).
The authors thank Karina Franco Topete for assistance with immunohistochemistry and Rogelio Troyo-Sanroman for helpful suggestions used in statistical analysis.
Literature Cited
- Bamberger AM, Kappes H, Methner C, Rieck G, Brummer J, Wagener C, Loning T, et al. (2002) Expression of the adhesion molecule CEACAM1 (CD66a, BGP, C-CAM) in breast cancer is associated with the expression of the tumor-suppressor genes Rb, Rb2, and p27. Virchows Arch 440:139–144 [DOI] [PubMed] [Google Scholar]
- Boulton IC, Gray-Owen SD. (2002) Neisserial binding to CEACAM1 arrests the activation and proliferation of CD4+ T lymphocytes. Nat Immunol 3:229–236 [DOI] [PubMed] [Google Scholar]
- Cavallaro U, Christofori G. (2004) Multitasking in tumor progression: signaling functions of cell adhesion molecules. Ann NY Acad Sci 1014:58–66 [DOI] [PubMed] [Google Scholar]
- Chen D, Iijima H, Nagaishi T, Nakajima A, Russell S, Raychowdhury R, Morales V, et al. (2004) Carcinoembryonic antigen-related cellular adhesion molecule 1 isoforms alternatively inhibit and costimulate human T cell function. J Immunol 172:3535–3543 [DOI] [PubMed] [Google Scholar]
- Comoglio PM, Boccaccio C, Trusolino L. (2003) Interactions between growth factor receptors and adhesion molecules: breaking the rules. Curr Opin Cell Biol 15:565–571 [DOI] [PubMed] [Google Scholar]
- Daneri-Navarro A, Del Toro-Arreola S, Sanchez-Hernandez PE, Ramirez-Duenas MG, Armendariz-Borunda J, Perez-Montfort R. (2005) Immunosuppressive activity of proteases in cervical carcinoma. Gynecol Oncol 98:111–117 [DOI] [PubMed] [Google Scholar]
- Dustin ML. (2001) Role of adhesion molecules in activation signaling in T lymphocytes. J Clin Immunol 21:258–263 [DOI] [PubMed] [Google Scholar]
- Estrera VT, Chen DT, Luo W, Hixson DC, Lin SH. (2001) Signal transduction by the CEACAM1 tumor suppressor. Phosphorylation of serine 503 is required for growth-inhibitory activity. J Biol Chem 276:15547–15553 [DOI] [PubMed] [Google Scholar]
- Gray-Owen SD, Blumberg RS. (2006) CEACAM1: contact-dependent control of immunity. Nat Rev Immunol 6:433–446 [DOI] [PubMed] [Google Scholar]
- Greicius G, Severinson E, Beauchemin N, Obrink B, Singer BB. (2003) CEACAM1 is a potent regulator of B cell receptor complex-induced activation. J Leukoc Biol 74:126–134 [DOI] [PubMed] [Google Scholar]
- Jimenez-Flores R, Mendez-Cruz R, Ojeda-Ortiz J, Munoz-Molina R, Balderas-Carrillo O, de la Luz Diaz-Soberanes M, Lebecque S, et al. (2006) High-risk human papilloma virus infection decreases the frequency of dendritic Langerhans' cells in the human female genital tract. Immunology 117:220–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalof AN, Evans MF, Simmons-Arnold L, Beatty BG, Cooper K. (2005) p16INK4A immunoexpression and HPV in situ hybridization signal patterns: potential markers of high-grade cervical intraepithelial neoplasia. Am J Surg Pathol 29:674–679 [DOI] [PubMed] [Google Scholar]
- Kammerer R, Riesenberg R, Weiler C, Lohrmann J, Schleypen J, Zimmermann W. (2004) The tumour suppressor gene CEACAM1 is completely but reversibly downregulated in renal cell carcinoma. J Pathol 204:258–267 [DOI] [PubMed] [Google Scholar]
- Markel G, Lieberman N, Katz G, Arnon TI, Lotem M, Drize O, Blumberg RS, et al. (2002) CD66a interactions between human melanoma and NK cells: a novel class I MHC-independent inhibitory mechanism of cytotoxicity. J Immunol 168:2803–2810 [DOI] [PubMed] [Google Scholar]
- Nakajima A, Iijima H, Neurath MF, Nagaishi T, Nieuwenhuis EE, Raychowdhury R, Glickman J, et al. (2002) Activation-induced expression of carcinoembryonic antigen-cell adhesion molecule 1 regulates mouse T lymphocyte function. J Immunol 168: 1028–1035 [DOI] [PubMed] [Google Scholar]
- Nittka S, Gunther J, Ebisch C, Erbersdobler A, Neumaier M. (2004) The human tumor suppressor CEACAM1 modulates apoptosis and is implicated in early colorectal tumorigenesis. Oncogene 23:9306–9313 [DOI] [PubMed] [Google Scholar]
- Oliveira-Ferrer L, Tilki D, Ziegeler G, Hauschild J, Loges S, Irmak S, Kilic E, et al. (2004) Dual role of carcinoembryonic antigen-related cell adhesion molecule 1 in angiogenesis and invasion of human urinary bladder cancer. Cancer Res 64:8932–8938 [DOI] [PubMed] [Google Scholar]
- Orbo A, Arnesen T, Arnes M, Straume B. (2004) Resection margins in conization as prognostic marker for relapse in high-grade dysplasia of the uterine cervix in northern Norway: a retrospective long-term follow-up material. Gynecol Oncol 93:479–483 [DOI] [PubMed] [Google Scholar]
- Pantelic M, Kim YJ, Bolland S, Chen I, Shively J, Chen T. (2005) Neisseria gonorrhoeae kills carcinoembryonic antigen-related cellular adhesion molecule 1 (CD66a)-expressing human B cells and inhibits antibody production. Infect Immun 73:4171–4179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan D, Cheng CJ, Galfione M, Vakar-Lopez F, Tunstead J, Thompson NE, Burgess RR, et al. (2004) Identification of Sp2 as a transcriptional repressor of carcinoembryonic antigen-related cell adhesion molecule 1 in tumorigenesis. Cancer Res 64: 3072–3078 [DOI] [PubMed] [Google Scholar]
- Sarian LO, Derchain SF, Pitta Dda R, Morais SS, Rabelo-Santos SH. (2004) Factors associated with HPV persistence after treatment for high-grade cervical intra-epithelial neoplasia with large loop excision of the transformation zone (LLETZ). J Clin Virol 31:270–274 [DOI] [PubMed] [Google Scholar]
- Schiffman M, Kjaer SK. (2003) Chapter 2: Natural history of anogenital human papillomavirus infection and neoplasia. J Natl Cancer Inst Monogr 31:14–19 [DOI] [PubMed] [Google Scholar]
- Schiffman MH, Castle P. (2003) Epidemiologic studies of a necessary causal risk factor: human papillomavirus infection and cervical neoplasia. J Natl Cancer Inst 95:E2. [DOI] [PubMed] [Google Scholar]
- Sienel W, Dango S, Woelfle U, Morresi-Hauf A, Wagener C, Brummer J, Mutschler W, et al. (2003) Elevated expression of carcinoembryonic antigen-related cell adhesion molecule 1 promotes progression of non-small cell lung cancer. Clin Cancer Res 9:2260–2266 [PubMed] [Google Scholar]
- Singer BB, Scheffrahn I, Obrink B. (2000) The tumor growth-inhibiting cell adhesion molecule CEACAM1 (C-CAM) is differently expressed in proliferating and quiescent epithelial cells and regulates cell proliferation. Cancer Res 60:1236–1244 [PubMed] [Google Scholar]
- Sundberg U, Obrink B. (2002) CEACAM1 isoforms with different cytoplasmic domains show different localization, organization and adhesive properties in polarized epithelial cells. J Cell Sci 115:1273–1284 [DOI] [PubMed] [Google Scholar]
- Virji M, Evans D, Griffith J, Hill D, Serino L, Hadfield A, Watt SM. (2000) Carcinoembryonic antigens are targeted by diverse strains of typable and non-typable Haemophilus influenzae . Mol Microbiol 36:784–795 [DOI] [PubMed] [Google Scholar]
- Waggoner SE. (2003) Cervical cancer. Lancet 361:2217–2225 [DOI] [PubMed] [Google Scholar]
- Wang SS, Hildesheim A. (2003) Chapter 5: Viral and host factors in human papillomavirus persistence and progression. J Natl Cancer Inst Monogr 31:35–40 [DOI] [PubMed] [Google Scholar]
- Yang M, Li CK, Li K, Hon KL, Ng MH, Chan PK, Fok TF. (2004) Hematological findings in SARS patients and possible mechanisms. Int J Mol Med 14:311–315 [PubMed] [Google Scholar]


