Abstract
SOX13 is a member of the SOX family of transcription factors. SOX proteins play essential roles in development, and some are associated with human genetic diseases. SOX13 maps to a multi-disease locus on chromosome 1q31-32, yet its function is unknown. Here we describe the temporal and spatial expression of SOX13 protein during mouse organogenesis. SOX13 is expressed in the three embryonic cell lineages, suggesting that it may direct various developmental processes. SOX13 is expressed in the developing central nervous system including the neural tube and the developing brain. Expression is also detected in the condensing mesenchyme and cartilage progenitor cells during endochondral bone formation in the limb as well as the somite sclerotome and its derivatives. SOX13 is also detected in the developing kidney, pancreas, and liver as well as in the visceral mesoderm of the extra-embryonic yolk sac and spongiotrophoblast layer of the placenta.
Keywords: SOX13, mouse embryonic development, expression pattern, neural tube, endochondral bone, sclerotome, visceral mesoderm, spongiotrophoblast
SOX [SRY-related high-mobility group (HMG) BOX] genes encode a family of transcription factors that bind and bend DNA through the HMG domain (Ferrari et al. 1992; Harley et al. 1992). SOX transcription factors have been implicated in a variety of developmental processes including sex determination, neurogenesis, and endochondral bone formation (Laudet et al. 1993; Pevny and Lovell-Badge 1997; Southard-Smith et al. 1998; Wegner 1999).
Spatial and temporal expression patterns of SOX genes during mammalian development support a role governing cell fate and differentiation. For instance, Sox9 is expressed in Sertoli cells of the developing testis as well as during mesenchymal condensation prior to chondrocyte development. Other tissues expressing Sox9 include the notochord, otocysts, vibrissae, tubular heart structures, and ventricular central nervous system (CNS) during embryonic development (Foster and Graves 1994; Bell et al. 1997; Lefebvre et al. 1998). Furthermore, mutations in SOX9 result in clinical pathologies consistent with an essential function in the development of these tissues. SOX9 is required for chondrogenesis and testis development as evidenced by mutations in campomelic dysplasia syndrome, characterized by severe skeletal malformation and autosomal sex reversal (Foster and Graves 1994; Wagner et al. 1994). Mutations in SOX10 result in the neurological disorder Hischsprung-Wardenberg syndrome (Inoue et al. 2002; Paratore et al. 2002), and mutations in SRY result in XY gonadal dysgenesis (Schmitt-Ney et al. 1995; Scherer et al. 1998; Canto et al. 2000; Uehara et al. 2002).
SOX13 belongs to group D of the SOX gene family. Murine Sox13 was cloned from a cDNA library of mouse embryonic tissue (Kido et al. 1998; Roose et al. 1998), whereas human SOX13 was cloned from a pancreatic islet cell cDNA expression library (Rabin et al. 1992; Argentaro et al. 2000; Kasimiotis et al. 2000). Human and mouse SOX13 share 99% amino acid sequence identity within the HMG domain and 90% overall amino acid identity (Kido et al. 1998; Argentaro et al. 2000; Kasimiotis et al. 2000), suggesting conserved function of SOX13 in mouse and human.
Human SOX13 mRNA has been detected in diverse tissues such as pancreas, heart, brain, placenta, lung, liver, and kidney (Kasimiotis et al. 2000). Furthermore, SOX13 maps to human chromosome 1q31.3–32.1 (Argentaro et al. 2000). This region is associated with insulin-dependent diabetes mellitus and other complex genetic disorders (Durand et al. 1995; Kraus et al. 1996b; Bahabri et al. 1998; Cornelis et al. 1998; Vollmer et al. 1998; Houdayer et al. 1999). Chromosome 1q31–32 also exhibits loss of heterozygosity in pancreatic cancer and hepatoblastoma (Kraus et al. 1996a) and various neoplastic diseases (Kraus et al. 1996a,b; Steiner et al. 1996; Benitez et al. 1997; Fogt et al. 1998). Given its unknown function and potential disease association, we examine here the expression of SOX13 protein during mouse embryonic development from embryonic day (E) 9.5 to E15.5, as well as in the yolk sac and placenta.
Materials and Methods
Mice
All procedures involving mice were approved by the Animal Ethics Committee of Monash University, Victoria, Australia. Embryos from E10.5 to E15.5 were removed from F1 (C57BL6 × CBA) natural mating.
Whole-Mount Immunohistochemistry (IHC)
Embryos were fixed overnight at 4C in 4% paraformaldehyde/PBS (pH 7) and dehydrated through a methanol series in PBS. Embryos were bleached in 5% hydrogen peroxide in methanol for 1 hr at room temperature, rehydrated through a reverse methanol series, and blocked by incubation in PBMT (3% skim milk, 0.1% Triton X-100/PBS) overnight at 4C. Embryos were then incubated overnight at 4C in PBMT containing 10% BSA and 4 μg/ml of affinity-purified rabbit polyclonal SOX13 antibody followed by five 1-hr washes in PBST (0.1% Triton X-100/PBS) at 4C. Antibody was raised against a human SOX13 peptide spanning amino acids 80–103 (Kasimiotis et al. 2000). As a negative control, 4 μg/ml of SOX13 antibody was preincubated with 3.6 × 10-5 M of SOX13 peptide (80–103) in 20% FBS/PBS on ice for 30 min. Primary antibody was detected by incubation with 2 μg/ml of horseradish peroxidase (HRP)-conjugated anti-rabbit anti-body (Chemicon; Temecula, CA) in PBMT overnight at 4C followed by PBST washes as above. For enzyme detection, embryos were preincubated with 0.3 mg/ml diaminobenzidine (DAB; Sigma, St Louis, MO) and 0.05% NiCl2 in PBST for 1 hr, and color development was activated by addition of 0.03% hydrogen peroxide. When optimal color development was achieved, the reaction was stopped by a PBST wash.
IHC Using Embryo Sections
Embryos were fixed for 2 hr to overnight at 4C in 4% paraformaldehyde/PBS (pH 7) and cryoprotected by incubation in 30% sucrose/PBS overnight at 4C. Embryos were embedded in optimal cutting temperature compound and cryo-sectioned into 8-μm sagittal or transversal sections on Super-frost Plus slides (Menzel-Glaser; Braunschweig, Germany).
Sections were air dried at room temperature and nuclear epitopes were unmasked by boiling in 0.01 M citrate buffer (pH 6) for 8 min followed by a wash in PBS (pH 7) with 0.1% Triton/X-100. Sections were blocked with 6% H2O2/PBS followed by CAS blocking solution (Zymed; South San Francisco, CA) for 30 min, respectively, at room temperature to block endogenous peroxidases and nonspecific antigens. Sections were then incubated with 1.2 μg/ml of affinity-purified rabbit polyclonal SOX13 antibody diluted in 20% fetal bovine serum/PBS (pH 7) at 4C overnight. Preimmune serum or peptide-blocked SOX13 antibody was used as negative control. Primary antibody was detected using a biotinylated goat anti-rabbit IgG secondary antibody (Vector Laboratories; Burlingame, CA) at a 1:200 dilution in PBS for 1 hr at room temperature, washed three times for 10 min each in PBST (0.1% Triton X-100/ PBS), and followed by incubation with 1:500 dilution of Streptavidin-HRP (Amersham Bioscience; Piscataway, NJ) at room temperature for 1 hr. Sections were incubated with liquid DAB substrate (DAKO; Carpinteria, CA) until optimal color development was achieved and then counterstained with hematoxylin.
Results
Temporal and Spatial Expression of SOX13
We examined the spatial and temporal expression of SOX13 during mouse organogenesis using whole-mount IHC from E9.5 to E12.5. As shown in Figure 1, SOX13 was broadly expressed throughout the embryo at all stages examined, with greatest abundance in neural tissues, somites, limb buds, and the oronasopharyngeal region (Figures 1A–1D). At E9.5, expression of SOX13 was evident in the brain and neural tube, limb buds, somites, and branchial arches (particularly the maxillary and mandibular arches) and in the naso-pharyngeal ectoderm (Figure 1A). At E10.5–E12.5, expression was maintained in these tissues, although dynamic changes were observed as the tissue became more complex (see the limb bud, for example, as shown in Figures 1B–1D and Figure 2).
Figure 1.
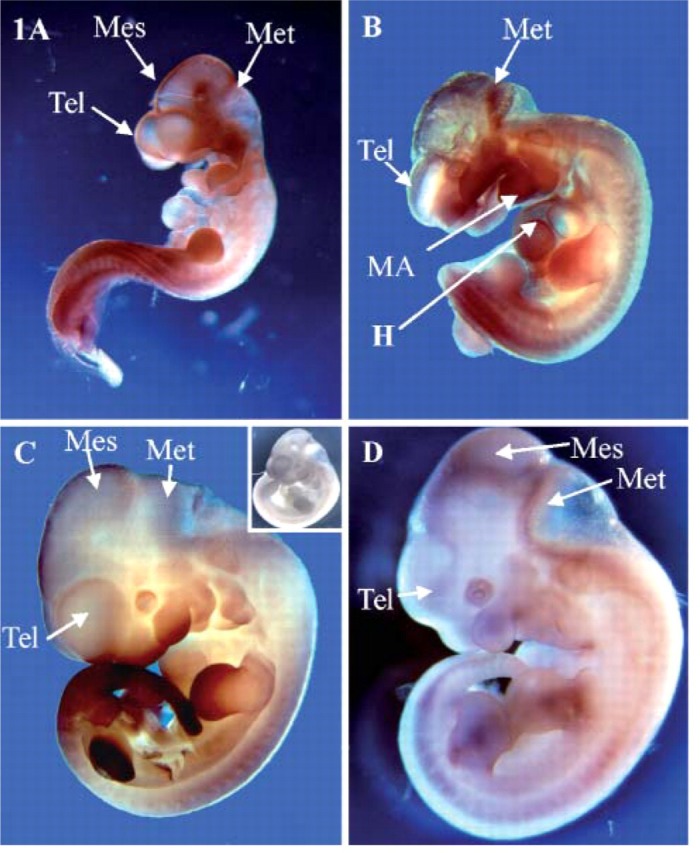
Expression of SOX13 during mouse embryonic organogenesis. Whole-mount immunohistochemistry (IHC) analysis of SOX13 was performed at (A) embryonic day (E) 9.5, (B) E10.5, (C) E11.5, (D) E12.5. Inset C, E11.5 is a negative control at E11.5. Tel, telencephalon; Mes, mesencephalon; Met, metencephalon; MA, mandibular arch; H, heart bulge.
Figure 2.
Expression of SOX13 revealed by whole-mount IHC in forelimb bud in (A) E9.5, (B) E10.5, (C) E11.5, and (D) E12.5 and in the hindlimb at E9.5 (E), E10.5 (F), E11.5 (G), and E12.5 (H). IHC on sagittal sections through the forelimb are shown in (I) E11.5 and (J) E13.5. r, rostral; p, proximal; d, distal; c, caudal; Ec, ectoderm. Bars: I = 200 μm; J = 50 μm; inset J = 25 μm.
Figures 1–2
Expression of SOX13 in the Developing Central Nervous System
At E9.5, as the brain vesicles begin to separate and the four major divisions of brain are defined from the surface, ectoderm SOX13 was detected in the prospective forebrain to the hindbrain region and along the length of the neural tube (Figure 1A). At E10.5–E11.5, expression of SOX13 was also detected in the neural tube (Figure 1B). By E12.5, when neuroepithelium actively undergoes differentiation and outward migration to form the lateral subventricular zone, expression of SOX13 is observed in all three regions of the developing brain and neural tube (Figure 1D). From E13.5 to E15.5, whereas neuronal genesis is ongoing, SOX13 is persistently highly expressed in the developing CNS where expression can be seen throughout the forebrain, midbrain, hindbrain, and spinal cord (Figures 1A–1D). Interestingly, expression of SOX13 is largely confined to the outer layer of the developing neuroepithelium where neural precursor cells stop dividing and begin to differentiate. At the subcellular level, SOX13 is located in the nucleus of neuroepithelial cells in the spinal cord and developing brain (Wang et al. 2005).
Dynamic Expression of SOX13 in the Developing Limb Bud and Somite
SOX13 expression is highly dynamic during limb bud development. At E9.5, as soon as the limb bud is discernible, SOX13 protein is first broadly expressed in the mesenchyme and surface ectoderm of the limb bud (Figures 2A, 2E, and 2I), and then SOX13 is progressively repositioned from the rostral perichondrial area to the mesenchyme of the paddle-shaped limb bud in the prospective arm region and hand plate (but is absent in the caudal surface ectoderm) (Figures 2B and 2F). As the prospective digits are formed in the hand plate, SOX13 expression is initially broadly detected in the distal limb bud mesenchyme with reduced expression toward the proximal mesenchyme (Figures 2C and 2G). At E12.5, expression of SOX13 is subsequently consolidated to the prospective arm regions. We examined sagittal sections from forelimb at E11.5 and E13.5 (Figure 3). Expression of SOX13 is confined to the cartilage progenitors in the developing tibia (Figure 2I) and digits (Figure 2J). Expression of SOX13 in the hindlimb bud is similar to that in the forelimb bud except for the developmental time delay (forelimb in Figures 2A–2D, 2I, and 2J; hindlimb in Figures 2E–2H). Dynamic expression of SOX13 in the developing limb bud suggests that SOX13 may play a role in limb patterning and later in chondrocyte differentiation.
Figure 3.
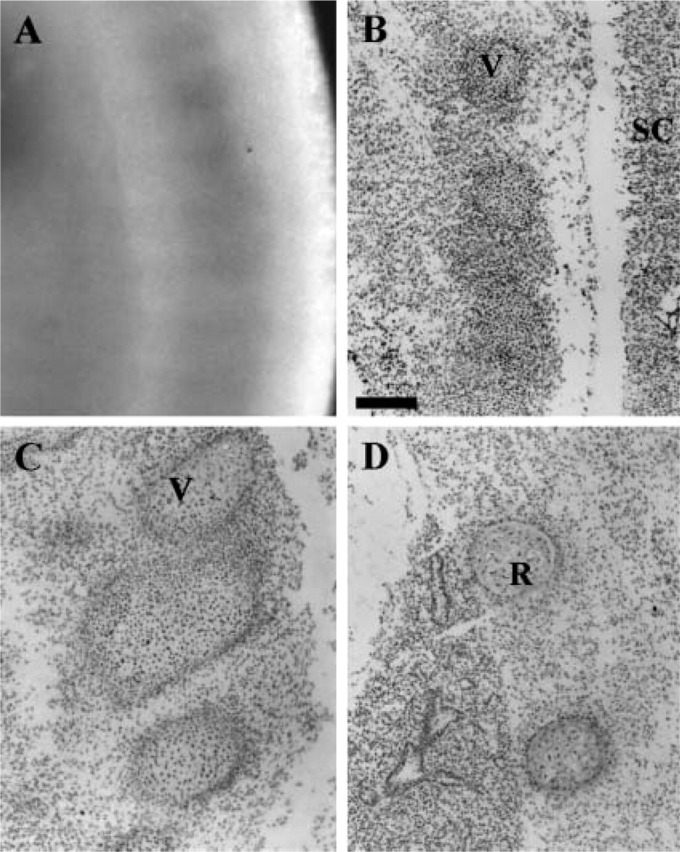
Expression of SOX13 in the developing somite revealed by whole-mount IHC in (A) E12.5 and by IHC on sagittal sections in (B) E12.5, (C) E13.5, and (D) E15.5. SC, spinal cord; V, vertebra; R, rib. Bar = 200 μm.
Parallel to the expression in limb bud, SOX13 is also detected in the somite as early as E9.5 (Figure 1A) and continues at E11.5 and E12.5 (Figures 1C and 1D). To determine the regional expression of SOX13 in the developing somites, we sectioned somites from E12.5 to E15.5 and examined them using IHC. SOX13 is expressed in the sclerotome of the somites and the derivative vertebrae and ribs (Figures 3A–3D). SOX13 is not detected in the dermatome and myotome of the somite.
Other Regions of SOX13 Expression During Embryogenesis
SOX13 expression is transiently detected in cardiac tissues at E10.5 (Figure 1B) but is not detected in these tissues at E11.5–E13.5. Later, SOX13 is weakly detected in the arterial wall of the heart at E15.5.
SOX13 Expression in the Developing Placenta and Yolk Sac
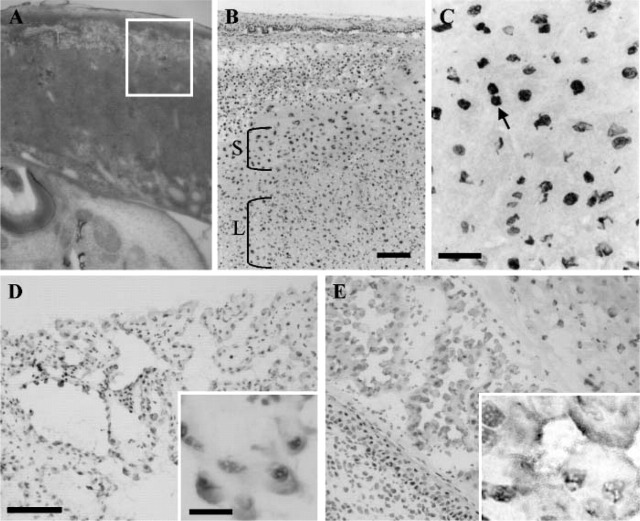
SOX13 is detected in the placenta and extra-embryonic yolk sac. At E15.5, SOX13 is strongly expressed in the spongiotrophoblast layer (Figure 4B), and expression of SOX13 is weakly detected in the labyrinth layer (Figure 4B). In addition, SOX13 is located in the spongiotrophoblast layer of the placenta (Figure 4) with subcellular localization in the nucleus of these cells (Figures 4B and 4C). In the E13.5 and E15.5 yolk sac, SOX13 is localized to the visceral mesoderm (Figures 4D and 4E).
Figure 4.
Expression of SOX13 in the placenta and yolk sac revealed by IHC on sections. (A) Hematoxylin-eosin staining reveals the overall appearance of placenta at E15.5, the boxed area indicating the approximate region in (B). SOX13 protein was observed in the spongiotrophoblast layer. Expression of SOX13 in the yolk sac is shown in (C) E13.5 and (D) E15.5. SOX13 protein localizes to the nucleus of giant trophoblast cells (arrow) and the cytoplasm of visceral mesodermal cells in yolk sac shown in insets in D,E at high magnification. S, spongiotrophoblast; L, labyrinth. Bars: B = 200 μm; C,D = 50 μm; inset D = 25 μm.
Discussion
We analyzed expression of SOX13 protein during mouse embryo organogenesis from E9.5 to E15.5 using whole-mount and section IHC. Our data demonstrated that SOX13 is widely expressed in a number of developing organs including the CNS, cartilage, and derivatives of neural crest such as the branchial arches. Apart from embryonic expression, SOX13 is also observed in the placenta and yolk sac, suggesting the possible role of SOX13 in both organogenesis and support of embryonic growth. Certain sites of SOX13 immunoreactivity are consistent with the sites by SOX13 RNA observed by in situ hybridization (Roose et al. 1998), such as cardiac tissues and the arterial wall of the heart.
SOX13 exhibits a dynamic expression profile during limb development and chondrogenesis. During limb development, expression of Sox8 and Sox9 occurs before morphological or structural changes in prechondrogenic mesoderm, and these proteins are required for mesenchymal condensation (Bi et al. 1999; Akiyama et al. 2002; Chimal-Monroy et al. 2003). Induction of Sox8, Sox9, and Sox10 precedes that of bone morphogenetic protein receptor 1b (Bmpr1b), suggesting that these Sox factors are upstream of BMP signaling during mesenchymal condensation. Conversely, BMPs have a positive influence on Sox gene expression in maintenance of the chondrocyte phenotype (Enomoto-Iwamoto et al. 1998; Chimal-Monroy et al. 2003). SOX13 is first broadly expressed in the condensed mesenchyme of the developing limb, becoming regionalized in the center of the condensed mesenchyme. This expression pattern is similar to that of L-Sox5 and Sox6 (Chimal-Monroy et al. 2003), which exhibit a loop-shaped domain of expression in cartilage. These Sox factors have been shown to be essential for overt chondrocyte differentiation (Smits et al. 2001; Chimal-Monroy et al. 2003). Furthermore, SOX5 and SOX6 cooperate with SOX9 to activate the Col2a1 enhancer. SOX13 together with L-Sox5 and Sox6 belong to SOX D subgroup and share high homology both within and outside the HMG domain, suggesting a conserved function during development in vertebrates. This overlapping expression suggests that SOX13 may also play a role in chondrocyte differentiation. Therefore, SOX proteins of the same group might act cooperatively where coexpressed.
SOX13 is highly expressed in the developing CNS. SOX13 is expressed in the prospective brain and neural tube and later in the outer layer of the neuroepithelium during active neuronal genesis. A number of Sox genes are known to function in the specification and differentiation of the CNS (Uwanogho et al. 1995; Collignon et al. 1996; Rex et al. 1997; Mizuseki et al. 1998; Pevny et al. 1998; Bylund et al. 2003; Graham et al. 2003). For instance, Sox4 and Sox11 (both group C) are predominantly expressed in differentiating neuronal cells, suggesting a role in neurogenesis. Restricted expression of SOX13 in the lateral region of the CNS suggests that SOX13 may function in the differentiation of the neuroepithelium. Given the similar expression of SOX13 with L-Sox5 and Sox6 during limb development, it might be important to compare the expression of these three group-D SOX members in the developing CNS.
In the placenta, high SOX13 expression was detected in the spongiotrophoblast layer within the nuclei of trophoblast giant cells. As far as we know, this is the first evidence of SOX protein expression in giant trophoblast cells. The trophoblast is involved in both blastocyst implantation and in fetoplacental growth and development. Trophoblast giant cells produce a number of angiogenic and vasoactive substances that may mediate uterine vascular remodeling (Cross et al. 2002b). So far, it has been shown that the basic helix-loop-helix transcription factor Hand1 is essential for differentiation of trophoblast giant cells in mice and for the regulation of the giant-cell-specific hormone, placental lactogen I gene promoter (Pl1) (Cross et al. 2002a). SOX13, as a nuclear transcription factor, may have a role in uterine vascular remodeling to support embryonic development.
In conclusion, we have demonstrated that SOX13, like other SOX factors, is expressed during embryonic development and may play a role in limb development, chondrogenesis, and neurogenesis. SOX proteins often display a widespread expression profile during different stages of embryogenesis, yet still play specific roles in development (Pevny and Lovell-Badge 1997; Wegner 1999). SOX13 is no exception, demonstrating widespread and dynamic expression during embryonic growth and development. The study of SOX13 at both the cellular and molecular levels in these developmental processes is being undertaken.
Acknowledgments
V.R.H is a Fellow of the National Health and Medical Research Council. The research was also supported by Diabetes Australia.
We thank Dr. Stefan Bagheri-Fam for helpful technical assistance and discussion.
Literature Cited
- Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B. (2002) The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev 16:2813–2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argentaro A, Olsson J, Critcher R, McDowall SG, Harley VR. (2000) Genomic characterisation and fine mapping of the human SOX13 gene. Gene 250:181–189 [DOI] [PubMed] [Google Scholar]
- Bahabri SA, Suwairi WM, Laxer RM, Polinkovsky A, Dalaan AA, Warman ML. (1998) The camptodactyly-arthropathy-coxa varapericarditis syndrome: clinical features and genetic mapping to human chromosome 1. Arthritis Rheum 41:730–735 [DOI] [PubMed] [Google Scholar]
- Bell DM, Leung KK, Wheatley SC, Ng LJ, Zhou S, Ling KW, Sham MH, et al. (1997) SOX9 directly regulates the type-II collagen gene. Nat Genet 16:174–178 [DOI] [PubMed] [Google Scholar]
- Benitez J, Osorio A, Barroso A, Arranz E, Diaz-Guillen MA, Robledo M, Rodriguez de Cordoba S, et al. (1997) A region of allelic imbalance in 1q31—32 in primary breast cancer coincides with a recombination hot spot. Cancer Res 57:4217–4220 [PubMed] [Google Scholar]
- Bi W, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. (1999) Sox9 is required for cartilage formation. Nat Genet 22:85–89 [DOI] [PubMed] [Google Scholar]
- Bylund M, Andersson E, Novitch BG, Muhr J. (2003) Vertebrate neurogenesis is counteracted by Sox1—3 activity. Nat Neurosci 6:1162–1168 [DOI] [PubMed] [Google Scholar]
- Canto P, de la Chesnaye E, Lopez M, Cervantes A, Chavez B, Vilchis F, Reyes E, et al. (2000) A mutation in the 5′ non-high mobility group box region of the SRY gene in patients with Turner syndrome and Y mosaicism. J Clin Endocrinol Metab 85:1908–1911 [DOI] [PubMed] [Google Scholar]
- Chimal-Monroy J, Rodriguez-Leon J, Montero JA, Ganan Y, Macias D, Merino R, Hurle JM. (2003) Analysis of the molecular cascade responsible for mesodermal limb chondrogenesis: Sox genes and BMP signaling. Dev Biol 257:292–301 [DOI] [PubMed] [Google Scholar]
- Collignon J, Sockanathan S, Hacker A, Cohen-Tannoudji M, Norris D, Rastan S, Stevanovic M, et al. (1996) A comparison of the properties of Sox-3 with Sry and two related genes, Sox-1 and Sox-2. Development 122:509–520 [DOI] [PubMed] [Google Scholar]
- Cornelis F, Faure S, Martinez M, Prud'homme JF, Fritz P, Dib C, Alves H, et al. (1998) New susceptibility locus for rheumatoid arthritis suggested by a genome-wide linkage study. Proc Natl Acad Sci USA 95:10746–10750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross JC, Anson-Cartwright L, Scott IC. (2002a) Transcription factors underlying the development and endocrine functions of the placenta. Recent Prog Horm Res 57:221–234 [DOI] [PubMed] [Google Scholar]
- Cross JC, Hemberger M, Lu Y, Nozaki T, Whiteley K, Masutani M, Adamson SL. (2002b) Trophoblast functions, angiogenesis and remodeling of the maternal vasculature in the placenta. Mol Cell Endocrinol 187:207–212 [DOI] [PubMed] [Google Scholar]
- Durand JB, Bachinski LL, Bieling LC, Czernuszewicz GZ, Abchee AB, Yu QT, Tapscott T, et al. (1995) Localization of a gene responsible for familial dilated cardiomyopathy to chromosome 1q32. Circulation 92:3387–3389 [DOI] [PubMed] [Google Scholar]
- Enomoto-Iwamoto M, Iwamoto M, Mukudai Y, Kawakami Y, Nohno T, Higuchi Y, Takemoto S, et al. (1998) Bone morphogenetic protein signaling is required for maintenance of differentiated phenotype, control of proliferation, and hypertrophy in chondrocytes. J Cell Biol 140:409–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Harley VR, Pontiggia A, Goodfellow PN, Lovell-Badge R, Bianchi ME. (1992) SRY, like HMG1, recognizes sharp angles in DNA. EMBO J 11:4497–4506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogt F, Zhuang Z, Linehan WM, Merino MJ. (1998) Collecting duct carcinomas of the kidney: a comparative loss of heterozygosity study with clear cell renal cell carcinoma. Oncol Rep 5:923–926 [DOI] [PubMed] [Google Scholar]
- Foster JW, Graves JA. (1994) An SRY-related sequence on the marsupial X chromosome: implications for the evolution of the mammalian testis-determining gene. Proc Natl Acad Sci USA 91:1927–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham V, Khudyakov J, Ellis P, Pevny L. (2003) SOX2 functions to maintain neural progenitor identity. Neuron 39:749–765 [DOI] [PubMed] [Google Scholar]
- Harley VR, Jackson DI, Hextall PJ, Hawkins JR, Berkovitz GD, Sockanathan S, Lovell-Badge R, et al. (1992) DNA binding activity of recombinant SRY from normal males and XY females. Science 255:453–456 [DOI] [PubMed] [Google Scholar]
- Houdayer C, Soupre V, Rosenberg-Bourgin M, Martinez H, Tredano M, Feldmann D, Feingold J, et al. (1999) Linkage analysis of 5 novel van der Woude syndrome kindreds to 1q32-q41 markers further supports locus homogeneity of the disease trait. Ann Genet 42:69–74 [PubMed] [Google Scholar]
- Inoue K, Shilo K, Boerkoel CF, Crowe C, Sawady J, Lupski JR, Agamanolis DP. (2002) Congenital hypomyelinating neuropathy, central dysmyelination, and Waardenburg-Hirschsprung disease: phenotypes linked by SOX10 mutation. Ann Neurol 52:836–842 [DOI] [PubMed] [Google Scholar]
- Kasimiotis H, Myers MA, Argentaro A, Mertin S, Fida S, Ferraro T, Olsson J, et al. (2000) Sex-determining region Y-related protein SOX13 is a diabetes autoantigen expressed in pancreatic islets. Diabetes 49:555–561 [DOI] [PubMed] [Google Scholar]
- Kido S, Hiraoka Y, Ogawa M, Sakai Y, Yoshimura Y, Aiso S. (1998) Cloning and characterization of mouse mSox13 cDNA. Gene 208:201–206 [DOI] [PubMed] [Google Scholar]
- Kraus JA, Albrecht S, Wiestler OD, von Schweinitz D, Pietsch T. (1996a) Loss of heterozygosity on chromosome 1 in human hepatoblastoma. Int J Cancer 67:467–471 [DOI] [PubMed] [Google Scholar]
- Kraus JA, Koch A, Albrecht S, Von Deimling A, Wiestler OD, Pietsch T. (1996b) Loss of heterozygosity at locus F13B on chromosome 1q in human medulloblastoma. Int J Cancer 67:11–15 [DOI] [PubMed] [Google Scholar]
- Laudet V, Stehelin D, Clevers H. (1993) Ancestry and diversity of the HMG box superfamily. Nucleic Acids Res 21:2493–2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V, Li P, de Crombrugghe B. (1998) A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type II collagen gene. EMBO J 17:5718–5733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuseki K, Kishi M, Matsui M, Nakanishi S, Sasai Y. (1998) Xenopus Zic-related-1 and Sox-2, two factors induced by chordin, have distinct activities in the initiation of neural induction. Development 125:579–587 [DOI] [PubMed] [Google Scholar]
- Paratore C, Eichenberger C, Suter U, Sommer L. (2002) Sox10 haplo-insufficiency affects maintenance of progenitor cells in a mouse model of Hirschsprung disease. Hum Mol Genet 11:3075–3085 [DOI] [PubMed] [Google Scholar]
- Pevny LH, Lovell-Badge R. (1997) Sox genes find their feet. Curr Opin Genet Dev 7:338–344 [DOI] [PubMed] [Google Scholar]
- Pevny LH, Sockanathan S, Placzek M, Lovell-Badge R. (1998) A role for SOX1 in neural determination. Development 125:1967–1978 [DOI] [PubMed] [Google Scholar]
- Rabin DU, Pleasic SM, Palmer-Crocker R, Shapiro JA. (1992) Cloning and expression of IDDM-specific human autoantigens. Diabetes 41:183–186 [DOI] [PubMed] [Google Scholar]
- Rex M, Uwanogho DA, Orme A, Scotting PJ, Sharpe PT. (1997) cSox21 exhibits a complex and dynamic pattern of transcription during embryonic development of the chick central nervous system. Mech Dev 66:39–53 [DOI] [PubMed] [Google Scholar]
- Roose J, Korver W, Oving E, Wilson A, Wagenaar G, Markman M, Lamers W, et al. (1998) High expression of the HMG box factor sox-13 in arterial walls during embryonic development. Nucleic Acids Res 26:469–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer G, Held M, Erdel M, Meschede D, Horst J, Lesniewicz R, Midro AT. (1998) Three novel SRY mutations in XY gonadal dysgenesis and the enigma of XY gonadal dysgenesis cases without SRY mutations. Cytogenet Cell Genet 80:188–192 [DOI] [PubMed] [Google Scholar]
- Schmitt-Ney M, Thiele H, Kaltwasser P, Bardoni B, Cisternino M, Scherer G. (1995) Two novel SRY missense mutations reducing DNA binding identified in XY females and their mosaic fathers. Am J Hum Genet 56:862–869 [PMC free article] [PubMed] [Google Scholar]
- Smits P, Li P, Mandel J, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B, et al. (2001) The transcription factors L-Sox5 and Sox6 are essential for cartilage formation. Dev Cell 1:277–290 [DOI] [PubMed] [Google Scholar]
- Southard-Smith EM, Kos L, Pavan WJ. (1998) Sox10 mutation disrupts neural crest development in Dom Hirschsprung mouse model. Nat Genet 18:60–64 [DOI] [PubMed] [Google Scholar]
- Steiner G, Cairns P, Polascik TJ, Marshall FF, Epstein JI, Sidransky D, Schoenberg M. (1996) High-density mapping of chromosomal arm 1q in renal collecting duct carcinoma: region of minimal deletion at 1q32.1—32.2. Cancer Res 56:5044–5046 [PubMed] [Google Scholar]
- Uehara S, Hashiyada M, Sato K, Nata M, Funato T, Okamura K. (2002) Complete XY gonadal dysgenesis and aspects of the SRY genotype and gonadal tumor formation. J Hum Genet 47:279–284 [DOI] [PubMed] [Google Scholar]
- Uwanogho D, Rex M, Cartwright EJ, Pearl G, Healy C, Scotting PJ, Sharpe PT. (1995) Embryonic expression of the chicken Sox2, Sox3 and Sox11 genes suggests an interactive role in neuronal development. Mech Dev 49:23–36 [DOI] [PubMed] [Google Scholar]
- Vollmer M, Jung M, Ruschendorf F, Ruf R, Wienker T, Reis A, Krapf R, et al. (1998) The gene for human fibronectin glomerulopathy maps to 1q32, in the region of the regulation of complement activation gene cluster. Am J Hum Genet 63:1724–1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, Pasantes J, et al. (1994) Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell 79:1111–1120 [DOI] [PubMed] [Google Scholar]
- Wang Y, Bagheri-Fam S, Harley VR. (2005) SOX13 is up-regulated in the developing mouse neuroepithelium and identifies a sub-population of differentiating neurons. Brain Res Dev Brain Res 157:201–208 [DOI] [PubMed] [Google Scholar]
- Wegner M. (1999) From head to toes: the multiple facets of Sox proteins. Nucleic Acids Res 27:1409–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]