Abstract
Chronic bone infection is associated with bone resorption. From animal studies, CD3/CD28-activated T cells are known to enhance osteoclastogenesis and bone resorption. Because CD28 is expressed constitutively on T cells and its expression is down-regulated by chronic exposure to the inflammatory environment, we characterized co-stimulatory molecule expression on T cells from chronically infected patients. We used cytofluorometric techniques to phenotypically characterize T cells, its co-stimulatory molecules and perforin secretion from infected and non-infected human bones. Chronic bone infection was defined as infection lasting for more than a month. We show a higher T cell activation [human leucocyte antigen D-related (HLA-DR+)] in infected compared to non-infected bones: median being 16 versus 7%, P = 0·009 for CD4 T cells, and 33 versus 15%, P = 0·038 for CD8 T cells, respectively. However, T cell proliferation (Ki67+) was lower for CD8 T cells in infected bones: 26 versus 34%, P = 0·045. In contrast, we detected no difference in apoptosis and regulatory T cells. In infected bone, we found higher CD28-negative CD4+ T cells compared to non-infected bone: 20 versus 8%, respectively (P = 0·005); this T cell subset had higher CD11b expression and perforin secretion. Chronically infected human bones are characterized by an increase of CD28-negative CD4+ T cells, indicating long-term activated cells with cytotoxic ability. Therefore, this alteration of co-stimulatory molecules may modify interactions with osteoclasts and impact bone resorption.
Keywords: apoptosis, bacterial, cell proliferation, regulatory T cells, T cells
Introduction
Increasing numbers of patients currently benefit from joint prosthesis, due to ageing, trauma and improved surgical techniques [1]. However, bone infection (BI) may complicate orthopaedic surgery, even when there are no prosthetic implants [2]. Infection of total hip arthroplasty or total knee arthroplasty occurs with an incidence of 1·5–2·5% for primary interventions, but higher rates (2–20%) have been reported after revision procedures [3].
BI, or osteomyelitis, is an inflammatory process accompanied by bone destruction. While in industrial countries the infecting microorganisms are usually Staphylococcus species, species such as Mycobacterium tuberculosis and Salmonella typhi are more common in other parts of the world [4]. It is noteworthy that bone resorption is associated with infection, regardless of the causative microbiological agent [5]. The role of immune activation at the site of infection is unclear, although it is thought to participate in bone resorption, as suggested by experimental and human studies, particularly in the case of periodontitis [6].
Osteoimmunology is an interdisciplinary research field focused on the molecular understanding of the interplay between the immune and skeletal systems. T lymphocytes and their products have been recognized as key regulators of osteoclast (OC) and osteoblast (OB) formation, lifespan and activity [7]. Interestingly, OC shows some characteristics of antigen-presenting cells (APC) in experimental models of inflammatory diseases [8], which imply an interaction of OC with T cells.
Accordingly, it has been demonstrated that activated T cells exert their effect via membrane-bound and secretory receptor activator of nuclear factor kappa B ligand (RANKL) [9]. In one in-vitro study it was found that CD4+ T cells can support osteoclastogenesis in the presence of macrophage colony-stimulating factor (M-CSF) alone, and the addition of RANKL led to increased bone resorption [10]. Conversely, CD8+ T cells did not support OC differentiation, but rather inhibited OC differentiation/activation induced by RANKL [11].
Of note, experimental models used CD3/CD28 stimulation to study lymphocyte–OC interactions [7,9,10]. However, co-stimulatory molecules, CD28, CD80, CD86, cytotoxic T-lymphocyte antigen-4 (CTLA-4)/CD152, CD40 and CD40L showed major alterations of expression in bacterial infection, which may be responsible for modulating cellular interactions [12]. Interestingly, analysis of synovium of patients with chronic septic arthritis showed dramatic T cell proliferation. This dramatic proliferation of T cells suggests the occurrence of some major cellular interactions [13].
Among other key immunological features studied in osteoimmunology is apoptosis of CD4 T cells, for which researchers have suggested an inverse CD4/CD8 ratio observed in patients with aseptic loosening of prosthesis [14]. Apoptosis might be related to regulatory T cells (Tregs), which are specialized to limit the magnitude of T cell effector responses [15].
To the best of our knowledge, all these T cell features have never been reported previously in human BI. Thus, our aim was to characterize the T cell phenotype infiltrating bacterial infected bones, focusing on the expression of co-stimulatory molecules.
Materials and methods
Patients
Inclusion criteria for our study subjects included ‘non-infected’ patients who presented with a definitive diagnosis of arthrosis and required prosthetic surgery. The ‘infected’ patients included those who presented with a diagnosis of BI, proved through positive bacteriological samples performed before and/or during surgery (septic loosening of the prosthetic implant). We considered the bone tissues as infected regardless of the bacterial species causing infection; i.e. in general we considered only bacterial infected bone tissues for our study. Exclusion criteria included primary or acquired immunodeficiency, autoimmune disease, cancer, cirrhosis and patients benefiting from immunosuppressive drugs.
This study was conducted in accordance with the ethical committee of our institution, for which consent was obtained from the patients.
Cortical bone tissue samples, both non-infected and infected, were from the compact bone region located at the periphery of diaphysis and just below the spongy bone of the femur. Because of the limited number of cells in infected bone tissues, not all tests could be performed in all patients; the number of patients included in a particular set of data is specified in the text.
Concerning blood experimentation, 12 healthy controls (20–25 years), four males and eight females, were from the hospital staff. Blood was collected in a tube containing acid citrate dextrose solution.
Cell preparation
Bone tissue samples were collected during standard operation procedures from the orthopaedic surgery room of Hôpital l'Archet, Nice, France. Immediately after surgery, the cortical part was removed and brought to the laboratory for analysis. The cortical bone tissues were cut further into small pieces and spongeous bone was removed, in order to retrieve those cells which were at the interface of bone marrow and bone tissue. They were then vortexed vigorously for 2–3 min with medium [RPMI-1640 + 2% fetal bovine serum (FBS)] to flush out cells in the medium. To avoid debris, the suspension was filtered using a BD Cell Strainer 40 μm nylon mesh (BD Biosciences, San Jose, CA, USA).
After centrifugation, the cell pellet was dissolved in 2 ml of red blood cell (RBC) lysing buffer (Sigma-Aldrich, St Louis, MO, USA). Control of viability and cell count were made in a final concentration of 10 million cells/ml, most of the cells being viable.
To characterize cell phenotypes, we used cytofluorometric techniques with a six-colour labelling pattern [fluorescein isothiocyanate (FITC)/phycoerythein (PE)/peridinin chlorophyll (PerCP) cyanin (Cy) 5·5/PE-Cy7/allophycocyanin (APC)/Pacific blue]. For intracellular labelling of Ki67, forkhead box protein 3 (FoxP3), interferon (IFN)-γ, interleukin (IL)-2 and perforin, cells were first labelled with extracellular antibodies, fixed, permeabilized and then labelled with intracellular antibodies.
Antibodies
The following conjugated anti-human monoclonal antibodies (mAbs) were purchased from BD Pharmingen (San Jose, CA, USA): CD3 PE-Cy7, CD4 FITC, CD4 PerCP-Cy5·5, CD4 APC, CD8 PerCP-Cy5·5, CD11b PE, CD25 PE, CD40 APC, CD45RA FITC, CD62L APC, CD80 FITC, CD86 PE, CTLA-4/CD152 PE, CD154 (CD40L) APC, human leucocyte antigen D-related (HLA-DR) APC, HLA-DR PE-Cy7, Ki67 PE, annexin V FITC, IFN-γ PE, IL-2 PE and propidium iodide. CD7 FITC, CD28 FITC, CD45 Pacific blue, FoxP3 APC and perforin PE were purchased from eBioscience, Inc. (San Diego, CA, USA). CD197/CCR7 was purchased from R&D Systems, Inc. (Minneapolis, MN, USA).
Multiparameter flow cytometry
Flow cytometry was performed using a fluorescence-activated cell sorter (FACS)-CANTO II flow cytometer (BD Biosciences). Instrument set-up, calibration and interpretation were performed using both blood and bone samples. At least 5000 lymphocytes were recorded each time. Data analysis was performed with FACS diva software.
Statistical analysis
In order to characterize immunological data in patients with BI, we performed the statistical analysis in normal blood and non-infected and infected bone tissue samples. Statistical analysis used the non-parametric Mann–Whitney U-test, as appropriate. A P-value of <0·05 was considered significant. Only significant values are indicated in the figures. The analysis was performed on Statview®F-4·5 software.
Results
The infected bone tissue samples were obtained from patients presenting with bacterial infection, as listed in Table 1. Most of the infected tissues were obtained during the chronic phase of the bacterial infection, i.e. lasting for more than 1 month [16].
Table 1.
Demographic data and bacterial species found in the patients presenting with bone infection
| Patients | Sex | Age (years) | Bacterial infection | Duration of the disease (months) |
|---|---|---|---|---|
| 1 | M | 69 | Prevotella oralis, Enterococcus avium | 18 |
| 2 | M | 71 | Staphylococcus aureus | 12 |
| 3 | M | 50 | Escherichia coli, Enterococcus avium, Klebsiella pneumonia | 21 |
| 4 | M | 52 | Staphylococcus epidermidis | 14 |
| 5 | M | 57 | Acinetobacter baumannii | 16 |
| 6 | M | 78 | Staphylococcus aureus | 6 |
| 7 | F | 59 | Staphylococcus coagulase | 24 |
| 8 | M | 39 | Propionibacterium acnes, Micrococcus species | 14 |
| 9 | M | 79 | Bacillus spp., Propionibacterium acnes | 6 |
| 10 | M | 47 | Staphylococcus aureus | 1 |
| 11 | M | 70 | Enterobacter aerogenes | 8 |
| 12 | M | 74 | Staphylococcus aureus | 18 |
| 13 | M | 40 | Staphylococcus aureus, Arcanobacterium haemolyticum | 2 |
All included patients benefited from bacterial investigations during surgical procedure. Herein are reported the patients' age, sex, duration of the infection and bacterial species isolated in the infected bone tissues. Among 13 infected bone tissues analysed, five of 13 patients had polymicrobial bacterial species infection. M: male; F: female.
On average, approximately 20 million mononuclear cells were obtained from the non-infected tissues. In infected patients, the number of cells depended mainly upon the size of bone tissues available, which varied from 2 to 10 million mononuclear cells from patient to patient. As there are very limited data in humans describing the lymphocyte phenotype in bone tissues, we first compared 12 blood controls and 25 non-infected bone tissues, in order to illustrate the immunological compartmentalization. Thereafter, we aimed to determine the particularities of infected bones in term of immune cell phenotypes compared to non-infected bones.
Comparison of blood and non-infected bones indicated immune compartmentalization
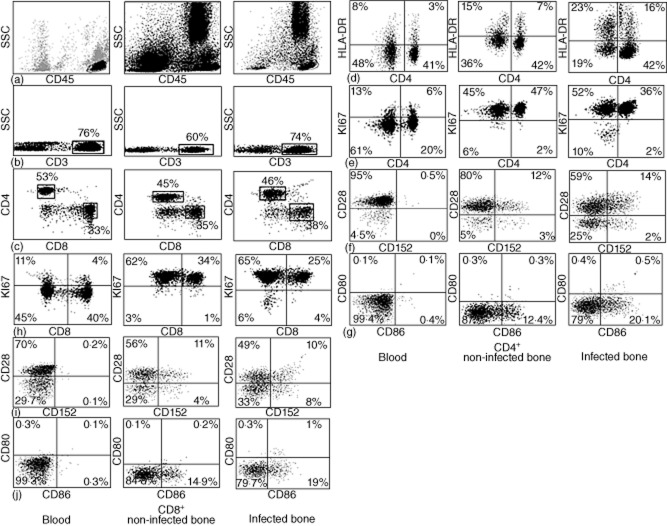
First, we analysed the different T cell subsets from blood and non-infected bone tissues. Although the cytometric cellular disposition in blood and bone tissue is quite different, we identified successfully the double-positive CD45+CD3+ lymphocytes, and thereafter co-stimulatory molecules on CD4 and CD8 T cell subsets in both samples. Figure 1 shows cellular distribution using cytofluorometric techniques. While comparing blood and non-infected bone, we observed that the T cell phenotype is significantly different, with an increase of activated and proliferating T cells in the bone. Regarding co-stimulatory molecules, T cell expression in bone tissue increased for CD152, CD80 and CD86; all values are given in Table 2. In contrast, no difference was observed in Treg (CD4+CD25+FoxP3+) cells.
Figure 1.
Cytometric data of T cell subsets and the co-stimulatory markers on blood, non-infected bone and infected bone. First the living population was gated on total cells analysed. Then, on living CD45+ cells, CD3 cells were gated and finally CD4+ and CD8+ cells were gated on CD3+ cells. (a) Morphology of cell population showing lymphocytes, monocytes and granulocytes. (b) CD3+ T cell population. (c) CD4 and CD8 T cell population. (d) Human leucocyte antigen D-related (HLA-DR) expression. In this figure, specifically, we considered that CD4-negative cells to be CD8-positive. (e,h) Ki67 expression. (f,j) CD28 and CD152 co-stimulatory markers. (g,j) CD80 and CD86 co-stimulatory marker.
Table 2.
Median values (%) of the markers expressed by T cell subsets in percentage in normal blood and non-infected and infected bone
| T cell phenotype | Blood | Standard deviation for blood | P-value between blood and non-infected bone | Non-infected bone | Standard deviation for non-infected bone | P-value between non-infected and infected bone | Infected bone | Standard deviation for infected bone |
|---|---|---|---|---|---|---|---|---|
| Total CD4 T cells | 53 (40–71) | 10·93 | 0·066 | 45 (23–67) | 12·51 | 0·988 | 46 (27–74) | 16·51 |
| CD4 T naive cells | 53 (35–74) | 13·11 | 0·001 | 33 (8–70) | 17·42 | 0·164 | 18 (2–63) | 20·54 |
| HLA-DR CD4 T cells | 3 (2–11) | 3 | 0·002 | 7 (3–24) | 5·86 | 0·009 | 16 (5–46) | 13·22 |
| Total CD8 T cells | 33 (21–41) | 7·19 | 0·372 | 35 (18–61) | 10·78 | 0·395 | 38 (21–65) | 16·06 |
| CD8 T naive cells | 74 (62–86) | 7·35 | <0·001 | 43 (30–79) | 14·56 | 0·790 | 47 (13–66) | 15·14 |
| HLA-DR CD8 T cells | 8 (3–25) | 6·8 | 0·03 | 15 (3–54) | 13·79 | 0·038 | 23 (9–60) | 17·66 |
| Treg cells | 4 (1–9) | 2·63 | 0·364 | 4 (0–8) | 1·76 | 0·880 | 4 (1–8) | 2·3 |
| PCD/proliferation | ||||||||
| Annexin-V CD4+ T cells | 0 (0–1) | 0 | 0·191 | 0 (0–1) | 0·11 | 0·076 | 0·2 (0–1) | 0·35 |
| Annexin-V CD8+ T cells | 0 (0–1) | 0·67 | 0·827 | 0 (0–1) | 0·88 | 0·071 | 0·2 (0–1) | 0·21 |
| Ki67+ CD4+ T cells | 6 (0·1–10) | 2·49 | <0·001 | 47 (25–67) | 12·43 | 0·187 | 34 (4–65) | 22·26 |
| Ki67+ CD8+ T cells | 4 (0–8) | 2·53 | <0·001 | 34 (20–53) | 8·45 | 0·045 | 26 (5–46) | 14·93 |
| Co-stimulatory molecules | ||||||||
| CD28+ CD4+ T cells | 96 (73–99) | 8·6 | 0·150 | 92 (61–99) | 9·81 | 0·005 | 70 (18–96) | 26·23 |
| CD28+ CD8+ T cells | 70 (59–81) | 7·08 | 0·305 | 67 (38–85) | 14·93 | 0·241 | 59 (27–78) | 12·63 |
| CD152+ CD4+ T cells | 0·5 (0·1–7) | 2 | <0·001 | 16 (4–32) | 8·03 | 0·642 | 15 (2–99) | 37·69 |
| CD152+ CD8+ T cells | 0·3 (0·1-0·8) | 0·28 | <0·001 | 15 (4–23) | 6·25 | 0·546 | 18 (2–98) | 35·87 |
| CD80+ CD4+ T cells | 0·2 (0–1) | 0·26 | 0·006 | 0·6 (0–6) | 1·58 | 0·824 | 0·8 (0·2–12) | 3·81 |
| CD80+ CD8+ T cells | 0·3 (0–1·3) | 0·45 | 0·660 | 0·4 (0–2·3) | 0·76 | 0·002 | 2 (0·3–14) | 4·33 |
| CD86+ CD4+ T cells | 0·5 (0·3–1·5) | 0·37 | <0·001 | 13 (2–28) | 6·94 | 0·444 | 21 (1–99) | 38·39 |
| CD86+ CD8+ T cells | 0·3 (0·1–4) | 1·24 | <0·001 | 15 (2–32) | 7·95 | 0·421 | 19 (0·3–99) | 38·18 |
| CD40+ CD4+ T cells | 0·10 (0–3·6) | 1·33 | 0·57 | 0·2 (0–5·1) | 4·11 | 0·09 | 1·5 (0–8·7) | 12·25 |
| CD40+ CD8+ T cells | 0·80 (0–16·2) | 10·71 | 0·12 | 0·2 (0–3·2) | 6·31 | 0·009 | 2 (0·1–11·6) | 4·11 |
| CD40L+CD4+ T cells | 0·15 (0–4·5) | 1·54 | 0·90 | 0·1 (0–3·2) | 7·93 | 0·10 | 2 (0–7·5) | 2·39 |
| CD40L+ CD8+ T cells | 1·05 (0–26) | 9·3 | 0·11 | 0·1 (0·03) | 5·95 | 0·03 | 1·5 (0–8·5) | 2·52 |
The median values (range) in percentage of the various markers expressed by the CD4 and CD8 T cell subsets in blood, non-infected bone and infected bone obtained through cytometric analysis are indicated, along with their standard deviation. Statistical comparison between two groups used the non-parametric Mann–Whitney U-test. P value < 0·05 was considered to be significant. HLA-DR: human leucocyte antigen D-related; PCD: programmed cell death; Treg: regulatory T cells.
In physiological immunology, as activation is synonymous with proliferation, we decided to measure proliferation as well as apoptosis, which are subsequent cellular events [17]. We observed a significant difference in the number of proliferating (Ki67+) cells for both CD4 and CD8 T cell subsets in non-infected bone compared to blood (Figs 1,2 and Table 2). In contrast, using annexin V staining no significant apoptosis was observed in peripheral blood mononuclear cells (PBMC) compared to bone tissues. It should be noted that, in parallel with this study, we worked on T cell apoptosis in HIV infection, where the detection of T cell apoptosis was observed using the same kit (data not shown).
Figure 2.
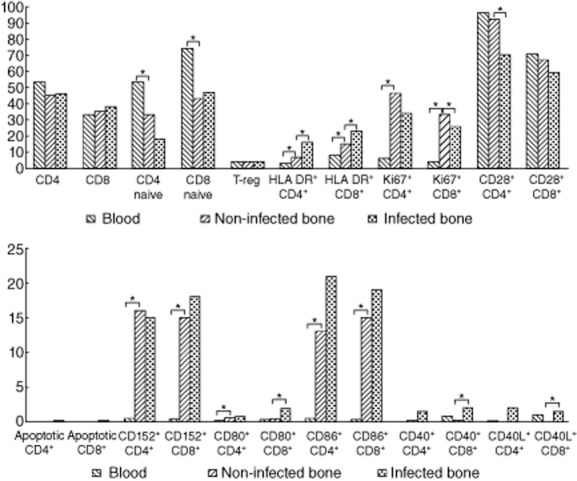
Graphic representation of the median values of all markers studied in blood, non-infected bone and infected bone. The figure is a graphic representation of the medium values of all markers obtained in blood, non-infected bone and infected bone. All significant (<0·005) P-values are marked with *.
Comparison of non-infected and infected bones
All the patients from whom the infected bone tissues were collected were in a state of chronic infection. In most of the cases we studied, the infection was caused by Staphylococcus spp. (Table 1).
For CD4 and CD8 T cell expression we analysed a total of 13 infected bone tissues. The median value of CD4 was 46% (27–74%) and that of CD8 T cells was 38% (21–65%), thus indicating that there was no significant alteration of the CD4/CD8 ratio. However, there was a trend towards fewer naive CD4 T cells in infected compared to non-infected bone (Table 2). For Treg cell expression we analysed 10 infected samples, which were 4% (1–8%) of the CD4 T cell population, and thus similar to non-infected bones. Activation marker HLA-DR was increased on T cell subsets in 13 infected bone tissues, the respective values being 16% (5–46%) for CD4 (P = 0·009) and 23% (9–60%) for CD8 T cells (P = 0·038). For the proliferation state of CD4 and CD8 T cells, we analysed 10 infected bone tissues. We observed a reduced proliferation in infected samples compared to non-infected bones, mainly for the CD8 T cell subset: 26% (5–46%) versus 44% (20–53%) (P = 0·045, see Table 2). Once again, we were unable to observe any significant T cell apoptosis in infected samples.
For the expression of co-stimulatory markers, we analysed 12 infected bone tissue samples. As shown in Fig. 1, we observed an increase of CD28−CD4 T cells in infected tissue, compared to non-infected bone (P = 0·005, see Table 2). Among other co-stimulatory molecules, CD40, CD40L and CD80 on CD8 T cells were modified in infected compared to non-infected bones (P = 0·002). The details of all the markers studied are given in Fig. 2 and Table 2.
Characterization of CD28−CD4+ and CD8+ T cells
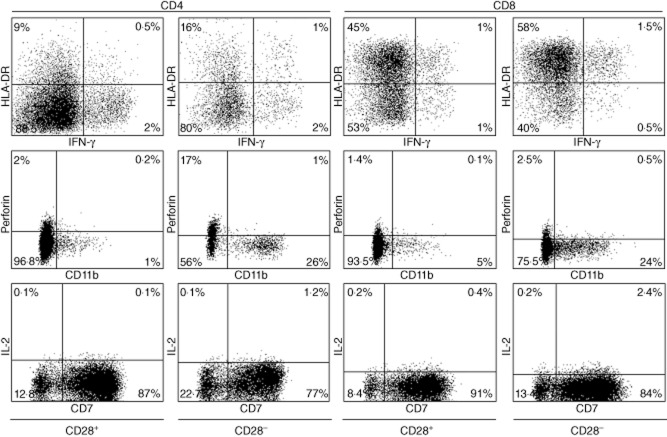
The observation of increased CD4 T cells negative for the CD28 molecule in infected bone tissue was an unexpected finding. Most previously published data concerning CD8+CD28− T cells have suggested a cytotoxic phenotype [18]. Therefore, we used infected bone tissues for labelling with HLA-DR, IFN-γ, perforin, CD11b, IL-2 and CD7 markers to characterize CD28+ and CD28− T cell subsets (Fig. 3). Due to a very small percentage of CD28− T cells in non-infected bone tissues, we used only infected bone tissues. As shown in Fig. 4, we observed a great difference in terms of CD11b expression and perforin secretion for the CD28− population compared to its positive counterpart (Table 3).
Figure 3.
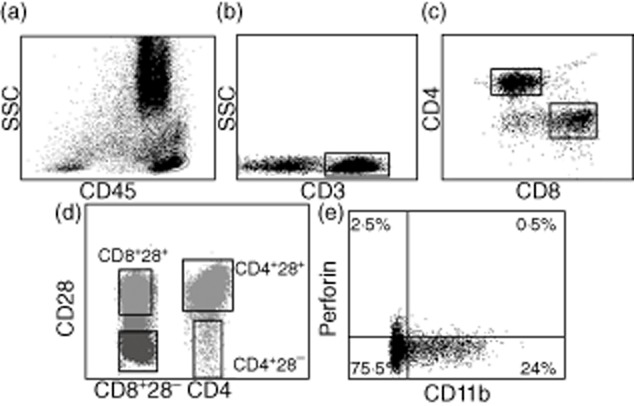
Stepwise analysis and gating of the population for the expression of CD11b and perforin secretion. CD11b expression and perforin secretion was observed on CD28− and CD28+ population only for CD4 and CD8-specific T cells. The figure represents the stepwise analysis of cytometric data for the expression of CD11b marker and perforin secretion on CD28− and CD28+ population of CD4 and CD8 T cell subsets. (a) First, the living population was gated on total cells analysed. (b) Then, on living CD45+ cells, CD3 cells were gated. (c) CD4+ and CD8+ T cells were gated on CD3+ cells. (d) By using the CD28 marker, the CD28− and CD28+ population was gated on the CD4 and CD8+ population. As the gating of the population was performed only on CD4 and CD8 T cells,the CD4− population was considered to be CD28+ T cells. (e) Finally, CD11b expression was observed on different specific populations of CD28 expression for CD4 and CD8 T cells separately.
Figure 4.
Characterization of CD28− T cells in patients with bone infection. CD28− or CD28+ cells were gated on both CD4 and CD8 T cells. For these subsets, we studied human leucocyte antigen D-related (HLA-DR), CD11b and CD7 membrane expression, and interferon (IFN)-γ, interleukin (IL)-2 and perforin secretions by intracellular staining. Over-expression of CD11b and perforin secretion and the absence of IL-2 secretion indicated a cytotoxic phenotype; data are provided in Table 3.
Table 3.
Median values (%) of the markers expressed by CD28-negative T cells and their counterparts CD28-positive cells
| Markers | CD4+CD28+ | CD4+CD28− | CD8+CD28+ | CD8+CD28− |
|---|---|---|---|---|
| HLA-DR | 10 (7–14) | 17 (13–24) | 46 (40–54) | 58 (25–74) |
| IFN-γ | 0·2 (0·1–3) | 1·2 (0–32) | 1·8 (1–3) | 2·1 (1–3) |
| Perforin | 1·2 (0·1–4) | 18 (1–36) | 1·5 (1–5) | 2·8 (1–12) |
| CD11b | 4 (3–8) | 27 (4–51) | 6 (4–9) | 25 (20–70) |
| IL-2 | 0·1 (0–0·2) | 0·3 (0–1·5) | 0·7 (0·4–1) | 2·6 (1–3) |
| CD7 | 87 (82–88) | 77 (72–89) | 91 (90–92) | 86 (84–91) |
The table shows the median values (range) in percentage of different markers expressed by negative or positive CD28 T cells from five infected patients. HLA-DR: human leucocyte antigen D-related; IL: interleukin; IFN: interferon.
Discussion
Very few studies characterizing the immune response in patients with bone infections have been published to date, as most of the literature is related to inflammatory diseases such as rheumatoid arthritis (RA) and/or osteoporosis [19,20]. In aseptic loosening after prosthesis replacement, an alteration of the CD4/CD8 ratio towards CD8 predominance has been reported [14]. In our present study, we thus investigated in detail the T cell phenotype that infiltrates tissue during human bacterial BI.
We first confirmed the immune compartmentalization comparing blood and non-infected bone, which showed higher differences in term of cellular phenotypes, regardless of subset studied. These initial results clearly indicated that osteoimmunology cannot be approached through blood samples and peripheral lymphocyte study, which necessitated the requirement of bone samples.
More importantly, we found that infected bones are characterized by higher T cell activation but fewer proliferations compared to non-infected bones, regardless of the T cell subset considered. Accordingly, we observed fewer down-regulated naive T cells and CD62L expression. Also, we found nearly undetectable level of apoptosis, while the percentage of Treg cells remained low.
Our results were obtained from well-characterized patients presenting with chronic BI only, from whom cellular extraction from bone tissue was based on microdissection, followed by robust reproducible cytofluorometric experiments using six concomitant markers to fully distinguish cellular phenotype.
It has been already shown that in mouse, bone marrow is highly enriched for the activated/memory phenotype [21]. Also, the population of naive CD4 T cells in bone marrow was 44%, which decreased after having microbial antigen exposure with subsequent increase of memory phenotype. These observations are obvious in accordance with immune physiology: T cells at inflammatory sites undergo an activation process, during which they lose the adhesion protein CD62L, facilitating the cell transmigration process [22]. In a mouse model of alveolar bone resorption induced by Porphyromonas gingivalis, only CD4 T cells appeared to be involved in the osteoclastogenic process [23]. In-vitro osteoclastogenesis by activated T cells through RANKL has also been shown using murine CD4 T cells or human PBMC-derived T cells [7,9,10]. In implant-associated post-traumatic osteomyelitis it has been shown that T cells down-regulate the expression of CD62L when infiltrating the inflamed site [18]. Thus, taken altogether, our results and others indicate T cell activation and differentiation in infected bone tissue.
We have also shown herein the association of a high level of T cell activation with less proliferation in human BI. Previous work on synovium in septic arthritis in humans have shown an increase in angiogenesis, but did not specifically study T cell fate [13]. Conversely, it has been shown that certain secretory products or toxins from Pseudomonas aeruginosa inhibited T cell proliferation through APC alteration [24]. Given that our patients presented with different pathogens, our results suggest that decreasing T cell proliferation may be a common response to bacterial challenge in human BI.
Decreased T cell proliferation in infected bone tissue might be the result of tissue infiltration by Tregs. One report indicated a high frequency of Treg cells in blood during human chronic osteomyelitis (2·06%) in comparison to normal subjects (1·43%), but this difference was quite low [25]. Accordingly, an increase of Treg cells in mice has been shown, mainly in the late stage of periodontitis [26]. However, in our study we did not observe such changes between non-infected and infected bones. Tregs are believed to induce anergy and ultimately lead to T cell apoptosis, which may be phagocytized rapidly, as seen in physiological conditions [27]. In our study, the absence of Tregs was well in accordance with the absence of cellular apoptotic features in infected tissue.
The CD28 molecule on T cells interacts with B7 molecules on APC for optimal expansion of the T cell response [28,29]. CD28 is expressed constitutively on T cells, being largely increased early in cellular activation, while CTLA-4 is expressed on the cell surface only after activation [30,31]. Our results indicate that infected bone exhibited a decrease in CD28 expression mainly on CD4 T cells compared to healthy bones, while CTLA-4 expression remained unchanged. The absence of CD28 expression is commonly observed on the CD8 T cell subset with advance in age [32]. Also, increase of CD28−CD8+ T cells has been reported in chronic inflammation, in both experimental and human diseases. As an example, CD28−CD4+ T cells have already been described in HIV infection as well as in Epstein–Barr virus infection [33,34]. Also, in patients with RA, the expansion of CD4+CD28− T cells has been described to be associated with cytomegalovirus infection [35,36]. To the best of our knowledge, this is the first study describing the increase of CD28−CD4+ T cells in human BI. Accordingly, these CD28− cells were shown to have a high expression of CD11b and synthesize higher amounts of perforin than their CD28+ counterparts, indicating cytotoxic activity.
We also observed an up-regulation of the CD40/CD40L pathway on both T cell subsets, suggesting a long process of T cell activation. CD40–CD40L interaction is required for both a prolonged T cell response against pathogens and antibody production, as shown in mouse models challenged with different kinds of pathogens [37]. To our knowledge, very little information in humans is available regarding this pathway and specifically no information in BI [38].
Our study has some limitations. First, the availability of small numbers of cells limited our study. We did not study some other important markers related to bone infection such as RANKL, etc. as the data on these markers have already been published [7,9,10]. Secondly, the bacteria involved in our cases of BIs were not always of the same genus, possibly leading to varying immune alterations. Thirdly, our results are mainly a phenotypic characterization of lymphocytes, without going through the kinetics of cellular and molecular interactions. Lastly, the donors of normal blood were quite young in comparison to the patients from whom the bone tissue samples were obtained.
In conclusion, bone tissue in humans appears to be a site of T cell activation and proliferation, the latter being decreased in chronic bacterial infections. Bone infections are associated with a high percentage of CD28− CD4 T cells exhibiting a cytotoxic phenotype as well as an up-regulated CD40/CD40L pathway on T cells. As activated T cells are already known to express RANKL, which is a crucial factor for osteoclastogenesis, our results suggest a creative line of work on the mechanisms of bone resorption in human BI. Moreover, we assume that biotherapies targeting co-stimulatory molecules might have significant but variable effects on bone resorption, depending on their membrane expression.
Disclosures
All authors report no conflicts of interest. All authors have submitted the ICMJE form for disclosure of potential conflicts of interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Acknowledgments
The work was supported by grants from Infectiopole Sud and Recherche Et Developpement en Pathologie Infectieuse et Tropicale (REDPIT), France.
References
- 1.Nasser S. The incidence of sepsis after total hip replacement arthroplasty. Semin Arthroplasty. 1994;5:153–159. [PubMed] [Google Scholar]
- 2.Lew DP, Waldvogel FA. Osteomyelitis. N Engl J Med. 1997;363:999–1007. doi: 10.1056/NEJM199704033361406. [DOI] [PubMed] [Google Scholar]
- 3.Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004;364:369–379. doi: 10.1016/S0140-6736(04)16727-5. [DOI] [PubMed] [Google Scholar]
- 4.Shirtliff ME, Cripps MW, Mader JT. 1999. Retrospective review of 728 patients with long bone osteomyelitis. American Society for Microbiology 99th General Meeting, Chicago.c410.
- 5.Potempa J, Banbula A, Travis J. Role of bacterial proteinases in matrix destruction and modulation of host responses. Periodontology. 2000;24:153–192. doi: 10.1034/j.1600-0757.2000.2240108.x. [DOI] [PubMed] [Google Scholar]
- 6.Graves DT, Cochran D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J Periodontol. 2003;74:391–401. doi: 10.1902/jop.2003.74.3.391. [DOI] [PubMed] [Google Scholar]
- 7.Horwood NJ, Kartsogiannis V, Quinn JM, Romas E, Martin TJ, Gillespie MT. Activated T lymphocytes support osteoclast formation in vitro. Biochem Biophys Res Commun. 1999;265:144–150. doi: 10.1006/bbrc.1999.1623. [DOI] [PubMed] [Google Scholar]
- 8.Li H, Hong S, Qian J, Zheng Y, Yang J, Yi Q. Cross talk between the bone and immune systems: osteoclasts function as antigen-presenting cells and activate CD4+ and CD8+ T cells. Blood. 2010;116:210–217. doi: 10.1182/blood-2009-11-255026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wyzga N, Varghese S, Wikel S, Canalis E, Sylvester FA. Effects of activated T cells on osteoclastogenesis depend on how they are activated. Bone. 2004;35:614–620. doi: 10.1016/j.bone.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 10.Kong YY, Feige U, Sarosi I, et al. Activated T-cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–309. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 11.Buchwald ZS, Kiesel JR, DiPaolo R, Pagadala MS, Aurora R. Osteoclast activated FoxP3+ CD8+ T-cells suppress bone resorption in vitro. PLOS ONE. 2012;7:e38199. doi: 10.1371/journal.pone.0038199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cacere CR, Mendes-Giannini MJS, Fontes CJ, Kono A, Duarte AJS, Benard G. Altered expression of the costimulatory molecules CD80, CD86, CD152, PD-1 and ICOS on T-cells from paracoccidioidomycosis patients: lack of correlation with T-cell hyporesponsiveness. Clin Immunol. 2008;129:341–349. doi: 10.1016/j.clim.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Pessler F, Dai L, Diaz-Torne C, et al. Increased angiogenesis and cellular proliferation as hallmarks of the synovium in chronic septic arthritis. Arthritis Rheum. 2008;59:1137–1146. doi: 10.1002/art.23915. [DOI] [PubMed] [Google Scholar]
- 14.Landgraeber S, von Knoch M, Löer F, et al. Association between apoptosis and CD4+/CD8+ T-lymphocyte ratio in aseptic loosening after total hip replacement. Int J Biol Sci. 2009;5:182–191. doi: 10.7150/ijbs.5.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh N, Yamamoto M, Takami M, et al. CD4+CD25+ regulatory T cells resist a novel form of CD28− and fas-dependent p53-induced T cell apoptosis. J Immunol. 2010;184:94–104. doi: 10.4049/jimmunol.0900753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henderson B, Nair SP. Hard labour: bacterial infection of the skeleton. Trends Microbiol. 2003;11:570–577. doi: 10.1016/j.tim.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Coates PJ, Hales SA, Hall PA. The association between cell proliferation and apoptosis: studies using the cell cycle-associated proteins Ki67 and DNA polymerase alpha. J Pathol. 1996;178:71–77. doi: 10.1002/(SICI)1096-9896(199601)178:1<71::AID-PATH456>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 18.Wagner C, Heck D, Lautenschläger K, et al. T lymphocytes in implant-associated posttraumatic osteomyelitis: identification of cytotoxic T effector cells at the site of infection. Shock. 2006;25:241–246. doi: 10.1097/01.shk.0000192119.68295.14. [DOI] [PubMed] [Google Scholar]
- 19.Morita Y, Yamamura M, Kawashima M, et al. Flow cytometric single-cell analysis of cytokine production by CD4+ T cells in synovial tissue and peripheral blood from patients with rheumatoid arthritis. Arthritis Rheum. 1998;41:1669–1676. doi: 10.1002/1529-0131(199809)41:9<1669::AID-ART19>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 20.Wagner C, Kondella K, Bernschneider T, Heppert V, Wentzensen A, Hansch GM. Post-traumatic osteomyelitis: analysis of inflammatory cells recruited into the site of infection. Shock. 2003;20:503–510. doi: 10.1097/01.shk.0000093542.78705.e3. [DOI] [PubMed] [Google Scholar]
- 21.Price PW, Cerny J. Characterization of CD4+ T cells in mouse bone marrow. I. Increased activated/memory phenotype and altered TCRVβ repertoire. Eur J Immunol. 1999;29:1051–1056. doi: 10.1002/(SICI)1521-4141(199903)29:03<1051::AID-IMMU1051>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 22.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 23.Baker PJ, Evans RT, Dufour L, Johnson E, Roopenian DC. CD4(+) T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect Immun. 1999;67:2804–2809. doi: 10.1128/iai.67.6.2804-2809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skindersoe ME, Zeuthen LH, Brix S, et al. Pseudomonas aeruginosa quorum-sensing signal molecules interfere with dendritic cell-induced T-cell proliferation. FEMS Immunol Med Microbiol. 2009;55:335–345. doi: 10.1111/j.1574-695X.2008.00533.x. [DOI] [PubMed] [Google Scholar]
- 25.Wu Y, Tang Y, Liang X, et al. The role of increased frequency of Treg cells in patients with chronic osteomyelitis. Orthopedics. 2011;34:98. doi: 10.3928/01477447-20101221-16. [DOI] [PubMed] [Google Scholar]
- 26.Garlet GP, Cardoso CR, Mariano FS, et al. Regulatory T cells attenuate experimental periodontitis progression in mice. J Clin Periodontol. 2010;7:591–600. doi: 10.1111/j.1600-051X.2010.01586.x. [DOI] [PubMed] [Google Scholar]
- 27.Belkaid Y, Tarbell K. Regulatory T cells in the control of host–microorganism interactions. Annu Rev Immunol. 2009;27:551–589. doi: 10.1146/annurev.immunol.021908.132723. [DOI] [PubMed] [Google Scholar]
- 28.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 29.Gray Parkin K, Stephan RP, Apilado RG, et al. Expression of CD28 by bone marrow stromal cells and its involvement in B lymphopoiesis. J Immunol. 2002;169:2292–2302. doi: 10.4049/jimmunol.169.5.2292. [DOI] [PubMed] [Google Scholar]
- 30.Wang S, Chen L. Co-signaling molecules of the B7-CD28 family in positive and negative regulation of T lymphocytes responses. Microbes Infect. 2004;6:759–766. doi: 10.1016/j.micinf.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 31.Vallejo AN, Weyand CM, Goronzy JJ. T-cell senescence: a culprit of immune abnormalities in chronic inflammation and persistent infection. Trends Mol Med. 2004;10:119–124. doi: 10.1016/j.molmed.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Filaci G, Fravega M, Negrini S, et al. Nonantigen specific CD8+ T suppressor lymphocytes originate from CD8+CD28− T cells and inhibit both T-cell proliferation and CTL function. Hum Immunol. 2004;65:142–156. doi: 10.1016/j.humimm.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Appay V, Zaunders JJ, Papagno L, et al. Characterizationof CD4 CTLs ex vivo. J Immunol. 2002;168:5954–5958. doi: 10.4049/jimmunol.168.11.5954. [DOI] [PubMed] [Google Scholar]
- 34.Hooper M, Kallas EG, Coffin D, Campbell D, Evans TG, Looney RJ. Cytomegalovirus seropositivity is associated with the expansion of CD4+CD28− and CD8+CD28− T cells in rheumatoid arthritis. J Rheumatol. 1999;26:1452–1457. [PubMed] [Google Scholar]
- 35.Khan N, Shariff N, Cobbold M, et al. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol. 2002;169:1984–1992. doi: 10.4049/jimmunol.169.4.1984. [DOI] [PubMed] [Google Scholar]
- 36.Looney RJ, Falsey A, Campbell D, et al. Role of cytomegalovirus in the T cell changes seen in elderly individuals. Clin Immunol. 1999;90:213–219. doi: 10.1006/clim.1998.4638. [DOI] [PubMed] [Google Scholar]
- 37.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229:152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schrum LW, Marriott I, Butler BR, Thomas EK, Hudson MC, Bost KL. Functional CD40 expression induced following bacterial infection of mouse and human osteoblasts. Infect Immun. 2003;3:1209–1216. doi: 10.1128/IAI.71.3.1209-1216.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]