Abstract
Background
With one quarter of the world population infected, the intestinal nematode Ascaris lumbricoides is one of the most common infectious agents, especially in the tropics and sub-tropics. Infection is caused by oral intake of eggs and can cause respiratory and gastrointestinal problems. To identify high risk areas for intervention, it is necessary to understand the effects of climatic, environmental and socio-demographic conditions on A. lumbricoides infection.
Methodology
Cross-sectional survey data of 6,366 study participants in the Mbeya region of South-Western Tanzania were used to analyze associations between remotely sensed environmental data and A. lumbricoides infection. Non-linear associations were accounted for by using fractional polynomial regression, and socio-demographic and sanitary data were included as potential confounders.
Principal Findings
The overall prevalence of A. lumbricoides infection was 6.8%. Our final multivariable model revealed a significant non-linear association between rainfall and A. lumbricoides infection with peak prevalences at 1740 mm of mean annual rainfall. Mean annual land surface temperature during the day was linearly modeled and negatively associated with A. lumbricoides infection (odds ratio (OR) = 0.87, 95% confidence interval (CI) = 0.78–0.97). Furthermore, age, which also showed a significant non-linear association (infection maximum at 7.7 years), socio-economic status (OR = 0.82, CI = 0.68–0.97), and latrine coverage around the house (OR = 0.80, CI = 0.67–0.96) remained in the final model.
Conclusions
A. lumbricoides infection was associated with environmental, socio-demographic and sanitary factors both in uni- and multivariable analysis. Non-linear analysis with fractional polynomials can improve model fit, resulting in a better understanding of the relationship between environmental conditions and helminth infection, and more precise predictions of high prevalence areas. However, socio-demographic determinants and sanitary conditions should also be considered, especially when planning public health interventions on a smaller scale, such as the community level.
Introduction
The intestinal nematode Ascaris lumbricoides is one of the most common causes of infection among the soil-transmitted helminths (STH). Common in the tropics and sub-tropics, it is estimated that more than one quarter of the world population is infected with this helminth [1]–[3].
The highest morbidity is found in children, especially in those with a high worm burden. A. lumbricoides can lead to reduced physical fitness, growth retardation, and respiratory and gastrointestinal problems [3]–[6]. Evidence if A. lumbricoides infection has a negative impact on cognitive function and educational achievement in school children is controversially debated [7]–[10].
Infection occurs through the oral intake of eggs, usually contained in soil or food. Adult worms live in the lumen of the small intestine where the female lays unembryonated eggs which are excreted with the feces. In the open, the eggs have to go through three stages of development in order to become infectious; a time during which they are exposed to environmental conditions [5], [11], [12]. When embryonated eggs are swallowed by a human host, the larvae hatch in the small intestine, have a short migratory phase (venous system, liver, lungs, trachea, esophagus) after which they return to the small intestine where they mature and mate [13], [14].
Recently, remotely sensed environmental data have increasingly been used to get a better understanding of the epidemiology and spatial distribution of STH [15]–[22]. The use of environmental data in combination with geographic information systems (GIS) has become a powerful tool for mapping and predicting STH, with the main purpose to identify high risk areas for intervention [23]–[26].
However, there are still challenges in the statistical modeling of environmental data. One problem is the consideration of non-linear relationships between outcome and predictor variables. Although non-linear relationships between environmental data and STH infection are a recognized fact [16], [22], this has rarely been taken into account in multivariable analysis. A further complication is the need to take care of potential confounders. Especially risk factors linked to transmission, such as poor sanitation facilities, crowding, and high population density [2], [6], [12], [14], [27]–[32], need to be considered when associations between environmental factors and STH infection are analyzed.
Therefore, the main objective of this study was to assess associations between remotely sensed environmental data and A. lumbricoides infection while considering potential non-linear relationships and confounders. A manuscript that examines associations of hookworm infection with environmental factors has recently been accepted [33], and manuscripts regarding Trichuris trichiura and schistosome infection are presently being prepared.
Methods
Ethics Statement
The study was approved by the ethics committee of the Tanzanian National Institute for Medical Research and conducted according to the principles expressed in the Declaration of Helsinki. All participants provided written informed consent before enrolment into the study; parents consented for their children below 18 years of age. Specifically, children who were old enough to understand the process were asked to participate in the consenting procedure, and children who were 12 years old or older were asked to sign/thumbprint the document in addition to their parent's signature/thumbprint.
Study Area and Epidemiological Data Collection
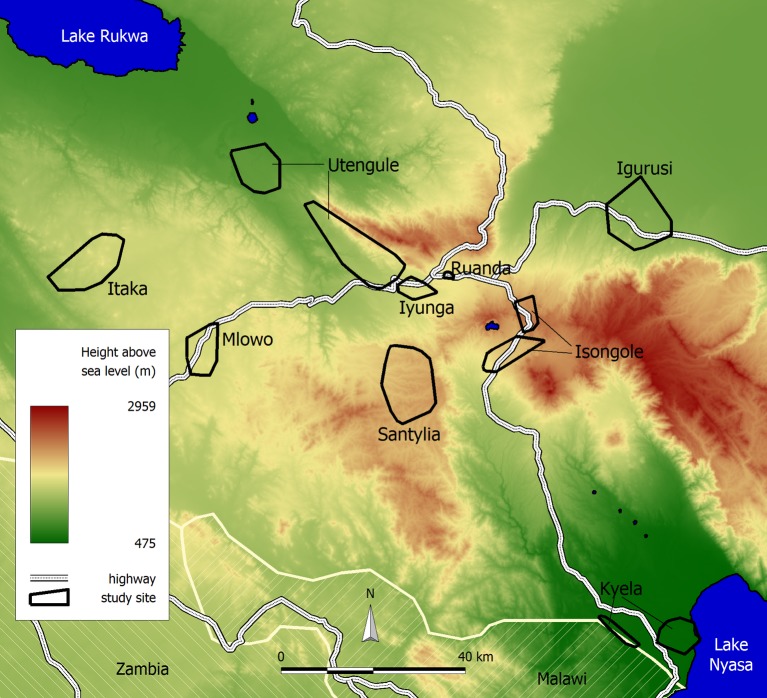
Data for this study were collected in nine study sites in the Mbeya Region in south-western Tanzania (Figure 1) from June 2008 until June 2009 during the third annual survey of the EMINI (Evaluating and monitoring the impact of new interventions) cohort study. The region is predominantly rural and most income generating activities are related to agriculture. During an initial population census in the nine sites, more than 42,000 households were identified and their geographical positions recorded, using handheld geographical positioning system (GPS) devices (SporTrak handheld GPS, Magellan Navigation Inc., Santa Clara, CA, USA). Geographically stratified random selection was used to choose 10% (4,283) of these households to participate in the main EMINI cohort study. During each annual survey these households were visited once to collect biological specimen and interview data. All participants provided written informed consent before inclusion into the study, with parents or care takers consenting for their minors.
Figure 1. Location and altitude of the EMINI study sites.
The large altitude range results in very diverse environmental conditions regarding temperature, vegetation, slope etc.
The collection of stool samples started in 2008. Due to logistic constraints, households were randomized into two groups of equal size of which only one was annually sampled for stool.
Interviews to characterize the socio-economic status (SES) of each household were conducted with the household head and included questions regarding infrastructure of the household, ownership of livestock, the availability of certain household assets, and materials that were used to build the house(s) in each homestead. Data on socio-demographic status (sex, age, marital status, religious denomination, education, occupation etc.), relevant behavior, knowledge and practices regarding various diseases were collected in interviews with each individual household member or – for children below 12 years – with their caretaker. All interviews and medical examinations were performed at the household and conducted in Kiswahili language.
Before stool collection started in the third survey round, intestinal nematodes were neither diagnosed nor treated as part of this study, and to our knowledge no other treatment programs had been conducted in the region. Stool samples were collected in pre-labeled screw-top containers, refrigerated at 4 °C directly after collection, using mobile refrigerators (WAECO CoolFreeze CF-50, WAECO, Emsdetten, Germany) and kept cool until slide preparation in the laboratory within two days of collection. The A. lumbricoides infection status of participants was established by Kato-Katz examination [34] of two sub-samples (41.7 mg each) from a single stool specimen, which was thoroughly mixed before slide preparation. Kato-Katz slides were examined for A. lumbricoides eggs by experienced staff within two days after slide preparation. A. lumbricoides infection was defined as the presence of at least one A. lumbricoides egg in any of the two slides and infection intensity was classified according to Montresor et al. [35]. To assure the quality of our lab results all Kato-Katz slides were archived and a sample of randomly selected slides were reexamined after at least one month by different lab staff.
Helminth infected participants were offered treatment with albendazole (for A. lumbricoides and other intestinal nematode infections) and/or praziquantel (for schistosome infections), according to their respective diagnosis.
Environmental Data
The following remotely sensed environmental data were considered for this analysis: Elevation was obtained using the NASA Shuttle Radar Topography Mission (SRTM) global digital elevation model (DEM) version 2.1 [36]. These elevation data were also used to calculate the slope. Mean annual rainfall and ambient temperature were downloaded from the WorldClim – Global Climate Data website [37]. Mean annual land surface temperature during day and night (LST-day and LST-night) and vegetation cover (Enhanced vegetation index (EVI)) which had been collected during NASA's Moderate-Resolution Imaging Spectroradiometer (MODIS) Terra mission, were downloaded from the Land Processes Distributed Active Archive Center (LP DAAC) [38], [39].
Household positions and inhabitant numbers from the initial population census were used to calculate population density around the household. Population density, ambient temperature, elevation, rainfall, LST, EVI, and slope were averaged for a buffer area within a 1,000 meter radius around each homestead in order to characterize the environmental situation around the household. This approach was preferred to using the respective spot values at the homestead position because spot data are more prone to random error than averages for a wider area. Latrine coverage in the surroundings of each household was calculated as the inverse distance weighted percentage of households with their own latrine within one kilometer around the household.
Initial processing of remotely sensed data was done in Idrisi GIS software v.32 (Clark Labs, Worcester, MA, USA). The GIS program Manifold System 8.0 Professional Edition (Manifold Net Ltd, Carson City, NV) was used to combine household positions and environmental data.
Socio-economic Status and Other Confounding Variables
Household income and expenditure data in developing countries, especially in rural areas, are often unreliable because many people do not have a regular cash income. To overcome this problem we employed a modification of a method initially proposed by Filmer and Pritchett (2001) that uses principal component analysis to generate an SES score using proxy variables [40]–[42]. The following proxy variables were used: Household assets (clock or watch, radio, television, mobile telephone, refrigerator, hand cart, bicycle, motor cycle, car, savings account), construction materials for the house, and sources of household fuels and drinking water. In addition to the above described SES score, age, sex, population density, latrine coverage around the household, and presence of a latrine in the household were considered as potential confounders.
Statistical Analysis
All statistical analyses were performed using Stata/SE (Version 11.2, StataCorp LP, College Station, TX). Because our environmental variables showed a high degree of correlation, multicollinearity was assessed using the variance inflation factor (VIF) (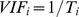 ) calculated with the tolerance (T) (
) calculated with the tolerance (T) (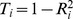 ).
). 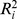 is the calculated variance of each covariate associated with the rest of the other independent variables. A VIF higher than 10 indicates a serious problem of multicollinearity [43]–[45].
is the calculated variance of each covariate associated with the rest of the other independent variables. A VIF higher than 10 indicates a serious problem of multicollinearity [43]–[45].
After multicollinearity analysis we performed univariable linear logistic regressions for each considered independent variable. All variables with a univariable Wald's p<0.2 were included in the multivariable analysis.
In our study design individual observations were clustered in households and these were clustered within study sites. Therefore, household clustering was accounted for by calculation of robust standard errors using Huber/White variance estimates [46]–[48] and the nine study sites were taken into account as dummy variables.
Multivariable logistic regression with the inclusion of fractional polynomials which is a flexible parametric approach for modeling continuous factors was applied to analyze non-linear associations between A. lumbricoides infection and environmental variables [49]. The power transformations 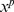 are found with a predefined set of powers S = −2,−1,−0.5,0,0.5,1,2,3 where
are found with a predefined set of powers S = −2,−1,−0.5,0,0.5,1,2,3 where 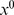 is defined as
is defined as 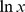 . A fractional polynomial model with one degree (FP1) takes the form
. A fractional polynomial model with one degree (FP1) takes the form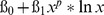 , with two degrees (FP2)
, with two degrees (FP2)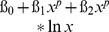 . The restriction of powers and the consideration of polynomials with degree 1 and 2 provide enough flexibility for statistical modeling [50], [51].
. The restriction of powers and the consideration of polynomials with degree 1 and 2 provide enough flexibility for statistical modeling [50], [51].
Multivariable fractional polynomial (MFP) models, an extended algorithm introduced by Royston and Sauerbrei, were used to detect non-linear associations. The MFP algorithm contains a function selection procedure which compares null, linear, and FP1 sub models for each covariate with an FP2 model based on the deviance [52]. A detailed description of the MFP algorithm is found in Ambler and Royston (2001) and in Sauerbrei and Royston (2008) [53], [54]. For the function selection procedure a lower p-value than 0.05 is recommended to avoid over fitting [50]. Therefore, a p-value of 0.01 was chosen as cut off when non-linear sub models were compared.
Our final model was calculated by removing variables with a p-value above 0.05. Changes of the Akaike Information Criterion (AIC) [55] and the Bayesian Information Criterion (BIC) [56], measuring the relative goodness of fit, were simultaneously considered. In order to asses spatial autocorrelation in the raw A. lumbricoides infection data and in the deviance residuals of our final logistic model the Stata module “spatcorr” was used to calculate Moran's I [57].
Results
Descriptive Results
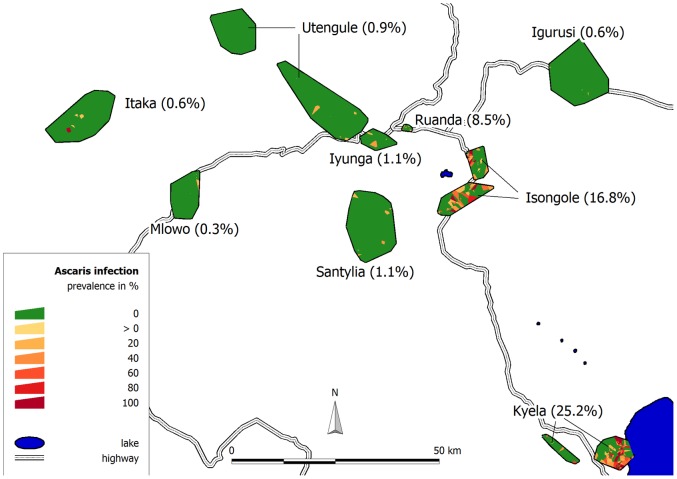
The overall prevalence of A. lumbricoides infection in the study population was 6.8% (n = 433/6,366). Most infections were of low intensity, moderate and high intensity infections were rare. The highest prevalences were found in Kyela (25.2%) and Isongole (16.9%). sites. Figure 2 demonstrates that A. lumbricoides infection was clustered both between and within sites.
Figure 2. A. lumbricoides prevalence in the study sites.
Color coding indicates household prevalence, labels indicate site name and site prevalence. A. lumbricoides infection is strongly clustered both between and within sites.
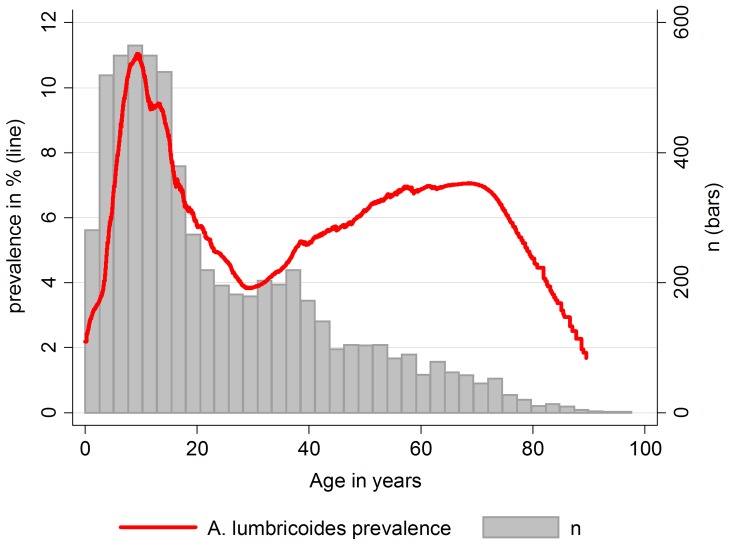
Men (47%) and women (53%) were almost equally represented in the study and the mean age was 23.6 years. Thus the majority of the study population were children and adolescents and the peak of A. lumbricoides infection occurred before the age of ten years (Figure 3). Nearly all households (97.5%) had their own latrine, which was a pit latrine in most cases (Table 1). The prevalence of A. lumbricoides infection was similar in female (6.57%) and male (7.11%) participants.
Figure 3. Lowess smoothed plot of unadjusted A. lumbricoides prevalence over age.
The main prevalence peak in childhood is in accordance with the age of maximum infection intensity mentioned in the literature [14], [67]. The second rise above the age of 30 with a less pronounced peak in older age seems less common.
Table 1. Description of variables.
| Variables | N | Mean or percentage a) | Std. Dev. | Min | Max |
| Ascaris infected | 6,366 | 6.8% | |||
| Ascaris infection intensity b): | |||||
| No infection (0 EPG) | 5,933 | 93.2% | |||
| Low intensity (1–1,999) | 317 | 4.98% | |||
| Moderate intensity (2,000–3,999 EPG) | 66 | 1.04% | |||
| Heavy intensity (≥4,000 EPG) | 50 | 0.97% | |||
| Elevation [m] | 6,366 | 1455 | 486 | 479 | 2313 |
| Mean annual ambient temperature [°C] | 6,366 | 19.8 | 2.8 | 14.7 | 25.0 |
| Mean annual rainfall [mm] | 6,366 | 1437 | 378 | 1013 | 2342 |
| Mean annual LST-day [°C] | 6,366 | 32.4 | 2.5 | 22.5 | 38.6 |
| Mean annual LST-night [°C] | 6,366 | 14.5 | 3.4 | 9.2 | 21.4 |
| Mean annual EVI | 6,366 | 0.288 | 0.058 | 0.151 | 0.472 |
| Slope [°] | 6,366 | 3.03 | 2.21 | 0.35 | 13.64 |
| Age [years] | 6,366 | 23.6 | 19.2 | 0 | 97.7 |
| Male gender | 6,317 | 47.0% | |||
| Household size [persons] | 6,363 | 6.5 | 3.6 | 1 | 30 |
| Population density [persons/km2] | 6,366 | 1875 | 3179 | 10 | 13133 |
| SES | 6,363 | −0.52 | 1.17 | −2.82 | 4.08 |
| Households with latrine | 6,363 | 97.5% | |||
| Latrine coverage in surroundings [%] c) | 6,366 | 95.8 | 8.2 | 29.6 | 100 |
N = number of observations; Std. Dev. = standard deviation; EPG = eggs per gram of feces; LST = land surface temperature; EVI = enhanced vegetation index; SES = socio-economic score.
Mean for continuous and % for categorical variables.
According to Montresor, 1998 [35].
Percentage of households with a latrine within one kilometer around the participant's household.
Univariable Logistic Regression and Multicollinearity Analysis
In univariable analysis, all considered environmental variables were significantly associated with A. lumbricoides infection (Table 2). Elevation, LST-day and slope showed an inverse association, whereas all other environmental variables were positively associated. Therefore, all environmental variables apart from elevation and ambient temperature were included in the multivariable analysis. Sex, household size, and population density were excluded because their p-values were above 0.2, the threshold which was chosen for the inclusion in multivariable analysis.
Table 2. Univariable association of environmental and socio-demographic factors with A. lumbricoides infection a).
| Variables | OR (95% CI) | p-value |
| Elevation, per 100 meters | 0.88 (0.84 to 0.91) | <0.001 |
| Mean annual ambient temperature, per 1°C | 1.23 (1.14 to 1.32) | <0.001 |
| Mean annual rainfall, per 1000 mm | 7.93 (5.85 to 10.75) | <0.001 |
| Mean annual LST-day, per 1°C | 0.73 (0.70 to 0.76) | <0.001 |
| Mean annual LST-night, per 1°C | 1.20 (1.13 to 1.27) | <0.001 |
| Mean annual EVI, per 0.1 units | 5.91 (4.10 to 8.50) | <0.001 |
| Slope, per 1 ° | 0.72 (0.63 to 0.83) | <0.001 |
| Age, per 10 years | 0.95 (0.90 to 1.00) | 0.060 |
| Sex | 1.09 (0.90 to 1.32) | 0.389 |
| Household size | 1.01 (0.96 to 1.07) | 0.603 |
| Population density, per 1000/km2 | 0.98 (0.94 to 1.03) | 0.379 |
| SES, per 1 unit | 0.59 (0.48 to 0.73) | <0.001 |
| Latrine in household (yes/no) | 0.24 (0.13 to 0.46) | <0.001 |
| Latrine coverage, per 10%b) | 0.57 (0.50 to 0.65) | <0.001 |
OR = odds ratio; 95% CI = confidence interval; LST = land surface temperature; EVI = enhanced vegetation index; SES = socio-economic score.
Results of logistic regression models adjusted for household clustering using Huber/White variance estimates.
Percentage of households with a latrine within one kilometer around the participant's household.
Due to high multicollinearity of the variables elevation (VIF = 116.65), ambient temperature (VIF = 71.05) and LST-night (VIF = 17.19), elevation and ambient temperature were excluded from multivariable analysis, since their VIFs by far exceeded the threshold of 10. We decided to include LST-night not only because of its lower VIF, but more importantly, because soil temperature appears to be more directly linked to helminth egg development than elevation or ambient temperature, as eggs develop in the soil or at the soil surface.
Multivariable Logistic Regression with Fractional Polynomials
In MFP analysis we found a non-linear relationship of rainfall and age with A. lumbricoides infection. In the full and in the final reduced model a fractional polynomial transformation with two degrees (FP2) was implemented.
For the other variables the linear assumption was retained and odds ratios (ORs) were calculated. The full and the final reduced multivariable regression models are shown in Table 3. Rainfall and LST-day were kept as significant environmental variables in the final model. LST-day showed an inverse association with A. lumbricoides infection. With every degree Celsius increase in LST-day the odds of being infected with A. lumbricoides decreased by about 13%.
Table 3. Multivariable association of environmental and socio-demographic factors with A. lumbricoides infection using logistic regression with fractional polynomials (n = 6,363).
| Full Model a) | Final Model after Backward Elimination a) | |||
| Covariables | β/OR (95% CI) | p | β/OR (95% CI) | p |
| Mean annual rainfall, per 1000 mm | β1 = 1.92 (0.33 to 3.51) | 0.018 | β1 = 1.58 (0.14 to 3.02) | 0.032 |
| (FP2 polynomial transformationb)) | β2 = −2.08 (−3.63 to −0.52) | 0.009 | β2 = −1.78 (−3.19 to −0.37) | 0.013 |
| Mean annual LST-day, per 1 °C | OR = 0.82 (0.73 to 0.93) | 0.002 | OR = 0.87 (0.78 to 0.97) | 0.012 |
| Mean annual LST-night, per 1 °C | OR = 1.24 (0.81 to 1.90) | 0.315 | . | . |
| Mean annual EVI, per 0.1 units | OR = 0.79 (0.42 to 1.50) | 0.471 | . | . |
| Slope, per 1 ° | OR = 0.90 (0.72 to 1.12) | 0.324 | . | . |
| Age, per 10 years | β1 = 2.57 (1.38 to 3.76) | <0.001 | β1 = 2.56 (1.38 to 3.74) | <0.001 |
| (FP2 polynomial transformationc)) | β2 = 1.13 (0.57 to 1.70) | <0.001 | β2 = 1.13 (0.56 to 1.69) | <0.001 |
| SES, per 1 unit | OR = 0.84 (0.70 to 1.00) | 0.052 | OR = 0.82 (0.68 to 0.97) | 0.024 |
| Latrine in household (yes/no) | OR = 0.68 (0.32 to 1.43) | 0.310 | . | . |
| Latrine coverage, per 10%d) | OR = 0.82 (0.68 to 0.99) | 0.043 | OR = 0.80 (0.67 to 0.96) | 0.018 |
| AIC | 2178 | 2178 | ||
| BIC | 2313 | 2287 | ||
β = beta coefficient; OR = odds ratio; 95% CI = confidence interval; LST = land surface temperature; EVI = enhanced vegetation index; SES = socio economic score, AIC = Akaike information criterion; BIC = Bayesian information criterion.
Adjusted for household clustering using Huber/White/Sandwich variance estimates and for study sites.
Fractional polynomial transformation with two degrees and powers p = 3: β1xp+β2xp*ln x.
Fractional polynomial transformation with two degrees and powers p = −0.5: β1xp+β2xp*ln x.
Percentage of households with a latrine within one kilometer around the participant's household.
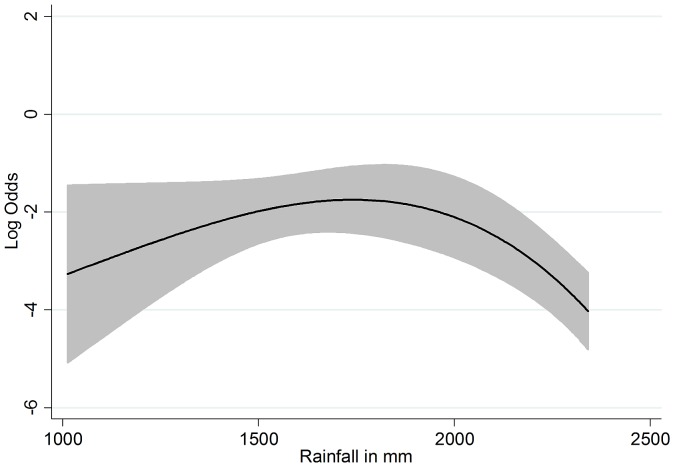
The calculated beta coefficients for the non-linear functions of rainfall and age are not directly interpretable as ORs. For rainfall, the odds of A. lumbricoides infection are increasing until a rainfall maximum of 1,740 mm and are decreasing again at higher values (Figure 4). For age there is a steep increase until an infection maximum at 7.7 years, after which the odds are decreasing (curve not shown). This is in agreement with the unadjusted age prevalence curve shown in figure 2.
Figure 4. Non-linear partial prediction of the log odds of A. lumbricoides infection by annual rainfall.
The partial predicted curve is adjusted for LST-day, slope, SES, age, latrine coverage and the nine study sites. The maximum is at 1740 mm of mean annual rainfall. Grey shadings indicate 95% confidence band.
Except for latrine ownership in the household, all included confounding variables (age, SES score and latrine coverage) were significantly negatively associated with A. lumbricoides infection and therefore retained in the final multivariable model.
In order to check our final model for plausible interactions we calculated interaction terms between each beta of rainfall and LST-day and between SES and latrine coverage. However, none of these interactions were significant (data not shown).
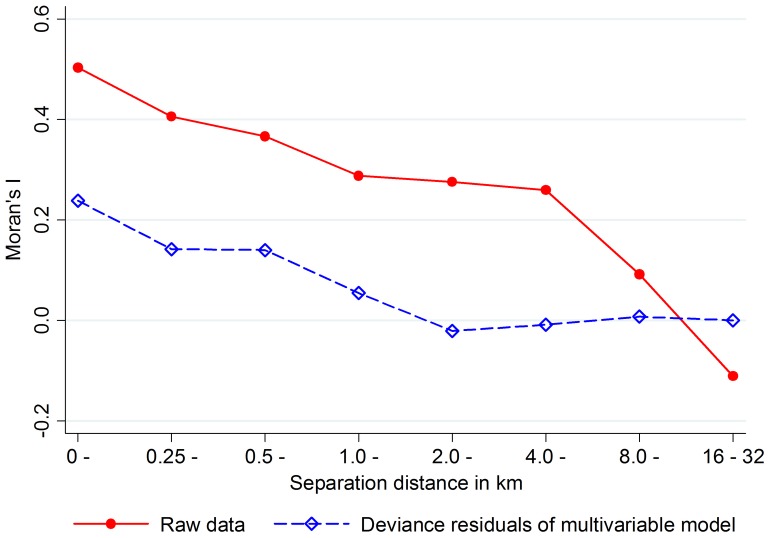
The raw A. lumbricoides infection data show strong positive spatial autocorrelation within separation distances of up to 8 km (Figure 5). The lower values for Moran's I in the deviance residuals of our final model indicate that the variables in the model account for a large part of this autocorrelation.
Figure 5. Spatial autocorrelation of A. lumbricoides infection within sites.
Moran's I for spatial autocorrelation of A. lumbricoides infection in the raw data and in the deviance residuals of the final multivariable model. Values above 0 indicate positive, values below 0 negative spatial autocorrelation. The figure only considers autocorrelation between households within the same sites.
Discussion
Our univariable results show that A. lumbricoides infection is significantly associated with several environmental factors. Of these, mean annual rainfall and mean annual LST-day remained significant in the multivariable model. LST-day had a linear negative association with A. lumbricoides infection, whereas the association of rainfall and age was non-linear with maximum infection odds at 1,740 mm of mean annual rainfall and at an age of 7.7 years. SES and latrine coverage around the household showed significant negative associations with A. lumbricoides infection, both in univariable and multivariable analyses.
Our results concerning LST-day are in line with the published literature [21], [23]–[25]. Two studies from Cameroon and Southeast Asia found that a higher LST was significantly associated with a lower risk of A. lumbricoides infection because high soil temperatures reduce humidity and thus lead to desiccation of Ascaris eggs [23], [24]. These studies considered mean, minimum and maximum LST, not LST for day and night as in our analysis. However, in both studies minimum LST was excluded from multivariable analysis which is in parallel to the exclusion of our LST-night variable. Maximum LST was significantly negatively associated with infection which is in agreement with the significant LST-day variable in our final model. One study from Uganda found that A. lumbricoides prevalence is <5% where maximum LST exceeds 36–37°C [21] and a study conducted in 20 schools in the Chad predicted no A. lumbricoides prevalence in areas where mean LST exceeds 37 °C [25].
Denser vegetation, as indicated by a higher EVI, showed a strong positive association with A. lumbricoides infection in univariable analysis, which, however, turned non-significant when including other variables in the multivariable model. Our significant univariable result for EVI is in line with multivariable results from former studies [23], [24], [58]. The non-significance of EVI in multivariable analysis is likely due to differences in local conditions compared to the former studies where EVI showed a significant association.
The non-linear relationship of rainfall with infection can be explained based on the life cycle of A. lumbricoides. Rainfall is an important determinant of larval development because humidity, soil moisture, and land surface temperature have a strong impact on embryonation [22]. Laboratory studies showed that higher humidity facilitates larval development [59], [60]. A multiple regression analysis of rainfall, number of wet days and ambient temperature in Sri Lanka found a significant association between increased number of wet days per month and higher rates of A. lumbricoides infections [61]. These findings are in line with the first part of our predicted non-linear curve showing higher infection odds when rainfall increases. However, another laboratory study showed that the development of eggs located on an extremely wet soil surface was delayed due to evaporation and the resulting low temperatures [59]. Since, in contrast to hookworm larvae, A. lumbricoides eggs are non-motile [12], they are directly exposed to rainfall and LST on the soil surface. Crompton states that eggs may be washed away by rainfall, too [5]. Increased rainfall can lead to a leaching effect and eggs are washed to deeper regions of the soil [62]. All these findings indicate that up to a certain amount, increasing rainfall supports larval development but that too much rain can delay larval development and thus reduce transmission.
Regarding the examined possible confounding variables, our univariable and multivariable results indicate that low SES and bad sanitary conditions in and around the household are risk factors for A. lumbricoides infection which is in line with former publications [12], [14], [27], [28], [30], [32]. The non-linear association between age and A. lumbricoides infection describing a higher risk in children and an infection peak in later childhood, is in line with former epidemiological studies [3]–. Although none of these variables substantially confounded the associations of A. lumbricoides infection with environmental variables, it is important to consider such factors when planning interventions or further studies on a smaller scale, e.g. the community level.
In the literature, non-linear associations between environmental variables and STH infection have rarely been analyzed in a multivariable context. Brooker et al. [21] predict the prevalence of various STH infections with generalized additive models in a spatial analysis in Uganda and found that the predicted prevalence of A. lumbricoides infection showed non-linear relationships with LST and rainfall. A case study in Cameroon observed non-linear associations between environmental variables (LST, rainfall and vegetation) and A. lumbricoides infection in scatterplots of prevalence and environmental data [22]. Our results suggest that the MFP procedure can be effectively used as a multivariable parametric approach to detect non-linear associations between environmental data and A. lumbricoides infection. Especially when a turning point within a non-linear prediction is detected, such as for rainfall in our study, the MFP procedure can provide new insights into the relationship between environmental conditions and STH infection. A more precise understanding of such relationships could play an important role in the future prediction of high prevalence areas to be targeted for interventions.
However, non-linear analysis also has its limitations. Transformed variables are not directly interpretable, and thus hard to generalize and compare between studies. Moreover, fractional polynomials are very sensitive to outliers and over fitting, and transformations can often be due to extreme observations. To avoid this, it is very important to analyze transformed variables for outliers and define lower p-values for the function selection procedure when non-linear models are compared. Besides that, it is recommended to analyze non-linearity only if prior knowledge for non-linear relationships exists [54].
One problem when assessing STH infection by Kato-Katz (and most other microscopy based techniques) is the low sensitivity of the method. This is best compensated by the examination of more than one stool specimen, which was logistically impossible in our study. Instead, we examined two separate Kato-Katz slides from the same sample. Although this should have increased sensitivity, we have inevitably missed some of the lighter infections. However, the Kato-Katz examination of a single stool specimen shows a better sensitivity for the detection of A. lumbricoides infection than for other STH infections [63], [64].
Another limitation of our study is the lack of information concerning soil types in our study sites, which could be important in the context of rainfall and the survival of Ascaris eggs. Beaver states that A. lumbricoides infections were more common in regions with clayey soils [65], [66]. Sandy soils are more permeable and are unable to keep moisture. Moreover, in sandy soils eggs are more likely to be washed down to deeper soil strata. Furthermore we are unable to account for seasonal aspects of rainfall or the intensity of rainfall within a short time. Gunawardena et al. [61] found out that the number of wet-days per month were more significantly associated with A. lumbricoides infection than the total amount of rainfall. In this context they state that heavy rains facilitate the scattering of eggs both vertically and horizontally, whereas steady rainfall helps to maintain soil moisture.
Although our final model was able to account for most of the spatial autocorrelation in the raw A. lumbricoides infection data (Figure 5), the figure also shows some remaining positive spatial autocorrelation in the deviance residuals of our final model. This might have influenced the variance estimates of the model and in turn might have led to spuriously low p-values for some of our environmental variables. Therefore, these p-values should be interpreted with caution.
To conclude, A. lumbricoides infection was associated with several environmental, socio-demographic and sanitary factors in univariable analyses. Of these, mean annual rainfall, mean annual LST-day, age, SES and latrine coverage remained significant in multivariable analysis. MFP models can be used as an effective statistical tool to get a better understanding of the – often non-linear - relationship between environmental factors and A. lumbricoides infection. Future studies should therefore consider potential non-linear relationships between environmental factors and STH in a multivariable context to yield more precise predictions. However, if data are available, socio-demographic and sanitary conditions should also be considered, especially when planning interventions.
Supporting Information
STROBE checklist.
(DOC)
Acknowledgments
We thank the participants of the EMINI study for their participation and continued support, and the field and laboratory teams at MMRP for their hard work.
Funding Statement
The EMINI study was funded by the European Commission (SANTE/2004/078-545/130 & SANTE/2006/129-931). Helminth data collection was supported by the German Science foundation DFG (SA 1878/1-1) and the German Federal Ministry of Education and Research BMBF (01 KA 0904). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, et al. (2006) Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet 367: 1521–1532. [DOI] [PubMed] [Google Scholar]
- 2. de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, et al. (2003) Soil-transmitted helminth infections: updating the global picture. Trends Parasitol 19: 547–551. [DOI] [PubMed] [Google Scholar]
- 3. de Silva NR, Chan MS, Bundy DA (1997) Morbidity and mortality due to ascariasis: re-estimation and sensitivity analysis of global numbers at risk. Trop Med Int Health 2: 519–528. [DOI] [PubMed] [Google Scholar]
- 4. de Silva NR, Guyatt HL, Bundy DA (1997) Worm burden in intestinal obstruction caused by Ascaris lumbricoides. Trop Med Int Health 2: 189–190. [DOI] [PubMed] [Google Scholar]
- 5.Crompton DWT, Pawlowski ZS (1985) Life history and development of Ascaris lumbricoides and the persistence of human ascariasis. In: Crompton DWT, Nesheim MC, Pawlowski ZS, editors. Ascariasis and its public health significance: a volume based on the agenda and discussions of the 1984 Banff conference, organized by WHO Parasitic Diseases Programme and Division of Nutritional Sciences, Cornell University, New York. London; Philadelphia: Taylor & Francis.pp. 9–23.
- 6. Scott ME (2008) Ascaris lumbricoides: A review of Its Epidemiology and Relationship to Other Infections. Annales Nestlé 66: 7–22. [Google Scholar]
- 7. Drake LJ, Jukes MCH, Sternberg RJ, Bundy DAP (2000) Geohelminth Infections (Ascariasis, Trichuriasis, and Hookworm): Cognitive and Developmental Impacts. Seminars in Pediatric Infectious Diseases 11: 245–251. [Google Scholar]
- 8.Abidin SAN, Hadidjaja P (2003) The effect of soil-transmitted helminth infection on the cognitive function of schoolchildren. In: Crompton DWT, Montresor A, Nesheim MC, Savioli L, editors. Controlling disease due to helminth infections: World Health Organization. pp. 67–71.
- 9. Dickson R, Awasthi S, Williamson P, Demellweek C, Garner P (2000) Effects of treatment for intestinal helminth infection on growth and cognitive performance in children: systematic review of randomised trials. BMJ 320: 1697–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taylor-Robinson DC, Maayan N, Soares-Weiser K, Donegan S, Garner P (2012) Deworming drugs for soil-transmitted intestinal worms in children: effects on nutritional indicators, haemoglobin and school performance. Cochrane Database Syst Rev 7: CD000371. [DOI] [PubMed] [Google Scholar]
- 11.Anderson RM (1982) The population dynamics and control of hookworm and roundworm infections. In: Anderson RM, editor. The Population dynamics of infectious diseases: theory and applications. London; New York: Chapman and Hall.pp. 68–108.
- 12.Crompton DWT, Savioli L (2007) Handbook of helminthiasis for public health. Boca Raton: CRC/Taylor & Francis. 362 p.p.
- 13. Crompton DW (2001) Ascaris and ascariasis. Adv Parasitol 48: 285–375. [DOI] [PubMed] [Google Scholar]
- 14. O'Lorcain P, Holland CV (2000) The public health importance of Ascaris lumbricoides. Parasitology 121 Suppl: S51–71 [DOI] [PubMed] [Google Scholar]
- 15. Brooker S, Clements AC, Bundy DA (2006) Global epidemiology, ecology and control of soil-transmitted helminth infections. Adv Parasitol 62: 221–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Magalhaes RJ, Clements AC, Patil AP, Gething PW, Brooker S (2011) The applications of model-based geostatistics in helminth epidemiology and control. Adv Parasitol 74: 267–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brooker S, Hay SI, Bundy DA (2002) Tools from ecology: useful for evaluating infection risk models? Trends Parasitol 18: 70–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brooker S, Clements AC (2009) Spatial heterogeneity of parasite co–infection: Determinants and geostatistical prediction at regional scales. Int J Parasitol 39: 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brooker S, Rowlands M, Haller L, Savioli L, Bundy DA (2000) Towards an atlas of human helminth infection in sub-Saharan Africa: the use of geographical information systems (GIS). Parasitol Today 16: 303–307. [DOI] [PubMed] [Google Scholar]
- 20. Brooker S, Kabatereine NB, Smith JL, Mupfasoni D, Mwanje MT, et al. (2009) An updated atlas of human helminth infections: the example of East Africa. Int J Health Geogr 8: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brooker S, Kabatereine NB, Tukahebwa EM, Kazibwe F (2004) Spatial analysis of the distribution of intestinal nematode infections in Uganda. Epidemiol Infect 132: 1065–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brooker S, Michael E (2000) The potential of geographical information systems and remote sensing in the epidemiology and control of human helminth infections. Adv Parasitol 47: 245–288. [DOI] [PubMed] [Google Scholar]
- 23. Brooker S, Hay SI, Tchuente LAT, Ratard R (2002) Using NOAA-AVHRR data to model human helminth distributions in planning disease control in Cameroon, West Africa. Photogramm Eng Rem S 68: 175–179. [Google Scholar]
- 24. Brooker S, Singhasivanon P, Waikagul J, Supavej S, Kojima S, et al. (2003) Mapping soil-transmitted helminths in Southeast Asia and implications for parasite control. Southeast Asian J Trop Med Public Health 34: 24–36. [PubMed] [Google Scholar]
- 25. Brooker S, Beasley M, Ndinaromtan M, Madjiouroum EM, Baboguel M, et al. (2002) Use of remote sensing and a geographical information system in a national helminth control programme in Chad. Bull World Health Organ 80: 783–789. [PMC free article] [PubMed] [Google Scholar]
- 26. Pullan RL, Gething PW, Smith JL, Mwandawiro CS, Sturrock HJ, et al. (2011) Spatial modelling of soil-transmitted helminth infections in Kenya: a disease control planning tool. PLoS Negl Trop Dis 5: e958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kan SP (1985) Ascaris lumbricoides in Malaysia. In: Crompton DWT, Nesheim MC, Pawlowski ZS, editors. Ascariasis and its public health significance: a volume based on the agenda and discussions of the 1984 Banff conference, organized by WHO Parasitic Diseases Programme and Division of Nutritional Sciences, Cornell University, New York. London; Philadelphia: Taylor & Francis.pp. 69–82.
- 28.Stephenson LS (1985) Factors affecting the prevalence and control of Ascaris lumbricoides infection in Kenya. In: Crompton DWT, Nesheim MC, Pawlowski ZS, editors. Ascariasis and its public health significance: a volume based on the agenda and discussions of the 1984 Banff conference, organized by WHO Parasitic Diseases Programme and Division of Nutritional Sciences, Cornell University, New York. London; Philadelphia: Taylor & Francis.pp. 113–127.
- 29. Tshikuka JG, Scott ME, Gray-Donald K (1995) Ascaris lumbricoides infection and environmental risk factors in an urban African setting. Ann Trop Med Parasitol 89: 505–514. [DOI] [PubMed] [Google Scholar]
- 30. Walker M, Hall A, Basanez MG (2011) Individual predisposition, household clustering and risk factors for human infection with Ascaris lumbricoides: new epidemiological insights. PLoS Negl Trop Dis 5: e1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Forrester JE, Scott ME, Bundy DA, Golden MH (1988) Clustering of Ascaris lumbricoides and Trichuris trichiura infections within households. Trans R Soc Trop Med Hyg 82: 282–288. [DOI] [PubMed] [Google Scholar]
- 32. Carneiro FF, Cifuentes E, Tellez-Rojo MM, Romieu I (2002) The risk of Ascaris lumbricoides infection in children as an environmental health indicator to guide preventive activities in Caparao and Alto Caparao, Brazil. Bull World Health Organ 80: 40–46. [PMC free article] [PubMed] [Google Scholar]
- 33. Riess H, Clowes P, Kroidl I, Kowuor DO, Nsojo A, et al. (2013) Hookworm infection and environmental factors in mbeya region, Tanzania: a cross-sectional, population-based study. PLoS Negl Trop Dis 7: e2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO (1991) Basic laboratory methods in medical parasitology. Geneva: World Health Organization. viii, 114 p.p.
- 35.Montresor A, Crompton DW, Bundy DAP, Hall A, Savioli L (1998) Guidelines for the evaluation of soil-transmitted helminthiasis and schistosomiasis at community level. Geneva: WHO. 45 p. [Google Scholar]
- 36.Farr TG, Rosen PA, Caro E, Crippen R, Duren R, et al. (2007) The shuttle radar topography mission. Reviews of Geophysics 45.. [Google Scholar]
- 37. Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25: 1965–1978. [Google Scholar]
- 38.NASA Land Processes Distributed Active Archive Center (LP DAAC) (2001) MODIS 11A2. USGS/Earth Resources Observation and Science (EROS) Center, Sioux Falls, South Dakota.
- 39.NASA Land Processes Distributed Active Archive Center (LP DAAC) (2001) MODIS 13Q1. Resources Observation and Science (EROS) Center, Sioux Falls, South Dakota.
- 40. Kolenikov S, Angeles G (2009) Socioeconomic Status Measurement with Discrete Proxy Variables: Is Principal Component Analysis a Reliable Answer? Rev Income Wealth 55: 128–165. [Google Scholar]
- 41. Filmer D, Pritchett LH (2001) Estimating wealth effects without expenditure data—or tears: an application to educational enrollments in states of India. Demography 38: 115–132. [DOI] [PubMed] [Google Scholar]
- 42. Vyas S, Kumaranayake L (2006) Constructing socio-economic status indices: how to use principal components analysis. Health Policy Plan 21: 459–468. [DOI] [PubMed] [Google Scholar]
- 43. Alin A (2010) Multicollinearity. WIREs Computational Statistics 2: 370–374. [Google Scholar]
- 44.Harrell FE (2001) Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York: Springer. xxii, 568 p. p.
- 45.Menard SW (2002) Applied logistic regression analysis. Thousand Oaks, Calif.: Sage Publications. viii, 111 p. p.
- 46. Huber PJ (1967) The behavior of maximum likelihood estimates under nonstandard conditions. Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability 1: 221–223. [Google Scholar]
- 47. White H (1980) A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica 48: 817–830. [Google Scholar]
- 48. Rogers WH (1993) Regression standard errors in clustered samples. Stata Technical Bulletin 3: 19–23. [Google Scholar]
- 49. Royston P, Altman DG (1994) Regression Using Fractional Polynomials of Continuous Covariates - Parsimonious Parametric Modeling. Appl Stat-J Roy St C 43: 429–467. [Google Scholar]
- 50. Royston P, Ambler G, Sauerbrei W (1999) The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol 28: 964–974. [DOI] [PubMed] [Google Scholar]
- 51. Royston P, Altman DG (1997) Approximating statistical functions by using fractional polynomial regression. Statistician 46: 411–422. [Google Scholar]
- 52. Sauerbrei W, Meier-Hirmer C, Benner A, Royston P (2006) Multivariable regression model building by using fractional polynomials: Description of SAS, STATA and R programs. Comput Stat Data An 50: 3464–3485. [Google Scholar]
- 53. Ambler G, Royston P (2001) Fractional polynomial model selection procedures: Investigation of type I error rate. J Stat Comput Sim 69: 89–108. [Google Scholar]
- 54.Royston P, Sauerbrei W (2008) Multivariable model-building: a pragmatic approach to regression analysis based on fractional polynomials for modelling continuous variables. Chichester, England; Hoboken, NJ: John Wiley. xvii, 303 p. p.
- 55. Akaike H (1974) A new look at the statistical model identification. IEEE Transactions on Automatic Control 19: 716–723. [Google Scholar]
- 56. Schwarz G (1978) Estimating the dimension of a model. Annals of Statistics 6: 461–464. [Google Scholar]
- 57. Pisati M (2001) Tools for spatial data analysis. Stata Technical Bulletin 60: 21–37. [Google Scholar]
- 58. Saathoff E, Olsen A, Kvalsvig JD, Appleton CC, Sharp B, et al. (2005) Ecological covariates of Ascaris lumbricoides infection in schoolchildren from rural KwaZulu-Natal, South Africa. Trop Med Int Health 10: 412–422. [DOI] [PubMed] [Google Scholar]
- 59. Otto GF (1929) A study of the moisture requirements of the eggs of the horse, the dog, human and pig ascarids. Am J Hyg 10: 0497–0520. [Google Scholar]
- 60. Spindler LA (1929) The relation of moisture to the distribution of human trichuris and ascaris. Am J Hyg 10: 0476–0496. [Google Scholar]
- 61. Gunawardena GS, Karunaweera ND, Ismail MM (2004) Wet-days: are they better indicators of Ascaris infection levels? J Helminthol 78: 305–310. [DOI] [PubMed] [Google Scholar]
- 62. Storey GW, Phillips RA (1985) The survival of parasite eggs throughout the soil profile. Parasitology 91 (Pt3): 585–590. [DOI] [PubMed] [Google Scholar]
- 63. Tarafder MR, Carabin H, Joseph L, Balolong E Jr, Olveda R, et al. (2010) Estimating the sensitivity and specificity of Kato-Katz stool examination technique for detection of hookworms, Ascaris lumbricoides and Trichuris trichiura infections in humans in the absence of a ‘gold standard’. Int J Parasitol 40: 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Knopp S, Mgeni AF, Khamis IS, Steinmann P, Stothard JR, et al. (2008) Diagnosis of soil-transmitted helminths in the era of preventive chemotherapy: effect of multiple stool sampling and use of different diagnostic techniques. PLoS Negl Trop Dis 2: e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Beaver PC (1975) Biology of Soil-Transmitted Helminths - Massive Infection. Health Lab Sci 12: 116–125. [PubMed] [Google Scholar]
- 66. Beaver PC (1952) Observation on the epidemiology of ascariasis in a region of high hookworm endemicity. Journal of Parasitology 38: 445–453. [PubMed] [Google Scholar]
- 67. Elkins DB, Haswell-Elkins M, Anderson RM (1986) The epidemiology and control of intestinal helminths in the Pulicat Lake region of Southern India. I. Study design and pre- and post-treatment observations on Ascaris lumbricoides infection. Trans R Soc Trop Med Hyg 80: 774–792. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
STROBE checklist.
(DOC)