Abstract
Jasmonates (JAs) are important signaling molecules in plants and play crucial roles in stress responses, secondary metabolites' regulation, plant growth and development. In this study, the promoter of AaAOC, which was the key gene of jasmonate biosynthetic pathway, had been cloned. GUS staining showed that AaAOC was expressed ubiquitiously in A. annua. AaAOC gene was overexpressed under control of 35S promoter. RT-Q-PCR showed that the expression levels of AaAOC were increased from 1.6- to 5.2-fold in AaAOC-overexpression transgenic A. annua. The results of GC-MS showed that the content of endogenous jasmonic acid (JA) was 2- to 4.7-fold of the control level in AaAOC-overexpression plants. HPLC showed that the contents of artemisinin, dihydroartemisinic acid and artemisinic acid were increased significantly in AaAOC-overexpression plants. RT-Q-PCR showed that the expression levels of FPS (farnesyl diphosphate synthase), CYP71AV1 (cytochrome P450 dependent hydroxylase) and DBR2 (double bond reductase 2) were increased significantly in AaAOC-overexpression plants. All data demonstrated that increased endogenous JA could significantly promote the biosynthesis of artemisinin in AaAOC-overexpression transgenic A.annua.
Introduction
Jasmonates (JAs) [jasmonic acid (JA) and methyl jasmonate (MeJA)] help to regulate diverse aspects of plant biology that range from stress responses to development [1]. As a signal molecule, JA plays an important role in wound response and pathogenesis [2]–[4]. Additionally, the stress hormone JAs can globally promote the production of multiple primary and secondary metabolites in plants [5]–[7].
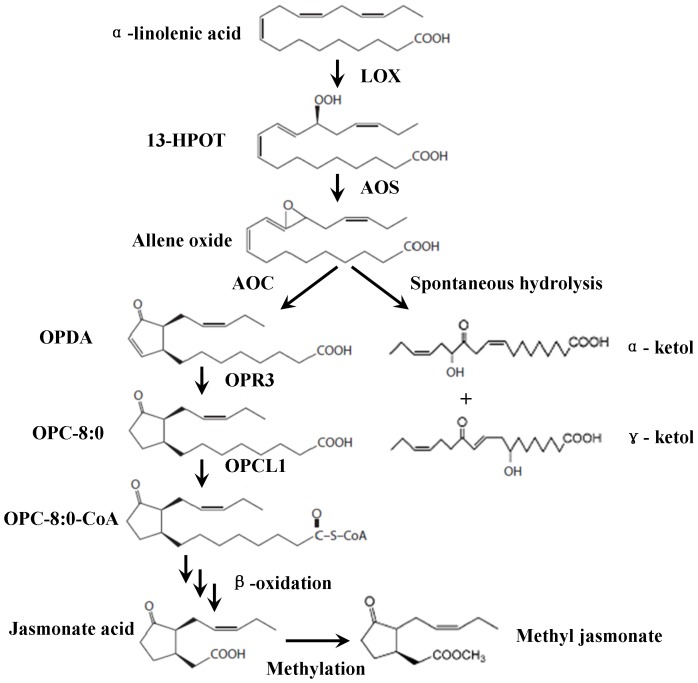
The jasmonate biosynthetic pathway in plants is from an oxylipin pathway (Figure 1) [8]. The α-linolenic acid (18∶3) is converted to 13-hydroperoxylinolenic acid (13-HPOT) by 13-lipoxygenase (LOX), and then allene oxide synthase (AOS) produces allene oxide [1], [9]. The key enzyme Allene oxide cyclase (AOC) catalyses the formation of (9S, 13S)-12-oxo-phytodienoic acid (OPDA) and establishes the stereochemical configuration of naturally occurring JA [10]. Spontaneous hydrolysis of the allene oxide also yields OPDA, although in its racemic form. An OPDA reductase (OPR3) catalyses OPDA to 3-oxo-2(2′-[Z]-pentenyl)cyclopentane-1-octanoic acid (OPC-8:0), which is activated by a carboxyl-CoA ligase encoded by OPCL1 [11]–[14]. Then, after three cycles of β-oxidation, (3R, 7S)-jasmonic acid is produced [15]. MeJA is the ester derivate of JA [16].
Figure 1. Jasmonate biosynthetic pathway in plants.
LOX, 13-lipoxygenase; AOS, allene oxide synthase; AOC, Allene oxide cyclase; OPR3, OPDA reductase 3; OPCL1, OPC-8:0 CoA ligase 1; 13-HPOT, 13-hydroperoxylinoleic acid; OPDA, (9S, 13S)-12-oxo-phytodienoic acid; OPC-8:0, 3-oxo-2(2′-[Z]-pentenyl)cyclopentane-1-octanoic acid; OPC-8:0-CoA, 3-oxo-2-(cis-2′-pentenyl)-cyclopentane-1-octanoyl CoA.
As allene oxide cyclase (AOC) is regarded as the most critical step in jasmonate biosynthetic pathway, the gene is preferentially chosen in metabolic engineering of jasmonates. Overexpression of HnAOC in tobacco resulted in the overexpression of nicotine biosynthetic pathway genes and higher yield of nicotine, with the maximum of 4.8-fold over control [6]. As well as in S. miltiorrhiza hairy roots, overexpression of SmAOC significantly enhanced the expression levels of key genes involved in the biosynthetic pathway of diterpenes and phenolic acids and yields of tanshinone IIA, rosmarinic acid (RA) and lithospermic acid B (LAB) [7]. Artemisinin, a functional secondary metabolite of Artemisia annua, is currently the best therapeutic against both drug-resistant and cerebral malaria-causing strains of Plasmodium falciparum [17]. In this study, we analyzed the results of overexpression AaAOC in transgenic A. Annua and demonstrated that the increase of endogenous JA resulted in the enhancing of artemisinin biosynthesis.
Results
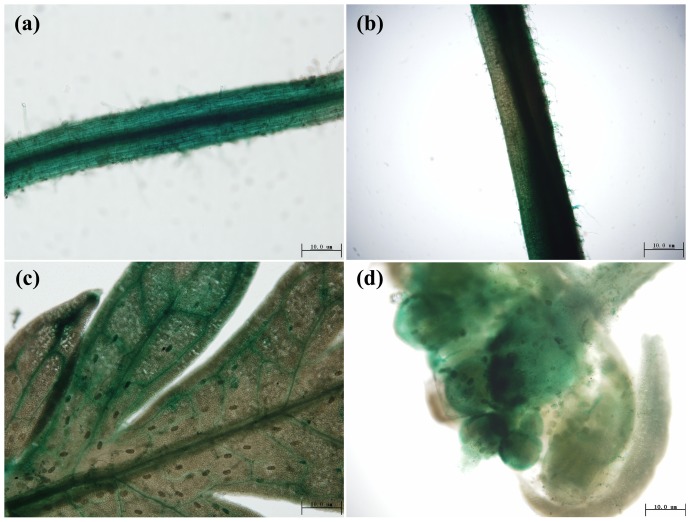
To examine the expression pattern of AaAOC in detail, a 2276 bp promoter sequence(KC477937) of AaAOC was cloned by genomic walking. After generated the AaAOC promoter-GUS transgenic plants, the expression pattern of AaAOC was investigated by GUS staining. GUS activity was detected in all examined tissues, including roots, stems, leaves and flower buds (Figure 2a, 2b, 2c and 2d). In 1-month-old plants, GUS activity was high in root tips, stems and leaves (Figure 2a, 2b and 2c), along with which can be detected in glandular trichomes and T-shaped trichomes (Figure 2b and 2c). During the flowering period, the GUS staining was observed in flower buds, too (Figure 2d). No GUS staining signals were observed in the negative control plants transformed with pCAMBIA1391Z empty vector (Figure S1 in File S1). All the data showed that AaAOC was ubiquitously expressed in A. annua.
Figure 2. Gus-staining in transgenic A. annua transformed using the pAOC-GUS plasmid.
(a) Gus-staining of roots in pAOC-GUS transgenic A. annua. (b) Gus-staining of stems in pAOC-GUS transgenic A. annua. (c) Gus-staining of leaves in pAOC-GUS transgenic A. annua. (d) Gus-staining of flower buds in pAOC-GUS transgenic A. annua.
Independent transformants were selected in kanamycin-containing medium and further confirmed by genomic PCR. Using forward primer of P35S and reverse primer AaAOC-RT-R, 945-bp products were amplified with five transgenic lines AOC-1, AOC-7, AOC-11, AOC13, AOC-17 and the plasmid pCAMBIA2300-35S::AaAOC::NOS as template (Figure S2a in File S1). 635-bp products could be amplified from all the transgenic A. annua with P35S and NPTII-R as primers (Figure S2b in File S1). The results showed that the existence of NPTII gene and exogenous AaAOC gene in AaAOC-overexpression transgenic plants.
The expression levels of AaAOC in AaAOC-overexpression A. annua were analyzed by RT-Q-PCR. The result indicated that expression profile of AaAOC gene varied from different transgenic lines. Compared to the control, the expression levels of AaAOC were increased from 1.6- to 5.2-fold in AaAOC-overexpression transgenic A. annua (Figure 3). The statistics analysis showed that the observed differences were statistically significant.
Figure 3. Analysis of AaAOC-overexpression transgenic A. annua by RT-Q-PCR.
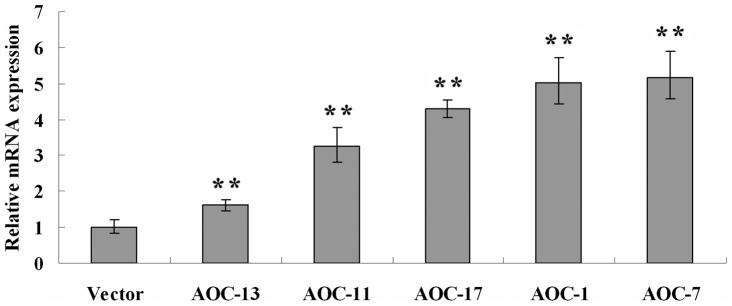
The expression analysis of AaAOC in transgenic A. annua by RT-Q-PCR. Error bars are SE (n = 3). ACTIN was used as a control for normalization. Statistical significance was determined by students't-test (**, P<0.01; *, P<0.05). These asterisks are the difference between the empty-vector and AaAOC-overexpression transgenic A. annua.
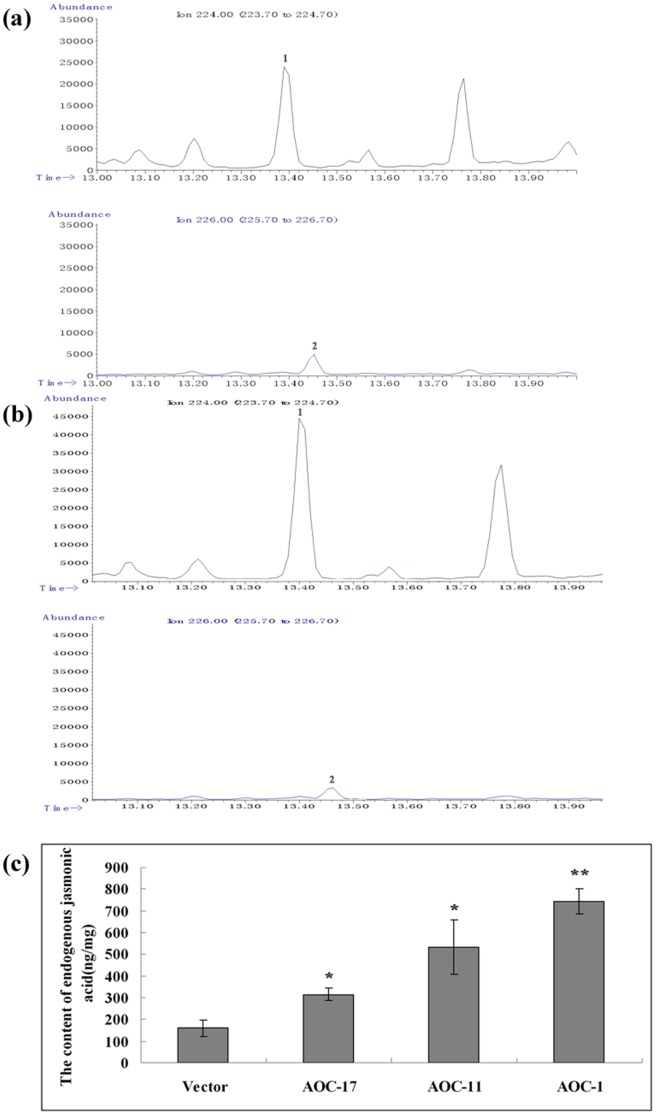
Three independent transgenic lines were chosen for further analysis. Comparing with the chromatograms and mass spectrometry of JA and DHJA standards, the special peaks of JA and DHJA were found. The retention time of JA was at 13.399 min, while the retention time of DHJA was at 13.456 min (Figure S3a and S3b in File S1). Then, the special peaks of 224 m/z and 226 m/z were extracted and integral, respectively. The ratio of those two peaks were used to count the concentrations of JA. The results showed that the contents of JA were increased 2- to 4.7-fold compared to the control (Figure 4). The statistics analysis showed that the observed differences were statistically significant. All the results demonstrated that the content of endogenous JA increased significantly in AaAOC-overexpression transgenic A. annua. The growth of AaAOC-overexpression lines were barely or only slightly changed versus the control (Figure S4 in File S1).
Figure 4. The measurement of endogenous JA by GC-MS.
(a) Chromatograms of JA and DHJA in empty-vector transgenic lines. (b) Chromatograms of JA and DHJA in AaAOC-overexpression transgenic lines. (c) The content of endogenous JA in empty-vector transgenic plants and AaAOC-overexpression transgenic plants. Error bars are SE (n = 3). Statistical significance was determined by students't-test (**, P<0.01; *, P<0.05). These asterisks are the difference between the empty-vector and AaAOC-overexpression transgenic A. annua.
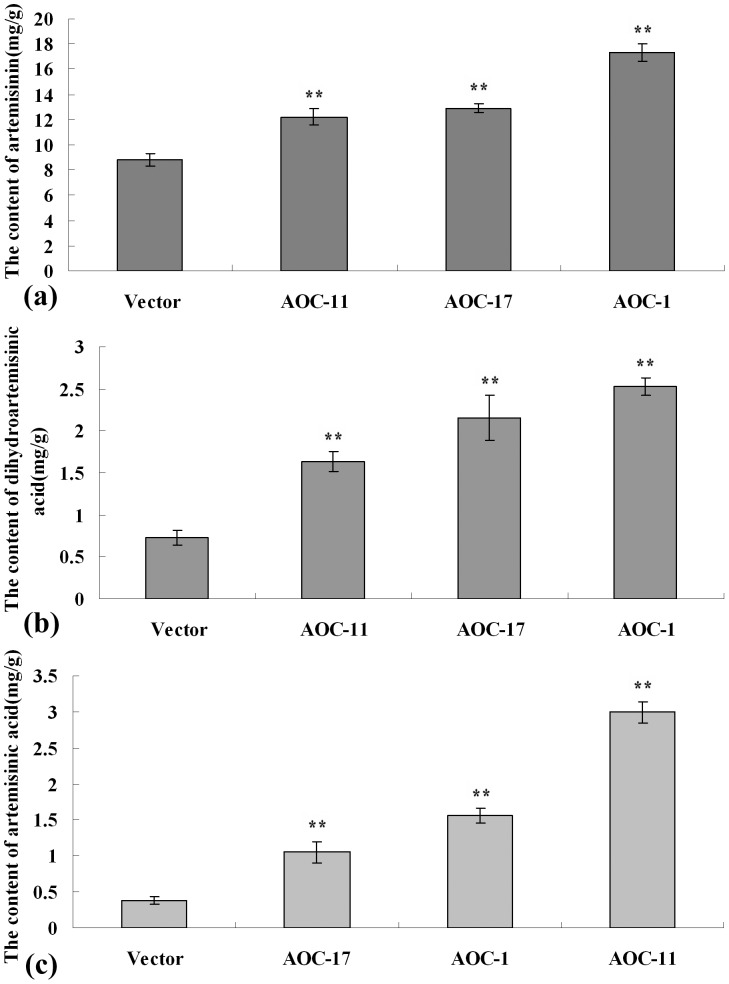
In view of exogenous JAs can increase the contents of sesquiterpenoids [18], to examine whether increased endogenous JA could enhance the biosynthesis of artemisinin, mature leaves of 5-month-old plants were analyzed by HPLC. Compared to the control, the content of artemisinin was increased by 38–97% in AaAOC-overexpression transgenic plants (Figure 5a). Compared to the control, the content of dihydroartemisinic acid was increased by 125–248%, while the content of artemisinic acid was increased by 172–675% (Figure 5b and 5c). The statistics analysis showed that the observed differences were statistically significant.
Figure 5. Measurements of artemisinin, dihydroartemisinic acid and artemisinic acid by HPLC in transgenic A. annua.
(a) The content of artemisinin in empty-vector and three independent AaAOC-overexpression transgenic A. annua. (b) The content of dihydroartemisinic acid in empty-vector and three independent AaAOC-overexpression transgenic A. annua. (c) The content of artemisinic acid in empty-vector and three independent AaAOC-overexpression transgenic A. annua. Error bars are SE (n = 3). Experiments were repeated triplicates.
The increased accumulation of sesquiterpenoids promoted us to analyse the reasons. Therefore, we analyzed the transcript levels of related genes in artemisinin biosynthesis by RT-Q-PCR (Figure S5 in File S1). The results were shown in figure 6. Briefly, compared to the control, the transcript levels of FPS were increased 1.7- to 4.3-fold, while the expression levels of CYP71AV1 and DBR2 were increased 5.8- to 17-fold and 1.5- to 5.1-fold, respectively (Figure 6). The statistics analysis showed that the observed differences were statistically significant. The expression levels of ADS (amorphadiene synthase), CPR (cytochrome P450 reductase) and ALDH1 (aldehyde dehydrogenase 1) were barely or only slightly changed (Figure 6).
Figure 6. Expression analyses of artemisinin biosynthetic pathway key genes in transgenic A. annua by RT-Q-PCR.
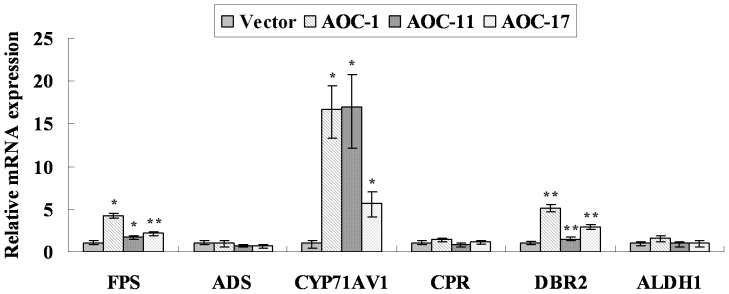
FPS, farnesyl diphosphate synthase gene; ADS, amorphadiene synthase gene; CYP71AV1, cytochrome P450 dependent hydroxylase gene; CPR, cytochrome P450 reductase gene; DBR2, double bond reductase 2 gene; Aldh1, aldehyde dehydrogenase 1 gene.
Discussion
Allene oxide cyclase (AOC) is the key enzyme of jasmonate biosynthetic pathway, which catalyses the formation of OPDA and establishes the stereochemical configuration of naturally occurring JA10. Here, our results of GC-MS demonstrated that overexpression of AaAOC gene could significantly increase the content of endogenous JA and artemisinin in transgenic A. annua plants. Mechanical wounding of Arabidopsis leaves led to an increase in JA-Ile, which is preceded by a large increase in free JA [1], [19], [20]. So, the wounding of A. annua plants could increase the content of endogenous JA, while the increased endogenous JA may promote the biosynthetic pathway of artemisinin. The presumption were consistent with the results of Liu et al., which showed that wounding stress can significantly elevate the artemisinin content by increasing the expression levels of some key genes in artemisinin biosynthetic pathway [21]. High level of JA can inhibit the root growth in growth medium [22]. We observed the phenotypes of the control and AaAOC-overexpression lines. The growth of AaAOC-overexpression lines were only slightly changed versus the control. So, we inferred that the concentrations of JA should reach to a high level which could result in the significant decrease of root growth and plants' growth.
Meas et al. showed that exogenous JA treatments promoted the expression levels of artemisinin biosynthetic pathway, which ultimately led to increased artemisinin accumulation in A. annua [17]. However, the significance and function of endogenous JA in artemisinin biosynthetic pathway remain unknown in A. annua. The results of RT-Q-PCR showed that, compared to the control level, the transcript levels of FPS, CYP71AV1 and DBR2 were increased significantly in AaAOC-overexpression transgenic A. annua, while ADS, CPR and ALDH1 were barely or only slightly changed. The results of HPLC showed that sesquiterpenoids accumulation significantly increased in AaAOC-overexpression transgenic A. annua. Interestingly, AOC-1, which has the highest content of endogenous JA, had the highest expression levels of FPS and DBR2 in transgenic A. annua plants. The contents of artemisinin and dihydroartemisinic acid in AOC-1 were also the highest in transgenic A. annua plants. From the above results, we concluded that the increased endogenous JA significantly promoted the expression levels of some key genes in aretmisinin biosynthetic pathway and resulted in the increase of sesquiterpenoids accumulation in AaAOC-overexpression transgenic A. annua. All above results demonstrated that the increased endogenous JA could positively regulate the biosynthesis of artemisinin in AaAOC-overexpression transgenic A. annua. So, endogenous JA also played an important role in the biosynthesis of artemisinin in A. annua.
In this study, 2276 bp AaAOC-promoter sequence was cloned by genomic walking. GUS staining showed that AaAOC was expressed ubiquitiously in A. annua plants. In AaAOC-overexpression transgenic A.annua, the content of endogenous JA was 2- to 4.7-fold of the control. The result of HPLC showed that sesquiterpenoids accumulation was increased significantly in AaAOC-overexpression transgenic A.annua. RT-Q-PCR showed that the expression levels of FPS, CYP71AV1 and DBR2 were increased significantly in AaAOC-overexpression transgenic A. annua. In a word, overexpression of AaAOC gene significantly increased the content of endogenous JA in transgenic A. annua. And the increased endogenous JA promoted the expression levels of some key genes in artemisinin biosynthetic pathway, which resulted in the increase of artemisinin biosynthesis in AaAOC-overexpression transgenic A. annua plants.
Materials and Methods
Materials
The seeds of A. annua were obtained from the School of Life Sciences, Southwest University in Chongqing, P.R. China. Leaves from A. annua plants were collected for RNA extraction using plant RNA isolation reagent (Tiangen Biotech, Beijing) following the manufacturer's instructions.
Clone of AaAOC promoter and β-glucuronidase (GUS) staining in transgenic A. annua
The upstream region of AaAOC was amplified from genomic DNA using the Genome Walker Kit (Clontech, Canada). The AaAOC-specific primers (AaAOC-sp1, AaAOC-sp2), Adaptor Primer 1 and Adaptor Primer 2 were used following the manufacturer's recommended procedures. The final reaction products were electrophoresed in 1% agarose gel, and a 2276 bp fragment was eluted from the gel and cloned into the pMD18-T simple vector. The insert DNA was sequenced by Shenzhen Genomics Institute.
To generate the AaAOC promoter-GUS construct, the 5′-flanking DNA of the AaAOC coding region was amplified with AaAOC-PF and AaAOC-PR. The 2.2 kb of PCR fragment was cloned into the pCAMBIA1391Z vector. The construct was transformed into A. annua plants as described previously [23]. Histochemical staining for GUS activity in transgenic plants was performed as the protocol described previously [24]. Plants transformed with pCAMBIA1391Z were used as a parallel negative control.
AaAOC-overexpression in A. annua
AaAOCF and AaAOCR were designed to amplify the ORF of AaAOC with A. annua cDNA as template. The pMD18-T-AaAOC vector was digested by BamHI and SacI. The full-length ORF of AaAOC was cloned into the BamHI and SacI sites of the pCAMBIA2300+ vector under the 35S promoter to generate pCAMBIA2300-35S::AaAOC::NOS. The construct was then transferred into A. tumefaciens strain EHA105 by the conventional freezing and melting method, and the resulted strains were used in the transformation of A. annua. The transformation of A. annua was performed following the protocol of Zhang et al [23]. All the transgenic plants were grown in the green house for 5 months.
PCR analysis in transgenic A. annua
The genomic DNA for PCR detective was isolated from fresh leaves by using the modified CTAB (cetylmethylammonium bromide) method [25]. The introduced AOC gene and NPTII gene were respectively detected by primers P35S, AaAOC-RT-R and NPTII-R. PCR reaction was carried out in a 20 µl reaction volume. The thermal cycling conditions was 94°C for 4 min, followed by 35 cycles of amplification (94°C for 30 s, 56°C for 30 s and 72°C for 1 min10 s) and 72°C for additional 10 min.
Real-time Quantitative PCR analysis (RT-Q-PCR)
The expression levels of AaAOC and key genes of artemisinin biosynthetic pathway in AaAOC-overexpression A. annua were analysed by RT-Q-PCR method. All RNA samples were digested with DNaseI prior to use. Aliquots of 0.4 µg total RNA were employed in the reverse transcriptase reaction using random hexamer primers for the synthesis of first strand cDNA. The amplification reactions of RT-Q-PCR were performed on an iCycler iQTM Real-Time PCR Machines (Bio-Rad, Watford, UK), and the SYBR ExScript RT-PCR kit (Takara, Shiga, Japan) protocol to confirm changes of gene expression. Procedures of RT-Q-PCR were performed as previously described [26]. The actin gene was used as the constitutive control gene. All the primers used in RT-Q-PCR were listed in Table S1 in File S1.
Extraction and qualitative analysis of endogenous JA by GC-MS
The extract procedure was done as described previously with some modifications [27], [28]. Leaves of transgenic and control plants were homogenised in liquid N2, then all the powder of samples were frozen dried; 50 mg of dry samples mixed with 1 ml of 2-propanol/water/concentrated HCL (2∶1∶0.002 v/v/v) and added 1000 ng of DHJA as the internal standard, then vortex for 1 min. After sonicate for 30 min, 1 ml of methylene chloride were added to the mixtures and vortex for 1 min; After centrifugation at 3500 rpm for 15 min at 5°C, the bottom methylene chloride/2-propanol layer was collected in new glass tubes. 2 µl of 2M trimethylsilyldiazomethane in hexane was added to the mixture. Cap the vials and allow them to stay at RT for 30 min. 2 µl of 2M acetic acid in hexane was added to the mixture. Dry under gaseous N2. Then, 200 µl of methylene chloride was added to reconstituted, then centrifugation at 10000 rpm for 3 min. The supernatant were collected to analyse by GC-MS.
The extracts were analysed using GC-MS (7890A GC/5975C MS, Agilent, USA). The column was DB-5MS (30 m×0.25 mm×0.25 µm). The program was 80°C (2 min hold), from 80°C to 200°C at 10°C/min, from 200°C to 300°C (15 min hold) at 20°C/min, with a splitless injector and electronic pressure control. The MS conditions were EI+ of 70 eV, full scan. The scan range was 33–500 m/z. The compounds were identificated by searching the NIST2011 library and the retention index (minutes) of JA and DHJA standards were obtained.
Metabolite analysis by High-performance liquid hromatography (HPLC)
Pooled leaves from 10th, 15th, 20th branches (counted from the apical meristem) of five month-old plants were harvested as samples. The leaves were dried at 45°C and grounded. The dried-leaf powder (0.1 g/sample) was extracted with methanol (2 ml) using a Shanghai Zhishun Instrument Co. Ltd model JYD-650 ultrasonic processor (two bursts of 30 min each). Then, the samples were centrifuged for 10 min at 4000 rpm to remove the suspended particles. The final supernatant was filtered through a 0.25-µm-pore-size filter.
Artemisinin, dihydroartemisinic acid and artemisinic acid were measured using a Waters Alliance 2695 HPLC system. The methods of measure these compounds were described as Ferreira and Gonzalez [29]. The results were analysed using Empower (Waters' chromatography data software).
Statistical analyses
All the experiments including GUS staining, PCR, real-time quantitative PCR analysis, HPLC analysis and measurement of endogenous JA by GC-MS were repeated three times. Results of metabolites content and real-time quantitative PCR analysis are presented as mean values ± S.D. The error bars are due to biological triplicates. Statistical significance was determined by students't-test (**, P<0.01; *, P<0.05).
Supporting Information
Contains the following files: Figure S1. Gus-staining of transgenic A. annua using the pCAMBIA1391Z empty vector. Figure S2. Analysis of AaAOC-overexpression transgenic A.annua by PCR. (a) PCR analysis using 35S forward primer and AaAOC reverse primer in AaAOC-overexpression transgenic A. annua. (b) PCR analysis using 35S forward primer and reverse primer of NPTII gene in AaAOC-overexpression transgenic A. annua. Figure S3. Chromatograms and mass spectrometry of JA and DHJA standards. (a) Chromatograms and mass spectrometry of JA. Peak1 is JA and the retention time is 13.399 min. (b) Chromatograms and mass spectrometry of DHJA. Peak2 is DHJA and the retention time is 13.456 min. Figure S4. The phenotypes of the transgenic A.annua and empty vector line. Figure S5. The synthetic pathway of artemisinin in A.annua. FPS, farnesyl diphosphate synthase; ADS, amorphadiene synthase; CYP, cytochrome P450 dependent hydroxylase (CYP71AV1); CPR, cytochrome P450 reductase; DBR2, double bond reductase 2; Aldh1, aldehyde dehydrogenase 1. Table S1. Primers used in this investigation.
(DOC)
Acknowledgments
We thank the Instrumental Analysis Center of the Shanghai Jiao Tong University for assistance with GC–MS analysis.
Note
The novel nucleotide sequence data published here have been deposited in the EMBL/DDBJ/GenBank databases under accession number KC477937.
Funding Statement
China “863” Program (grant no. 2011AA100605), the URL of funder's website: http://www.most.gov.cn/eng/programmes1/200610/t20061009_36225. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Browse J (2009) Jasmonate Passes Muster: A Receptor and Targets for the Defense Hormone. Annu Rev Plant Biol 60: 183–205. [DOI] [PubMed] [Google Scholar]
- 2. Vijayan P, Shockey J, Levesque CA, Cook RJ, Browse J (1998) A role for jasmonate in pathogen defense of Arabidopsis. Proc Natl Acad Sci USA 95: 7209–7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang DH, Hettenhausen C, Baldwin IT, Wu JQ (2011) BAK1 regulates the accumulation of jasmonic acid and the levels of trypsin proteinase inhibitors in Nicotiana attenuata's responses to herbivory. J Exp Bot 62: 641–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu WS, Mazarei M, Rudis MR, Fethe MH, Peng YH, et al. (2013) Bacterial pathogen phytosensing in transgenic tobacco and Arabidopsis plants. Plant Biotechnol J 11: 43–52. [DOI] [PubMed] [Google Scholar]
- 5. Van der Fits L, Memelink J (2000) ORCA3, a jasmonate-responsive transcriptional regulator of plant primary and secondary metabolism. Science 289: 295–297. [DOI] [PubMed] [Google Scholar]
- 6. Jiang KJ, Pi Y, Hou R, Jiang LL, Sun XF, et al. (2009) Promotion of nicotine biosynthesis in transgenic tobacco by overexpressing allene oxide cyclase from Hyoscyamus niger . Planta 229: 1057–1063. [DOI] [PubMed] [Google Scholar]
- 7. Gu XC, Chen JF, Xiao Y, Di P, Xuan HJ, et al. (2012) Overexpression of allene oxide cyclase promoted tanshinone phenolic acid production in Salvia miltiorrhiza . Plant Cell Rep 31: 2247–2259. [DOI] [PubMed] [Google Scholar]
- 8. Tretner C, Huth U, Hause B (2008) Mechanostimulation of Medicago truncatula leads to enhanced levels of jasmonic acid. J Exp Bot 59: 2847–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee DS, Nioche P, Hamberg M, Raman CS (2008) Structural insights into the evolutionary paths of oxylipin biosynthetic enzymes. Nature 455: 363–368. [DOI] [PubMed] [Google Scholar]
- 10. Ziegler J, Stenzel I, Hause B, Maucher H, Miersch O, et al. (2000) Molecular cloning of allene oxide cyclase – the enzyme establishing the stereochemistry of octadecanoids and jasmonates. J Biol Chem 275: 19132–19138. [DOI] [PubMed] [Google Scholar]
- 11. Sanders PM, Lee PY, Biesgen C, Boone JD, Beals TP, et al. (2000) The Arabidopsis DELAYED DEHISCENCE1 gene encodes an enzyme in the jasmonic acid synthesis pathway. Plant Cell 12: 1041–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schaller F, Biesgen C, Müssig C, Altmann T, Weiler EW (2000) 12-Oxophytodienoate reductase 3 (OPR3) is the isoenzyme involved in jasmonate biosynthesis. Planta 210: 797–984. [DOI] [PubMed] [Google Scholar]
- 13. Koo AJ, Chung HS, Kobayashi Y, Howe GA (2006) Identification of a peroxisomal acyl-activating enzyme involved in the biosynthesis of jasmonic acid in Arabidopsis. J Biol Chem 281: 33511–33520. [DOI] [PubMed] [Google Scholar]
- 14. Andersson MX, Hamberg M, Kourtchenko O, Brunnström A, McPhail KL, et al. (2006) Oxylipin profiling of the hypersensitive response in Arabidopsis thaliana. Formation of a novel oxo-phytodienoic acid-containing galactolipid, Arabidopside E. J Biol Chem 281: 31528–31537. [DOI] [PubMed] [Google Scholar]
- 15. Vick BA, Zimmerman DC (1983) The biosynthesis of jasmonic acid: a physiological role for plant lipoxygenase. Biochem Biophys Res Commun 111: 470–477. [DOI] [PubMed] [Google Scholar]
- 16. Turner JG, Ellis C, Devoto A (2002) The jasmonate signal pathway. Plant Cell 14 Supplement: 153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weathers PJ, Elkholy S, Wobbe KK (2006) Artemisinin: the biosynthetic pathway and its regulation in Artemisia annua, a terpenoid-rich species. In Vitro Cell Dev-PL 42: 309–317. [Google Scholar]
- 18. Maes L, Van Nieuwerburgh FCW, Zhang Y, Reed DW, Pollier J, et al. (2011) Dissection of the phytohormonal regulation of trichome formation and biosynthesis of the antimalarial compound artemisinin in Artemisia annua plants. New Phytol 189: 176–189. [DOI] [PubMed] [Google Scholar]
- 19. Chung HS, Koo AJ, Gao X, Jayanty S, Thines B, et al. (2008) Regulation and function of Arabidopsis Jasmonate ZIM-domain genes in response to wounding and herbivory. Plant Physiol 146: 952–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Suza WP, Staswick PE (2008) The role of JAR1 in Jasmonoyl-l-isoleucine production during Arabidopsis wound response. Planta 227: 1221–1232. [DOI] [PubMed] [Google Scholar]
- 21. Liu DH, Zhang LD, Li CX, Yang K, Wang YY, et al. (2010) Effect of wounding on gene expression involved in artemisinin biosynthesis and artemisinin production in Artemisia annua . Russ J Plant Physiol 57: 882–886. [Google Scholar]
- 22. Staswick PE, Su W, Howell SH (1992) Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. PNAS 89: 6837–6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang L, Jing FY, Li FP, Li MY, Wang YL, et al. (2009) Development of transgenic Artemisia annua (Chinese wormwood) plants with an enhanced content of artemisinin, an effective anti-malarial drug, by hairpin-RNA-mediated gene silencing. Biotechnol Appl Bioc 52: 199–207. [DOI] [PubMed] [Google Scholar]
- 24. Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stewart CN Jr, Via LE (1993) A Rapid CTAB DNA Isolation Technique Useful for RAPD Fingerprinting and Other PCR Applications. BioTechniques 14: 748–749. [PubMed] [Google Scholar]
- 26. Lu X, Jiang WM, Zhang L, Zhang FY, Shen Q, et al. (2012) Characterization of a novel ERF transcription factor in Artemisia annua and its induction kinetics after hormones and stress treatments. Mol Biol Rep 39: 9521–9527. [DOI] [PubMed] [Google Scholar]
- 27. Engelberth J, Schmelz EA, Alborn HT, Cardozab YJ, Huang J, et al. (2003) Simultaneous quantification of jasmonic acid and salicylic acid in plants by vapor-phase extraction and gas chromatography-chemical ionization-mass spectrometry. Anal Biochem 312: 242–250. [DOI] [PubMed] [Google Scholar]
- 28. Müller A, Düchting P, Weiler EW (2002) A multiplex GC-MS/MS technique for the sensitive and quantitative single-run analysis of acidic phytohormones and related compounds, and its application to Arabidopsis thaliana . Planta 216: 44–56. [DOI] [PubMed] [Google Scholar]
- 29. Ferreira JFS, Gonzalez JM (2009) Analysis of underivatized artemisinin and related sesquiterpene lactones by high-performance liquid chromatography with ultraviolet detection. Phytochem Anal 20: 91–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Contains the following files: Figure S1. Gus-staining of transgenic A. annua using the pCAMBIA1391Z empty vector. Figure S2. Analysis of AaAOC-overexpression transgenic A.annua by PCR. (a) PCR analysis using 35S forward primer and AaAOC reverse primer in AaAOC-overexpression transgenic A. annua. (b) PCR analysis using 35S forward primer and reverse primer of NPTII gene in AaAOC-overexpression transgenic A. annua. Figure S3. Chromatograms and mass spectrometry of JA and DHJA standards. (a) Chromatograms and mass spectrometry of JA. Peak1 is JA and the retention time is 13.399 min. (b) Chromatograms and mass spectrometry of DHJA. Peak2 is DHJA and the retention time is 13.456 min. Figure S4. The phenotypes of the transgenic A.annua and empty vector line. Figure S5. The synthetic pathway of artemisinin in A.annua. FPS, farnesyl diphosphate synthase; ADS, amorphadiene synthase; CYP, cytochrome P450 dependent hydroxylase (CYP71AV1); CPR, cytochrome P450 reductase; DBR2, double bond reductase 2; Aldh1, aldehyde dehydrogenase 1. Table S1. Primers used in this investigation.
(DOC)