Abstract
Little information is available regarding changes in immune status for patients with Mycobacterium avium complex (MAC) lung disease during antibiotic therapy. Serum immunomolecules from 42 patients with MAC lung disease were assayed comparatively using an array-based system according to (i) patients with MAC lung disease at the time of diagnosis versus healthy controls and (ii) alterations after 12 months of antibiotic therapy in the MAC lung disease group. In addition, cytokine analyses were performed to determine whether cytokine responses were associated specifically with the disease phenotype, treatment outcome and aetiological agent. Notably, the serum concentrations of type 1 cytokine-associated molecules, such as CD40L, interferon (IFN)-γ, interleukin (IL)-8 and IL-23, were decreased significantly in patients at the time of diagnosis, suggesting that these molecules may serve as indicators of host susceptibility to MAC disease. Although the overall serum level of T helper type 1 (Th1)-related molecules, such as CD40L and IFN-γ, was restored after treatment, Th17-related cytokines, such as IL-17 and IL-23, were down-regulated significantly at 12 months post-treatment compared to pretreatment. Furthermore, these cytokine patterns differed among patient subgroups. Decreased serum concentrations of IL-17 and/or IL-23 were associated with failure of sputum conversion, the fibrocavitary disease phenotype and M. intracellulare lung disease. Thus, the reciprocal balance between Th1 and Th17 immunity during antibiotic therapy for MAC lung disease is critical for dictating the treatment response. In conclusion, a low level of Th1-related immunomolecules may perpetuate MAC lung disease, and the serum concentrations of Th17-related cytokines can reflect the treatment outcome, disease phenotype and aetiological agent.
Keywords: immunomolecules, lung disease, Mycobacterium avium complex, Th1 immunity, Th17 immunity
Introduction
Non-tuberculous mycobacteria (NTM) lung diseases are becoming more prevalent worldwide [1]–[4]. The Mycobacterium avium complex (MAC) consists predominantly of M. avium and M. intracellulare and is the most common aetiology of NTM lung disease [1],[5]. Because MAC is widespread in the environment and is readily isolated from soil and water, exposure to these organisms is inevitable [6]. However, it is generally believed that normal host defence mechanisms are sufficient to prevent infection [7]. Therefore, otherwise healthy individuals who develop NTM lung disease probably have specific susceptibility factors that make them vulnerable to these infections [8]. In addition, treatment regimens for MAC lung disease are still largely undefined and outcomes remain disappointing, despite substantial improvements in laboratory diagnosis and the availability of new anti-microbials. Treatment success is impaired by the long duration of treatment, side effects and drug interactions, which prevent patients from being fully compliant [1],[9].
Understanding molecular immunity to MAC infection is critical for the development of effective strategies to treat MAC lung disease. Although host defence mechanisms against mycobacteria are, on the whole, poorly understood, immunomolecules such as cytokines and chemokines have been firmly established to play a major role in determining the outcomes of infection with these important intracellular pathogens. Additionally, studies on cytokine expression profiles have shown that a dominant T helper type 1 (Th1)-like response is associated with control of the infection [10]. Furthermore, cytokines and chemokines both act in the early stages of infection to initiate the immune response and, at later stages, to sustain and regulate it. Therefore, determining the function of these immunomolecules is important for understanding how host resistance is induced, maintained and regulated.
Of particular note is that host resistance to mycobacterial infection is dependent upon T cell and macrophage activation by various cytokines and chemokines [11]. Previous studies have reported that peripheral blood mononuclear cells (PBMCs) from patients with MAC lung disease produce fewer protective cytokines, including interferon (IFN)-γ, interleukin (IL)-12 and tumour necrosis factor (TNF)-α [12]–[15], in comparison to control subjects. In addition, reduced IL-17 production has been related to susceptibility to MAC lung disease compared with adult offspring [16]. IL-17-producing Th17 cell-mediated immunity is thus important for the development of protective responses against MAC lung disease. These findings suggest that decreased production of protective cytokines could be associated with host susceptibility to the development of MAC lung disease. In addition, accumulating evidence indicates that antigen-specific Th1 cells and their associated cytokines, such as TNF-α, IFN-γ, IFN-γ inducible protein (IP)-10 and IL-12, orchestrate these protective features of mycobacterial lung disease [17]. Additionally, recent studies have shown that Th17 cells and IL-23, an IL-12-related cytokine that is essential for the survival and functional maturation of Th17 cells, are involved in protection against mycobacterial infections [18]–[20].
Circulating levels of cytokines in the sera are reflective of local immune responses at the disease sites and are therefore representative of endogenous activation within the host as a result of innate and adaptive immune mechanisms [21]. However, little information is available regarding the relationship between immunomolecule levels in sera from patients with MAC lung disease and the treatment response among these patients. Changes in serum immunomolecules at pre-and post-treatment could be used as markers or indicators of successful treatment, disease progression or treatment strategies for MAC infection.
Because little is known about alterations in serum immunomolecule levels during antibiotic therapy in patients with MAC lung disease, we investigated the dynamics of serum immunomolecules before and after 12 months of antibiotic therapy. Furthermore, immunomolecule levels were analysed cross-sectionally in relation to disease characteristics and longitudinally in relation to treatment outcomes.
Materials and methods
Ethics statement
The data in this study are part of an ongoing prospective observational cohort study investigating NTM lung disease (ClinicalTrials.gov Identifier: NCT00970801). The study protocol was approved by the Institutional Review Board (IRB) of the Samsung Medical Center (IRB approval 2008-09-016), and written informed consent was obtained from all the participants. Reporting of the study conforms to the STROBE (STrengthening the Reporting of OBservational Studies in Epidemiology) statement along with the broader EQUATOR (Enhancing the QUAlity and Transparency of health Research) guidelines [22],[23].
Study subjects
This study enrolled 42 patients with MAC lung disease who were diagnosed and treated at the Samsung Medical Center (Seoul, South Korea) between November 2002 and June 2009. All the patients met the diagnostic criteria for MAC lung disease according to the standards set by the American Thoracic Society [1]. None of the patients had been treated previously for NTM lung disease before visiting our hospital. In addition, 18 unrelated healthy adult volunteers (nine male and nine female) were recruited. No control subjects had any history of pulmonary disease.
All the patients received standardized combination antibiotic therapy, consisting of clarithromycin, rifampicin and ethambutol [1],[9]. Streptomycin was administered intramuscularly three times per week for the first several months to patients with advanced disease such as fibrocavitary disease. Sputum conversion was defined as three consecutive negative cultures [24],[25]. Sputum conversion was achieved in 34 patients (81%), and eight patients (19%) demonstrated failure of sputum conversion. Serum samples were obtained from the patients before antibiotic therapy and at 12 months after antibiotic treatment and were stored at −80°C until tested. A total of 93 sera samples, including 18 samples from the healthy control group and 75 pre-and post-treatment samples from 42 patients with MAC lung disease (42 samples at month 0 and 33 samples at month 12 after the start of antibiotic treatment), were examined to generate the data used in our analyses.
We classified chest radiography and high-resolution computed tomography findings obtained at the time of diagnosis as showing either fibrocavitary disease or nodular bronchiectatic disease. When the disease was neither the fibrocavitary form nor the nodular bronchiectatic form, it was deemed unclassifiable [24],[26].
Cytokine array
The cytokine array contained 36 molecules involved in biological processes, such as T helper type 1 (Th1), Th2 and Th17 immunity; innate and adaptive immunity; cell migration; cell survival; disease progression; and inflammation. A total of 36 molecules were screened simultaneously for each serum sample using the Proteome Profiler™ Array (human cytokine array panel A; R&D Systems Inc., Minneapolis, MN, USA), according to the manufacturer's instructions. Additional details on the 36 molecules are provided in the Supporting information, Table S1. Each unique target molecule was assessed twice in duplicate. Briefly, the membranes were placed individually in chambers of a four-well multi-dish and were blocked with blocking buffer. While blocking, 250 μl of serum was diluted 1:5 in blocking buffer and was incubated with 15 μl of a reconstituted detection antibody cocktail at room temperature (RT) for 1 h. Following blocking, the sample mixture was added and incubated overnight at 4°C. The membranes were washed repeatedly and incubated with streptavidin–horseradish peroxidase (HRP) for 30 min, followed by a final set of washes. The membranes were developed using a chemiluminescence assay (Hybond ECL; Amersham Pharmacia Biotech, Amersham, UK) and were subsequently exposed to X-ray film (Kodak Biomax ML; Eastman Kodak, New Haven, CT, USA). The arrays were scanned into a computer, and the densitometric value of each locus on the array was measured using ArrayGauge software (Fujifilm, Tokyo, Japan). The background was subtracted from the value of each spot on the array. The calculated numeric data were transformed as relative density according to each standard.
Statistical analysis
The data are presented as the medians and ranges. The data were analysed by repeated-measures analysis using proc mixed in sas statistical software, version 9·1 (SAS Institute, Inc., Cary, NC, USA). The objectives and methods of the statistical analysis are listed in the Supporting information, Table S2. For the comparison of pre-and post-treatment levels, Dunnett's test was used for comparisons with baseline. For comparison in the patient subgroup, P-value is corrected by Bonferroni's method. A P-value < 0·05 was considered significant.
Results
Baseline patient characteristics
The baseline characteristics of the patients with MAC lung disease are presented in Table 1. The median age of the 42 patients (24 males and 18 females) was 59 years (interquartile range 50–70 years). The aetiological organisms included M. intracellulare in 25 patients (60%) and M. avium in 17 patients (40%). Twenty-eight patients (67%) demonstrated the nodular bronchiectatic form, and 14 patients (33%) demonstrated the fibrocavitary form. None of the patients were immunodeficient or had a malignancy, and all of them tested negative for antibodies to HIV.
Table 1.
Demographic and baseline characteristics of 42 study patients with Mycobacterium avium complex lung disease.
| No. of patients (%) or median (IQR) | |
|---|---|
| Age (years) | 59 (50–70) |
| Male | 24 (57) |
| Body mass index, kg/m2 | 20·0 (17·9–21·0) |
| Smoking | |
| Non-smoker | 28 (67) |
| Current or ex-smoker | 14 (33) |
| Co-morbidity | |
| Cardiovascular disease | 5 (12) |
| DM | 1 (2) |
| Chronic liver disease | 2 (5) |
| Rheumatic disease | 3 (7) |
| Previous history of TB treatment | 26 (62) |
| Aetiology | |
| M. avium | 17 (40) |
| M. intracellulare | 25 (60) |
| Type | |
| Nodular bronchiectatic form | 28 (67) |
| Fibrocavitary form | 14 (33) |
| Time from diagnosis to the start of treatment, days | 80 (25–389) |
| Laboratory test at treatment | |
| CRP, mg/dl | 0·51 (0·12–1·59) |
| ESR, mm/h | 39 (26–56) |
IQR = interquartile ranges; TB = tuberculosis; CRP = C-reactive protein; ESR = erythrocyte sedimentation rate. Categorical variables were denoted by no. of patients (%). Continuous variable was denoted by median (IQR).
Baseline differences in serum immunomolecules between healthy controls and patients with MAC lung disease
To investigate host susceptibility factors, serum immunomolecules were first compared between healthy controls and patients with MAC lung disease prior to antibiotic treatment. Notably, CD40 ligand (CD40L; P < 0·001), IFN-γ (P = 0·005), IL-6 (P = 0·003), IL-8 (P = 0·031) and IL-23 (P = 0·005) were decreased significantly in patients with MAC lung disease compared to healthy controls (Fig. 1). No other significant differences were found in the baseline serum levels of the 36 tested immunomolecules between patients with MAC lung disease and healthy controls (data not shown).
Figure 1.
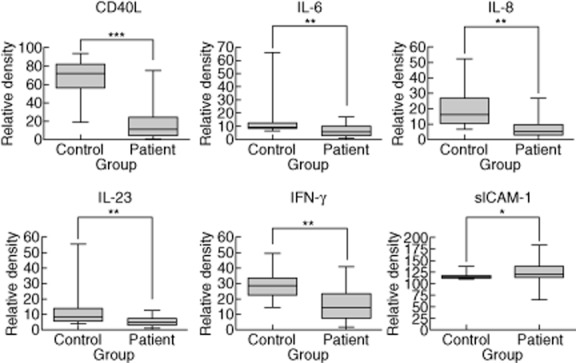
Overall comparison of serum immunomolecules between patients with Mycobacterium avium complex (MAC) lung disease and healthy controls. The data for down-regulated serum immunomolecules are presented as the medians and ranges with P-values: **P < 0·01; ***P < 0·001 for patients with MAC lung disease compared to healthy controls.
Alterations of serum immunomolecule levels in patients with MAC lung disease during antibiotic therapy
Next, the serum immunomolecule level was compared before and after treatment in patients with MAC lung disease to identify potential markers of treatment effects. The levels of CD40L (P = 0·029) and granulocyte colony-stimulating factor (G-CSF; P = 0·035) were up-regulated at 12 months after the start of antibiotic therapy compared to the pretreatment levels (Fig. 2a), while the levels of IL-1α (P = 0·040), IL-17 (P < 0·001) and IL-23 (P = 0·036) were significantly down-regulated (Fig. 2b). The levels of IFN-γ, IL-6 and IL-8 were unchanged throughout the treatment period (data not shown), although IFN-γ, IL-6 and IL-8 were decreased in patients before treatment in comparison to the healthy controls.
Figure 2.
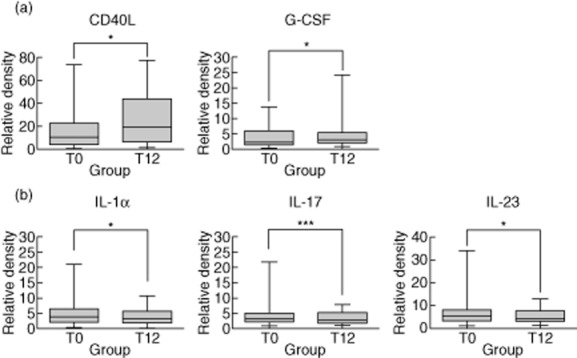
Alteration of serum immunomolecule levels in patients with Mycobacterium avium complex (MAC) lung disease during antibiotic therapy. The data for up-regulated (a) and down-regulated (b) serum immunomolecules are presented as the medians and ranges with P-values: *P < 0·05; ***P < 0·001 for the results at 12 months after the start of treatment compared to pretreatment in patients with MAC lung disease.
Comparison of serum immunomolecule levels in the patient subgroups according to three criteria
To investigate whether the changes in immunomolecule levels were inter-related, all the possible correlations were analysed extensively. The patients were divided into two subgroups based on three criteria: success versus failure of sputum conversion; type of disease (nodular bronchiectatic form versus fibrocavitary form); and aetiology (M. avium versus M. intracellulare infection). In this analysis, we accordingly compared the levels of serum immunomolecules at two time-points, pre-and post-treatment (T0 versus T0 and T12 versus T12), in accordance with the previous three criteria using the immunomolecules with significantly altered levels after 12 months of antibiotic therapy (T0 versus T12).
Comparison of serum immunomolecule levels in the patient subgroups according to sputum culture conversion
To identify potential candidates as surrogate markers for sputum culture conversion, we analysed the serum immunomolecule levels in the patient subgroups. IL-13 (P = 0·023) and IFN-γ-inducible T cell α chemoattractant (I-TAC; P = 0·003) were expressed more highly in patients with failure of sputum conversion than in patients with successful sputum conversion at pretreatment; furthermore, at 12 months post-treatment, the decrease in immunomolecule levels was larger among patients with failure of sputum conversion than in those with successful sputum conversion (IL-13, P = 0·007; I-TAC, P = 0·001; Fig. 3a). Additionally, complement component 5a (C5a; P = 0·030) and macrophage inflammatory protein (MIP)-1α (P = 0·010) were expressed more highly in patients with failure of sputum conversion than in patients with successful sputum conversion at pretreatment; at post-treatment, no significant changes in these immunomolecule levels were detected (Fig. 3b). The levels of IL-17 (P < 0·001) and IL-23 (P < 0·001) were also decreased significantly after 12 months of antibiotic therapy compared to pretreatment in patients with failure of sputum conversion (Fig. 3c).
Figure 3.
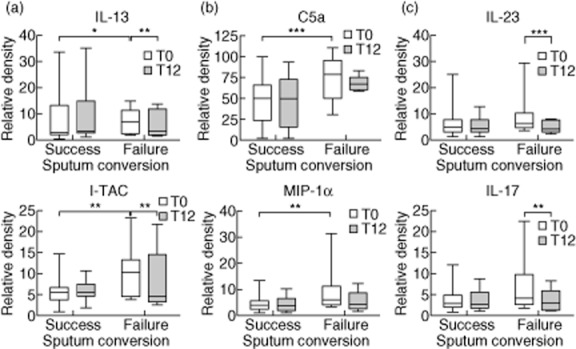
Comparison of serum immunomolecule levels in the patient subgroups according to sputum culture conversion [successes (n = 34) versus failures (n = 8)]. (a) Up-regulated immunomolecules in patients with failure of sputum conversion compared to patients with successful sputum conversion at pretreatment; these immunomolecules were decreased post-treatment. (b) Up-regulated immunomolecules in patients with failure of sputum conversion compared to patients with successful sputum conversion at pretreatment; no significant changes were detected post-treatment. (c) Decreased immunomolecules at post-treatment compared to pretreatment in patients with failure of sputum conversion. The data are presented as the medians and ranges. *P < 0·05; **P < 0·01; ***P < 0·001 for the results of the comparisons between subgroups at each time-point or during treatment in patients with Mycobacterium avium complex (MAC) lung disease.
Comparison of serum immunomolecule levels in the patient subgroups according to disease type
NTM lung disease can be classified into two distinct types: the nodular bronchiectatic form and the fibrocavitary form. Moreover, the disease type caused by clinical strains with different virulence levels can influence the cytokine levels. In the fibrocavitary form subgroup, C5a and CD40L were increased after 12 months of antibiotic therapy compared to pretreatment (P = 0·024 and P < 0·001, respectively). In addition, IL-17 (P < 0·001) was decreased significantly after treatment. No significant changes in immunomolecule levels were detected in the nodular bronchiectatic form subgroup (Fig. 4a). For comparison of the two disease types, IL-13 (P = 0·019) was decreased at pretreatment in the fibrocavitary form subgroup, whereas growth-related oncogene (GRO)-α (P = 0·035) was expressed more highly at 12 months post-treatment in the fibrocavitary form subgroup (Fig. 4b).
Figure 4.
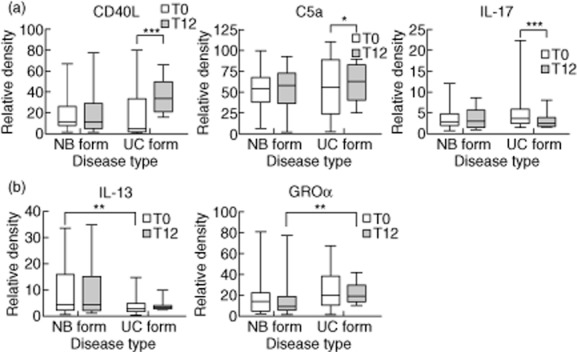
Comparison of serum immunomolecule levels in the patient subgroups according to disease type [nodular bronchiectatic form (n = 28) versus fibrocavitary form (n = 14)]. (a) Up-or down-regulated immunomolecules during treatment in the comparison of the two disease types. (b) Up-or down-regulated immunomolecules at pretreatment or post-treatment in the comparison of the two disease types. The data are presented as medians and ranges. *P < 0·05; **P < 0·01; ***P < 0·001 for the results of the comparisons between subgroups at each time-point or during treatment in patients with Mycobacterium avium complex (MAC) lung disease.
Comparison of serum immunomolecule levels in the patient subgroups according to aetiology
Species differentiation between M. avium and M. intracellulare may have prognostic and therapeutic implications. IP-10 (P < 0·001) and IL-17 (P = 0·001) levels were decreased after treatment compared to pretreatment in patients with M. intracellulare lung disease (Fig. 5a). In addition, IL-12p70 (P = 0·014), IL-1β (P = 0·042) and IL-10 (P = 0·001) levels were decreased after treatment in patients with M. intracellulare lung disease; these immunomolecule levels at post-treatment were decreased significantly in patients with M. intracellulare lung disease compared to patients with M. avium lung disease (P = 0·003, P = 0·037 and P = 0·018, respectively; Fig. 5b).
Figure 5.
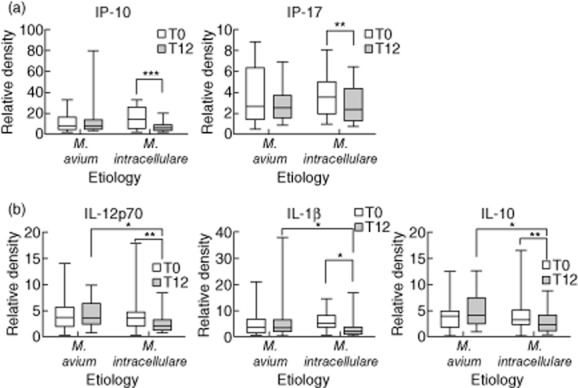
Comparison of serum immunomolecule levels in the patient subgroups according to aetiology [Mycobacterium avium (n = 17) versus M. intracellulare (n = 25)]. (a) Down-regulated immunomolecules during treatment in the comparison of the two aetiologies. (b) Down-regulated immunomolecules both during treatment and at post-treatment in the comparison of the two aetiologies. The data are presented as medians and ranges. *P < 0·05; **P < 0·01; ***P < 0·001 for the results of the comparisons between subgroups at each time-point or during treatment in patients with M. avium complex (MAC) lung disease.
Discussion
In this study, we investigated the profiles of 36 serum immunomolecules in patients with MAC lung disease and healthy controls to identify promising markers for treatment effects and baseline differences between responder phenotypes.
We first investigated the distinct immunomolecule profiles between healthy controls and patients with MAC lung disease. The levels of CD40L, IFN-γ, IL-6, IL-8 and IL-23 were significantly lower in the MAC lung disease group compared to healthy controls. Moreover, the levels of IFN-γ, IL-6 and IL-8 observed during antibiotic therapy in patients with MAC infections did not differ from those observed at baseline. Therefore, we believe that decreased levels of immunomolecules represent host susceptibility factors and may trigger the initiation of MAC infections. The Th1 cytokine (IL-12, IL-23, IFN-γ and IL-17) pathway is important for innate and adaptive immune responses to infection by intracellular pathogens. Individuals with defects in Th1 cytokine pathway genes have enhanced susceptibilities to infections with otherwise poorly pathogenic, mainly environmental mycobacteria or vaccine-associated M. bovis BCG. Collectively, the genetic defects underlying this increased susceptibility are referred to as Mendelian susceptibility to mycobacterial disease.
The decreased levels of serum immunomolecules may also be associated with antigen-presenting cells (APCs). Macrophages/monocytes infected with MAC down-regulate CD40L expression on T cells, and this may prevent optimal induction of protective immunity to MAC infection by CD40–CD40L interactions. Additionally, IL-6, IL-8 and IL-23 are produced by APCs [27], and activated APCs thereby influence the type of T cell response and participate in the recruitment and activation of other effector cells [28]. However, M. tuberculosis can inhibit the maturation of APCs and impair their ability to stimulate T cell proliferation, and the effector functions of APCs may be suppressed in patients with MAC lung disease. The reduced level of IFN-γ, which is produced primarily by T cells during mycobacterial infections, may also be a consequence of MAC-driven APC suppression. Therefore, the reduced production of cytokines and chemokines associated with APCs leads to increased susceptibility to the development of MAC lung disease.
Upon comparing the levels of immunomolecules before and after treatment in patients, marked reductions in IL-1α, IL-17 and IL-23 were observed at 12 months after antibiotic therapy compared to pretreatment. Interestingly, the decreases in IL-17 and IL-23 were correlated significantly with the failure of sputum conversion at 12 months post-treatment; in addition, the decreased levels of IL-17 were correlated significantly with the fibrocavitary form of disease and M. intracellulare lung disease at 12 months post-treatment. IL-17 is recognized as an inflammatory cytokine capable of inducing chemokine gradients and initiating inflammation, particularly in the lungs [29]. As IL-23 is responsible for the persistence and function of Th17 cells, it may also be a key player in inflammation [30]. Furthermore, IL-23 and IL-17 act in a complex manner in the control of mycobacteria-induced inflammation [31]. The absence of IL-23 and IL-17 in the lungs leads to an increased severity of inflammation, which may be related to recent reports showing that IL-17 alters neutrophil survival and that IL-17 and IL-23 alter the functional profile of neutrophils [32],[33]. During MAC infection, reduced early neutrophil infiltration into infected tissues resulting from both IL-23 and IL-17 deficiency may be associated with the failure of sputum conversion. Th17 responses are suppressed in patients with active TB disease compared with healthy donors. The association between Th17 response and the clinical outcome of M. tuberculosis infection suggests that Th17 cells are more likely to play an important role in prevention of disease development and progression, but these cells have a less important role in resistance to the primary infection [34]. In addition, Lim et al. reported that NTM lung disease may be associated with reduced Th17 immunity [16]. However, as far as we know, the link between Th17 immunity and the antibiotic therapy in the field of mycobacterial diseases remains unknown. Thus, further studies will be required to investigate the link between Th17 immunity and antibiotics.
Regarding the comparison between patient subgroups with and without sputum conversion, the levels of I-TAC and MIP-1α were increased significantly at the pretreatment stage in the subgroup of patients who failed to achieve sputum conversion. I-TAC, which is produced by macrophages and monocytes in response to IFN-γ, is important for the recruitment of lymphocytes to the site of infection for granuloma formation [35]. MIP-1α induces the activation and proliferation of T cells and macrophages, promotes Th1 cell differentiation and plays a significant role in granuloma formation [36]. Once formed, granulomas prevent bacterial clearance [37], and the progression of granulomas via increased production of I-TAC and MIP-1α may impact sputum conversion negatively.
CD40L was decreased significantly in patients with MAC lung disease; however, the levels of CD40L were increased at 12 months after antibiotic treatment when compared to pretreatment levels, particularly in the fibrocavitary form subgroup. The disease phenotype observed at the time of therapy initiation may reflect alterations in host immune responses during treatment.
Patients with M. intracellulare lung disease exhibit a more severe presentation and have a worse prognosis than patients with M. avium lung disease with regard to disease progression and treatment response [24]. Therefore, species differentiation between M. avium and M. intracellulare may have prognostic and therapeutic implications. Interestingly, low levels of IL-1β, IL-10 and IL-12p70 were detected in patients with M. intracellulare lung disease at 12 months after treatment. The proinflammatory cytokine IL-1β is a key mediator of inflammation and plays an important role in the host resistance to mycobacterial infections [38]. IL-12 is a cytokine produced by APCs and contributes to protection against MAC [15]. IL-10 is immunosuppressive and inhibits IL-12 production by monocytes exposed to mycobacteria [39]. A decrease in the number of live intracellular bacilli after therapy leads to a decrease in both Th1 and Th2 immunity. The different pathogenicities of M. avium and M. intracellulare may be related to the different host immune responses and may reflect alterations in host immune status according to the treatment.
This study has several limitations. First, this study was conducted at a single centre and performed on a referral bias, with the analysis of only a small number of Korean patients; therefore, caution should be taken when attempting to generalize our findings. Secondly, our methodological approach may represent a limitation, because our array system compared the qualitative concentrations of the molecules rather than their definite concentrations. Thirdly, the decision to initiate long-term combination antibiotic therapy did not depend upon firmly established objective criteria. However, we believe that the probability of bias was distributed equally among the patients because information regarding the immunological status of the patients was not available to the attending physician during the study period. Finally, this study was preliminary, because we did not investigate the precise mechanism of the specific molecules associated with clinical outcome. A better understanding of the cytokines detected in the study and their function roles in the host immune response may help in the rational design of more effective therapeutic strategies against MAC pulmonary disease.
In summary, we analysed the profiles of 36 serum immunomolecules using a cytokine array method in patients with MAC lung disease before and after 12 months of antibiotic therapy to identify specific immune markers. The deficiency of immunomolecules associated with Th1 immunity may lead to increased susceptibility to MAC lung disease and, importantly for MAC infection, the serum concentrations of Th17-related cytokines may serve as key markers reflecting the treatment outcome, disease phenotype and aetiological agent. Moreover, the reciprocal balance between Th1 and Th17 immunity during antibiotic therapy for MAC lung disease is important, and understanding these features of immunomolecules in host–MAC interactions will provide major insight into the pathogenesis of MAC lung disease and lead to new approaches for immunological treatment interventions.
Acknowledgments
We thank Ms Shin Hye Lee and Hyeji Kim (Samsung Medical Center) for their assistance and technical support. This work was supported by a grant from the Korean Health Technology R&D Project, Ministry for Health and Welfare, Republic of Korea (A120647) and the Mid-career Researcher Program through a National Research Foundation grant funded by the Ministry of Education, Science and Technology (2011–0015546). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Disclosures
None of the authors has any potential financial conflicts of interest related to this study.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web-site:
Cytokine array coordinates of screened cytokines and chemokines.
Comparative analysis of immunomolecule levels investigated between designated groups.
References
- 1.Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 2.Kendall BA, Winthrop KL. Update on the epidemiology of pulmonary nontuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013;34:87–94. doi: 10.1055/s-0033-1333567. [DOI] [PubMed] [Google Scholar]
- 3.Koh WJ, Kwon OJ, Jeon K, et al. Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens in Korea. Chest. 2006;129:341–348. doi: 10.1378/chest.129.2.341. [DOI] [PubMed] [Google Scholar]
- 4.Prevots DR, Shaw PA, Strickland D, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med. 2010;182:970–976. doi: 10.1164/rccm.201002-0310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daley CL, Griffith DE. Pulmonary non-tuberculous mycobacterial infections. Int J Tuberc Lung Dis. 2010;14:665–671. [PubMed] [Google Scholar]
- 6.Falkinham JO., III Reducing human exposure to Mycobacterium avium. Ann Am Thorac Soc. 2013;10:378–382. doi: 10.1513/AnnalsATS.201301-013FR. [DOI] [PubMed] [Google Scholar]
- 7.Guide SV, Holland SM. Host susceptibility factors in mycobacterial infection. genetics and body morphotype. Infect Dis Clin North Am. 2002;16:163–186. doi: 10.1016/s0891-5520(03)00051-5. [DOI] [PubMed] [Google Scholar]
- 8.Sexton P, Harrison AC. Susceptibility to nontuberculous mycobacterial lung disease. Eur Respir J. 2008;31:1322–1333. doi: 10.1183/09031936.00140007. [DOI] [PubMed] [Google Scholar]
- 9.Philley JV, Griffith DE. Management of nontuberculous mycobacterial (NTM) lung disease. Semin Respir Crit Care Med. 2013;34:135–142. doi: 10.1055/s-0033-1333575. [DOI] [PubMed] [Google Scholar]
- 10.Cooper AM, Mayer-Barber KD, Sher A. Role of innate cytokines in mycobacterial infection. Mucosal Immunol. 2011;4:252–260. doi: 10.1038/mi.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper AM, Khader SA. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunol Rev. 2008;226:191–204. doi: 10.1111/j.1600-065X.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greinert U, Schlaak M, Rusch-Gerdes S, Flad HD, Ernst M. Low in-vitro production of interferon-γ and tumor necrosis factor-α in HIV-seronegative patients with pulmonary disease caused by non-tuberculous mycobacteria. J Clin Immunol. 2000;20:445–452. doi: 10.1023/a:1026407815946. [DOI] [PubMed] [Google Scholar]
- 13.Kartalija M, Ovrutsky AR, Bryan CL, et al. Patients with nontuberculous mycobacterial lung disease exhibit unique body and immune phenotypes. Am J Respir Crit Care Med. 2013;187:197–205. doi: 10.1164/rccm.201206-1035OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwon YS, Kim EJ, Lee SH, et al. Decreased cytokine production in patients with nontuberculous mycobacterial lung disease. Lung. 2007;185:337–341. doi: 10.1007/s00408-007-9040-z. [DOI] [PubMed] [Google Scholar]
- 15.Vankayalapati R, Wizel B, Samten B, et al. Cytokine profiles in immunocompetent persons infected with Mycobacterium avium complex. J Infect Dis. 2001;183:478–484. doi: 10.1086/318087. [DOI] [PubMed] [Google Scholar]
- 16.Lim A, Allison C, Price P, Waterer G. Susceptibility to pulmonary disease due to Mycobacterium avium–intracellulare complex may reflect low IL-17 and high IL-10 responses rather than Th1 deficiency. Clin Immunol. 2010;137:296–302. doi: 10.1016/j.clim.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Damsker JM, Hansen AM, Caspi RR. Th1 and Th17 cells: adversaries and collaborators. Ann NY Acad Sci. 2010;1183:211–221. doi: 10.1111/j.1749-6632.2009.05133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cruz A, Khader SA, Torrado E, et al. Cutting edge: IFN-gamma regulates the induction and expansion of IL-17-producing CD4 T cells during mycobacterial infection. J Immunol. 2006;177:1416–1420. doi: 10.4049/jimmunol.177.3.1416. [DOI] [PubMed] [Google Scholar]
- 19.Khader SA, Bell GK, Pearl JE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 20.Khader SA, Pearl JE, Sakamoto K, et al. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J Immunol. 2005;175:788–795. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- 21.Hasan Z, Jamil B, Khan J, et al. Relationship between circulating levels of IFN-gamma, IL-10, CXCL9 and CCL2 in pulmonary and extrapulmonary tuberculosis is dependent on disease severity. Scand J Immunol. 2009;69:259–267. doi: 10.1111/j.1365-3083.2008.02217.x. [DOI] [PubMed] [Google Scholar]
- 22.Simera I, Moher D, Hoey J, Schulz KF, Altman DG. A catalogue of reporting guidelines for health research. Eur J Clin Invest. 2010;40:35–53. doi: 10.1111/j.1365-2362.2009.02234.x. [DOI] [PubMed] [Google Scholar]
- 23.Elm von E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 24.Koh WJ, Jeong BH, Jeon K, et al. Clinical significance of the differentiation between Mycobacterium avium and Mycobacterium intracellulare in M. avium complex lung disease. Chest. 2012;142:1482–1488. doi: 10.1378/chest.12-0494. [DOI] [PubMed] [Google Scholar]
- 25.Sim YS, Park HY, Jeon K, Suh GY, Kwon OJ, Koh WJ. Standardized combination antibiotic treatment of Mycobacterium avium complex lung disease. Yonsei Med J. 2010;51:888–894. doi: 10.3349/ymj.2010.51.6.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koh WJ, Yu CM, Suh GY, et al. Pulmonary TB and NTM lung disease: comparison of characteristics in patients with AFB smear-positive sputum. Int J Tuberc Lung Dis. 2006;10:1001–1007. [PubMed] [Google Scholar]
- 27.Zhu K, Shen Q, Ulrich M, Zheng M. Human monocyte-derived dendritic cells expressing both chemotactic cytokines IL-8, MCP-1, RANTES and their receptors, and their selective migration to these chemokines. Chin Med J (Engl) 2000;113:1124–1128. [PubMed] [Google Scholar]
- 28.Jong de JM, Schuurhuis DH, Ioan-Facsinay A, et al. Dendritic cells, but not macrophages or B cells, activate major histocompatibility complex class II-restricted CD4+ T cells upon immune-complex uptake in vivo. Immunology. 2006;119:499–506. doi: 10.1111/j.1365-2567.2006.02464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sergejeva S, Ivanov S, Lotvall J, Linden A. Interleukin-17 as a recruitment and survival factor for airway macrophages in allergic airway inflammation. Am J Respir Cell Mol Biol. 2005;33:248–253. doi: 10.1165/rcmb.2004-0213OC. [DOI] [PubMed] [Google Scholar]
- 30.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Khader SA, Cooper AM. IL-23 and IL-17 in tuberculosis. Cytokine. 2008;41:79–83. doi: 10.1016/j.cyto.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dragon S, Saffar AS, Shan L, Gounni AS. IL-17 attenuates the anti-apoptotic effects of GM-CSF in human neutrophils. Mol Immunol. 2008;45:160–168. doi: 10.1016/j.molimm.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 33.Zelante T, De Luca A, Bonifazi P, et al. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol. 2007;37:2695–2706. doi: 10.1002/eji.200737409. [DOI] [PubMed] [Google Scholar]
- 34.Chen X, Zhang M, Liao M, et al. Reduced Th17 response in patients with tuberculosis correlates with IL-6R expression on CD4+ T Cells. Am J Respir Crit Care Med. 2010;181:734–742. doi: 10.1164/rccm.200909-1463OC. [DOI] [PubMed] [Google Scholar]
- 35.Fuller CL, Flynn JL, Reinhart TA. In situ study of abundant expression of proinflammatory chemokines and cytokines in pulmonary granulomas that develop in cynomolgus macaques experimentally infected with Mycobacterium tuberculosis. Infect Immun. 2003;71:7023–7034. doi: 10.1128/IAI.71.12.7023-7034.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chensue SW, Warmington KS, Allenspach EJ, et al. Differential expression and cross-regulatory function of RANTES during mycobacterial (type 1) and schistosomal (type 2) antigen-elicited granulomatous inflammation. J Immunol. 1999;163:165–173. [PubMed] [Google Scholar]
- 37.Russell DG, Cardona PJ, Kim MJ, Allain S, Altare F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat Immunol. 2009;10:943–948. doi: 10.1038/ni.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamada H, Mizumo S, Horai R, Iwakura Y, Sugawara I. Protective role of interleukin-1 in mycobacterial infection in IL-1 alpha/beta double-knockout mice. Lab Invest. 2000;80:759–767. doi: 10.1038/labinvest.3780079. [DOI] [PubMed] [Google Scholar]
- 39.Gong JH, Zhang M, Modlin RL, et al. Interleukin-10 downregulates Mycobacterium tuberculosis-induced Th1 responses and CTLA-4 expression. Infect Immun. 1996;64:913–918. doi: 10.1128/iai.64.3.913-918.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cytokine array coordinates of screened cytokines and chemokines.
Comparative analysis of immunomolecule levels investigated between designated groups.


