Abstract
Through the successful implementation of policies to prevent mother-to-child-transmission (PMTCT) of HIV-1 infection, children born to HIV-1-infected mothers are now much less likely to acquire HIV-1 infection than previously. Nevertheless, HIV-1-exposed uninfected (HEU) children have substantially increased morbidity and mortality compared with children born to uninfected mothers (unexposed uninfected, UU), predominantly from infectious causes. Moreover, a range of phenotypical and functional immunological differences between HEU and UU children has been reported. As the number of HEU children continues to increase worldwide, two questions with clear public health importance need to be addressed: first, does exposure to HIV-1 and/or ART in utero or during infancy have direct immunological consequences, or are these poor outcomes simply attributable to the obvious disadvantages of being born into an HIV-affected household? Secondly, can we expect improved maternal care and ART regimens during and after pregnancy, together with optimized infant immunization schedules, to reduce the excess morbidity and mortality of HEU children?
Keywords: AIDS, host–pathogen interactions, vaccination
Introduction
The widespread implementation of prevention of mother-to-child transmission (PMTCT) programmes has been one of the great public health success stories of the 21st century. The combination of tailored anti-retroviral therapy (ART) to mother and infant, together with improved obstetric management and the avoidance of breastfeeding, has virtually eliminated infant HIV-1 infection in much of the developed world. Even though considerable logistic challenges to full PMTCT coverage in developing countries remain, the past decade has seen remarkable progress, with millions of babies born free from HIV-1 infection who would otherwise have acquired the infection from their mothers. However, compared to infants of uninfected mothers, these children are nevertheless reported to face a significantly higher early life burden of predominantly infectious diseases, associated with a range of phenotypical and functional immunological abnormalities. Even after the introduction of PTMCT programmes that should lead to improved maternal health and reduced viral loads during pregnancy, an increased disease burden continues to be reported in HEU infants: whether this reflects exposure to ART rather than HIV-1 is difficult to tease out from the literature.
Given that HEU children may now account for as many as 30% of all births in parts of southern Africa [1], two questions with clear public health importance need to be addressed: first, does in-utero exposure to HIV-1 have direct immunological consequences, or are these poor outcomes simply attributable to the obvious disadvantages of being born into an HIV-affected household? Secondly, can we expect improved ART regimens during pregnancy together with optimized infant immunization schedules to reduce morbidity and mortality of HEU children, or will increased exposure to ART cancel out the benefits to the infant of reduced HIV exposure and better maternal health?
Here we will review the available literature on HEU children and attempt to dissect out those features that might be attributed to exposure to HIV or anti-retroviral drugs, rather than the impact of starting life in an HIV-afflicted household. We will also explore the responses to infant vaccination recorded in this vulnerable group, as this may provide functional evidence of immune dysfunction.
Increased disease burden among HIV-exposed uninfected children
Many groups have reported on the outcomes of infected and uninfected children born to HIV+ mothers compared with unexposed uninfected (UU) infants, both before and after the institution of PTMCT programmes. One of the largest was the ZVITAMBO (Zimbabwe Vitamin A for Mothers and Babies project) cohort in Zimbabwe, which recruited more than 14 000 children born between 1997 and 2000, before PMTCT strategies or infant ART were widely used in sub-Saharan Africa. The 2-year mortality of HEU children was more than three times greater than that of UU children (9·2% versus 2·9%), although considerably lower than for HIV-infected children (which ranged from 33% for those acquiring postnatal HIV infection to more than 65% for those infected in utero or at birth) [2]. Mortality for HEU children peaked at 3–6 months and was associated predominantly with lower respiratory tract infection (LRTI). Sick clinic visits and hospitalizations were also significantly higher in the HEU than the UU group [3], and varied inversely according to the CD4 count of the mother.
Similarly, in another study performed before maternal ART was widely used, HEU infants in rural Uganda were reported to have twice the mortality of UU infants in the first 2 years of life [4]. More recently in Malawi, when mothers were provided with single-dose Nevirapine (sdNVP) prophylaxis for PMTCT, the mortality rate of HEU infants at 20 months was 18·7% compared to 4·3% in UU controls [5]. In Zambian HEU infants, whose mothers had also had sdNVP, the cumulative mortality in HEU children was 13·6% between 1 and 24 months of age (with 4% already having died in the first month of life); the mortality risk increased during weaning from breast milk [6]. A recent case–control study in South Africa, in the post-PMTCT era, reported that HEU children were significantly more likely to be admitted to hospital for infections throughout the first year of life [7]. In addition, HEU children are more likely to have growth stunting than their UU counterparts, which is not corrected by nutritional supplements [8].
Further studies in Africa have confirmed that mortality in HEU children is caused predominantly by lower respiratory tract infections (LRTI) [9] of varied aetiology. A study of 358 children with severe pneumonia from South Africa showed that the chances of failing to respond to first-line antibiotic therapy within 48 h were increased sixfold in HEU children. Intriguingly, the infectious agents found in bronchoalveolar lavage (BAL) fluid were similar between HIV+ and HEU children [i.e. Pneumocystis jirovecii and cytomegalovirus (CMV)] [10]. Occasionally, severe infections may be accompanied by clinical evidence of immune deficiency [11]. Although most reports of HEU children come from Africa, a similarly high disease burden was also reported in India [12], where gastroenteritis, sepsis and pneumonia were the major causes of morbidity in the HEU group. Even in the setting of a European teaching hospital, HEU infants in Brussels (mainly the children of African families) were shown to have a dramatically increased risk of invasive group B streptococcal infection [13].
Taken together, these data suggest strongly that HEU children have an increased susceptibility to infection, leading occasionally to severe disease, and mediated potentially by an impaired immune system. Whether the increased morbidity and mortality in these infants might also be explained by an increased frequency of exposure to maternally derived pathogens, particularly drug-resistant strains, remains to be determined.
Exposure of the HEU infant to maternal HIV-1 virions and antigens
Despite separation of the fetal and maternal circulations by the villous trophoblastic layers of the placenta, considerable trafficking of cells between the fetus and mother has been reported in both health and disease [14]. It is also clear that viral particles and parasites can cross the placental barrier, demonstrated by congenital infections with Herpes simplex virus, rubella, CMV, parvovirus and Toxoplasma gondii [15,16]. Vertical transmission of HIV is thought to occur most commonly during delivery, when infants are exposed through the oral mucosal route to infected cervico–vaginal secretions [17]. Transmission in utero has also been reported: in some reports widespread HIV-1 infection has been detected in aborted fetuses [18]. Breastfeeding is the other common route of MTCT, whereby HIV-1 infection and exposure occurs through the oral mucosa, either through infection with cell-free HIV-RNA [19] mediated by interactions between HIV envelope gp120 and tonsillar DC-SIGN (dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin) [20] or from maternal T cell-associated provirus [21]. Breast milk viral load has been reported to correlate inversely with maternal CD4 T cell count [22,23], implying that maternal disease status will affect the extent of infant HIV exposure through breast milk.
Twenty years ago we reported the detection of HIV-specific cytotoxic T lymphocyte (CTL) responses in an HEU infant [24]: the presence of virus-specific immune responses may be regarded as further evidence of infant HIV exposure. Although not found in all studies, several research groups in the United States, South America, Europe and Africa have described a broad range of HIV-1-specific T cell responses in HEU infants, which are thought to have been primed by exposure to HIV antigens in utero or at delivery [25–30] (reviewed in [31]). Both HIV-specific cytotoxic lymphocyte (CTL) activity [lysis of infected cells and interferon (IFN)-γ secretion] and CD4 helper T cell responses [proliferation, interleukin (IL)-2 and IFN-γ secretion] have been detected in HEU infants, directed against structural and core proteins. The greatest breadth and magnitude of responses have been reported soon after birth [32], and responses have not been detected in older HEU children (aged 7 years) [33], suggesting that cellular responses wane in the absence of continued exposure. More recent studies have suggested that the magnitude of HIV-1-specific T cell responses are associated with protection from postnatal HIV-1 transmission [30,31], and that these responses can be revealed or enhanced by depletion of regulatory T cells [29]. Similarly, natural killer (NK) cells responding to HIV-1 peptides were described in HEU infants (and their mothers) and these responses also correlated with significantly reduced vertical transmission [34].
Taken together, these data suggest that substantial HIV exposure takes place in the infants of HIV-infected mothers and that the immune responses mounted in the HEU infant potentially contribute to protection from infection.
Immunological findings in HEU infants
Several research groups [11,35–37] have reported significant phenotypical differences between HEU and UU infants; the most consistent finding is that of a more antigen-experienced cellular phenotype, which could be driven by exposure to HIV or its proteins. If phenotypical differences in immune cell populations are mirrored by impaired function, this could potentially contribute to increased susceptibility to infections in early life (summarized in Table 1). However, most previous studies have been relatively small and cross-sectional, undertaken predominantly in the pre-ART era, and generally lacking detailed information about maternal disease status, treatment and co-infections (particularly human cytomegalovirus (CMV), which is associated with dramatic phenotypical changes in the infant immune system and infects African infants very early in life [38,39]). Hence, caution is needed before drawing definitive conclusions about the significance of the reported immunological perturbations that we describe below.
Table 1.
Maternal factors that may affect the immune health of HIV-1-exposed uninfected infants
| Factor | Example(s) | Controlled by maternal ART? |
|---|---|---|
| HIV-1 viraemia | Exposure without infection could induce ‘tolerance’ or lead to antigen-specific immune responses in the infant (Legrand et al. [29]) | Yes |
| HIV-1 antigenaemia | Circulating gp120 protein may be immunosuppressive (Weinhold et al. [61]; Chougnet et al. [62]) | Yes |
| Colonization or infection with a broad range of potential pathogens | Group B streptococcus more likely in vaginal flora of HIV+ mothers (Epalza et al. [13]) | Not known |
| HIV+ women more likely to be CMV viraemic at delivery (Slyker et al. [104]) | Probably | |
| Increased likelihood of infectious TB | Probably | |
| Immune dysregulation including chronic immune activation and high levels of proinflammatory cytokines | Increased levels of TNF-α production in mothers of HEU infants undergoing PMTCT (Borges-Almeida et al. [126]) | Partially |
| Impaired placental function | Reduced maternal antibody transfer (de Moraes-Pinto et al. [40]; Farquhar et al. [46]; Cumberland et al. [42]; Scott et al. [45]; Bunders et al. [44]; Jones et al. [93]) | Not known |
| ART exposure | Micronutrient deficiencies in infant? | |
| May affect mitochondrial function (see Table 2) | Yes | |
| Reduced breastfeeding | May lead to nutritional deficiency and increased risk of gastrointestinal and/or respiratory infections (Mwiru et al. [127]) | Yes |
ART = anti-retroviral therapy; CMV = cytomegalovirus; HEU = HIV-1-exposed uninfected; PMTCT = prevent mother-to-child-transmission; TNF = tumour necrosis factor.
The most unequivocal immune difference between HEU and UU infants is the reduced levels of specific maternal antibodies transferred to the infant from HIV-1-infected mothers [40–42]. During the last trimester, infants accumulate maternal immunoglobulin (Ig)G antibodies that actively cross the placenta through Fc-receptor-mediated transport and usually persist for several months, providing protection from infection according to the immunological experiences of the mother [43]. In HEU infants, although the overall levels of immunoglobulin (IgA, IgM and IgG) are higher than in unexposed children [44], neonatal levels of antibodies towards tetanus are approximately 50% lower in the infants of HIV-1-infected women [42]. Similarly, transferred measles antibodies are reported to be significantly lower in HEU than UU infants [42,45], particularly in the infants of mothers with high viral load [46]. The probable explanation is thought to be a combination of impaired maternal B cell function and less efficient IgG transfer across the placentas of HIV-1-infected women. It is not yet clear whether the use of combination ART in pregnancy will improve maternal B cell and placental function sufficiently to restore infant levels of maternal antibodies to normal. To date, no studies have looked at how reduced levels of maternal antibodies might be related to the morbidity and mortality of HEU infants.
The use of cord blood mononuclear cells (CBMC) from HEU neonates provides the earliest non-invasive time-point to investigate the potential impact of the intrauterine environment on immune development. Differences between HEU and UU infants could arise because of exposure to HIV or other pathogens in utero, ART exposure or an altered placental cytokine milieu (Fig. 1), but are less likely to be confounded by co-infection with CMV. Few studies have compared immunological parameters between the HEU infants of women with ART-mediated viral control with those of untreated women. In one study, cord blood from women with viral load (VL) below detection at delivery was compared with that from women with elevated viral load, most of whom had not taken ART during pregnancy. CBMC from the HEU infants of untreated women showed increased proliferation in response to mitogens, together with reduced levels of the anti-inflammatory cytokine IL-10 [47]. Others have shown that polyclonally activated CBMC from mothers with high VL generated higher levels of proinflammatory cytokines [IFN-γ and tumour necrosis factor (TNF)-α] together with T helper type 17 (Th17) cytokines, including IL-17 [48]. Exposure to heightened levels of maternal immune activation could predispose infant lymphocytes to apoptosis and potentially drive immune senescence in the infant immune system. Both spontaneous apoptosis and activation-induced apoptosis (following anti-CD3 stimulation) were increased significantly in the CBMCs of HEU infants [49]. These findings suggest that uncontrolled HIV replication is associated with a significantly activated, proinflammatory intrauterine immune environment, in contrast to the state of immunological tolerance normally present during pregnancy.
Figure 1.
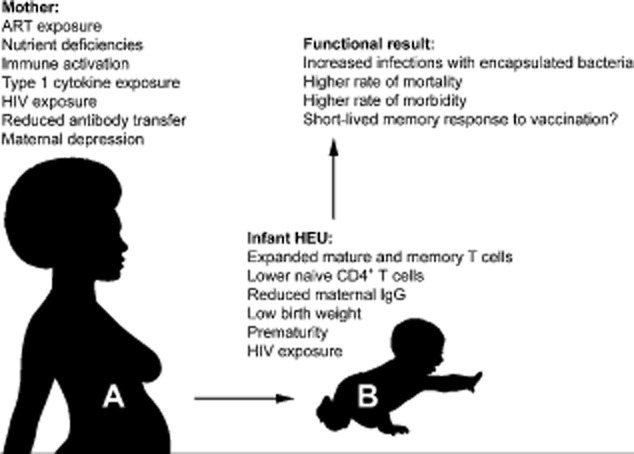
HIV infection and anti-retroviral therapy (ART) exposure affect the maternal gestational environment and consequently the immune system of the HIV-1-exposed uninfected (HEU) infant, potentially leading to increased morbidity and mortality.
Neonatal dendritic cells (DCs) are less polyfunctional than those of adults and produce lower levels of type I interferons and IL-12, in response to Toll-like receptor (TLR) ligands (reviewed in [50]). Studies of CBMC production of IL-12, a predominantly type 1 cytokine produced by antigen-presenting cells (APCs), showed that HEU CBMCs produced significantly less IL-12 than UU CBMCs following stimulation with Staphylococcus aureus Cowan (SAC), which was not restored by co-stimulation with IFN-γ and soluble trimeric CD40 ligand (CD40L) [51,52]. Cord blood-derived myeloid DC (mDC) in HEU infants appear to be expanded and have increased inhibitory (B7-H1) and activation (CD80 and CD86) markers following mitogen stimulation compared with UU infants [53].
Immunological studies during infancy and in older HEU children have also revealed significant differences from unexposed children. In a detailed cross-sectional study of HEU and UU infants from a European cohort, older children (mean age 7 years) not exposed to ART were compared to infants (mean age 1 month) who had received AZT prophylaxis [33]. While the B and NK cell compartments were similar between UU controls and HEU infants, absolute CD4+ T cells were reduced in HEU children in both age groups. Moreover, naive CD8+ T cells were reduced, while activated (CD38+) and memory (CD45RO+) CD8+ T cell populations were augmented. A striking finding was a significant expansion of immature double-negative (CD4–CD8–) T cells, which may indicate disturbed thymic function. Both infant and older HEU groups showed significantly higher levels of serum IL-7 than uninfected controls, which may also indicate a response to impaired T cell homeostasis. HIV exposure in the newborns (despite AZT prophylaxis) was indicated by the presence of HIV-specific CD4+ T cell responses. The expansion of memory and activated compartments in this study is consistent with an antigen-exposed, activated immune profile that persists to some extent in older children. In one adolescent HEU cohort, significantly increased populations of activated and terminally differentiated CD8+ T cells, increased CD19 B cells and reduced effector memory CD4+ and CD16+ NK cell populations were reported compared to age-matched controls [54].
In keeping with the suggestion of homeostatic dysregulation, impaired thymic function, with lower numbers of naive CD4+ T cells and reduced T cell receptor excision circles (TRECs), as well as reduced progenitor function, were described in cord blood mononuclear cells (CBMCs) from 19 HEU infants compared to age-matched UU controls [55]. In this cohort all but one of the mothers had received ART during pregnancy. These data suggest that HIV exposure could have a direct impact on T cell differentiation, homeostasis and possibly thymic function even up to early childhood, independently of ART regimen. In contrast, no differences were found in a Danish cohort when comparing T cell populations, including regulatory T cells (Tregs), cytokine profiles or the antibody response to Haemophilus influenzae type b (Hib) vaccination between 20 HEU children (aged 15 months) and UU controls [56]. Nevertheless, in this study HEU infants had reduced thymic size, known to predict increased susceptibility to infection and mortality in HIV-negative infants [57,58].
Further evidence that HIV exposure is associated with activation of the infant immune system was demonstrated in a study measuring the expression of CD154 (CD40L), an inducible co-stimulatory molecule from the TNF superfamily of ligands that is expressed on activated cells and is an important mediator of T cell/B cell cross-talk. High levels of CD154 were seen on T cells from ART-exposed HEU infants [59], even compared to age-matched controls infected congenitally with CMV, toxoplasmosis, hepatitis C or rubella. CD154 was up-regulated on more than 95% of CD4+ and CD8+ T cells in HEU infants, but the levels of expression did not correlate with maternal CD4+ T cell count or viral load.
As discussed previously, it is difficult to disentangle the relative contributions of HIV and ART exposure in the reported immunological differences between HEU and UU children. In one older cohort of HEU children (aged 6–18 years), 11 (44%) mothers had received some form of ART during their pregnancy. The HEU children had a significantly reduced CD4+ T cell count; however, this was confined to those whose mothers received ART, whereas the CD4+ T cell count of children without ART exposure did not differ from controls. Interestingly, an increase in B lymphocyte apoptosis (assessed through caspase-3+ detection on CD19+ cells) was also noted in the HEU adolescents, regardless of ART exposure [60].
It is plausible that HIV-1 could affect the infant immune system by transfer of viral products across the placenta without productive infection. For example, even in the absence of viral infection, purified HIV-1 gp120 shows a dose-dependent immunosuppressive effect on the antigen-driven proliferation of cloned CD4+ T cells and the cytolytic activity of Epstein–Barr virus (EBV)-specific cytotoxic lymphocytes (CTL) [61]. Other investigators have suggested a role for gp120 in impaired DC function through direct contact, leading to defective CD4 T cell–DC interactions [62]. Similarly, soluble HIV-1 negative regulatory factor (nef) can be transferred from macrophages to B cells and directly affects the generation of antibody responses through impairment of class-switching [63].
In summary, the key phenotypical findings in HEU children are of expanded memory T cell subsets and increased immune activation with increased apoptosis, reduced thymic function and fewer naive T cells, accompanied by functional differences consistent with Th1/Th17 polarization and impaired APC function, in a setting of reduced transfer of maternal antibodies. These abnormalities could have a negative effect on the response to infection and to T cell-dependent antigens during routine vaccination in early life. As B cells rely on T cell ‘help’ to generate antigen-specific memory B cells, the responses generated against encapsulated pathogens that require antibody-dependent clearance may also be disturbed.
Immunological consequences of ART exposure
The use of ART in pregnancy and breastfeeding has enabled the elimination of vertical HIV transmission to become an achievable goal. Although most ART-exposed children are in good health, some concerns remain about the secondary effects of ART and their impact on early infant development. As prolonged ART exposure during infancy becomes more widespread, it will be important to dissect out the specific health risks posed to HEU infants by the different classes of ART from those that arise through exposure to a maternal environment perturbed by HIV infection. Most of the drugs used in pregnancy, including the nucleoside analogue reverse transcriptase inhibitors (NRTIs), and particularly zidovudine (AZT), readily cross the placenta [64].
A number of biological alterations in HEU infants have been linked to ART-exposure during PMTCT (Table 2), with varying effects on infant health. Mitochondrial toxicity (MT) has emerged as central to the aetiology of many of these disorders (reviewed in [65]). The NRTI class of ART is thought to mediate MT principally through mitochondrial γ polymerase inhibition and the accumulation of somatic point mutations in mtDNA (reviewed in [66]). A few cases of severe neuropathy and an increased 18-month incidence of neuromitochondrial disease were observed in HEU infants from the French perinatal cohort exposed in utero to NRTIs [67,68]. The rarity of these severe disorders was highlighted by the absence of similar overt cases in several US [69] and European [70] cohorts. However, the retrospective analysis of 20 suspected cases of infant MT from two large US cohorts found lower levels of mtDNA and higher oxidative phosphorylation enzyme activities compared with controls [71]. Similar analyses have shown mtDNA depletion following NRTI exposure [72,72–75], but the implications of these findings are not entirely clear. Aside from the rare clinically severe cases of MT, subclinical MT is likely to contribute to some of the longer-lasting biological alterations reported in ART-exposed infants, such as the development of lactic acidaemia [76–79], cardiac growth and function abnormalities [80] and, with particular relevance to immune health, haematological perturbations.
Table 2.
Immune abnormalities in HIV-1-exposed uninfected (HEU) infants
| Feature | Reference |
|---|---|
| Reduced transfer of maternal antibody including IgG | de Moraes-Pinto et al. [40]; Farquhar et al. [46]; Cumberland et al. [42]; Scott et al. [45]; Bunders et al. [44]; Jones et al. [93] |
| Altered CD4+ and CD8+ T cell counts | Clerici et al. [33]; Miles et al. [98]; Slogrove et al. [11]; Borges-Almeida, et al. [126] |
| Increased proinflammatory responses in cord blood to polyclonal stimulation | Hygino et al. [47]; Hygino et al. [48] |
| Increased T cell immune activation | Rich et al. [36]; Clerici et al. [33]; Romano et al. [59]; Vigano et al. [54] |
| Skewed T cell memory and differentiation subset distributions | Rich et al. [36]; Clerici et al. [33]; Nielson et al. [128]; Vigano et al. [54]; Miles et al. [98] |
| Increased susceptibility to T cell apoptosis | Economides et al. [49] |
| Increased plasma IL-7 levels | Clerici et al. [33] |
| Altered DC phenotype and in-vitro IL-12 production | Chougnet et al. [51]; Velilla et al. [53] |
| Reduced thymic size | Kolte et al. [56] |
| Reduced TREC levels in periphery | Nielson et al. [128] |
| Skewed maturation of B cell subsets and susceptibility to apoptosis | Bunders et al. [44]; Miyamoto et al. [129]; Borges-Almeida et al. [126] |
IgG = immunoglobulin G; IL = interleukin; TREC = T cell receptor excision circles.
Changes in haematological parameters in HEU infants exposed to various combinations of ARTs (Table 2) have been reported consistently in large European and North American cohorts [81]. Transient decreases in haemoglobin levels and more sustained decreases in platelets, total lymphocytes and CD4+ and CD8+ T cell subsets that persisted to at least 2 years of age were observed in French [81] and US cohorts [82]. In addition, the European Collaborative Study found subclinical but sustained reductions in total lymphocyte and CD8+ T cell counts [83] and, strikingly, reduced neutrophil counts during the first 8 years of life [84]. Furthermore, exposure to more complex drug regimens (compared to monotherapy) has been associated with greater depletion of haematological cell lineages [81,85]. In vitro, AZT has been shown to reduce myeloid and erythroid CD34+ haematopoietic stem progenitor proliferation [86,87]. Low thymic CD4+ T cell counts and impaired CD34+ progenitor cell function were reported in a cohort of NRTI-exposed HEU infants [55]. A recent report showed striking differences in the transcriptional profiles of cord blood-derived CD34+ progenitor cells between the HEU infants of mothers who received AZT in pregnancy and unexposed controls, particularly in genes involved in the cell cycle, cell death and in DNA replication, recombination and repair [88]. These findings were coupled with a threefold increase in the rate of aneuploidy (cells containing an abnormal number of chromosomes following cell division) in AZT-exposed cord blood, which could be an indication of AZT-induced DNA damage. Parallel toxicological findings in animal models implicate ART, rather than HIV exposure, in the aetiology of these changes [89]. These sustained effects highlight the potential impact of ART exposure on haematopoeisis, with potential consequences for both innate and adaptive immunity.
In addition to these fundamental ontological effects, direct immunomodulation was shown to occur after maternal administration of single-dose nevirapine (sdNVP) to reduce MTCT. Unexpectedly, sdNVP was associated with an increase in soluble plasma immune activation markers in HEU infants, above the heightened level of immune activation detected as a result of HIV exposure alone [90]. The immunomodulatory properties of AZT–3TC dual therapy were investigated using samples from a randomized PMTCT drug trial [91]. Attenuated HIV-specific T helper responses to envelope antigens, but not to allogeneic stimulation, were detected among infants whose mothers had received ART versus those who were unexposed. However, HIV infection among the ART-unexposed group may have been a confounding factor in this report.
Further studies are needed to clarify the extent of direct immunomodulation by ART and assess the functional significance of the various haematological alterations that have been reported. However, in the PMTCT era, distinguishing the effect of individual anti-retroviral agents, singly or in combination, from the effect of HIV exposure remains challenging. Recent advances in the development of the Erythrocebus patas monkey model [89,92], and in methods of assessing cumulative in utero and postpartum ART exposure in infant hair samples (Mwesigwa et al., AIDS 2012 poster), are likely to be instrumental in addressing these issues. Overall, these strategies in pharmacovigilance should inform efforts to develop optimal ART regimens that enhance the health of HEU infants and minimize the toxic of ART exposure in early life.
Do HEU infants make normal vaccine responses?
If HIV and/or ART exposure has significant immunological consequences (summarised in table 3), are these sufficient to affect vaccine responses to infant immunization? A large cross-sectional study of South African infants reported robust antibody responses to routine Expanded Programme on Immunization (EPI) vaccines in HEU infants, often to a greater magnitude than unexposed controls (except in the case of HBV) [93]. In keeping with previous studies, the investigators noted lower levels of transferred maternal antibodies in the HEU infants; reduced maternal antibody interference with the vaccine may have contributed to their strong vaccine responses. Detailed longitudinal studies in a birth cohort showed no significant impairment of antibody levels following EPI vaccines between HEU and UU infants [94]; other studies have reported conflicting results [95,96]. In one of these studies, low poliovirus vaccine titres was associated with reduced duration of breastfeeding in the HIV-exposed group [96]. Thus it remains an open question if and when EPI vaccines elicit adequate antibody responses in HEU infants; in particular, long-lasting B cell memory and antibody quality have not been addressed in detail.
Table 3.
Summary of biological changes induced by exposure to anti-retroviral therapy (ART) in HIV-1-exposed uninfected (HEU) infants
| Feature | Incidence | Probable cause | Reference |
|---|---|---|---|
| Metabolic | |||
| Altered mitochondrial function leading to severe neuropathy | 0·3% [67]; 0·26% [68] | MT | Blanche et al. [67]; Barret et al. [68]; Blanche et al. [130] |
| Transient alterations in mDNA levels | Not known | MT | Poirier et al. [73]; Shiramizu et al. [75]; Divi et al. [74]; Aldrovandi et al. [131]; Brogly et al. [71]; |
| Uncomplicated reversible hyperlactataemia; symptomatic lactic acidosis | 50–92% [76–78]; ∼5% [77] | MT | Alimenti et al. [77]; Noguera et al. [78] Ekouevi et al. [79]; Giaquinto et al. [76] |
| Altered cardiac growth and function | Not known. Effect more pronounced in girls versus boys | MT | Lipshultz et al. [80] |
| Haematological | |||
| Altered CD34+ progenitor cell proliferation/function | Not known | gDNA damage | Nielsen et al. [55]; Andre-Schmutz et al. [88] |
| Subclinical low lymphocytes, neutrophil and platelet counts | Not known | HSC alterations | Pacheco et al. [82]; ECS [84]; Bunders et al. [83]; Le Chenadec et al. [81] |
| Increased aneuploidy in CB lymphocytes | Not known. Threefold increase in aneuploidy rate | gDNA damage | Andre-Schmutz et al. [88] |
CB = cord blood; HSC = haematopoietic stem cell; MT = mitochondrial toxicity.
The data on T cell responses to vaccines in HEU children are even less clear-cut. A number of studies have compared immune responses to the live attenuated bacillus Calmette–Guérin (BCG) vaccine – given to infants at birth in tuberculosis-endemic areas – in HEU and UU controls, with contradictory results. Impairment of BCG immunogenicity was suggested in an early study in The Gambia, which reported a significantly reduced occurrence of BCG scars after vaccination in HEU children [97]. More recently, altered proliferative responses following in-vitro BCG stimulation were reported in HEUs at 10 weeks [98] and at a median of 7 months of age [99]. A trend towards lower IFN-γ production was seen in whole blood stimulated with purified protein derivative (PPD) from 6-week-old HEU infants [100]. By contrast, the same study, in which the entire HEU cohort presented a BCG scar, found no demonstrable effect of maternal HIV-infection status on IFN-γ secretion after stimulation with BCG or early secreted antigenic target protein 6 (ESAT-6). This finding is in accordance with others in which neither IFN-γ alone [98] nor IFN-γ, IL-13, IL-5 or IL-10 production [101] following in-vitro stimulation with mycobacterial antigens was compromised in HEUs. Finally, the cytokine profiles of T cells responsive to mycobacterial antigens were explored in HEU infants longitudinally by flow cytometry during the first year of life. A complex pattern of expression profiles dominated by CD4 T cells expressing one or more of the Th1 cytokines, IFN-γ, IL-2 and TNF-α, was found that did not differ statistically between HEU infants and UU controls. Collectively, these results suggest that, for the most part, immune responses to BCG in HEU are within normal limits; however, as the correlates of protection by BCG vaccination are still not defined [102], studies to assess the relative risk of TB infection in BCG-vaccinated HEU infants are needed to confirm that these immune responses are adequate for protection.
What is the impact of CMV infection on HEU children?
HIV-1-infected mothers have an increased likelihood of co-infections that could affect the health of their infants, independently of HIV-1 exposure. Probably the most significant of these is CMV infection, which is acquired in early life by the majority of children in sub-Saharan Africa [103]. CMV reactivation appears to be common in pregnant HIV-1-infected women: CMV viraemia was found at delivery in 17% of Kenyan HIV+ women who had received short-course AZT in the third trimester, leading to an increased likelihood of early transmission of CMV to the infant [104]. In this study, maternal CMV viraemia at delivery was associated with significantly increased mortality in both mother and infant. Improved maternal ART regimens may reduce the risk of early CMV transmission to the infant, but this has not been assessed formally in Africa. In one study in the United States, in which all the mothers were provided with combination ART during pregnancy, the prevalence of congenital CMV infection in HEU newborns was 3% [105]. Two studies in Zambia have suggested an increased burden of CMV infection in HEU infants, who were more likely than UU infants to experience high CMV viral loads [106]. A large study of infant nutritional supplementation showed that CMV viraemia at 6 months in HEU (but not UU) infants was associated significantly with growth stunting, together with reduced head size and retarded psychomotor development [107]; the absence of similar associations in UU children suggests that the control of CMV infection is impaired in HEU children, potentially because of earlier infection with high viral loads.
The acquisition of primary CMV infection after birth is associated with profound changes in the phenotype of CD8+ and CD4+ T cells, which show increased levels of activation and differentiation [38,39,108]. In HIV-1 co-infected children, these phenotypical changes are far more substantial than those caused by HIV-1 alone [109]. Therefore, early CMV infection could be a major confounding factor when analysing the immune phenotype of HEU infants, and could certainly account for the increased proportion of activated and memory T cells described in these children.
Are there parallels between HIV exposure and exposure to other maternal infections?
Some maternal infections during pregnancy can alter the development of fetal immune responses, not only to the specific pathogen but also to unrelated pathogens. Thus, the potential in utero modulation of immune responses by HIV virions or soluble antigens is not unique, but parallels outcomes observed with other maternal chronic infections, as reviewed recently [110].
In malaria infection, it has been suggested that soluble parasite components transferred transplacentally induce fetal immune modulation, which may influence infant immune responses to malaria antigen stimuli [111–115]. Poor control of placental malaria can lead to reduced Th1-type responses in the infant, thereby affecting fetal growth and infant survival [116].
Similarly, maternal infection with various helminths may skew infant immunity to a dominant Th2 response, with corresponding down-regulation of Th1 responses, a process mediated by the generation of regulatory T cells and anti-inflammatory cytokines [110,117,118]. This sensitization may persist into childhood even in the absence of infant infection [119], and may also diminish responses to other unrelated infections [119].
Cells from infants born to mothers infected with T. cruzi may produce an altered repertoire of proinflammatory cytokines [120], even though they are not themselves infected [110,121], suggesting that they have hyperactivated monocytes like their mothers. These infants also have T. cruzi-specific IgA and IgM in cord blood, implying that a B cell response has been generated in utero [122].
Following hepatitis B virus (HBV) vaccination, HBV-exposed uninfected infants were more likely to generate potent virus-specific polyfunctional CD4+ responses than infants from uninfected mothers [123]. In utero sensitization by transplacental transfer of mycobacterial antigens during gestation has also been reported in a mouse model. Subsequent postnatal immunization with the homologous antigen resulted in antigen-specific recall responses and protection against mycobacterial infection [124].
Summary and discussion
The overall benefits of PMTCT programmes are undeniable: maternal ART in pregnancy has prevented millions of children from acquiring HIV-1 infection. Nevertheless, there is compelling evidence that uninfected children born to HIV-1-infected mothers, particularly in the developing world, can experience increased morbidity and mortality, predominantly from infectious diseases. Accompanying these epidemiological observations, a number of immunological perturbations have been described in HEU infants that may, in some cases, presumably be sufficient to lead to increased susceptibility to infection. Immune abnormalities could potentially be a consequence of HIV exposure in utero and early life, but could also be due to exposure to anti-retroviral drugs, as well as early transmission of persistent viral infections such as CMV. It is likely that in resource-poor settings these three factors have a synergistic effect that undermines the developing immune system of young infants. In such settings it is also important to recognize that immune recovery from these insults may be impaired by other frequent infections such as malaria and measles, together with malnutrition and poor socioeconomic circumstances [125]. The suggestion from some studies that HEU infants may have impaired T cell responses to infant vaccines is of obvious public health relevance. While the satisfactory antibody levels attained in HEU infants following EPI vaccines in most studies is reassuring, the longevity of these responses also needs to be assessed.
Although the increasing use of combination ART to improve maternal health might be expected to lead to better health in their uninfected infants, this has not yet been demonstrated formally. Moreover, while exposure to HIV and co-infections from healthier mothers should be reduced as a consequence of this policy, their children will inevitably have more prolonged ART exposure, often from early in gestation. The mechanisms underlying the apparent immunodeficiency in HEU need to be identified, particularly in resource-poor settings where the burden of HIV and other life-threatening infection is high. Well-designed prospective longitudinal studies of HIV-1-infected mothers and their infants are needed, with accurate records of ART use, accompanied by detailed assessment of the transmission of maternal co-infections and the transfer of maternal antibodies, alongside phenotypical and functional studies of the infant immune system. A central component of these studies should be an assessment of the long-term responses of HEU infants to EPI vaccines. The vaccine schedule of HEU infants may need to be adjusted to provide optimal protection from infection for these vulnerable children.
Disclosure
None.
References
- 1.Shapiro RL, Lockman S. Mortality among HIV-exposed infants: the first and final frontier. Clin Infect Dis. 2010;50:445–447. doi: 10.1086/649887. [DOI] [PubMed] [Google Scholar]
- 2.Marinda E, Humphrey JH, Iliff PJ, et al. Child mortality according to maternal and infant HIV status in Zimbabwe. Pediatr Infect Dis J. 2007;26:519–526. doi: 10.1097/01.inf.0000264527.69954.4c. [DOI] [PubMed] [Google Scholar]
- 3.Koyanagi A, Humphrey JH, Ntozini R, et al. Morbidity among human immunodeficiency virus-exposed but uninfected, human immunodeficiency virus-infected, and human immunodeficiency virus-unexposed infants in Zimbabwe before availability of highly active antiretroviral therapy. Pediatr Infect Dis J. 2011;30:45–51. doi: 10.1097/INF.0b013e3181ecbf7e. [DOI] [PubMed] [Google Scholar]
- 4.Brahmbhatt H, Kigozi G, Wabwire-Mangen F, et al. Mortality in HIV-infected and uninfected children of HIV-infected and uninfected mothers in rural Uganda. J Acquir Immune Defic Syndr. 2006;41:504–508. doi: 10.1097/01.qai.0000188122.15493.0a. [DOI] [PubMed] [Google Scholar]
- 5.Landes M, van Lettow M, Chan AK, Mayuni I, Schouten EJ, Bedell RA. Mortality and health outcomes of HIV-exposed and unexposed children in a PMTCT cohort in Malawi. PLoS ONE. 2012;7:e47337. doi: 10.1371/journal.pone.0047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fawzy A, Arpadi S, Kankasa C, et al. Early weaning increases diarrhea morbidity and mortality among uninfected children born to HIV-infected mothers in Zambia. J Infect Dis. 2011;203:1222–1230. doi: 10.1093/infdis/jir019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slogrove A, Reikie B, Naidoo S, et al. HIV-exposed uninfected infants are at increased risk for severe infections in the first year of life. J Trop Pediatr. 2012;58:505–508. doi: 10.1093/tropej/fms019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filteau S, Baisley K, Chisenga M, Kasonka L, Gibson RS. Provision of micronutrient-fortified food from 6 months of age does not permit HIV-exposed uninfected Zambian children to catch up in growth to HIV-unexposed children: a randomized controlled trial. J Acquir Immune Defic Syndr. 2011;56:166–175. doi: 10.1097/QAI.0b013e318201f6c9. [DOI] [PubMed] [Google Scholar]
- 9.Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 10.McNally LM, Jeena PM, Gajee K, et al. Effect of age, polymicrobial disease, and maternal HIV status on treatment response and cause of severe pneumonia in South African children: a prospective descriptive study. Lancet. 2007;369:1440–1451. doi: 10.1016/S0140-6736(07)60670-9. [DOI] [PubMed] [Google Scholar]
- 11.Slogrove AL, Cotton MF, Esser MM. Severe infections in HIV-exposed uninfected infants: clinical evidence of immunodeficiency. J Trop Pediatr. 2010;56:75–81. doi: 10.1093/tropej/fmp057. [DOI] [PubMed] [Google Scholar]
- 12.Singh HK, Gupte N, Kinikar A, et al. High rates of all-cause and gastroenteritis-related hospitalization morbidity and mortality among HIV-exposed Indian infants. BMC Infect Dis. 2011;11:193. doi: 10.1186/1471-2334-11-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epalza C, Goetghebuer T, Hainaut M, et al. High incidence of invasive group B streptococcal infections in HIV-exposed uninfected infants. Pediatrics. 2010;126:e631–638. doi: 10.1542/peds.2010-0183. [DOI] [PubMed] [Google Scholar]
- 14.Lo YM, Lo ES, Watson N, et al. Two-way cell traffic between mother and fetus: biologic and clinical implications. Blood. 1996;88:4390–4395. [PubMed] [Google Scholar]
- 15.Rombaldi RL, Serafini EP, Mandelli J, Zimmermann E, Losquiavo KP. Transplacental transmission of human papillomavirus. Virol J. 2008;5:106. doi: 10.1186/1743-422X-5-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright HT., Jr Congenital anomalies and viral infections in infants. The etiologic role of maternal viral infections. Calif Med. 1966;105:345–351. [PMC free article] [PubMed] [Google Scholar]
- 17.Gaillard P, Verhofstede C, Mwanyumba F, et al. Exposure to HIV-1 during delivery and mother-to-child transmission. AIDS. 2000;14:2341–2348. doi: 10.1097/00002030-200010200-00015. [DOI] [PubMed] [Google Scholar]
- 18.Mano H, Chermann JC. Fetal human immunodeficiency virus type 1 infection of different organs in the second trimester. AIDS Res Hum Retroviruses. 1991;7:83–88. doi: 10.1089/aid.1991.7.83. [DOI] [PubMed] [Google Scholar]
- 19.Van de Perre P, Simonon A, Karita E, et al. Infective and anti-infective properties of breastmilk from HIV-1-infected women. Lancet. 1993;341:914–918. doi: 10.1016/0140-6736(93)91210-d. [DOI] [PubMed] [Google Scholar]
- 20.Cameron PU, Lowe M, Sotzik F, Coughlan AF, Crowe SM, Shortman K. The interaction of macrophage and non-macrophage tropic isolates of HIV-1 with thymic and tonsillar dendritic cells in vitro. J Exp Med. 1996;183:1851–1856. doi: 10.1084/jem.183.4.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shapiro RL, Ndung'u T, Lockman S, et al. Highly active antiretroviral therapy started during pregnancy or postpartum suppresses HIV-1 RNA, but not DNA, in breast milk. J Infect Dis. 2005;192:713–719. doi: 10.1086/432489. [DOI] [PubMed] [Google Scholar]
- 22.Pillay K, Coutsoudis A, York D, Kuhn L, Coovadia HM. Cell-free virus in breast milk of HIV-1-seropositive women. J Acquir Immune Defic Syndr. 2000;24:330–336. doi: 10.1097/00126334-200008010-00006. [DOI] [PubMed] [Google Scholar]
- 23.Richardson BA, John-Stewart GC, Hughes JP, et al. Breast-milk infectivity in human immunodeficiency virus type 1-infected mothers. J Infect Dis. 2003;187:736–740. doi: 10.1086/374272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rowland-Jones SL, Nixon DF, Aldhous MC, et al. HIV-specific CTL activity in an HIV-exposed but uninfected infant. Lancet. 1993;341:860–861. doi: 10.1016/0140-6736(93)93063-7. [DOI] [PubMed] [Google Scholar]
- 25.Cheynier R, Langlade-Demoyen P, Marescot M-R, et al. Cytotoxic T lymphocyte responses in the peripheral blood of children born to HIV-1-infected mothers. Eur J Immunol. 1992;22:2211–2217. doi: 10.1002/eji.1830220905. [DOI] [PubMed] [Google Scholar]
- 26.Clerici M, Sison AV, Berzofsky JA, et al. Cellular immune factors associated with mother-to-infant transmission of HIV. AIDS. 1993;7:1427–1433. doi: 10.1097/00002030-199311000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Aldhous MC, Watret KC, Mok JY, Bird AG, Froebel KS. Cytotoxic T lymphocyte activity and CD8 subpopulations in children at risk of HIV infection. Clin Exp Immunol. 1994;97:61–67. doi: 10.1111/j.1365-2249.1994.tb06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Maria A, Cirillo C, Moretta L. Occurrence of HIV-specific CTL activity in apparently uninfected children born to HIV-1-infected mothers. J Infect Dis. 1994;170:1296–1299. doi: 10.1093/infdis/170.5.1296. [DOI] [PubMed] [Google Scholar]
- 29.Legrand FA, Nixon DF, Loo CP, et al. Strong HIV-1-specific T cell responses in HIV-1-exposed uninfected infants and neonates revealed after regulatory T cell removal. PLOS ONE. 2006;1:e102. doi: 10.1371/journal.pone.0000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.John-Stewart GC, Mbori-Ngacha D, Payne BL, et al. HIV-1-specific cytotoxic T lymphocytes and breast milk HIV-1 transmission. J Infect Dis. 2009;199:889–898. doi: 10.1086/597120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuhn L, Coutsoudis A, Moodley D, et al. T-helper cell responses to HIV envelope peptides in cord blood: protection against intrapartum and breast-feeding transmission. AIDS. 2001;15:1–9. doi: 10.1097/00002030-200101050-00003. [DOI] [PubMed] [Google Scholar]
- 32.Holditch SJ, Eriksson EM, Tarosso LF, et al. Decay kinetics of HIV-1 specific T cell responses in vertically HIV-1 exposed seronegative infants. Front Immunol. 2011;2:94. doi: 10.3389/fimmu.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clerici M, Saresella M, Colombo F, et al. T-lymphocyte maturation abnormalities in uninfected newborns and children with vertical exposure to HIV. Blood. 2000;96:3866–3871. [PubMed] [Google Scholar]
- 34.Tiemessen CT, Shalekoff S, Meddows-Taylor S, et al. Natural killer cells that respond to human immunodeficiency virus type 1 (HIV-1) peptides are associated with control of HIV-1 infection. J Infect Dis. 2010;202:1444–1453. doi: 10.1086/656535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gesner M, Papaevangelou V, Kim M, et al. Alteration in the proportion of CD4 T lymphocytes in a subgroup of human immunodeficiency virus-exposed-uninfected children. Pediatrics. 1994;93:624–630. [PubMed] [Google Scholar]
- 36.Rich KC, Chang BH, Mofenson L, et al. Elevated CD8+DR+ lymphocytes in HIV-exposed infants with early positive HIV cultures: a possible early marker of intrauterine transmission. Women and Infants Transmission Study Group. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15:204–210. doi: 10.1097/00042560-199707010-00004. [DOI] [PubMed] [Google Scholar]
- 37.Ono E, Nunes dos Santos AM, de Menezes Succi RC, et al. Imbalance of naive and memory T lymphocytes with sustained high cellular activation during the first year of life from uninfected children born to HIV-1-infected mothers on HAART. Braz J Med Biol Res. 2008;41:700–708. doi: 10.1590/s0100-879x2008000800011. [DOI] [PubMed] [Google Scholar]
- 38.Miles DJ, van der Sande M, Jeffries D, et al. Cytomegalovirus infection in Gambian infants leads to profound CD8 T-cell differentiation. J Virol. 2007;81:5766–5776. doi: 10.1128/JVI.00052-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miles DJ, van der Sande M, Jeffries D, et al. Maintenance of large subpopulations of differentiated CD8 T-cells two years after cytomegalovirus infection in Gambian infants. PLOS ONE. 2008;3:e2905. doi: 10.1371/journal.pone.0002905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Moraes-Pinto MI, Verhoeff F, Chimsuku L, et al. Placental antibody transfer: influence of maternal HIV infection and placental malaria. Arch Dis Child Fetal Neonatal Ed. 1998;79:F202–205. doi: 10.1136/fn.79.3.f202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de MoraesPinto MI, Almeida ACM, Kenj G, et al. Placental transfer and maternally acquired neonatal IgG immunity in human immunodeficiency virus infection. J Infect Dis. 1996;173:1077–1084. doi: 10.1093/infdis/173.5.1077. [DOI] [PubMed] [Google Scholar]
- 42.Cumberland P, Shulman CE, Maple PA, et al. Maternal HIV infection and placental malaria reduce transplacental antibody transfer and tetanus antibody levels in newborns in Kenya. J Infect Dis. 2007;196:550–557. doi: 10.1086/519845. [DOI] [PubMed] [Google Scholar]
- 43.Zinkernagel RM. Maternal antibodies, childhood infections, and autoimmune diseases. N Engl J Med. 2001;345:1331–1335. doi: 10.1056/NEJMra012493. [DOI] [PubMed] [Google Scholar]
- 44.Bunders M, Pembrey L, Kuijpers T, Newell ML. Evidence of impact of maternal HIV infection on immunoglobulin levels in HIV-exposed uninfected children. AIDS Res Hum Retroviruses. 2010;26:967–975. doi: 10.1089/aid.2009.0241. [DOI] [PubMed] [Google Scholar]
- 45.Scott S, Moss WJ, Cousens S, et al. The influence of HIV-1 exposure and infection on levels of passively acquired antibodies to measles virus in Zambian infants. Clin Infect Dis. 2007;45:1417–1424. doi: 10.1086/522989. [DOI] [PubMed] [Google Scholar]
- 46.Farquhar C, Nduati R, Haigwood N, et al. High maternal HIV-1 viral load during pregnancy is associated with reduced placental transfer of measles IgG antibody. J Acquir Immune Defic Syndr. 2005;40:494–497. doi: 10.1097/01.qai.0000168179.68781.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hygino J, Lima PG, Filho RG, et al. Altered immunological reactivity in HIV-1-exposed uninfected neonates. Clin Immunol. 2008;127:340–347. doi: 10.1016/j.clim.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 48.Hygino J, Vieira MM, Guillermo LV, et al. Enhanced Th17 phenotype in uninfected neonates born from viremic HIV-1-infected pregnant women. J Clin Immunol. 2011;31:186–194. doi: 10.1007/s10875-010-9485-3. [DOI] [PubMed] [Google Scholar]
- 49.Economides A, Schmid I, Anisman-Posner DJ, Plaeger S, Bryson YJ, Uittenbogaart CH. Apoptosis in cord blood T lymphocytes from infants of human immunodeficiency virus-infected mothers. Clin Diagn Lab Immunol. 1998;5:230–234. doi: 10.1128/cdli.5.2.230-234.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prendergast AJ, Klenerman P, Goulder PJ. The impact of differential antiviral immunity in children and adults. Nat Rev Immunol. 2012;12:636–648. doi: 10.1038/nri3277. [DOI] [PubMed] [Google Scholar]
- 51.Chougnet C, Kovacs A, Baker R, et al. Influence of human immunodeficiency virus-infected maternal environment on development of infant interleukin-12 production. J Infect Dis. 2000;181:1590–1597. doi: 10.1086/315458. [DOI] [PubMed] [Google Scholar]
- 52.Kuhn L, Kasonde P, Sinkala M, et al. Does severity of HIV disease in HIV-infected mothers affect mortality and morbidity among their uninfected infants? Clin Infect Dis. 2005;41:1654–1661. doi: 10.1086/498029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Velilla PA, Montoya CJ, Hoyos A, Moreno ME, Chougnet C, Rugeles MT. Effect of intrauterine HIV-1 exposure on the frequency and function of uninfected newborns' dendritic cells. Clin Immunol. 2008;126:243–250. doi: 10.1016/j.clim.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 54.Vigano A, Saresella M, Schenal M, et al. Immune activation and normal levels of endogenous antivirals are seen in healthy adolescents born of HIV-infected mothers. AIDS. 2007;21:245–248. doi: 10.1097/QAD.0b013e328011d7d3. [DOI] [PubMed] [Google Scholar]
- 55.Nielsen SD, Jeppesen DL, Kolte L, et al. Impaired progenitor cell function in HIV-negative infants of HIV-positive mothers results in decreased thymic output and low CD4 counts. Blood. 2001;98:398–404. doi: 10.1182/blood.v98.2.398. [DOI] [PubMed] [Google Scholar]
- 56.Kolte L, Rosenfeldt V, Vang L, et al. Reduced thymic size but no evidence of impaired thymic function in uninfected children born to human immunodeficiency virus-infected mothers. Pediatr Infect Dis J. 2011;30:325–330. doi: 10.1097/INF.0b013e3182019bc3. [DOI] [PubMed] [Google Scholar]
- 57.Aaby P, Marx C, Trautner S, et al. Thymus size at birth is associated with infant mortality: a community study from Guinea-Bissau. Acta Paediatr. 2002;91:698–703. doi: 10.1080/080352502760069142. [DOI] [PubMed] [Google Scholar]
- 58.Garly ML, Trautner SL, Marx C, et al. Thymus size at 6 months of age and subsequent child mortality. J Pediatr. 2008;153:683–688. doi: 10.1016/j.jpeds.2008.04.069. 8 e1–3. [DOI] [PubMed] [Google Scholar]
- 59.Romano MF, Buffolano W, Bisogni R, et al. Increased CD154 expression in uninfected infants born to HIV-positive mothers exposed to antiretroviral prophylaxis. Viral Immunol. 2006;19:363–372. doi: 10.1089/vim.2006.19.363. [DOI] [PubMed] [Google Scholar]
- 60.Miyamoto M, Pessoa SD, Ono E, et al. Low CD4+ T-cell levels and B-cell apoptosis in vertically HIV-exposed noninfected children and adolescents. J Trop Pediatr. 2010;56:427–432. doi: 10.1093/tropej/fmq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weinhold KJ, Lyerly HK, Stanley SD, Austin AA, Matthews TJ, Bolognesi DP. HIV-1 GP120-mediated immune suppression and lymphocyte destruction in the absence of viral infection. J Immunol. 1989;142:3091–3097. [PubMed] [Google Scholar]
- 62.Chougnet C, Gessani S. Role of gp120 in dendritic cell dysfunction in HIV infection. J Leukoc Biol. 2006;80:994–1000. doi: 10.1189/jlb.0306135. [DOI] [PubMed] [Google Scholar]
- 63.Xu W, Santini PA, Sullivan JS, et al. HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nat Immunol. 2009;10:1008–1017. doi: 10.1038/ni.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Else LJ, Taylor S, Back DJ, Khoo SH. Pharmacokinetics of antiretroviral drugs in anatomical sanctuary sites: the fetal compartment (placenta and amniotic fluid) Antivir Ther. 2011;16:1139–1147. doi: 10.3851/IMP1918. [DOI] [PubMed] [Google Scholar]
- 65.Venhoff N, Walker UA. Mitochondrial disease in the offspring as a result of antiretroviral therapy. Expert Opin Drug Saf. 2006;5:373–381. doi: 10.1517/14740338.5.3.373. [DOI] [PubMed] [Google Scholar]
- 66.Kohler JJ, Lewis W. A brief overview of mechanisms of mitochondrial toxicity from NRTIs. Environ Mol Mutagen. 2007;48:166–172. doi: 10.1002/em.20223. [DOI] [PubMed] [Google Scholar]
- 67.Blanche S, Tardieu M, Rustin P, et al. Persistent mitochondrial dysfunction and perinatal exposure to antiretroviral nucleoside analogues. Lancet. 1999;354:1084–1089. doi: 10.1016/S0140-6736(99)07219-0. [DOI] [PubMed] [Google Scholar]
- 68.Barret B, Tardieu M, Rustin P, et al. Persistent mitochondrial dysfunction in HIV-1-exposed but uninfected infants: clinical screening in a large prospective cohort. AIDS. 2003;17:1769–1785. doi: 10.1097/00002030-200308150-00006. [DOI] [PubMed] [Google Scholar]
- 69.The Perinatal Safety Review Working Group. Nucleoside exposure in the children of HIV-infected women receiving antiretroviral drugs: absence of clear evidence for mitochondrial disease in children who died before 5 years of age in five United States cohorts. J Acquir Immune Defic Syndr. 2000;25:261–268. doi: 10.1097/00126334-200011010-00009. [DOI] [PubMed] [Google Scholar]
- 70.European Collaborative Study. Exposure to antiretroviral therapy in utero or early life: the health of uninfected children born to HIV-infected women. J Acquir Immune Defic Syndr. 2003;32:380–387. doi: 10.1097/00126334-200304010-00006. [DOI] [PubMed] [Google Scholar]
- 71.Brogly SB, DiMauro S, Van Dyke RB, et al. Short communication: transplacental nucleoside analogue exposure and mitochondrial parameters in HIV-uninfected children. AIDS Res Hum Retroviruses. 2011;27:777–783. doi: 10.1089/aid.2010.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aldrovandi GM, Chu C, Shearer WT, et al. Antiretroviral exposure and lymphocyte mtDNA content among uninfected infants of HIV-1-infected women. Pediatrics. 2009;124:e1189–1197. doi: 10.1542/peds.2008-2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Poirier MC, Divi RL, Al-Harthi L, et al. Long-term mitochondrial toxicity in HIV-uninfected infants born to HIV-infected mothers. J Acquir Immune Defic Syndr. 2003;33:175–183. doi: 10.1097/00126334-200306010-00010. [DOI] [PubMed] [Google Scholar]
- 74.Divi RL, Walker VE, Wade NA, et al. Mitochondrial damage and DNA depletion in cord blood and umbilical cord from infants exposed in utero to Combivir. AIDS. 2004;18:1013–1021. doi: 10.1097/00002030-200404300-00009. [DOI] [PubMed] [Google Scholar]
- 75.Shiramizu B, Shikuma KM, Kamemoto L, et al. Placenta and cord blood mitochondrial DNA toxicity in HIV-infected women receiving nucleoside reverse transcriptase inhibitors during pregnancy. J Acquir Immune Defic Syndr. 2003;32:370–374. doi: 10.1097/00126334-200304010-00004. [DOI] [PubMed] [Google Scholar]
- 76.Giaquinto C, De Romeo A, Giacomet V, et al. Lactic acid levels in children perinatally treated with antiretroviral agents to prevent HIV transmission. AIDS. 2001;15:1074–1075. doi: 10.1097/00002030-200105250-00023. [DOI] [PubMed] [Google Scholar]
- 77.Alimenti A, Burdge DR, Ogilvie GS, Money DM, Forbes JC. Lactic acidemia in human immunodeficiency virus-uninfected infants exposed to perinatal antiretroviral therapy. Pediatr Infect Dis J. 2003;22:782–789. doi: 10.1097/01.inf.0000086400.93257.74. [DOI] [PubMed] [Google Scholar]
- 78.Noguera A, Fortuny C, Munoz-Almagro C, et al. Hyperlactatemia in human immunodeficiency virus-uninfected infants who are exposed to antiretrovirals. Pediatrics. 2004;114:e598–603. doi: 10.1542/peds.2004-0955. [DOI] [PubMed] [Google Scholar]
- 79.Ekouevi DK, Toure R, Becquet R, et al. Serum lactate levels in infants exposed peripartum to antiretroviral agents to prevent mother-to-child transmission of HIV: agence Nationale de Recherches Sur le SIDA et les Hepatites Virales 1209 study, Abidjan, Ivory Coast. Pediatrics. 2006;118:e1071–1077. doi: 10.1542/peds.2006-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lipshultz SE, Shearer WT, Thompson B, et al. Cardiac effects of antiretroviral therapy in HIV-negative infants born to HIV-positive mothers: NHLBI CHAART-1 (National Heart, Lung, and Blood Institute Cardiovascular Status of HAART Therapy in HIV-Exposed Infants and Children cohort study) J Am Coll Cardiol. 2011;57:76–85. doi: 10.1016/j.jacc.2010.08.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Le Chenadec J, Mayaux MJ, Guihenneuc-Jouyaux C, Blanche S. Perinatal antiretroviral treatment and hematopoiesis in HIV-uninfected infants. AIDS. 2003;17:2053–2061. doi: 10.1097/00002030-200309260-00006. [DOI] [PubMed] [Google Scholar]
- 82.Pacheco SE, McIntosh K, Lu M, et al. Effect of perinatal antiretroviral drug exposure on hematologic values in HIV-uninfected children: an analysis of the women and infants transmission study. J Infect Dis. 2006;194:1089–1097. doi: 10.1086/507645. [DOI] [PubMed] [Google Scholar]
- 83.Bunders M, Thorne C, Newell ML. Maternal and infant factors and lymphocyte, CD4 and CD8 cell counts in uninfected children of HIV-1-infected mothers. AIDS. 2005;19:1071–1079. doi: 10.1097/01.aids.0000174454.63250.22. [DOI] [PubMed] [Google Scholar]
- 84.Study EC. Levels and patterns of neutrophil cell counts over the first 8 years of life in children of HIV-1-infected mothers. AIDS. 2004;18:2009–2017. doi: 10.1097/00002030-200410210-00005. [DOI] [PubMed] [Google Scholar]
- 85.Feiterna-Sperling C, Weizsaecker K, Buhrer C, et al. Hematologic effects of maternal antiretroviral therapy and transmission prophylaxis in HIV-1-exposed uninfected newborn infants. J Acquir Immune Defic Syndr. 2007;45:43–51. doi: 10.1097/QAI.0b013e318042d5e3. [DOI] [PubMed] [Google Scholar]
- 86.Lewis LD, Amin S, Civin CI, Lietman PS. Ex vivo zidovudine (AZT) treatment of CD34+ bone marrow progenitors causes decreased steady state mitochondrial DNA (mtDNA) and increased lactate production. Hum Exp Toxicol. 2004;23:173–185. doi: 10.1191/0960327104ht437oa. [DOI] [PubMed] [Google Scholar]
- 87.Dainiak N, Worthington M, Riordan MA, Kreczko S, Goldman L. 3′-Azido-3′-deoxythymidine (AZT) inhibits proliferation in vitro of human haematopoietic progenitor cells. Br J Haematol. 1988;69:299–304. doi: 10.1111/j.1365-2141.1988.tb02366.x. [DOI] [PubMed] [Google Scholar]
- 88.Andre-Schmutz I, Dal-Cortivo L, Six E, et al. Genotoxic signature in cord blood cells of newborns exposed in utero to a zidovudine-based antiretroviral combination. J Infect Dis. 2013;208:235–243. doi: 10.1093/infdis/jit149. [DOI] [PubMed] [Google Scholar]
- 89.Olivero OA, Torres LR, Gorjifard S, et al. Perinatal exposure of Patas monkeys to antiretroviral nucleoside reverse-transcriptase inhibitors induces genotoxicity persistent for up to 3 years of age. J Infect Dis. 2013;208:244–248. doi: 10.1093/infdis/jit146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schramm DB, Kuhn L, Gray GE, Tiemessen CT. In vivo effects of HIV-1 exposure in the presence and absence of single-dose nevirapine on cellular plasma activation markers of infants born to HIV-1-seropositive mothers. J Acquir Immune Defic Syndr. 2006;42:545–553. doi: 10.1097/01.qai.0000225009.30698.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kuhn L, Meddows-Taylor S, Gray G, et al. Reduced HIV-stimulated T helper cell reactivity in cord blood with short-course antiretroviral treatment for prevention of maternal–infant transmission. Clin Exp Immunol. 2001;123:443–450. doi: 10.1046/j.1365-2249.2001.01460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Olivero OA. Relevance of experimental models for investigation of genotoxicity induced by antiretroviral therapy during human pregnancy. Mutat Res. 2008;658:184–190. doi: 10.1016/j.mrrev.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jones CE, Naidoo S, De Beer C, Esser M, Kampmann B, Hesseling AC. Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants. JAMA. 2011;305:576–584. doi: 10.1001/jama.2011.100. [DOI] [PubMed] [Google Scholar]
- 94.Reikie BA, Naidoo S, Ruck CE, et al. Antibody responses to vaccination among South African HIV-exposed and unexposed uninfected infants during the first 2 years of life. Clin Vaccine Immunol. 2013;20:33–38. doi: 10.1128/CVI.00557-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Abramczuk BM, Mazzola TN, Moreno YM, et al. Impaired humoral response to vaccines among HIV-exposed uninfected infants. Clin Vaccine Immunol. 2011;18:1406–1409. doi: 10.1128/CVI.05065-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sanz-Ramos M, Manno D, Kapambwe M, et al. Reduced poliovirus vaccine neutralising-antibody titres in infants with maternal HIV-exposure. Vaccine. 2013;31:2042–2049. doi: 10.1016/j.vaccine.2013.02.044. [DOI] [PubMed] [Google Scholar]
- 97.Ota MO, O'Donovan D, Marchant A, et al. HIV-negative infants born to HIV-1 but not HIV-2-positive mothers fail to develop a bacillus Calmette–Guérin scar. AIDS. 1999;13:996–998. doi: 10.1097/00002030-199905280-00020. [DOI] [PubMed] [Google Scholar]
- 98.Miles DJ, Gadama L, Gumbi A, Nyalo F, Makanani B, Heyderman RS. Human immunodeficiency virus (HIV) infection during pregnancy induces CD4 T-cell differentiation and modulates responses to bacille Calmette–Guérin (BCG) vaccine in HIV-uninfected infants. Immunology. 2010;129:446–454. doi: 10.1111/j.1365-2567.2009.03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mazzola TN, da Silva MT, Abramczuk BM, et al. Impaired bacillus Calmette–Guérin cellular immune response in HIV-exposed, uninfected infants. AIDS. 2011;25:2079–2087. doi: 10.1097/QAD.0b013e32834bba0a. [DOI] [PubMed] [Google Scholar]
- 100.Van Rie A, Madhi SA, Heera JR, et al. Gamma interferon production in response to Mycobacterium bovis BCG and Mycobacterium tuberculosis antigens in infants born to human immunodeficiency virus-infected mothers. Clin Vaccine Immunol. 2006;13:246–252. doi: 10.1128/CVI.13.2.246-252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Elliott AM, Mawa PA, Webb EL, et al. Effects of maternal and infant co-infections, and of maternal immunisation, on the infant response to BCG and tetanus immunisation. Vaccine. 2010;29:247–255. doi: 10.1016/j.vaccine.2010.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kagina BM, Abel B, Scriba TJ, et al. Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus Calmette–Guérin vaccination of newborns. Am J Respir Crit Care Med. 2010;182:1073–1079. doi: 10.1164/rccm.201003-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kaye S, Miles D, Antoine P, et al. Virological and immunological correlates of mother-to-child transmission of cytomegalovirus in The Gambia. J Infect Dis. 2008;197:1307–1314. doi: 10.1086/586715. [DOI] [PubMed] [Google Scholar]
- 104.Slyker JA, Lohman-Payne BL, Rowland-Jones SL, et al. The detection of cytomegalovirus DNA in maternal plasma is associated with mortality in HIV-1-infected women and their infants. AIDS. 2009;23:117–124. doi: 10.1097/QAD.0b013e32831c8abd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Duryea EL, Sanchez PJ, Sheffield JS, et al. Maternal human immunodeficiency virus infection and congenital transmission of cytomegalovirus. Pediatr Infect Dis J. 2010;29:915–918. doi: 10.1097/INF.0b013e3181e0ce05. [DOI] [PubMed] [Google Scholar]
- 106.Bates M, Monze M, Bima H, Kapambwe M, Kasolo FC, Gompels UA. High human cytomegalovirus loads and diverse linked variable genotypes in both HIV-1 infected and exposed, but uninfected, children in Africa. Virology. 2008;382:28–36. doi: 10.1016/j.virol.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 107.Gompels UA, Larke N, Sanz-Ramos M, et al. Human cytomegalovirus infant infection adversely affects growth and development in maternally HIV-exposed and unexposed infants in Zambia. Clin Infect Dis. 2012;54:434–442. doi: 10.1093/cid/cir837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Miles DJ, Sande M, Kaye S, et al. CD4(+) T cell responses to cytomegalovirus in early life: a prospective birth cohort study. J Infect Dis. 2008;197:658–662. doi: 10.1086/527418. [DOI] [PubMed] [Google Scholar]
- 109.Slyker JA, Rowland-Jones SL, Dong T, et al. Acute cytomegalovirus infection is associated with increased frequencies of activated and apoptosis-vulnerable T cells in HIV-1-infected infants. J Virol. 2012;86:11373–11379. doi: 10.1128/JVI.00790-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dauby N, Goetghebuer T, Kollmann TR, Levy J, Marchant A. Uninfected but not unaffected: chronic maternal infections during pregnancy, fetal immunity, and susceptibility to postnatal infections. Lancet Infect Dis. 2012;12:330–340. doi: 10.1016/S1473-3099(11)70341-3. [DOI] [PubMed] [Google Scholar]
- 111.Broen K, Brustoski K, Engelmann I, Luty AJ. Placental Plasmodium falciparum infection: causes and consequences of in utero sensitization to parasite antigens. Mol Biochem Parasitol. 2007;151:1–8. doi: 10.1016/j.molbiopara.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 112.Metenou S, Suguitan AL, Jr, Long C, Leke RG, Taylor DW. Fetal immune responses to Plasmodium falciparum antigens in a malaria-endemic region of Cameroon. J Immunol. 2007;178:2770–2777. doi: 10.4049/jimmunol.178.5.2770. [DOI] [PubMed] [Google Scholar]
- 113.King CL, Malhotra I, Wamachi A, et al. Acquired immune responses to Plasmodium falciparum merozoite surface protein-1 in the human fetus. J Immunol. 2002;168:356–364. doi: 10.4049/jimmunol.168.1.356. [DOI] [PubMed] [Google Scholar]
- 114.Dent A, Malhotra I, Mungai P, et al. Prenatal malaria immune experience affects acquisition of Plasmodium falciparum merozoite surface protein-1 invasion inhibitory antibodies during infancy. J Immunol. 2006;177:7139–7145. doi: 10.4049/jimmunol.177.10.7139. [DOI] [PubMed] [Google Scholar]
- 115.Malhotra I, Dent A, Mungai P, et al. Can prenatal malaria exposure produce an immune tolerant phenotype? A prospective birth cohort study in Kenya. PLOS Med. 2009;6:e1000116. doi: 10.1371/journal.pmed.1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Steketee RW, Wirima JJ, Bloland PB, et al. Impairment of a pregnant woman's acquired ability to limit Plasmodium falciparum by infection with human immunodeficiency virus type-1. Am J Trop Med Hyg. 1996;55:42–49. doi: 10.4269/ajtmh.1996.55.42. [DOI] [PubMed] [Google Scholar]
- 117.van Riet E, Hartgers FC, Yazdanbakhsh M. Chronic helminth infections induce immunomodulation: consequences and mechanisms. Immunobiology. 2007;212:475–490. doi: 10.1016/j.imbio.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 118.Malhotra I, Mungai P, Wamachi A, et al. Helminth-and bacillus Calmette–Guérin-induced immunity in children sensitized in utero to filariasis and schistosomiasis. J Immunol. 1999;162:6843–6848. [PubMed] [Google Scholar]
- 119.Malhotra I, Mungai PL, Wamachi AN, et al. Prenatal T cell immunity to Wuchereria bancrofti and its effect on filarial immunity and infection susceptibility during childhood. J Infect Dis. 2006;193:1005–1013. doi: 10.1086/500472. [DOI] [PubMed] [Google Scholar]
- 120.Cuna WR, Choque AG, Passera R, Rodriguez C. Pro-inflammatory cytokine production in chagasic mothers and their uninfected newborns. J Parasitol. 2009;95:891–894. doi: 10.1645/GE-1927.1. [DOI] [PubMed] [Google Scholar]
- 121.Vekemans J, Truyens C, Torrico F, et al. Maternal Trypanosoma cruzi infection upregulates capacity of uninfected neonate cells to produce pro-and anti-inflammatory cytokines. Infect Immun. 2000;68:5430–5434. doi: 10.1128/iai.68.9.5430-5434.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Truyens C, Mjihdi K, Lambot MA, Rivera MT, Noel JC, Carlier Y. Effects of acute and chronic Trypanosoma cruzi infection in pregnant mice. Rev Soc Bras Med Trop. 2005;38(Suppl. 2):68–72. [PubMed] [Google Scholar]
- 123.Koumbi L, Bertoletti A, Anastasiadou V, et al. Hepatitis B-specific T helper cell responses in uninfected infants born to HBsAg+/HBeAg− mothers. Cell Mol Immunol. 2010;7:454–458. doi: 10.1038/cmi.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rahman MJ, Degano IR, Singh M, Fernandez C. Influence of maternal gestational treatment with mycobacterial antigens on postnatal immunity in an experimental murine model. PLOS ONE. 2010;5:e9699. doi: 10.1371/journal.pone.0009699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Glennie SJ, Williams NA, Heyderman RS. Mucosal immunity in resource-limited setting: is the battle ground different? Trends Microbiol. 18:487–493. doi: 10.1016/j.tim.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 126.Borges-Almeida E, Milanez HM, Vilela MM, et al. The impact of maternal HIV infection on cord blood lymphocyte subsets and cytokine profile in exposed non-infected newborns. BMC Infect Dis. 2011;11:38. doi: 10.1186/1471-2334-11-38. doi: 10.1186/1471-2334-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mwiru R, Spiegelman D, Hertzmark E, et al. Nutritional predictors of acute respiratory infections among children born to HIV-infected women in Tanzania. Journal of tropical pediatrics. 2013;59:203–208. doi: 10.1093/tropej/fmt004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nielsen SD, Jeppesen DL, Kolte L, et al. Impaired progenitor cell function in HIV-negative infants of HIV-positive mothers results in decreased thymic output and low CD4 counts. Blood. 2001;98:398–404. doi: 10.1182/blood.v98.2.398. [DOI] [PubMed] [Google Scholar]
- 129.Miyamoto M, Pessoa SD, Ono E, et al. Low CD4+ T-cell levels and B-cell apoptosis in vertically HIV-exposed noninfected children and adolescents. Journal of tropical pediatrics. 2010;56:427–432. doi: 10.1093/tropej/fmq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Blanche S, Tardieu M, Benhammou V, Warszawski J, Rustin P. Mitochondrial dysfunction following perinatal exposure to nucleoside analogues. Aids. 2006;20:1685–1690. doi: 10.1097/01.aids.0000242814.42344.77. [DOI] [PubMed] [Google Scholar]
- 131.Aldrovandi GM, Chu C, Shearer WT, et al. Antiretroviral exposure and lymphocyte mtDNA content among uninfected infants of HIV-1-infected women. Pediatrics. 2009;124:e1189–1197. doi: 10.1542/peds.2008-2771. [DOI] [PMC free article] [PubMed] [Google Scholar]


