Abstract
Expression of membrane-bound carbonic anhydrases (CAs) of CA IV, CA IX, CA XII, and CA XIV has been investigated in the mouse heart. Western blots using microsomal membranes of wild-type hearts demonstrate a 39-, 43-, and 54-kDa band representing CA IV, CA IX, and CA XIV, respectively, but CA XII could not be detected. Expression of CA IX in the CA IV/CA XIV knockout animals was further confirmed using matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Cardiac cells were immunostained using anti-CA/FITC and anti-α-actinin/TRITC, as well as anti-CA/FITC and anti-SERCA2/TRITC. Subcellular CA localization was investigated by confocal laser scanning microscopy. CA localization in the sarcolemmal (SL) membrane was examined by double immunostaining using anti-CA/FITC and anti-MCT-1/TRITC. CAs showed a distinct distribution pattern in the sarcoplasmic reticulum (SR) membrane. CA XIV is predominantly localized in the longitudinal SR, whereas CA IX is mainly expressed in the terminal SR/t-tubular region. CA IV is present in both SR regions, whereas CA XII is not found in the SR. In the SL membrane, only CA IV and CA XIV are present. We conclude that CA IV and CA XIV are associated with the SR as well as with the SL membrane, CA IX is located in the terminal SR/t-tubular region, and CA XII is not present in the mouse heart. Therefore, the unique subcellular localization of CA IX and CA XIV in cardiac myocytes suggests different functions of both enzymes in excitation-contraction coupling.
Keywords: carbonic anhydrase, cardiomyocytes, sarcoplasmic reticulum, sarcolemma
Within THE MAMMALIAN α-carbonic anhydrase (CA) gene family, 11 enzymatically active CA isoforms have been identified so far. Five isoforms are cytosolic (CA I, CA II, CA III, CA VII, CA XIII), four are membrane-bound (CA IV, CA IX, CA XII, CA XIV), two are mitochondrial (CA VA and CA VB), and one is a secretory form (CA VI). Three proteins are characterized by a homologous “CA-like” domain but are catalytically inactive (CA-related proteins, CA-RP VIII, CA-RP X, CA-RP XI). Lehtonen et al. (2004) demonstrated a CA 13 gene transcript in the mouse heart, but neither Lehtonen et al. (2004) nor Geers et al. (1992) has found a cytosolic CA protein or activity in the heart. With the dansylsulfonamide (DNSA) technique, Bruns and Gros (1992) histochemically demonstrated a sarcolemmal (SL) CA staining in the rabbit heart as well as a CA staining at intracellular structures, which they could not further identify. Vandenberg et al. (1996) confirmed the presence of an SL CA in the ferret heart by the modified Hansson technique. Additionally, they found CA to be located at capillary endothelial membranes. Neither the DNSA nor the Hansson technique can distinguish among the different CA isoforms. In 1998, Sender et al. was able to identify the isoform of the SL as well as the endothelial CA, namely, CA IV. Using electron microscopy and staining of ultrathin sections with anti-CA IV/immunogold, they found a weak intracellular staining, which indicated that CA IV might also be associated with the sarcoplasmic reticulum (SR). Mori et al. (1999), as well as Fujikawa-Adachi et al. (1999), identified a novel membrane-bound isoform, CA XIV, and found CA XIV mRNA in the heart muscle. No evidence for any expression of CA IX as well as of CA XII in the mouse heart has been reported so far (Ivanov et al. 2001; Hilvo et al. 2004). Therefore, we intended to elucidate (1) whether there is an intracellular, membrane-bound CA isoform present in the heart and (2) whether CA IX, CA XII, and/or CA XIV are expressed in the cardiac muscle membranes. By confocal laser scanning microscopy (CLSM), we found CA IV and CA XIV to be located in the SR as well as in the SL membranes of adult cardiomyocytes of mice. The presence of CA IX in mouse heart was also confirmed by proteomics using matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS/MS). CA IX is solely expressed in the terminal SR and/or t-tubule membrane, and CA XII is not present in the mouse heart.
Materials and Methods
Preparation of Microsomal Membranes
Wild-type mice, mice deficient for CA IV and CA XIV individually, or CA IV/CA XIV double-knockout (KO) mice were sacrificed by an overdose of diethyl ether. Hearts or kidneys were rapidly dissected out and kept in 0.75 M KCl, 5 mM imidazole, pH 7.4, 4C or flash frozen in liquid nitrogen before use. Microsomal membranes or total tissue membranes were prepared as described (Wetzel and Gros 1990; Waheed et al. 1992b). Protein concentration was measured by micro-Lowry protein assay (Lowry et al. 1951). All experiments were carried out in accordance with the guidelines of the Bezirksregierung Hannover.
Antibodies
Rabbit anti-mouse CA IV and CA XIV antibodies were described earlier (Parkkila et al. 2001,2002; Kaunisto et al. 2002). Rabbit anti-mouse CA XII antibody was described by Kyllönen et al. (2003). Rabbit anti-human CA IX antibody was produced using affinity-purified human CA IX from the secretions of Chinese hamster ovary cells expressing the secretory form of CA IX cDNA (Kivelä et al. 2000). CA IX cDNA was a kind gift from Dr. J. Pastorek, Academy of Sciences of Prague, Czech Republic. Goat anti-mouse SERCA2 antibody and goat anti-mouse MCT-1 antibody, as well as all secondary anti-rabbit IgG and anti-goat IgG antibodies, were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal goat anti-mouse α-actinin antibody was obtained from Sigma (St Louis, MO).
Purification and Characterization of Mouse CA IX From CA IV/XIV KO Mice Heart Tissues
Total tissue membranes equivalent to 50 mg protein in 10 ml 10 mM HEPES, pH 7.5, with 1 mM each of PMSF, benzamidine HCl, and o-phenanthroline and 1% NP-40 were homogenized with polytron for three 10-sec bursts to extract the enzyme. Membrane homogenates were centrifuged at 30,000 × g for 30 min at 4C. The clear supernatant was loaded onto the pAMBS-Sepharose (Sigma) affinity column in a 5-ml volume. Flow-through was recycled five times to ensure complete binding of the enzyme to the affinity resin. Unbound material was washed out using 10 mM HEPES, pH 7.5, with 0.1% NP-40 until OD 280 nm was close to zero. Protein bound to the affinity column was eluted with 0.1 M sodium acetate, pH 5.6, with 0.5 M sodium perchlorate and 0.05% NP-40 in 5-ml fractions. The fractions containing protein were pooled, concentrated, and dialyzed against 10 mM Tris-SO4, pH 7.5. Affinity-purified enzyme equivalent to 100 μg protein was subjected to large SDS-PAGE (16 × 14 cm) under reducing conditions (Waheed et al. 1992a). The gel was stained with Coomassie blue stain (Sigma) and destained with water. Several polypeptide bands were cut out and subjected to MALDI-TOF MS/MS analyses. In parallel, a gel was subjected to Western blot using CA IX-specific antibodies. Polypeptides corresponding to CA IX Western blot bands were also cut out from the Coomassie-stained gel and analyzed by MALDI-TOF MS/MS.
MALDI-TOF MS/MS Analysis
Gel pieces were dehydrated with two washes in 50% acetonitrile (ACN) in 200 mM NH4HCO3 followed by one wash in 100% ACN and were further dehydrated in a vacuum centrifuge for 30 min, then rehydrated in 50 mM NH4HCO3/1 mM CaCl2 containing 1 μg/ml modified tryspin (Promega; Madison, WI). Digestion was performed overnight (16-24 hr) at 37C (Terry et al. 2004).
Peptide extraction was performed as previously described (Chen 2006), except with three volumes of 2% ACN/1% formic acid. The pooled extract was lyophilized and resuspended in 2% ACN/1% formic acid. ZipTip μC18 (Millipore; Billerica, MA) clean-up was performed according to the manufacturer's instruction, except the peptides were eluted with 60% ACN/0.1% formic acid. An aliquot of the ZipTip eluent was spotted onto a stainless steel MALDI target plate (Applied Biosystems; Foster City, CA). An equal aliquot of α-cyan-o-4-hydroxycinnamic acid (Resolution Systems; Holland, MI) was mixed with the eluent on the target plate. Spots were allowed to dry completely.
A QSTAR-XL equipped with an oMALDI ion source (Applied Biosystems/MDS Sciex; Boston, MA) was used for protein identification. TOF-MS and product ion spectra were generated automatically using the IDA feature of the Analyst QS Software in conjunction with the oMALDI server and the following parameters: TOF-MS range of 700-3500 m/z and MS/MS range of 50-2000 m/z. Approximately 25 cycles of one TOF-MS scan followed by product ion scans of the three most intense peaks were acquired.
For protein identification, MALDI MS/MS spectra were searched against the SWISS-PROT database using the MASCOT search engine www.matrixscience.com) as previously described (Chen 2006), except with a mass tolerance of 50 ppm. Unambiguous identification was judged by the MASCOT mowse score, quality of MS/MS spectra, and the number of peptides matched.
Western Blot Analysis
The mouse heart or kidney membranes containing 50 μg proteins were analyzed by SDS-PAGE under reducing conditions. Western blot analysis was performed as described (Waheed et al. 1992a). After SDS-PAGE, the polypeptides were transferred to an Immobilon-P membrane (Millipore). The polypeptides for CAs were characterized using primary anti-mouse CA IV, XII, and XIV, as well as anti-human CA IX antibodies at a dilution of 1:3000 and secondary antibodies, anti-rabbit IgG, conjugated with peroxidase (1 ng/ml; Calbiochem, Merck Biosciences, Schwalbach, Germany). The blots were developed using a luminol/peroxide buffer kit from Pierce Biotechnology (SuperSignal West Femto; Rockford, IL). Low molecular mass standard proteins were from Bio-Rad Laboratories (Hercules, CA).
Cell Culture
Adult mice were sacrificed by an overdose of diethyl ether and immediately dissected out. The heart muscle was cut into small pieces and treated with collagenase. A primary cell culture was established according to a modified method as described by Freimueller et al. (1983). Cells were seeded on glass coverslips and cultured for 3 hr, 24 hr, or for 3 days at 37C in DMEM with 10% fetal calf serum and 5% CO2.
Immunofluorescence Staining
Cardiomyocytes were washed and fixed with 3% paraformaldehyde and 100% methanol and permeabilized in 0.1% Triton X-100 for 5 min as described earlier (Meissner et al. 2001). Cells were incubated with primary antibodies for 30 min. The primary anti-mouse CA XIV antibody was diluted 1:1600-fold; the anti-mouse CA IV, the anti-mouse CA XII, and the anti-human CA IX antibodies were diluted 1:300-fold; and the anti-mouse α-actinin, the anti-mouse SERCA2, and anti-mouse MCT 1 antibodies were diluted 1:300-fold. Incubation with the primary antibody was followed by 30-min incubation with FITC-labeled anti-rabbit IgG secondary antibody and with tetramethyl-rhodamine (TRITC)-labeled anti-goat IgG secondary antibody. Intracellular localization of CA, α-actinin, and SERCA2, respectively, was examined by CLSM (Leica DMIRBE; Wetzlar, Germany) and analyzed with Image Space software (Leica TCS-NT). In the case of extracellular localization of CA and MCT-1, fixation and permeabilization of cardiomyocytes were omitted, and cells were visualized by epifluorescence.
CA KO Mice
The CA IX KO mouse as well as the CA IV KO, CA XIV KO, and CA IV/CA XIV KO mice have been characterized earlier (Gut et al. 2002; Shah et al. 2005).
Results
CA Isoforms in Mice Heart
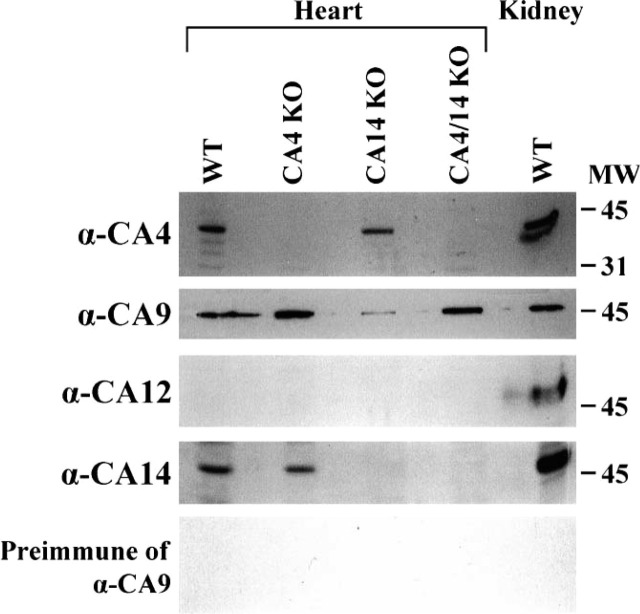
Expression patterns of different CA isozymes in wild-type, CA IV KO, CA XIV KO, and CA IV/XIV KO hearts are shown in Figure 1. CA IV was expressed in wild-type and CA XIV KO heart. There were no CA IV polypeptides observed in CA IV KO or CA IV/CA XIV KO mice hearts, as expected. For positive control, kidney tissue membranes were used for CA IV expression in wild-type mice. Similarly, CA XIV was expressed in wild-type and CA IV KO mice; however, heart from CA XIV or CA IV/CA XIV KO mice did not show any CA XIV polypeptide. Mouse CA XII was not detected in any heart. There was a positive polypeptide staining for CA XII in the wild-type kidney, as expected (Halmi et al. 2004). CA IX isozyme was expressed in heart tissues of all mice used. Preimmune serum for CA IX did not show any reaction, suggesting a specific reaction of the polypeptide with CA IX antiserum.
Figure 1.
CA isoforms in mouse heart. Total heart tissue membranes containing 50 μg protein from wild-type (WT), CA IV KO, CA XIV KO, and CA IV/CA XIV KO mice were analyzed by SDS-PAGE followed by Western blot using αCA IV, CA IX, CA XII, and CA XIV antisera, and preimmune serum for CA IX. Polypeptides for CA IV, CA IX, CA XII, and CA XIV are indicated. Preimmune serum for CA IX did not show any reaction.
Identification of CA IX in CA IV/CA XIV KO Mice Heart
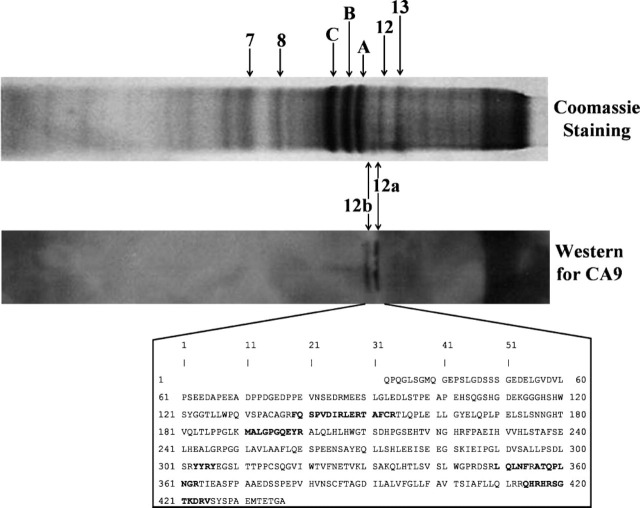
Using CA-inhibitor affinity purification and separation of the polypeptides over SDS-PAGE, we found several polypeptide bands stained with Coomassie blue. We also identified the presence of CA IX polypeptide on Western blot. The gel pieces corresponding to CA IX polypeptides, as well as several prominent polypeptide bands, were further subjected to MALDI-TOF MS/MS analyses. These results are shown in Figure 2. Both polypeptides reactive to CA IX antiserum were identified to be CA IX. In the box, the complete amino acid sequence of mouse CA IX is shown, and the polypeptides identified by MALDI-TOF-MS/MS are shown in bold letters.
Figure 2.
Identification of CA IX in CA IV/CA XIV KO mice heart. Affinity-purified enzyme for CA IV/CA XIV KO mouse heart was analyzed on SDS-PAGE. The gels were stained for protein using Coomassie blue or subjected to Western blot for CA IX using CA IX-specific antibodies. Several major polypeptides (7, 8, 12, 13, A, B, and C) were removed from the Coomassie-stained gel and analyzed by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS/MS). Two minor polypeptides corresponding to Western blot polypeptides 12a and 12b were also removed from the Coomassie-stained gel and analyzed by MALDI-TOF MS/MS. Polypeptides 12a and 12b were characterized as mouse CA IX. The amino acid sequence of the enzyme is shown, and the polypeptides identified by MALDI-TOF MS/MS are indicated in bold letters.
Other major polypeptides were identified as follows: A. ATP synthase β-chain (P56480), B. ATP synthase β-chain (P56480), C. mouse actin, aortic smooth muscle (A, α-actin 2) (P62737), 7. ATP synthase β-chain (P56480), 8. ATP synthase β-chain (P56480), 12. cytochrome C (Q980M3), 13. mouse actin, aortic smooth muscle (P62737), 12a. carbonic anhydrase IX precursor (Q8VHB5), and 12b. carbonic anhydrase IX precursor (Q8VHB5).
Cardiomyocytes in Culture
Figure 3A shows a freshly isolated, rod-shaped cardiac myocyte. The cell shows positive α-actinin staining as visualized by the red fluorescence of rhodamine and a staining of the nucleus by 4′-6-diamidino-2-phenylindole. α-Actinin protein is located on the Z discs. Therefore, α-actinin staining indicates the localization of the Z discs. The latter defines the localization of the myofibrils, which are surrounded by a network of SR membranes. After 24 hr in culture, cardiac cells still display a nearly rod-shaped morphology without presenting extensions (Figure 3B). After 3 days in culture, cardiomyocytes are no longer rod-shaped and do show some extensions (Figure 3C). In contrast to 3-hr and 24-hr cultured cells, 3-day cultured cells contracted spontaneously and were more tightly attached to the coverslips so that only a few cells were lost during the immunostaining procedure.
Figure 3.
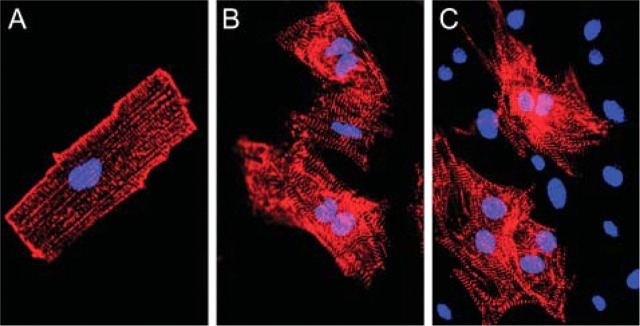
Cardiomyocytes of adult hearts from mouse in cell culture. (A) Cardiomyocytes 3 hr in culture. (B) Cardiomyocytes 24 hr in culture. (C) Cardiomyocytes 3 days in culture. Cardiomyocytes show a positive α-actinin staining as visualized by incubation with tetramethyl-rhodamine-labeled anti-rabbit IgG antibody. The α-actinin protein is located in the Z discs. Nuclei are stained by 4′-6-diamidino-2-phenylindole (bisbenzimide H 33258; Sigma). Epifluorescence.
Intracellular CA Associated With the SR Membrane
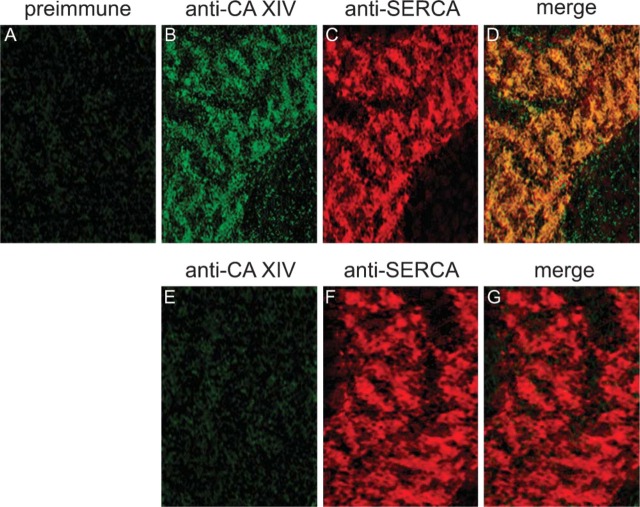
CA XIV. Intracellular CA staining was examined by CLSM. As seen in Figure 4A, the green fluorescence signal of FITC shows the intracellular staining of CA XIV. Anti-CA XIV staining is located in between the anti-α-actinin staining (see Figures 4B and 4C). This distribution pattern of anti-CA XIV and anti-α-actinin labeling does not change among 3-hr (Figures 4A-4C), 24-hr (Figures 4D-4F), and 3-day (Figures 4G-4I) cultured cells. Because the distribution pattern of CA XIV and α-actinin was not affected by 3-day cultivation, and because 3-day cultured cells contracted spontaneously and were more tightly attached to the coverslips, immunohistochemical studies were performed with 3-day cultured cardiac cells. Double immunofluorescence staining with anti-SERCA2 antibody clearly demonstrates that CA XIV is associated with the SR membrane (Figures 5B-5D). Preimmune serum applied to cardiomyocytes of wild-type mice (Figure 5A) as well as anti-mouse CA XIV serum applied to cardiomyocytes of CA XIV KO mice (Figures 5E-5G) result in negative controls.
Figure 4.
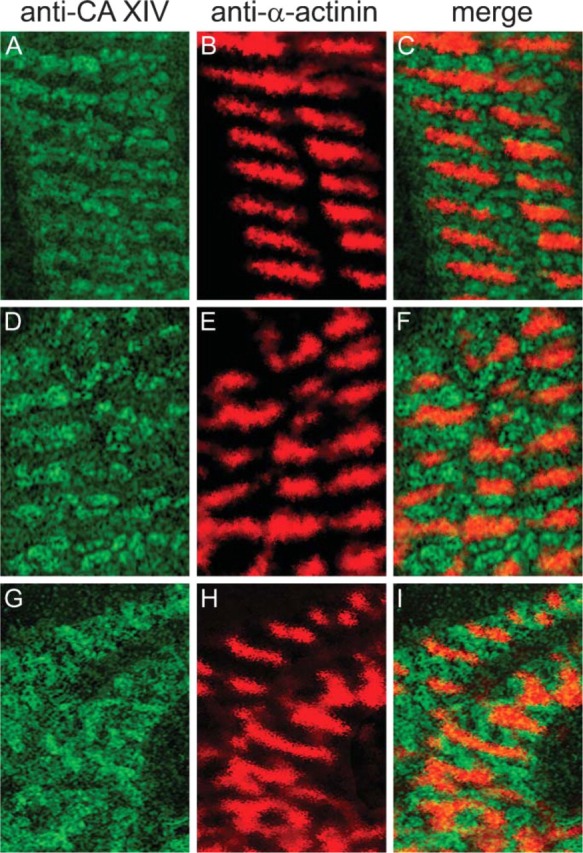
Double-immunofluorescence staining of CA XIV and α-actinin in adult cardiomyocytes of mouse. (A-C) Cardiomyocytes 3 hr in culture. (A) Anti-mouse CA XIV/FITC. (B) Anti-mouse α-actinin/TRITC. (C) Merge of A and B. (D-F) Cardiomyocytes 24 hr in culture. (D) Anti-mouse CA XIV/FITC. (E) Anti-mouse α-actinin/TRITC. (F) Merge of D and E. (G-I) Cardiomyocytes 3 days in culture. (G) Anti-mouse CA XIV/FITC. (H) Anti-mouse α-actinin/TRITC. (I) Merge of G and H. Confocal laser scanning microscopy.
Figure 5.
Double-immunofluorescence staining of CA XIV and anti-SERCA2 in adult cardiomyocytes of mouse. (A-D) Cardiomyocytes of wild-type mouse. (A) Incubation with preimmune serum and anti-rabbit IgG/FITC. (B) Anti-mouse CA XIV/FITC. (C) Anti-mouse SERCA2/TRITC. (D) Merge of B and C. (E-G) Cardiomyocytes of CA XIV KO mouse. (E) Anti-mouse CA XIV/FITC. (F) Anti-mouse SERCA2/TRITC. (G) Merge of E and F. Confocal laser scanning microscopy.
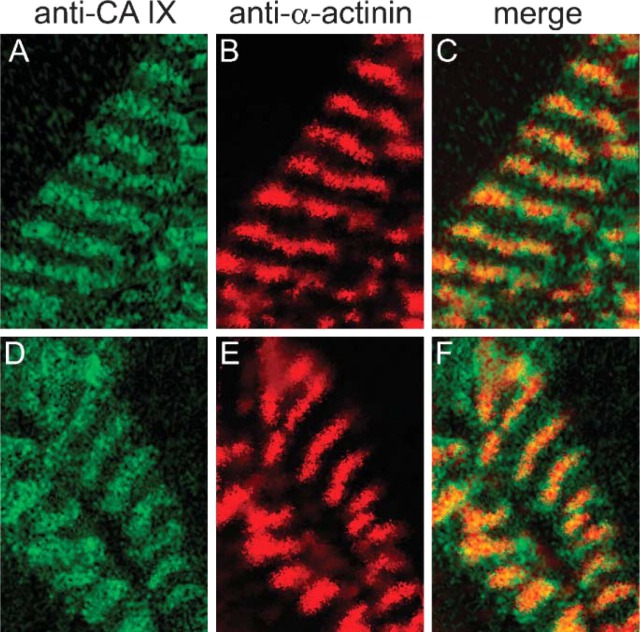
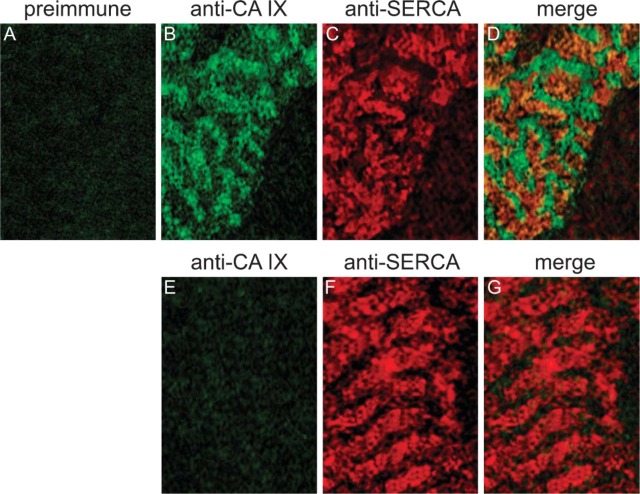
CA IX. Figure 6 shows the double immunostaining with anti-CA IX and anti-α-actinin. In contrast to the anti-CA XIV/anti-α-actinin staining, anti-CA IX staining (Figure 6A) predominantly appears in the region of anti-α-actinin staining (Figure 6B), which is clearly seen in the overlapping signal (Figure 6C). As seen in Figures 6D-6F, the distribution pattern of anti-CA IX/anti-α-actinin staining of 3-day cultured cardiac myocytes does not differ from the pattern of freshly isolated cells (Figures 6A-6C). Double immunostaining with anti-SERCA demonstrates that the green CA IX-positive fluorescence signals are by far not colocalized with the red SERCA-positive fluorescence signals in a size as it is true for CA XIV and SERCA (Figures 7B-7D). CA IX-positive fluorescence signals are mainly located in between SERCA-positive fluorescence signals. Both controls, preimmune serum applied to wild-type cells and anti-CA IX serum applied to CA IX KO cardiac myocytes, were negative (Figures 7A, 7E-7G).
Figure 6.
Double-immunofluorescence staining of CA IX and α-actinin in adult cardiomyocytes of mouse. (A-C) Cardiomyocytes 3 hr in culture. (A) Anti-mouse CA IX/FITC. (B) Anti-mouse α-actinin/TRITC. (C) Merge of A and B. (D-F) Cardiomyocytes 3 days in culture. (D) Anti-mouse CA IX/FITC. (E) Anti-mouse α-actinin/TRITC. (F) Merge of D and E. Confocal laser scanning microscopy.
Figure 7.
Double-immunofluorescence staining of CA IX and SERCA2 in adult cardiomyocytes of mouse. (A-D) Cardiomyocytes of wild-type mouse. (A) Incubation with preimmune serum and anti-rabbit IgG/FITC. (B) Anti-mouse CA IX/FITC. (C) Anti-mouse SERCA2/TRITC. (D) Merge of B and C. (E-G) Cardiomyocytes of CA IX KO mouse. (E) Anti-mouse CA IX/FITC. (F) Anti-mouse SERCA2/TRITC. (G) Merge of E and F. Confocal laser scanning microscopy.
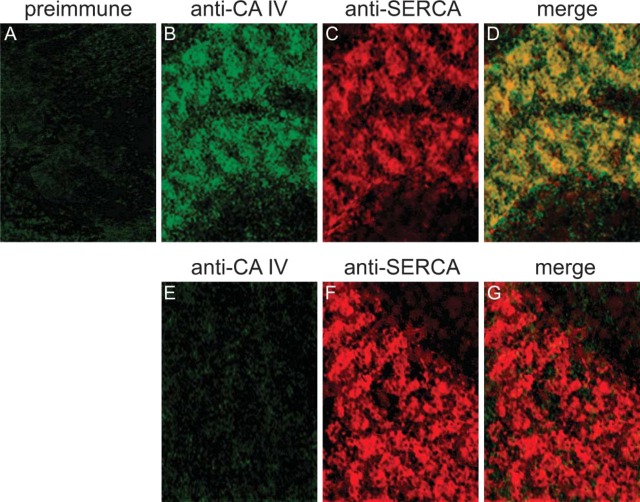
CA IV. Double immunostaining with anti-CA IV and anti-α-actinin showed that the fluorescent sites of CA IV and α-actinin overlapped partially (data not shown). Figure 8B shows the intracellular staining of CA IV, which is also localized at the SR membranes as indicated by double immunostaining with anti-SERCA2 antibody (Figures 8C and 8D). Using preimmune serum instead of anti-CA IV serum, cardiomyocytes of wild-type mice remain unstained (Figure 8A). Using anti-CA IV serum, cardiomyocytes of CA IV KO mice also remain unstained (Figures 8E-8G).
Figure 8.
Double-immunofluorescence staining of CA IV and SERCA2 in adult cardiomyocytes of mouse. (A-D) Cardiomyocytes of wild-type mouse. (A) Incubation with preimmune serum and anti-rabbit IgG/FITC. (B) Anti-mouse CA IV/FITC. (C) Anti-mouse SERCA2/TRITC. (D) Merge of B and C. (E-G) Cardiomyocytes of CA IV KO mouse. (E) Anti-mouse CA IV/FITC. (F) Anti-mouse SERCA2/TRITC. (G) Merge of E and F. Confocal laser scanning microscopy.
CA XII. Although rabbit anti-mouse CA XII serum was applied in a dilution of 1:300, cardiomyocytes did not show any specific CA XII immunostaining (data not shown). Using the same antiserum, Kyllönen et al. (2003) and Halmi et al. (2004) found positive immunohistochemical staining of mouse kidney and mouse colon, respectively.
Are CA XIV, CA IX, and CA IV localized in the same regions along the SR membrane or do their expression patterns differ from each other? Figure 4I displays no overlap of CA XIV-positive and α-actinin-positive fluorescence signals. But, as seen in Figure 5D, fluorescence signals indicating CA XIV and SERCA, respectively, overlap nearly perfectly, indicating expression of CA XIV and SERCA in the same regions of the SR. In heart, one finds diads or triads in the region of the Z discs consisting of the terminal SR membrane and of the t-tubule membrane. Figure 6F displays a nearly perfect overlap of CA IX-positive and α-actinin-positive fluorescence signals, whereas overlap of CA IX- and SERCA-positive fluorescence signals is very weak (Figure 7D). This indicates that CA IX is predominantly located near the Z discs and is very likely membrane-bound to the terminal SR membrane and/or to the t-tubule membrane. Distribution of CA IV is less clear-cut. The fluorescent sites indicating the expression of CA IV and α-actinin overlap partially (data not shown). This finding is confirmed by the double immunostaining of CA IV and SERCA (Figure 8D). Regions in yellow demonstrate the overlap of CA IV and SERCA fluorescence signals. Regions of overlapping fluorescence alternate with regions of pure CA IV fluorescence (green, Figure 8D). CA IV and SERCA are also expressed in the same membrane regions, but CA IV is also localized in the region of the Z discs, in the membrane of the terminal SR or the t-tubular system.
CA Associated With the SL Membrane
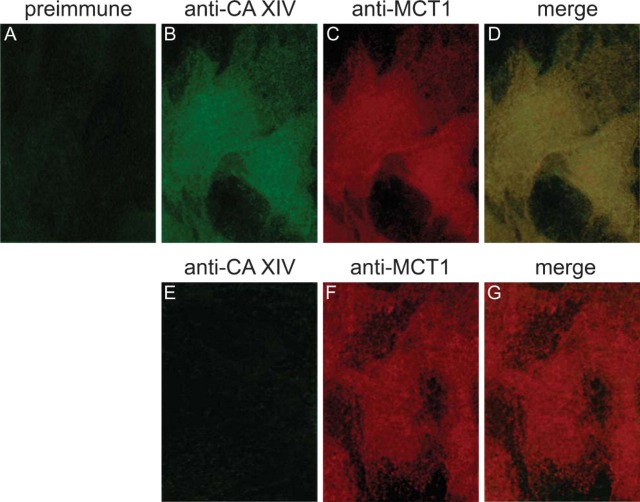
CA XIV. Localization of the SL CA was investigated by epifluorescence studies. A CA XIV-positive staining is seen in Figure 9B. The marker molecule MCT-1, the H+/lactate cotransporter, is located in the SL membrane (Johannsson et al. 1997). Double-immunofluorescence staining with the anti-CA XIV and anti-MCT-1 antibodies reveals that both proteins, CA XIV and MCT-1, are localized in the SL membrane (Figures 9C and 9D). Controls using preimmune serum in cardiomyocytes of wild-type mice and controls using anti-CA XIV serum in cardiomyocytes of CA XIV KO mice remain negative (Figures 9A, 9E, and 9F).
Figure 9.
Double-immunofluorescence staining of CA XIV and MCT-1 in cardiomyocytes of mouse. (A-D) Cardiomyocytes of wild-type mouse. (A) Incubation with preimmune serum and anti-rabbit IgG/FITC. (B) Anti-mouse CAXIV/FITC. (C) Anti-mouse MCT-1/TRITC. (D) Merge of B and C. (E-G) Cardiomyocytes of CA XIV KO mouse. (E) Anti-mouse CA XIV/FITC. (F) Anti-mouse MCT-1/TRITC. (G) Merge of E and F. Epifluorescence.
CA IV. Double immunostaining with anti-CA IV and anti-MCT-1 antibodies demonstrates the expression of CA IV in the SL (Figure 10B), confirming the report of Sender et al. (1998). Controls using preimmune serum in cardiomyocytes of wild-type mice and controls using anti-CA IV serum in cardiomyocytes of CA IV KO mice show negative results (data not shown).
Figure 10.
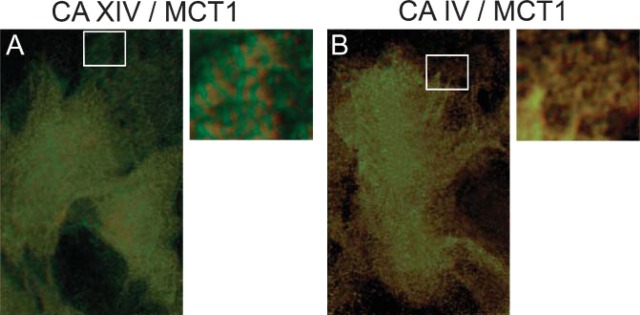
Merge of double-immunofluorescence staining of CA XIV and MCT-1 as well as of CA IV and MCT-1 in cardiomyocytes of mouse. As seen in the regions at higher magnification, CA IV and MCT-1 (B) are more closely coexpressed than CA XIV and MCT-1 (A). Epifluorescence. The sections were zoomed in by Adobe Photoshop (Adobe Systems; Palo Alto, CA).
The SL region observed in the merged picture at higher magnification (Figure 10A) shows a patchy distribution pattern of CA XIV fluorescence (green) and MCT-1 fluorescence (red), whereas the merge of CA IV and MCT-1 fluorescence signals displays a seemingly perfect overlap (Figure 10B). This result indicates that CA IV and MCT-1 are more closely co-expressed in the same membrane regions than CA XIV and MCT-1.
CA IX and CA XII. Double immunostaining with anti-human CA IX and anti-MCT-1 antibodies reveals no specific CA IX staining in the SL membrane (Figures 11B-11D).
Figure 11.
Double immunofluorescence staining of CA IX and MCT-1 in cardiomyocytes of mouse. (A-D) Cardiomyocytes of wild-type mouse. (A) Incubation with preimmune serum and anti-rabbit IgG/FITC. (B) Anti-mouse CA IX/FITC. (C) Anti-mouse MCT-1/TRITC. (D) Merge of B and C. Epifluorescence.
Controls using preimmune serum in cardiomyocytes of wild-type mice (Figure 11A) and controls using anti-human CA IX serum in cardiomyocytes of CA IX KO mice show negative results (data not shown). Rabbit anti-mouse CA XII serum did not result in a positive CA XII staining of SL membranes (data not shown).
Discussion
Extracellularly Active SL CA
By positive fluorescence staining of CA XIV and CA IV in the SL and by negative controls using preimmune serum in wild-type cardiomyocytes and anti-CA serum in cardiomyocytes of KO animals, our studies give unequivocal evidence for an expression of CA XIV and CA IV in SL membranes of the heart. This result is in accordance with the studies of Bruns and Gros (1992), who determined CA activities in isolated SL vesicles of the bovine heart. Microsomal preparations of cardiomyocytes of pig and human hearts showed that ∼90% of their total CA activity was due to a membrane-bound CA activity, but only a minor portion of this membrane-bound CA was constituted by CA IV (Knüppel-Ruppert et al. 2000). From the present data, it is now clear that CA IX and CA XIV contributed to the total CA activity of their microsomal preparations.
What might be the function of an extracellularly active CA in the heart? DeHemptinne et al. (1986) demonstrated on cardiac muscle preparations that an extracellularly active CA increases the availability of the extracellular CO2/HCO3 - buffer system. Vanheel et al. (1985,1986) reported that the extracellular buffer capacity affects the surface pH in cardiac muscle. Their studies demonstrated that a reduced extracellular buffer capacity leads to a more acidic surface pH, which subsequently leads to a more acidic intracellular pH (pHi) and results in a decrease in twitch tension. This finding has been confirmed by the studies of Geers and Gros (1995). In the presence of CA inhibitors, they found a decrease in force and a reduction of rise and relaxation times in rabbit papillary muscles, findings that were explained by an intracellular acidosis. Because the extracellular protein concentration in vivo is low (1 g%), proteins contribute only a little to the total extracellular buffer capacity. This makes the CO2/HCO3 - system the most important buffer system in the extracellular space. By accelerating the reversible CO2 hydration reaction, extracellularly active CA increases its effective buffer capacity and thus plays an essential role in surface pH regulation, which in turn affects pHi regulation. In our previous study (Wetzel et al. 2001), we demonstrated in isolated rat skeletal muscle fibers that the extracellular CA accelerates the influx as well as the efflux of lactic acid. During the influx of lactic acid, the extracellular CO2/HCO3 - system catalyzed by CA provides a fast delivery of protons, which are cotransported with lactate in the stoichiometry of 1:1. Very likely, this effect will also be important in the heart, for which lactate is an important substrate.
Why are two membrane-bound CA isoforms expressed in the SL membrane? One possible explanation might be the idea that these different CAs interact with different membrane proteins. Sterling et al. (2002) demonstrated that CA IV interacts with the extracellular loop 4 of the AE1 transporter and thus accelerates the movement of HCO3 - across the SL membrane. Furthermore, CA IV binds to the extracellular loop 4 of the electrogenic NBC1 transporter (Alvarez et al. 2003). Both transporters are also present in the heart. Therefore, CA IV may be involved in the transport of HCO3 - by AE1 and NBC1, respectively.
Our studies might indicate an alternative interaction of CA IV: it is perfectly colocalized with the H+/lactate cotransporter (Figure 10B). This would be compatible with a functional interaction of CA IV and MCT-1 and would be in line with the observed reduction of the lactate transport rate and with the reduced appearance of lactate in blood after exercise as seen under CA inhibition (Kowalchuk et al. 2000; Wetzel et al. 2001). In contrast, CA XIV deviates from a perfect colocalization with MCT-1 (Figure 10A). CA XIV may be associated with another membrane protein, but evidence for this has not yet been obtained.
Intracellularly Active CA
Histochemical studies of Bruns and Gros (1992) exhibited a CA staining associated with intracellular structures, and Sender et al. (1998) found a weak intracellular anti-CA IV/immunogold staining by electron microscopy using ultrathin sections, but not by light microscopy. Now, by CLSM using double immunofluorescence staining with anti-CA and anti-SERCA2 antibodies, it is shown here that CA XIV and CA IV are associated with the longitudinal SR membrane and that CA IV and CA IX are associated with the terminal SR/t-tubule membranes.
Functions of an Intracellularly Active CA
Evidence has been presented that heart muscle from rabbit does not possess cytosolic CA (Geers et al. 1992). On the other hand, there are numerous functional observations that can only be explained in terms of an intracellularly accessible CA (for review, see Swenson 1997). We suggest that the CAs associated with the SR as reported here are responsible for these observations.
An intracellular CA catalyzes the reaction CO2 + H2O ↔ H+ + HCO3 - and, by this, CA enables the CO2/HCO3 - system to act as a fast-reacting intracellular buffer system. Thus, intracellular CA and the CO2/HCO3 - buffer system are involved in the regulation of pHi. Several studies have reported an involvement of CA in pHi regulation (see Ellis and Thomas 1970; Lagadic-Gossmann et al. 1992; Vandenberg et al. 1996; Leem and Vaughan-Jones 1998). In all cases studied, CA inhibition had attenuated the kinetics of the intracellular CO2/HCO3 - buffer system and consequentially the kinetics of pHi regulation. Romanowski et al. (1992) demonstrated an additional role of intracellular CA in the heart: intracellular CO2 diffusion in the rabbit heart was 2.7 times accelerated by facilitated diffusion, and this effect was almost abolished by CA inhibition. A CA-facilitated diffusion of CO2 in the heart will lower the intracellular pCO2 and H+ concentrations. Spitzer et al. (2002) reported that intracellular CA enhances the effective intracellular H+ mobility and hence prevents the occurrence of spatiotemporal pHi gradients. This would be useful because pHi gradients could negatively affect pHi-dependent processes in the cardiomyocyte.
A function of cardiac intracellular CA that specifically requires its localization in the SR membrane has not yet been demonstrated. It is conceivable, however, that SR CA in the heart, as shown for skeletal muscle (Wetzel et al. 2002), is involved in electromechanical coupling, e.g., the mechanisms of Ca2+ release and reuptake. Several studies have shown a transport of H+ into the SR during the release of Ca2+ by the ryanodine receptor as well as a H+ ejection coupled to the uptake of Ca2+ by SERCA (see Somlyo et al. 1981; Levy et al. 1990; Kamp et al. 1998). The different distribution patterns of CA IV, CA IX, and CA XIV as seen in Figure 4-Figure 8 may be related to different functional associations of these enzymes. CA IV is present in the terminal SR, which is the region of Ca2+ release, as well as in the longitudinal SR, which is the region of Ca2+ uptake. By its glycosylphosphatidylinositol anchor, it can be predicted that the membrane orientation of CA IV is directed to the inside of the SR so that CA IV is catalytically active within the SR. Therefore, by accelerating the CO2/HCO3 - buffer system inside the SR, CA IV might provide fast buffering of H+ during Ca2+ release as well as fast delivery of H+ during Ca2+ uptake. CA IX and CA XIV are type 1 transmembrane proteins. If these isoforms are oriented toward the cytoplasmic side of the SR membrane, they will be catalytically active in the cytoplasm. By virtue of the proximity of CA XIV to SERCA in the longitudinal SR, as suggested by their apparent perfect colocalization, CA XIV might provide a fast buffering of the H+ ejected during Ca2+ uptake and by this might protect SERCA from a negative feedback mechanism by H+. CA IX is predominantly located in the terminal SR/t-tubule membrane. Therefore, CA IX may provide a fast delivery at the cytoplasmic side of the SR membrane of H+, which can then be transported into the SR during Ca2+ release. Further studies are necessary to test this hypothesis of the different functions of CA IV, IX, and XIV in the SR.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft, Grant We1962/4-1 (to PW) and National Institutes of Health, Grant DK-40163 (to WSS).
We thank Dr. Yie-Hwa Chang and Kristi Stubbert for proteomic analyses. We also thank Tracey Baird and Shirley Bratcher for editorial help in preparing this manuscript.
Literature Cited
- Alvarez BV, Loiselle FB, Supuran CT, Schwartz GJ, Casey JR. (2003) Direct extracellular interaction between carbonic anhydrase IV and the human NBC 1 sodium/bicarbonate co-transporter. Biochemistry 42:12321–12329 [DOI] [PubMed] [Google Scholar]
- Bruns W, Gros G. (1992) Membrane-bound carbonic anhydrase in the heart. Am J Physiol 262:H577–584 [DOI] [PubMed] [Google Scholar]
- Chen S. (2006) Rapid protein identification using direct infusion nano-electrospray ionization mass spectrometry. Proteomics 16:16–25 [DOI] [PubMed] [Google Scholar]
- DeHemptinne A, Marrannes R, Vanheel B. (1986) Surface pH and the control of intracellular pH in cardiac and skeletal muscle. Can J Physiol Pharmacol 65:970–977 [DOI] [PubMed] [Google Scholar]
- Ellis D, Thomas RC. (1970) Direct measurement of the intracellular pH of mammalian cardiac muscle. J Physiol 262:755–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freimueller B, Nasheuer HP, Mueller WH. (1983) Induction of alkaline phosphatase in rat brain cells by retinoic acid. Biol Chem HS 346:1125 [Google Scholar]
- Fujikawa-Adachi K, Nishimori I, Taguchi T, Onishi S. (1999) Human carbonic anhydrase XIV (CA14): cDNA cloning, mRNA expression, and mapping to chromosome 1. Genomics 61:74–81 [DOI] [PubMed] [Google Scholar]
- Geers C, Gros G. (1995) Contractile function of papillary muscles with carbonic anhydrase inhibitors. Life Sci 57:591–597 [DOI] [PubMed] [Google Scholar]
- Geers C, Krueger D, Siffert W, Schmid A, Bruns W, Gros G. (1992) Carbonic anhydrase in skeletal and cardiac muscle from rabbit and rat. Biochem J 282:165–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gut MO, Parkkila S, Vernerová Z, Rohde E, Závada J, Höcker M, Pastorek J, et al. (2002) Gastric hyperplasia in mice with targeted disruption of the carbonic anhydrase gene Car9. Gastroenterology 123:1889–1903 [DOI] [PubMed] [Google Scholar]
- Halmi P, Lehtonen J, Waheed A, Sly WS, Parkkila S. (2004) Expression of hypoxia-inducible, membrane-bound carbonic anhydrase isozyme XII in mouse tissues. Anat Rec A Discov Mol Cell Evol Biol 277:171–177 [DOI] [PubMed] [Google Scholar]
- Hilvo M, Rafajova M, Pastorekova S, Pastorek S, Parkkila S. (2004) Expression of carbonic anhydrase IX in mouse tissues. J Histochem Cytochem 52:1313–1322 [DOI] [PubMed] [Google Scholar]
- Ivanov S, Liao SY, Ivanova A, Danilkovitch-Miagkova A, Tarasova N, Weirich G, Merrill MJ, et al. (2001) Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol 158:905–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsson E, Nagelhus EA, McCullagh KJ, Sejersted OM, Blackstad TW, Bonen A, Ottersen OP. (1997) Cellular and subcellular expression of the monocarboxylate transporter MCT1 in rat heart. A high-resolution immunogold analysis. Circ Res 80:400–407 [PubMed] [Google Scholar]
- Kamp F, Donoso P, Hidalgo C. (1998) Changes in luminal pH caused by calcium release in sarcoplasmic reticulum vesicles. Biophys J 74:290–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaunisto K, Parkkila S, Rajaniemi H, Waheed A, Grubb J, Sly WS. (2002) Carbonic anhydrase XIV: luminal expression suggests key role in renal acidification. Kidney Int 61:2111–2118 [DOI] [PubMed] [Google Scholar]
- Kivelä AJ, Parkkila S, Saarnio J, Kartunen TJ, Kivelä J, Parkkila AK, Pastoreková S, et al. (2000) Expression of transmembrane carbonic anhydrase isoenzymes IX and XII in normal human pancreas and pancreatic tumors. Histochem Cell Biol 114:197–204 [DOI] [PubMed] [Google Scholar]
- Knüppel-Ruppert AS, Gros G, Harringer W, Kubis HP. (2000) Immunochemical evidence for a unique GPI-anchored carbonic anhydrase isozyme in human cardiomyocytes. Am J Physiol 278:H1335–H1344 [DOI] [PubMed] [Google Scholar]
- Kowalchuk JM, Smith SA, Weening BS, Marsh GD, Paterson DH. (2000) Forearm muscle metabolism studied using 31P-MRS during progressive exercise to fatigue after Acz administration. J Appl Physiol 89:200–209 [DOI] [PubMed] [Google Scholar]
- Kyllönen MS, Parkkila S, Rajaniemi H, Waheed A, Grubb JH, Shah GN, Sly WS, et al. (2003) Localization of carbonic anhydrase XII to the basolateral membrane of H+-secreting cells of mouse and rat kidney. J Histochem Cytochem 51:1217–1224 [DOI] [PubMed] [Google Scholar]
- Lagadic-Gossmann D, Buckler KJ, Vaughan-Jones RD. (1992) Role of bicarbonate in pH recovery from intracellular acidosis in the guinea pig ventricular myocyte. J Physiol 458:361–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leem CH, Vaughan-Jones RD. (1998) Out-of-equilibrium pH transients in the guinea pig ventricular myocyte. J Physiol 509:471–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtonen J, Shen B, Vihinen M, Casini A, Scozzafava A, Supuran CT, Parkkila AK, et al. (2004) Characterization of CA XIII, a novel member of the carbonic anhydrase isozyme family. J Biol Chem 279:2719–2727 [DOI] [PubMed] [Google Scholar]
- Levy D, Seigneuret M, Bluzat A, Rigaud JL. (1990) Evidence for proton countertransport by the sarcoplasmic reticulum Ca++-ATPase during calcium transport in reconstituted proteoliposomes with low ionic permeability. J Biol Chem 265:19524–19534 [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275 [PubMed] [Google Scholar]
- Meissner JD, Kubis HP, Scheibe RJ, Gros G. (2001) Calcineurin regulates slow myosin, but not fast myosin or metabolic enzymes, during fast-to-slow transformation in muscle cell culture. J Physiol 533:215–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Ogawa Y, Ebihara K, Tamura N, Tashiro K, Kuwahara T, Mukoyama M, et al. (1999) Isolation and characterization of CA XIV, a novel membrane-bound carbonic anhydrase from mouse kidney. J Biol Chem 274:15701–15705 [DOI] [PubMed] [Google Scholar]
- Parkkila S, Kivelä AJ, Kaunisto K, Parkkila AK, Hakkola J, Rajaniemi H, Waheed A, et al. (2002) The plasma membrane carbonic anhydrase in murine hepatocytes identified as isozyme XIV. BMC Gastroenterology 2:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkila S, Parkkila AK, Rajaniemi H, Shah GN, Grubb JH, Waheed A, Sly WS. (2001) Expression of membrane-associated carbonic anhydrase XIV on neurons and axons in mouse and human brain. Proc Natl Acad Sci USA 98:1918–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanowski F, Schierenbeck J, Gros G. (1992) Facilitated CO2 diffusion in various striated muscles. In Kessler M, Frank K, eds. On Quantitative Spectroscopy in Tissue. Frankfurt am Main, Springer-Verlag, 205–211 [Google Scholar]
- Sender S, Decker B, Fenske CD, Sly WS, Carter ND, Gros G. (1998) Localization of carbonic anhydrase IV in rat and human heart muscle. J Histochem Cytochem 46:855–861 [DOI] [PubMed] [Google Scholar]
- Shah GN, Ulmasov B, Waheed A, Becker T, Makani S, Svichar N, Chesler M, et al. (2005) Carbonic anhydrase IV and XIV knockout mice: roles of the reseptive carbonic anhydrases in buffering the extracellular space in brain. Proc Natl Acad Sci USA 102:16771–16776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo AV, Gonzalez-Serratos H, Shuman H, McClellan G, Somlyo AP. (1981) Calcium release and ionic changes in the sarcoplasmic reticulum of tetanized muscle: an electron-probe study. J Cell Biol 90:577–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer KW, Skolnick RL, Peercy BE, Keener JP, Vaughan-Jones RD. (2002) Facilitation of intracellular H+ ion mobility by CO2/HCO3 - in rabbit ventricular myocytes is regulated by carbonic anhydrase. J Physiol 541:159–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling D, Alvarez BV, Casey JR. (2002) The extracellular component of a transport metabolon. J Biol Chem 277:25239–25246 [DOI] [PubMed] [Google Scholar]
- Swenson ER. (1997) Carbonic anhydrase and the heart. Cardiologia 42:453–462 [PubMed] [Google Scholar]
- Terry DE, Umstot E, Desiderio DM. (2004) Optimized sample-processing time and peptide recovery for the mass spectrometric analysis of protein digests. J Am Soc Mass Spectrom 15:784–789 [DOI] [PubMed] [Google Scholar]
- Vandenberg JI, Carter ND, Bethell HWL, Nogradi A, Ridderstrale Y, Metcalfe JC, Grace AA. (1996) Carbonic anhydrase and cardiac pH regulation. Am J Physiol 271:C1838–1846 [DOI] [PubMed] [Google Scholar]
- Vanheel B, DeHemptinne A, Leusen I. (1985) Intracellular pH and contraction of isolated rabbit and cat papillary muscle: effect of superfusate buffering. J Mol Cell Cardiol 17:23–29 [DOI] [PubMed] [Google Scholar]
- Vanheel B, DeHemptinne A, Leusen I. (1986) Influence of surface pH on intracellular pH regulation in cardiac and skeletal muscle. Am J Physiol 250:C748–760 [DOI] [PubMed] [Google Scholar]
- Waheed A, Zhu XL, Sly WS. (1992a) Membrane-associated carbonic anhydrase from rat lung. J Biol Chem 267:3308–3311 [PubMed] [Google Scholar]
- Waheed A, Zhu XL, Sly WS, Wetzel P, Gros G. (1992b) Rat skeletal muscle membrane associated carbonic anhydrase is 39-kDa, glycolysated, GPI-anchored CA IV. Arch Biochem Biophys 294:550–556 [DOI] [PubMed] [Google Scholar]
- Wetzel P, Gros G. (1990) Sarcolemmal carbonic anhydrase in red and white rabbit skeletal muscle. Arch Biochem Biophys 279:345–354 [DOI] [PubMed] [Google Scholar]
- Wetzel P, Hasse A, Papadopoulos S, Voipio J, Kaila K, Gros G. (2001) Extracellular carbonic anhydrase activity facilitates lactic acid transport in rat skeletal muscle fibres. J Physiol 531:743–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel P, Kleinke T, Papadopoulos S, Gros G. (2002) Inhibition of muscle carbonic anhydrase slows the Ca2+ transient in rat skeletal muscle fibers. Am J Physiol 283:C1242–1253 [DOI] [PubMed] [Google Scholar]