Reason for posting: Second-generation antipsychotic drugs, which are felt to have fewer extrapyramidal adverse effects than the first-generation drugs,1 are sometimes used to control behavioural disturbances in elderly patients with dementia. However, similar to warnings issued in the last 2 years about risperidone and associations with hyperglycemia and cerebrovascular events,2 a warning was recently issued for another second-generation drug, olanzapine.3
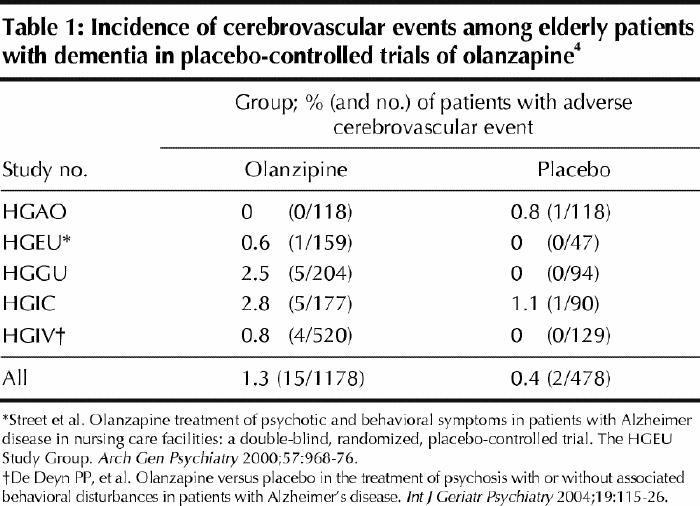
The drug: Olanzapine is an antipsychotic agent with a novel mechanism of action. The US Food and Drug Administration recently issued a warning about an increased incidence of hyperglycemia and diabetes among patients taking the drug.3 In a separate letter to physicians, Eli Lilly Canada also recently reported some summary data on cerebrovascular event rates in 5 placebo-controlled trials of olanzapine in elderly patients (ages not specified) who had dementia (including Alzheimer's, vascular dementia and mixed dementia) (Table 1).4 According to information supplied by the manufacturer, only 2 of the 5 studies have been published in full (Table 1). The duration of the published trials was between 6 and 10 weeks, with doses of the drug ranging from 1 to 15 mg. Overall, the relative risk of a cerebrovascular event among patients taking olanzapine in the 5 trials was 3.1 (95% confidence interval [CI] 0.7– 13.5) and the absolute increased risk was 0.9%. The rates of cerebrovascular events were similar to those reported for risperidone.2
Table 1
What to do: The behavioural disturbances associated with dementia are distressing for caregivers and patients alike. Before prescribing antipsychotic medications, physicians should consider nondrug options such as education of caregivers about the behavioural disturbances and assisting them in obtaining increased home care or placement in long-term care facilities with special floors for patients with dementia-related behavioural problems. If medications are considered necessary, they should be prescribed at as low a dose as possible. Olanzapine is not licensed for use in elderly patients with dementia. Patients taking the drug should be monitored for hyperglycemia, and patients and caregivers should be informed of possible cerebrovascular risks, risks that may apply to all second-generation antipsychotic drugs.
Eric Wooltorton CMAJ
Supplementary Material
References
- 1.Choice of an antipsychotic. Med Letter 2003;45:102-4. [PubMed]
- 2.Wooltorton E. Risperidone (Risperdal): increased rate of cerebrovascular events in dementia trials. CMAJ 2004;167 (11): 1269-70. [PMC free article] [PubMed]
- 3.2004 safety alert: Zyprexa (olanzapine). Washington: US Food and Drug Administration; 2004 Mar 1. Available: www.fda.gov/medwatch/SAFETY/2004/zyprexa.htm (accessed 2004 Apr 1).
- 4.Olanzapine (Zyprexa) and cerebrovascular adverse events in placebo-controlled elderly dementia trials [Dear Healthcare Professional Letter]. Toronto: Elli Lilly Inc.; 2004 Mar 10. Available: www.hc-sc.gc.ca/hpfb-dgpsa/tpd-dpt/zyprexa_hpc_e.pdf (accessed 2004 Apr 1).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


