Abstract
The antimicrobial activity of the bacteriocin-like substance (BLS) P34 against Listeria monocytogenes was investigated in chicken sausage. The BLS was applied to chicken sausages (256 AU g−1) previously inoculated with a suspension of 102 cfu g−1 of L. monocytogenes. BLS P34 inhibited the indicator microorganism in situ in all incubation times for up to 10 days at 5 °C. The effectiveness of BLS P34 was increased when it was added in combination with nisin. The bacteriocin was also tested in natural eatable natural bovine wrapping (salty semi-dried tripe) against the same indicator microorganism, also showing inhibitory capability in vitro. BLS P34 showed potential to control L. monocytogenes in refrigerated meat products.
Keywords: bacteriocin, chilled chicken sausage, Listeria monocytogenes, biopreservation
Introduction
Raw meat is highly sensitive to microbial spoilage because of its natural properties (aw, pH and nutrients). In addition, meat and meat products are responsible for many foodborne illness and outbreaks. Listeria monocytogenes is a foodborne pathogen of public health importance due to the high mortality rate it causes in infected vulnerable individuals. Foodborne outbreaks involving products such as pasteurized milk, soft Mexican style cheeses, pork tongue in jelly and delicatessen meats, have raised concerns about L. monocytogenes on both dairy and meat industry (Gandhi and Chikindas, 2007). The occurrence of a number of listeriosis outbreaks has further raised its report among producers, consumers and regulators. L. monocytogenes is a ubiquitous microorganism that can grow at refrigeration temperatures and relatively low pH, making its control extremely difficult. This bacterium can be considered an in house bacterium in processing plants of meat products (Chasseignaux et al., 2002). The development of innovative methods to control L. monocytogenes in foods is therefore a current priority.
Some bacteriocins like nisin, enterocins A and B, sakacin, or pediocin AcH, have been tested, alone or in combination with several physico-chemical treatments (modified atmosphere packaging, high hydrostatic pressure, heat, pH and chemical preservatives) as an additional hurdle to control proliferation of Listeria in meat products (Cleveland et al., 2001; Garriga et al., 2002; Työppönen et al., 2003). Also, several bacteriocinogenic lactic acid bacteria have been used as protective cultures for food manufacturing processes in attempts to control this bacterium (De Martinis et al., 2002; Mataragas et al., 2003; Kouakou et al., 2009). Currently, nisin is the only bacteriocin widely used as a food preservative, but its successful application in meat system has been limited due the interaction with phospholipids, low solubility and inactivation by meat endogenous enzymes (Cleveland et al., 2001).
Bacillus sp. P34 produces a bacteriocin-like substance (BLS) with bactericidal activity against several Gram-positive bacteria, including pathogens like L. monocytogenes and Bacillus cereus. BLS P34 presents a broad spectrum of antimicrobial activity, stability in a wide range of temperature and pH and sensitivity to proteases (Motta et al., 2007). In a recent study, BLS P34 and nisin showed similar effects on eukaryotic cells, indicating its potential to be used as a biopreservative in foods (Vaucher et al., 2010). The aim of this study was to investigate the ability of BLS P34 to control L. monocytogenes in chilled chicken sausage.
Materials and Methods
Bacterial cultures
Bacillus sp. strain P34 was used for production of BLS P34 (Motta et al., 2007). The indicator strain used in the study was Listeria monocytogenes ATCC 7644 (American Type Culture Collection, Rockville, USA). These microorganisms were stored in Brain Heart Infusion Broth (BHI; Oxoid, Basingstoke, UK) containing 20% glycerol (v/v) at −21 °C, and propagated twice in BHI broth before use.
Production of bacteriocin-like substance P34
For the production of antibacterial substance, Bacillus sp. P34 was grown in 100 mL BHI medium at 30 °C in a rotary shaker at 180 rpm for desired times. After cultivation for 24 h, the cells were harvested by centrifugation at 10,000 × g for 15 min and the culture supernatant was filtered through 0.22 μm membranes (Millipore, Bedford, USA). The filtrate was precipitated with ammonium sulfate at 20% saturation. The precipitate was dissolved in 10 mmol L−1 phosphate buffer pH 7.0. This solution was further purified by gel filtration chromatography on a Sephadex G-100 column, and active fractions were pooled (Motta et al., 2007). The BLS P34 was stored in sterile flasks at 4 °C until used.
Antimicrobial activity
An aliquot of 20 μL of partially purified BLS P34 was applied on discs (6 mm) onto BHI agar plates previously inoculated with a swab submerged in an indicator strain suspension which corresponded to a 0.5 McFarland turbidity standard solution. Plates were incubated for 24 h at 37 °C and inhibition zones were measured. The antimicrobial activity titre was determined by the serial twofold dilution method. Activity was defined as the reciprocal of the dilution after the last serial dilution giving an inhibition zone and expressed as activity unit (AU) per milliliter (Motta and Brandelli, 2002). The AU mL−1 was determined in each experiment against the indicator strain L. monocytogenes ATCC 7644.
Anti-listerial activity of BLS P34 in chilled chicken sausage
Prior to experiment, L. monocytogenes ATCC 7644 was cultured in BHI-medium at 37 °C for 18 h. Chicken sausage was treated with BLS P34 (final concentration 256 AU g−1) or 10 mmol L−1 phosphate buffer pH 7.0, and then inoculated with 102 cfu g−1 of L. monocytogenes. Non-inoculated chicken sausage added of 10 mmol L−1 phosphate buffer pH 7.0 was used as a control. After inoculation, the packages were sealed and kept at 5 °C until analysis.
When tested in combination with nisin, samples of chicken sausage were separately treated with 12.5 μg g−1 nisin, 128 AU g−1 P34, or 6.5 μg g−1 nisin + 64 AU g−1 P34. Nisin (Nisaplin®; Danisco, Copenhagen, Denmark) was suspended in 0.02 mol L−1 HCl to obtain a 12.5 mg mL−1 stock solution and further diluted in phosphate buffer saline (PBS; 35 mmol L−1 phosphate, 150 mmol L−1 NaCl, pH 7.4) for application in sausages.
For recovery of P34 activity, 5 g samples were homogenized with 10 mmol L−1 phosphate buffer (pH 6.4) in presence or absence 0.2% (w/v) Tween 80 and the mixture was heated for 15 min at 60 °C in a water bath. Then, the homogenates were centrifuged at 10,000 × g for 30 min, and the antimicrobial activity of the supernatants was determined by the agar diffusion assay.
Enumeration of Listeria monocytogenes
For enumeration of L. monocytogenes population, 10 g of each sample of chilled chicken sausage were added to 90 mL of 1 g L−1 peptone water and then homogenized in a blender. Decimal dilutions were prepared and plated on MOX Agar (Oxford Listeria agar base added of Oxford Listeria Selective Supplement; Merck, Darmstadt, Germany). Colony forming unit counting was performed after 48 h incubation at 37 °C.
Anti-listerial activity of BLS P34 incorporated to natural edible bovine wrapping
Aliquots of 500 μL of BLS P34 (3200 AU mL−1) were applied to 1.5 cm2 natural eatable natural bovine wrapping. After completely dryness, the pieces were submerged in hot water (100 °C for 30 s or 10 min) or cold water (0 °C for 30 s or 10 min). Samples inoculated with 10 mM sodium phosphate buffer pH 7.0 were used as control in this study. The dried samples were placed over BHI agar previously inoculated with 0.5 McFarland suspensions of L. monocytogenes. The plates were incubated at 37 °C for 24 h, and inhibition zones around natural eatable natural bovine wrapping samples were observed.
Statistical analysis
Each experiment was run two separate times with duplicate analysis in each replicate. Data were submitted to analysis of variance (ANOVA) and Tukey’s test using the software Statistic 5.0 for Windows (SPSS Inc, Chicago, IL). Differences were considered significant each other at p < 0.05.
Results
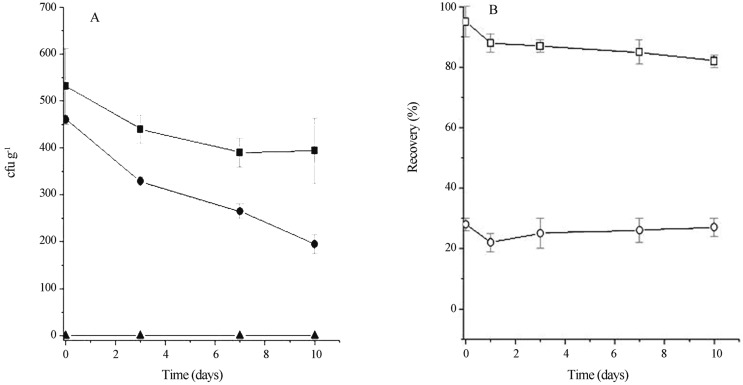
To evaluate the effect of BLS P34 in foods, this substance was added to fresh chicken sausage artificially inoculated with L. monocytogenes and to a natural eatable bovine wrapping. The addition of BLS P34 decreased the viable counts of L. monocytogenes in chilled chicken sausage in all incubation time investigated. Maximal reduction of the L. monocytogenes population was detected between 0 and 3 days (Figure 1A). A reduction of about 260 cfu g−1 was observed after 10 days of incubation showing that the BLS P34 has anti-listerial activity in chicken sausage. The activity of BLS P34 recovered from sausages was above 20%, but increased to more than 80% when extraction was carried out in the presence of Tween 80 (Figure 1B). The activity was maintained at this level for the incubation period of 10 days.
Figure 1.
Effect of BLS P34 on growth of Listeria monocytogenes in chilled chicken sausage. (A) Chicken sausage was treated with 256 AU g−1 BLS P34 (●) or 10 mmol L−1 phosphate buffer pH 7.0 (■) and then inoculated with 102 UFC g−1 L. monocytogenes; (▲) non-inoculated chicken sausage. After inoculation, samples were sealed and kept at 5 °C until analysis. (B) Recovery of BLS P34 activity extracted in the presence (¨) or absence (¡) of Tween 80 (Sant’Anna et al.).
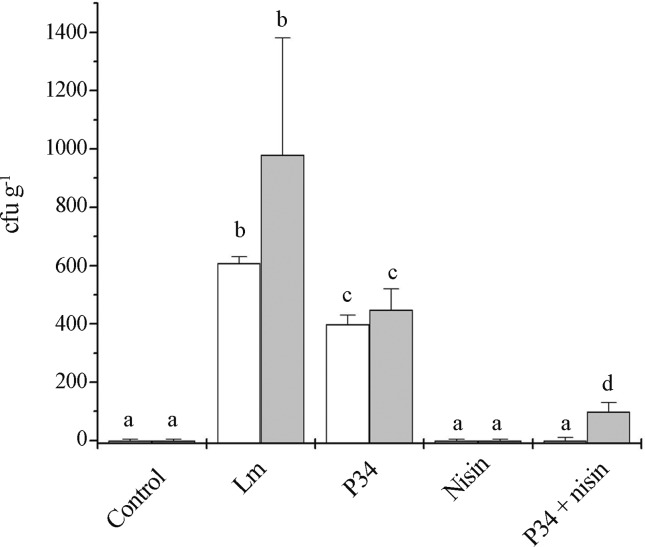
The capability of BLS P34 to control L. monocytogenes in chicken sausage was also tested in combination with nisin. Nisin alone was able to inhibit L. monocytogenes for up to 10 days at 5 °C in chicken sausage, whereas BLS P34 caused a significant reduction in viable counts. Combination of P34 with nisin was more effective in reducing viable counts of L. monocytogenes than P34 alone (Figure 2).
Figure 2.
Counts of Listeria monocytogenes in chilled chicken sausage. Chicken sausage was treated with 128 AU g−1 BLS P34, 12.5 μg g−1 nisin, or 64 AU g−1 BLS P34 + 6.5 μg g−1 nisin, and then inoculated with 102 UFC g−1 L. monocytogenes. Non-inoculated (control) and inoculated samples without bacteriocin addition (Lm) were used as controls. Samples were stored at 5 °C and analyzed at day 1 (white bars) and day 10 (gray bars) (Sant’Anna et al.).
The antimicrobial activity of BLS P34 was also tested in natural eatable natural bovine wrapping, which is a usual enclose material for sausages. This material incorporated with BLS P34 was able to inhibit L. monocytogenes (Table 1). In addition, the antilisterial activity was maintained after different thermal treatments, indicating that the bacteriocin-containing wrapping could be submitted to boiling or refrigeration and still has antimicrobial activity.
Table 1.
Inhibitory activity of BLS P34 inoculated in natural eatable bovine wrapping against Listeria monocytogenes ATCC 7644.
| Wrapping treatment | Inhibition zone (mm) |
|---|---|
| Control | 0 |
| P34 | 4.7 ± 0.6 |
| P34 + 100 °C/30″ | 4.2 ± 1.0 |
| P34 + 100 °C/10′ | 4.3 ± 0.8 |
| P34 + 4 °C/30″ | 4.3 ± 1.2 |
| P34 + 4 °C/10′ | 4.0 ± 0.5 |
Discussion
The BLS P34 produced by Bacillus sp. strain P34 showed inhibitory activity when tested in chicken sausage artificially inoculated with L. monocytogenes at refrigeration temperature during 10 days. This pathogen was also inhibited when BLS P34 was incorporated in natural eatable natural bovine wrapping.
BLS P34 has a bactericidal and bacteriolytic effect on L. monocytogenes as previously observed by a marked decreased of viable cells during the exponential growth phase (Motta et al., 2008). However, viable cells were observed in this work during all incubation period, showing the potential of L. monocytogenes to persist viable for a long time at 5 °C, which has been also reported in modified atmosphere, presence of organic acids, NaCl and bacteriocins (Gandhi and Chikindas, 2007). Therefore, if the storage temperature was sufficiently low, the lag phase of L. monocytogenes would be longer than the shelf-life of the product and, even if the organism was hypothetically able to grow, no significant growth would occur before consumption. However, in a product with a long shelf-life such as vacuum packed cooked ham, growth even at low temperatures could occur before spoilage of the product. Besides, it is unrealistic to think of maintaining those low temperature values throughout the distribution chain (Giannakourou et al., 2005). Temperature measurements from domestic and retail refrigerators have proved that food products are commonly stored at abusive temperatures. It has been observed that combination of different hurdles (pressurization, storage at 1 °C, and natural antimicrobials) are necessary to reduce population of L. monocytogenes (Marcos et al., 2008).
The improvement of anti-listerial activity of BLS P34 by combination with nisin makes more interesting the application of this BLS in meat products, since it might enhance the antimicrobial activity of autochthonous lactic acid bacteria. With respect to the application of bacteriocin in fat rich foods, bacteriocins would be more efficient when applied to a meat surface, than in a food system where the fat, water and the bacteriocins are mixed, such as in sausages or homogenized whole milk. Studies on the use of bacteriocins in milk have shown reduced efficiency with increasing fat content (Zapico et al., 1999; Bizani et al., 2008). Also the interaction of bacteriocins with components of the food matrix influence in the antimicrobial activity (Kouakou et al., 2009). Bacteriocins are compounds that often present a high content of hydrophobic amino acids. Binding to charged or hydrophobic macromolecules in foods can therefore be expected and is one of the disadvantages of the use of bacteriocins as preservatives. Indeed, the effectiveness of nisin in meat products may be affected by combination with meat phospholipids or inactivation by γ-glutamyl transferase (Cleveland et al., 2001). This was not observed in chilled chicken sausages, since nisin and P34 were able to inhibit L. monocytogenes for up to 10 days at 5 °C.
In previous studies on inhibition of Listeria in smoked salmon by nisin, the applied concentrations (10–30 μg g−1) were not sufficient for complete inhibition for up to 3 weeks (Katla et al., 2001). Otherwise, the growth of L. monocytogenes was completely inhibited for a period of 3 weeks in both chicken cold cuts and smoked salmon by addition of 3.5 μg/g sakacin P (Aasen et al., 2003). Although nisin has been used to control L. monocytogenes in meat and dairy, strains presenting resistance to conventional bacteriocins have been reported (Martinez et al., 2005), thus the investigation for alternative BLS is a subject of greatest importance.
L. monocytogenes, the causal agent of listerosis, is a bacterium widely distributed in natural environment and consequently present in animal products and vegetables. Considering that L. monocytogenes can contaminate meat products during the animal slaughter or the product processing, and it can survive and grow within a broad pH range and at refrigeration temperature, the discovery of novel antimicrobial substances to combat this pathogen in foods is a topic of utmost importance. This work indicates that BLS P34 may be an attractive additional hurdle against L. monocytogenes in meat products.
Acknowledgments
This work received financial support of CNPq and CAPES, Brazil.
References
- Aasen IM, Markussen S, Moretro T, Katla T, Axelsson L, Naterstad K. Interactions of the bacteriocins sakacin P and nisin with food constituents. Int J Food Microbiol. 2003;87:35–43. doi: 10.1016/s0168-1605(03)00047-3. [DOI] [PubMed] [Google Scholar]
- Bizani D, Morrissy JAC, Dominguez APM, Brandelli A. Inhibition of Listeria monocytogenes in dairy products using the bacteriocin-like peptide cerein 8A. Int J Food Microbiol. 2008;121:229–233. doi: 10.1016/j.ijfoodmicro.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Chasseignaux E, Gerault P, Toquin MT, Salvat G, Colin P, Ermel G. Ecology of Listeria monocytogenes in the environment of raw poultry meat and raw pork meat processing plants. FEMS Microbiol Lett. 2002;210:271–275. doi: 10.1111/j.1574-6968.2002.tb11192.x. [DOI] [PubMed] [Google Scholar]
- Cleveland J, Montville TJ, Nes IF, Chikindas ML. Bacteriocins: safe, natural antimicrobials for food preservation. Int J Food Microbiol. 2001;71:1–20. doi: 10.1016/s0168-1605(01)00560-8. [DOI] [PubMed] [Google Scholar]
- De Martinis ECP, Alves VF, Franco BDG. Fundamentals and perspectives of the use of bacteriocins by lactic acid bacteria in meat products. Food Rev Int. 2002;18:191–208. [Google Scholar]
- Gandhi M, Chikindas ML. Listeria: A foodborne pathogen that knows how to survive. Int J Food Microbiol. 2007;113:1–15. doi: 10.1016/j.ijfoodmicro.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Garriga M, Aymerich MT, Costa S, Monfort JM, Hugas M. Bactericidal synergism through bacteriocins and high pressure in meat model system during storage. Food Microbiol. 2002;19:509–518. [Google Scholar]
- Giannakourou MC, Koutsoumanis K, Nychas GJE, Taoukis PS. Field evaluation of the application of time temperature integrators for monitoring fish quality in the chill chain. Int J Food Microbiol. 2005;102:323–336. doi: 10.1016/j.ijfoodmicro.2004.11.037. [DOI] [PubMed] [Google Scholar]
- Katla T, Moretro T, Aasen IM, Holck A, Axelsson L, Naterstad K. Inhibition of Listeria monocytogenes in cold smoked salmon by addition of sakacin P and/or live Lactobacillus sakei cultures. Food Microbiol. 2001;18:431–439. [Google Scholar]
- Kouakou P, Ghalfi H, Destain J, Dubois-Dauphin R, Evrard P, Thonart P. Effects of curing sodium nitrite additive and natural meat fat on growth control of Listeria monocytogenes by the bacteriocin-producing Lactobacillus curvatus strain CWBI-B28. Food Microbiol. 2009;26:623–628. doi: 10.1016/j.fm.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Marcos B, Jofré A, Aymerich T, Monfort JM, Garriga M. Combined effect of natural antimicrobials and high pressure processing to prevent Listeria monocytogenes growth after a cold chain break during storage of cooked ham. Food Control. 2008;19:76–81. [Google Scholar]
- Martinez B, Bravo D, Rodriguez A. Consequences of the development of nisin-resistant Listeria monocytogenes in fermented dairy products. J Food Protec. 2005;68:2383–2388. doi: 10.4315/0362-028x-68.11.2383. [DOI] [PubMed] [Google Scholar]
- Mataragas M, Drosinos EH, Metaxopoulos J. Antagonistic activity of lactic acid bacteria against Listeria monocytogenes in slice cooked cured pork shoulder stored under vacuum or modified atmosphere at 4 ± 2 °C. Food Microbiol. 2003;20:259–265. [Google Scholar]
- Motta AS, Brandelli A. Characterization of an antibacterial peptide produced by Brevibacterium linens. J Appl Microbiol. 2002;92:63–71. doi: 10.1046/j.1365-2672.2002.01490.x. [DOI] [PubMed] [Google Scholar]
- Motta AS, Cannavan FS, Tsai SM, Brandelli A. Characterization of a broad range antibacterial substance from a new Bacillus species isolated from Amazon basin. Arch Microbiol. 2007;188:367–375. doi: 10.1007/s00203-007-0257-2. [DOI] [PubMed] [Google Scholar]
- Motta AS, Flores FS, Souto AA, Brandelli A. Antibacterial activity of a bacteriocin-like substance produced by Bacillus sp. P34 that targets the bacterial cell envelope. Antonie van Leeuwenhoek. 2008;93:275–284. doi: 10.1007/s10482-007-9202-2. [DOI] [PubMed] [Google Scholar]
- Tienungoon S, Rarkowsky DA, McMeekin TA, Ross T. Growth limits of Listeria monocytogenes as a function of temperature, pH, NaCl, and lactic acid. Appl Environ Microbiol. 2000;66:4979–4987. doi: 10.1128/aem.66.11.4979-4987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Työppönen S, Petäjä E, Mattila-Sandholm T. Bioprotectives and probiotics for dry sausages. Int J Food Microbiol. 2003;83:233–244. doi: 10.1016/s0168-1605(02)00379-3. [DOI] [PubMed] [Google Scholar]
- Vaucher RA, Motta AS, Brandelli A. Evaluation of the in vitro cytotoxicity of the antimicrobial peptide P34. Cell Biol Int. 2010;34:317–323. doi: 10.1042/CBI20090025. [DOI] [PubMed] [Google Scholar]
- Zapico P, De Paz M, Medina M, Nunez M. The effect of the homogenization of whole milk, skim milk and milk fat on nisin activity against Listeria innocua. Int J Food Microbiol. 1999;46:151–157. doi: 10.1016/s0168-1605(98)00190-1. [DOI] [PubMed] [Google Scholar]