Abstract
Lung cancer is one of the most common cancers and is the leading cause of death worldwide. Platinum-based chemotherapy is the main treatment method in lung cancer patients. Our previous studies indicated that single nucleotide polymorphisms (SNPs) in some transporter genes played important role in platinum-based chemotherapy efficacy. The aim of this study was to investigate the association of SNPs in transporter genes and platinum-based chemotherapy efficacy. The main polymorphisms on transporters OCT2, LRP, AQP2, AQP9 and TMEM205 genes were genotyped in 338 lung cancer patients. The rs195854 in genotypic model, rs896412 in genotypic and recessive models for all subjects showed significant association with chemotherapy response. In stratification analysis, TMEM205 rs896412, OCT2 rs1869641 and rs195854, AQP9 rs1516400 and AQP2 rs7314734 showed significant relation to chemotherapy response. In conclusion, the genetic polymorphisms in OCT2, AQP2, AQP9 and TMEM205 may contribute to chemotherapy response in lung cancer patients.
Introduction
Lung cancer is one of the most common cancers and is the leading cause of death worldwide [1]. The percentage of five-years survival was about 15 which is much lower than other cancers [2]. Lung cancer consists of two types: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). Surgery is the mainstay of treatment for early NSCLC patients, while the later stage NSCLC and SCLC patients were mainly treated by platinum-based chemotherapy [3], [4]. Cisplatin, carboplatin were the most commonly used first-line clinical therapeutic drugs, but the tumors were always resistant to them and decreased their efficacy [4].
The chemotherapy resistance has several mechanisms, including reduced platinum compounds accumulation (decrease intake or increase efflux by transporters), detoxification, or increased level of DNA damage repair and so on [3], [5]. Several transporters contribute to platinum accumulation in the cancer cells. Aquaporins(AQPs)are members of a family of transmembrane proteins that have the function of transporting molecular water channels. The AQPs family contains at least 11 different types [6]. The expression of AQP2 and AQP9 were reduced in Pt resistant lines presenting that they are the potential new Pt drug transporters [7]. TMEM205 was a novel transmembrane protein, having four transmembrane domains, is predicted to be a secretion-related protein by its nucleotide sequences [5]. The resistance to cisplatin was increased by transfecting TMEM205 gene in cisplatin-resistant cells [8], [9]. Transmembrane protein 205 (TMEM205) may decrease the accumulation of platinum compound by increasing efflux [5]. The organic cation transporter 2 (OCT2), encoded by SLC22A2 gene, had a potential role of increasing platinum uptake and sensitivity [10]–[12]. Resistance-related protein (MVP/LRP) can up-regulate sensitivity by increasing cellular cisplatin accumulation and/or by decreasing cisplatin efflux from nuclei in ovarian cancer cells [13]. To sum up, increasing the expression of AQP2, AQP9, OCT2 and MVP/LRP or decreasing the expression of TMEM205 might lead to platinum sensitivity.
Using the same drug and dose patients will have different response to chemotherapy because of comparable internal or external differences such as patient age, smoking status, their overall health and genetic variants [14]. Many studies found that SNPs in drug transporters play important role in metabolism and transport of therapeutic drugs, and affecting the response to therapy [15]. Daniele Campa et.al [16] investigated the relationship of ABCB1, ABCC2 and ABCG2 polymorphisms with platinum-based chemotherapy efficacy and demonstrated that a SNP of ABCC2 was significantly linked with chemotherapy response. Our previous studies showed that several copper transporter protein1 SNPs may be significantly associated with platinum-based chemotherapy efficacy and toxicity in lung cancer patients [17], [18]. Oliver Zolk et.al indicated that the c.808 G>T SNP in OCT2 significantly altered uptake of endogenous compounds and drugs [19]. Therefore we hypothesized that some other transporter gene polymorphisms may also be related with platinum-based chemotherapy efficacy.
In this work, we used the tagging method to analysis the relationship of 26 polymorphisms of AQP2, AQP9, LRP/MVP, TMEM205 and OCT2 genes with platinum-based chemotherapy response (Table 1). This the first study to investigate association of these genes polymorphisms with lung cancer chemotherapy response.
Table 1. The 26 gene polymorphisms examined in this study.
| Gene | Location | dbSNP | Category | Call Rate (%) | Polymorphism | MAF |
| LRP | 16q13.3 | rs7204252 | 3′ downstream | 98.52 | C/T | 0.081 |
| rs4788186 | Intron | 99.11 | A/G | 0.27 | ||
| rs4788184 | nearGene-5 | 100.00 | C/T | 0.00 | ||
| rs1057451 | Intron | 99.70 | G/T | 0.088 | ||
| OCT2 | 6q25.3 | rs195862 | 5′ downstream | 99.11 | A/C | 0.036 |
| rs195854 | 5′ downstream | 90.23 | A/T | 0.27 | ||
| rs3823036 | 5′ downstream | 99.11 | C/T | 0.30 | ||
| rs2444933 | 5′ downstream | 98.82 | C/T | 0.27 | ||
| rs1883306 | 5′ downstream | 93.20 | A/C | 0.32 | ||
| rs1869641 | 5′ downstream | 98.52 | C/T | 0.17 | ||
| TMEM205 | 19p13.2 | rs7251786 | 3′ downstream | 99.41 | C/T | 0.25 |
| rs896412 | 5′ downstream | 99.41 | C/G | 0.19 | ||
| rs172731 | 5′ downstream | 98.22 | C/T | 0.22 | ||
| AQP2 | 12p13.2 | rs461872 | Intron | 99.70 | A/G | 0.23 |
| rs3759125 | nearGene-5 | 99.70 | A/C | 0.40 | ||
| rs7305534 | nearGene-5 | 98.82 | C/T | 0.46 | ||
| rs296766 | UTR-3 | 100.00 | C/T | 0.14 | ||
| rs3759126 | nearGene-5 | 99.11 | A/G | 0.38 | ||
| rs7314734 | nearGene-5 | 100.00 | C/T | 0.095 | ||
| rs10875989 | UTR-3 | 98.82 | C/T | 0.40 | ||
| AQP9 | 15q21.3 | rs1867380 | Exon | 99.11 | A/G | 0.18 |
| rs1516400 | nearGene-5 | 98.22 | A/G | 0.47 | ||
| rs1554203 | nearGene-5 | 99.70 | A/G | 0.16 | ||
| rs9920375 | 3′ downstream | 99.11 | C/T | 0.42 | ||
| rs2077737 | nearGene-3 | 99.70 | C/T | 0.34 | ||
| rs8023369 | 3′ downstream | 98.52 | G/T | 0.39 |
Materials and Methods
Study subjects
The study protocol has been approved by the Ethics Committee of Xiangya School of Medicine, Central South University with a registration number of CTXY-110008-2. The clinical research admission was approved by Chinese Clinical Trial Registry and the registration number is ChiCTR-RO-12002873 (http://www.chictr.org/usercenter/project/edit.aspx?proj=4039). Lung cancer patients were quantified and enrolled between September 2011 and March 2013 at Xiangya Hospital of Central South University and Hunan province tumor Hospital in Changsha Hunan. All the patients were provided written informed consent in compliance with the code of ethics of the World Medical Association (Declaration of Helsinki) before this study was initiated. The basic clinical characteristics were collected including age, smoking status, histology, gender and TNM stage, Eastern Cooperative Oncology Group Performance Status (ECOG PS).
The inclusion criteria were listed as followed: (1) Patients who were diagnosed lung cancer by histology or cytology; (2) Patients who had never received any radical or biological therapy before and during chemotherapy; (3) All patients who were received at least two cycle of first line chemotherapy; (4) All patients who were received throughout follow up among six months. The exclusion criteria were listed as followed: (1) Patients who were in pregnancy or feeding period; (2) Patients who had been diagnosed with other malignancies; (3) Patients who had brain metastases; (4) Patients who had active infection.
The enrolled patients in this study received first-line platinum-based chemotherapy including cisplatin and carboplatin. Chemotherapy response was assessed after first two cycles of chemotherapy according to the RECIST guideline (version 1.1) for solid tumors [20]. The patients that showed complete response (CR) or partial response (PR) were regarded as platinum sensitivity, while progressive disease (PD) or stable disease (SD) were regarded as platinum resistance [20].
Tagging SNPs selection
We investigated all of the common genetic variants in TMEM205, AQP2, AQP9, LRP and OCT2. All SNPs of the 5 kb upstream of the first exon and 5 kb downstream of the last exon of the five genes were selected as the candidate SNPs. In total, 26 SNPs were selected in this work (Table 1). All these 26 SNPs satisfied the following criteria: (1) The SNPs were chosen from the International HapMap Project Phase II database of Chinese population (http://www.hapmap.org/); (2) All SNPs were haplotype tagger SNPs; (3) Minor allele frequency (MAF) was larger than 5% in Beijing Han population China; (4) The pairwise linkage disequilibrium was squared correlation coefficient (r2)>0.8. This work was performed by using Haploview version 4.2 [21].
There were 3 tagger SNPs for TMEM205, mean r2 of 0.988, 4 tagger SNPs for LRP, mean r2 of 1.000, 7 tagger SNPs for AQP2, mean r2 of 0.997, 6 tagger SNPs for AQP9, mean r2 of 0.964, 6 tagger SNPs for OCT2, mean r2 of 1.000.
DNA extraction and genotyping procedure
Approximately 5 ml venous blood was collected from each patient for genetic studies. Genomic DNA was extracted from whole peripheral blood using either the DNeasy Blood & Tissue Kit (Qiagen, Shanghai China) or Genomic DNA Purification Kit (Promega, USA) according to the standard protocols.
Poymorphisms were detected by using Sequenom Mass Array Genotype Platform (Sequenom, San Diego, California, USA). Primers were designed by using AssayDesigner 3.1 software. Primer sequences were shown in Table S1. Procedures of genotyping are the following five main steps: (1) Polymerase chain reaction (PCR) amplification; Components and reagents were as following: 1.8 μL HPLC grade Water, 0.5 μL 10 × PCR Buffer with 15 mM MgCl2, 0.4 μL 25 mM MgCl2, 0.1 μL 25 mM dNTP Mix, 0.2 μL 0.5 mM Primer Mix, 0.2 μL 5 U/μL HotStar Taq and 1 μL 10 ng/μL DNA in an final volume 5 μL. PCR was ran at 94°C for 15 minutes, thermo cycling 45 cycles (94°C for 20 seconds, 56°C for 30 seconds and 72°C for 1 minutes), extension at 72°C for 3 min. (2) SAP treatment; Components and reagents were as following: 1.53 μL Nanopure Water, 0.17 μL SAP Buffer, 0.3 μL SAP Enzyme(1.7 U/μL) in an final volume 2 μL. The conditions were 37°C for 40 minutes, 85°C for 5 minutes. (3) Extension reaction;Components and reagents were as following: 0.619 μL Nanopure water, 0.2 μL iPLEX Buffer Plus, 0.2 μL iPLEX Termination mix, 0.94 μL iPLEX Extend Primer Mix, 0.041 μL iPLEX Enzyme, 2 μL SAP reagent and 5 μL PCR reagent in an final volume 9 μL. The conditions were 94°C for 30 seconds, 94°C for 5 seconds, 40 cycles of 52°C for 5 seconds, 5 cycles of 80°C for 5 seconds, and 72°C for 3 minutes. (4) Ion Exchange Clean-up; (5) Fragment analysis; Polymorphisms calls were analyzed by using Sequenom's Spectro Typer 4.0 software.
Statistical analysis
All SNPs obeyed Hardy - Weinberg Equilibrium (HWE) by using the chi - square test. The association of genotypes and chemotherapy response were tested with logistic regression analysis.The potential covariates on chemotherapy response were selected by using binary logistic regression. Age, Sex, smoking status, PS and histology type were considered as covariates. All the above analysis were performed by plink (version 1.07, http://pngu.mgh.harvard.edu/purcell/plink/) and/or SPSS 13.0 (SPSS Inc, Chicago, Illinois, USA). Odds ratios (OR) and 95% confidence intervals (CI) were used to assess response to chemotherapy. P<0.05 was considered as significant. The figure was performed using StataSE version 12 (StataCorp, College Station, TX, USA). All data can be provided to anyone who wants via email.
Results
A total of 338 patients had been studied. The basic clinical characteristics of these patients were shown in Table 2. The responders(CR+PR)were 154 and non-responders were 184 with the median age of 55.6±8.9 years (range 36–77 years) and 55.3±8.8 years (range 31–76 years), respectively. There were no significant differences between responders and non-responders in sex, age and smoking status indicated that the populations were adequately matched. The non-small cell lung carcinoma patients were more likely to be non-responders than responders (79.9 vs 57.8%, P<0.0001). The ratio of ECOG 0–1 was lower in responders than non-responders (96.8 vs 99.5%, P = 0.047).
Table 2. Main clinical characteristics of lung cancer patients.
| Characteristics | Responders | Non-responders | ||
| N | (%) | N | (%) | |
| Total | 154 | 100.0 | 184 | 100.0 |
| Age(years) | ||||
| ≤55 | 72 | 53.2% | 84 | 45.7% |
| >55 | 82 | 46.8% | 100 | 54.3% |
| Gender | ||||
| Male | 131 | 85.1% | 140 | 76.1% |
| Female | 23 | 14.9% | 44 | 23.9% |
| Histology | ||||
| NSCLC | 89 | 57.8% | 147 | 79.9% |
| SCLC | 65 | 42.2% | 37 | 20.1% |
| Stage (non-small cell lung carcinoma) | ||||
| I–II | 3 | 2.0% | 6 | 3.3% |
| III–IV | 86 | 55.8% | 141 | 76.6% |
| Stage (small cell lung carcinoma) | ||||
| Limited | 33 | 21.4% | 14 | 7.6% |
| Extensive | 32 | 20.8% | 23 | 12.5% |
| Smoking | ||||
| Yes | 105 | 68.2% | 106 | 57.6% |
| No | 49 | 31.8% | 78 | 42.4% |
| ECOG PS | ||||
| 0–1 | 149 | 96.8% | 183 | 99.5% |
| >1 | 5 | 3.2% | 1 | 0.5% |
| Chemotherapeutic regiment | ||||
| Cisplatin | 119 | 77.3% | 152 | 82.6% |
| Carboplatin | 35 | 22.7% | 32 | 17.4% |
Covariates analysis showed that histology and ECOG might be statistically significant risk factors on platinum-based chemotherapy efficacy in all population, female and NSCLC patients. Histology was a statistically significant factor for male, no-smoking and smoking patients. Histology, gender and smoking or not might be statistically significant factors for patents above 55 years. All possible covariates were adjusted before data analysis. All 26 polymorphisms that probably were associated with platinum-based chemotherapy efficacy were detected and analyzed. The basic information of the 26 SNPs were shown in Table 1. However, to make the table not too complicate, only the most significant 5 SNPs' data were listed in the published version. The others results were listed in Table S2. We analyzed the association in genotypic, dominant and recessive models, respectively. The rs195854 (OR = 0.60, 95% CI, 0.36–1.00, P = 0.049) of OCT2 might be associated with chemotherapy efficacy in genotypic model, rs896412 of TMEM205 presented significance in genotypic (OR = 0.29, 95% CI, 0.10–0.81, P = 0.019) and recessive (OR = 0.082, 95% CI, 0.010–0.66, P = 0.019) models. There may be no polymorphisms significantly associated with chemotherapy efficacy in dominant model.
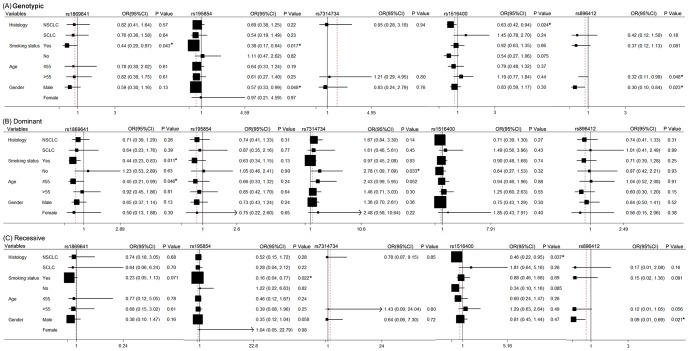
Non-small cell and small cell lung cancer were very different biologically. Smokers and non-smokers, younger and elders, male and female also may be different in chemotherapy response. To further investigate the relationship of these SNPs with response of chemotherapy in these different categories, we performed the stratification analysis. Results of the five SNPs were shown in Figure 1. The others were listed in Table S3. The results showed that OCT2 rs1869641 in genotypic and dominant models for smoke subjects and in dominant model for age ≤ 55 populations was likely significantly associated with chemotherapy efficacy. OCT2 rs195854 in genotypic model for smoking and male subgroup, as well as in recessive model for smoking subgroup might be associated with chemotherapy response. The rs7314734 of AQP2 only showed significant association with chemotherapy response for non-smokers in dominant model. The rs1516400 of AQP9 might be significantly associated with chemotherapy for NSCLC patients in genotypic and recessive models. The rs896412 of TMEM205 showed significant association for subgroup of age >55 in genotypic model, male subgroup in genotypic and recessive models.
Figure 1. Stratification analysis of the associations of 5 SNPs and chemotherapy efficacy in Genotypic (A), Dominant (B), Recessive (C) models.
Each box and horizontal line represents the OR and 95% CI. NSCLC: non-small cell lung carcinoma, SCLC: small cell lung carcinoma. *P<0.05.
In this study, the number of independent variables for OCT2, LRP, AQP2, AQP9 and TMEM205 are 6, 4, 7, 6 and 3, respectively. Each SNP was analyzed in three genetic models. To take into account the problem of multiple testing, the significance threshold is therefore 0.05/3(6+4+7+6+3) = 0.0006. Using this threshold, no one association remained significant.
Discussion
In this study, we investigate the association of 5 transporter genes (OCT2, AQP2, AQP9, MVP and TMEM205) SNPs with platinum-based chemotherapy response. Our results showed that SNPs rs195854 (OCT2) and rs186941 (OCT2), rs7314734 (AQP2), rs1516400 (AQP9), and rs896412 (TMEM205) might be related with chemotherapy response in lung cancer patients.
OCT2 was mainly expressed on the basolateral membrane which was the main site of cisplatin-induced renal toxicity [7], [22], [23]. Studies suggested that stably transfected OCT2 into cell lines, the intracellular accumulation of platinum and the formation of Pt-DNA adducts were significantly increased [24]. These studies suggested that OCT2 might be a key transporter for cisplatin and regulator in renal elimination of cisplatin. Based on the present study, the SNPs rs195854 and rs186941 of OCT2 are both showed association with chemotherapy in smoking subgroup and/or in male populations. This result suggested that the OCT2 gene polymorphisms might play a critical role in the platinum-based chemotherapy efficacy. However, the significant association was only occurred in rs195854 in genotypic model in all patients. Genotypic model is comparing the wild-type homozygous, heterozygous and mutant homozygous subjects. However, the other two models were comparing two genotypes and the remaining genotype. We hypothesis the possible reason is that the difference may be bigger in genotypic model if the number of one genotype subjects is significant different in two groups. However, the real reason should be investigated in the future studies. In smoking subgroup, rs195854 and rs1869641 both showed more chemotherapy resistance in two of the three models (Figure 1). There is possibility that smoking is overriding the potential effect of genetic variation in OCT2 in regulation of transport of platinum. Smoking may lead to poor chemotherapy response in many reasons. Such as, cigarette smoke may be related to alter the lung metabolism of many endogenous compounds, the activities of many biotransforming enzymes in lung tissues and affect the rates of metabolism for several drugs [25]. And those light or never-smoking lung cancer patients had a higher ratio of EGFR mutations that had been linked to EGFR-TKI therapy response [26], [27]. In future treatment of lung cancer, we might distinguish subjects according to their smoking habits and apply different chemotherapeutic agents.
The SNP rs195854 might be significantly associated with chemotherapy response for male patients in genotypic model and had the trend in recessive model although there was no significant association. This result was consistent with retarder drug elimination in women and that the expression of OCT2 was significantly lower in females in rabbits, mice, and rats [28]–[30]. It is thus tempting to speculate sex-dependent role for OCT2 in chemotherapy response contributes to the different elimination of cisplatin. A significant association was also occurred in subgroup of age ≤ 55 for rs1869641. Kelly K. Filip ski et.al suggested that rs316019 of OCT2 might be not associated with their studied pharmacokinetic variables [24]. Our results showed that OCT2 might be associated with platinum-based chemotherapy efficacy, but the specific mechanisms need to be studied in the further work.
AQP9 was not only important to arsenic resistance in human lung cancer cells by enhancing arsenic uptake [31], but also play a critical role in development of platinum-based chemotherapy response. AQP9 rs1516400 might be significantly associated with chemotherapy response for NSCLC patients in genotypic and recessive models but not for SCLC patients. Those results may be due to the NSCLC and SCLC differential various points, for example they had different molecular genetic abnormalities: the p53 mutation is higher in SCLC [32] and NSCLC are often over-expression in COX-2 [33]. Moreover, there are different in loss of cell cycle controls for the two types of histology [34], [35]. The different effect for AQP9 rs1516400 in NSCLC and SCLC may reinforce the fact that they have different genetic prognostic markers. This studies identified that AQP9 might be a potential predictive biomarkers for platinum-based chemotherapy response in NSCLC patients. However, the mechanism how AQP9 play a role in platinum-based chemotherapy efficacy for lung cancer patients requires to be studied in the next work.
AQP2 is the most important water channel in the apical plasma membrane [6]. It was found to be associated with resistance of cisplatin and might be a membrane transporter/binding protein/carrier for it [36]. The SNP rs7314734 of AQP2 showed more association with chemotherapy response in non-smoking subgroup and the reason could be explained similar to OCT2.
Ding-Wu Shen et.al showed that the expression of TMEM205 was increased in cisplatin resistant cells [8]. However, once the gene was overexpressed cells got more resistant to cisplatin [9]. This study showed that TMEM205 rs896412 might be significantly associated with chemotherapy response in all lung cancer patients (genotypic and recessive models) (Table 3), male subgroup (genotypic and recessive models) and subgroup of age >55 (genotypic model) (Figure 1). The different results in male and female subgroups may be linked with differ elimination of platinum similar to OCT2. Studies suggested that elder patients (>70 years) especially ones that had no coexistent diseases can get similar results from treatment as younger patients (<70 years) [37]–[39]. We found a statistically significant different between rs1869641 of OCT2 in subgroup of age ≤ 55 and rs896412 of TMEM205 in subgroup of age > 55. That was different comparing with the previous studies. The possible reason may be the different borderline (55, 70, respectively), but it should be further investigated. We thought that TMEM205 may play an important role in lung cancer platinum-based chemotherapy response and would also be a possible biomarker for lung cancer chemotherapy.
Table 3. Association of 5-based chemotherapy response in all lung cancer patients.
| Gene | Polymorphisms | Genotype | Responders | Non-responders | Genotypic | Dominant | Recessive | |||
| N(%) | N(%) | OR(95%CI) | P value | OR(95%CI) | P value | OR(95%CI) | P value | |||
| OCT2 | rs1869641 | GG | 112(75.1) | 120(65.22) | 0.78(0.44–1.40) | 0.41 | 0.67(0.40–1.10) | 0.11 | 0.68(0.21–2.16) | 0.51 |
| AG | 32(21.48) | 55(29.89) | ||||||||
| AA | 5(3.36) | 9(4.89) | ||||||||
| rs195854 | AA | 81(55.86) | 81(50.63) | 0.60(0.36–1.00) | 0.049* | 0.78(0.48–1.25) | 0.30 | 0.38(0.14–1.02) | 0.056 | |
| AT | 58(40.00) | 63(39.38) | ||||||||
| TT | 6(4.14) | 16(10.00) | ||||||||
| AQP2 | rs7314734 | CC | 122(79.22) | 155(84.24) | 0.93(0.28–3.12) | 0.90 | 1.49(0.84–2.67) | 0.17 | 0.80(0.071–8.99) | 0.86 |
| CT | 31(20.13) | 27(14.67) | ||||||||
| TT | 1(0.65) | 2(1.09) | ||||||||
| AQP9 | rs1516400 | TT | 46(30.67) | 49(29.92) | 0.80(0.58–1.10) | 0.18 | 0.84(0.51–1.38) | 0.49 | 0.66(0.38–1.15) | 0.14 |
| CT | 73(48.67) | 86(47.25) | ||||||||
| CC | 31(20.67) | 47(25.82) | ||||||||
| TMEM205 | rs896412 | GG | 103(67.32) | 115(62.84) | 0.29(0.10–0.81) | 0.019* | 0.81(0.50–1.29) | 0.37 | 0.082(0.010–0.66) | 0.019* |
| CG | 49(32.03) | 56(30.60) | ||||||||
| CC | 1(0.65) | 12(6.56) | ||||||||
*P<0.05.
The rs195854 and rs1869641 of OCT2, rs896412 of TMEM205, rs7314734 of AQP2, located in 5 kb upstream of the gene regions, the possible mechanism of regulating the expression were not known at present. We hypothesize that they locate in the enhancer area and the variant can regulate the expression. The rs1516400 located in the neargene-5 of AQP9. It may be in the promoter area and its mutation may regulate the activity of the promoter of AQP9, and then the expression of the AQP9 was changed. However, the possible mechanisms need to be studied in the further studies.
This study has some limitations. On one hand, to get more credible conclusion, we should do an independent validation for these SNPs. We are thus trying to amplify the sample size and will validate these SNPs in the future study. However, the samples are very difficult to collect and not enough for another independent population validation now. On the other hand, evaluating the chemotherapy response after every two cycles of chemotherapy treatment is likely to get more reliable results. However, we can only unify the two cycles of chemotherapy for these patients. Many patients didn't receive more than four cycles of chemotherapy because of many reasons, such as financial condition and so on.
There was not any study indicated whether polymorphisms of these five genes were related to platinum-based chemotherapy response in lung cancer patients before. This study was the first one to investigate their associations with platinum-based chemotherapy response. The results of TMEM205 rs896412, OCT2 rs1869641 and rs195854, AQP9 rs1516400 and AQP2 rs7314734 suggested that these SNPs might be associated with platinum-based chemotherapy response. Further studies how these SNPs impact the platinum-based chemotherapy response for lung cancers should be warranted.
Supporting Information
Primers of all the selected SNPs.
(DOCX)
Association of the other single nucleotide polymorphisms and platinum-based chemotherapy response in all lung cancer patients.
(DOCX)
Stratification analyses of the associations of the other polymorphisms and chemotherapy efficacy in genotypic, dominant, recessive models.
(DOCX)
Acknowledgments
We thank all the funds that supported the study, and all patients who participated in the study, and Bio Miao Biological Technology Co., Ltd. Beijing, China for their technical assistance, Kamila Śmieszkol corrected the manuscript.
Funding Statement
This work was supported in part by National High-tech R&D Program of China 863 Program Grant 2012AA02A517 (ZYC)(http://www.863.gov.cn/), National Natural Science Foundation of China Grants 81173129 and 81373490 (ZYC), and 81202595 (JY)(http://scholarmate.nsfc.gov.cn/scm/), Program for the Special Scientific Research Foundation of Doctor Disciplines in University of Ministry of Education of China Grant 20110162110034 (ZYC), Hunan Provincial Natural Science Foundation of China Grant 12JJ7006 (ZYC)(http://www.hnst.gov.cn/zxgz/zkjj/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. (2011) Global cancer statistics. CA Cancer J Clin 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 2. Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60: 277–300. [DOI] [PubMed] [Google Scholar]
- 3. Wernyj RP, Morin PJ (2004) Molecular mechanisms of platinum resistance: still searching for the Achilles' heel. Drug Resist Updat 7: 227–232. [DOI] [PubMed] [Google Scholar]
- 4. Spira A, Ettinger DS (2004) Multidisciplinary management of lung cancer. N Engl J Med 350: 379–392. [DOI] [PubMed] [Google Scholar]
- 5. Shen DW, Pouliot LM, Hall MD, Gottesman MM (2012) Cisplatin resistance: a cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol Rev 64: 706–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Knepper MA, Wade JB, Terris J, Ecelbarger CA, Marples D, et al. (1996) Renal aquaporins. Kidney Int 49: 1712–1717. [DOI] [PubMed] [Google Scholar]
- 7. Hall MD, Okabe M, Shen DW, Liang XJ, Gottesman MM (2008) The role of cellular accumulation in determining sensitivity to platinum-based chemotherapy. Annu Rev Pharmacol Toxicol 48: 495–535. [DOI] [PubMed] [Google Scholar]
- 8. Shen DW, Ma J, Okabe M, Zhang G, Xia D, et al. (2010) Elevated expression of TMEM205, a hypothetical membrane protein, is associated with cisplatin resistance. J Cell Physiol 225: 822–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shen DW, Gottesman MM (2012) RAB8 enhances TMEM205-mediated cisplatin resistance. Pharm Res 29: 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rabik CA, Dolan ME (2007) Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev 33: 9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ludwig T, Riethmuller C, Gekle M, Schwerdt G, Oberleithner H (2004) Nephrotoxicity of platinum complexes is related to basolateral organic cation transport. Kidney Int 66: 196–202. [DOI] [PubMed] [Google Scholar]
- 12. Ciarimboli G, Ludwig T, Lang D, Pavenstadt H, Koepsell H, et al. (2005) Cisplatin nephrotoxicity is critically mediated via the human organic cation transporter 2. Am J Pathol 167: 1477–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang W, Ke S, Chen G, Gao Q, Wu S, et al. (2004) Effect of lung resistance-related protein on the resistance to cisplatin in human ovarian cancer cell lines. Oncol Rep 12: 1365–1370. [PubMed] [Google Scholar]
- 14. Innocenti F, Kroetz DL, Schuetz E, Dolan ME, Ramirez J, et al. (2009) Comprehensive pharmacogenetic analysis of irinotecan neutropenia and pharmacokinetics. J Clin Oncol 27: 2604–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Efferth T, Volm M (2005) Pharmacogenetics for individualized cancer chemotherapy. Pharmacol Ther 107: 155–176. [DOI] [PubMed] [Google Scholar]
- 16. Campa D, Muller P, Edler L, Knoefel L, Barale R, et al. (2012) A comprehensive study of polymorphisms in ABCB1, ABCC2 and ABCG2 and lung cancer chemotherapy response and prognosis. Int J Cancer 131: 2920–2928. [DOI] [PubMed] [Google Scholar]
- 17. Xu X, Ren H, Zhou B, Zhao Y, Yuan R, et al. (2012) Prediction of copper transport protein 1 (CTR1) genotype on severe cisplatin induced toxicity in non-small cell lung cancer (NSCLC) patients. Lung Cancer 77: 438–442. [DOI] [PubMed] [Google Scholar]
- 18. Xu X, Duan L, Zhou B, Ma R, Zhou H, et al. (2012) Genetic polymorphism of copper transporter protein 1 is related to platinum resistance in Chinese non-small cell lung carcinoma patients. Clin Exp Pharmacol Physiol 39: 786–792. [DOI] [PubMed] [Google Scholar]
- 19. Zolk O, Solbach TF, Konig J, Fromm MF (2009) Functional characterization of the human organic cation transporter 2 variant p.270Ala>Ser. Drug Metab Dispos 37: 1312–1318. [DOI] [PubMed] [Google Scholar]
- 20. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, et al. (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 21. The International HapMap Project. Nature 426: 789–796. [DOI] [PubMed] [Google Scholar]
- 22. Fujita T, Urban TJ, Leabman MK, Fujita K, Giacomini KM (2006) Transport of drugs in the kidney by the human organic cation transporter, OCT2 and its genetic variants. J Pharm Sci 95: 25–36. [DOI] [PubMed] [Google Scholar]
- 23. Wright SH, Dantzler WH (2004) Molecular and cellular physiology of renal organic cation and anion transport. Physiol Rev 84: 987–1049. [DOI] [PubMed] [Google Scholar]
- 24. Filipski KK, Loos WJ, Verweij J, Sparreboom A (2008) Interaction of Cisplatin with the human organic cation transporter 2. Clin Cancer Res 14: 3875–3880. [DOI] [PubMed] [Google Scholar]
- 25. Gupta A, Srivastava S, Prasad R, Natu SM, Mittal B, et al. (2009) Smoking intensity, oxidative stress and chemotherapy in nonsmall cell lung cancer: a correlated prognostic study. Biosci Trends 3: 191–199. [PubMed] [Google Scholar]
- 26. Pao W, Miller V, Zakowski M, Doherty J, Politi K, et al. (2004) EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 101: 13306–13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pham D, Kris MG, Riely GJ, Sarkaria IS, McDonough T, et al. (2006) Use of cigarette-smoking history to estimate the likelihood of mutations in epidermal growth factor receptor gene exons 19 and 21 in lung adenocarcinomas. J Clin Oncol 24: 1700–1704. [DOI] [PubMed] [Google Scholar]
- 28. Groves CE, Suhre WB, Cherrington NJ, Wright SH (2006) Sex differences in the mRNA, protein, and functional expression of organic anion transporter (Oat) 1, Oat3, and organic cation transporter (Oct) 2 in rabbit renal proximal tubules. J Pharmacol Exp Ther 316: 743–752. [DOI] [PubMed] [Google Scholar]
- 29. Alnouti Y, Petrick JS, Klaassen CD (2006) Tissue distribution and ontogeny of organic cation transporters in mice. Drug Metab Dispos 34: 477–482. [DOI] [PubMed] [Google Scholar]
- 30. Slitt AL, Cherrington NJ, Hartley DP, Leazer TM, Klaassen CD (2002) Tissue distribution and renal developmental changes in rat organic cation transporter mRNA levels. Drug Metab Dispos 30: 212–219. [DOI] [PubMed] [Google Scholar]
- 31. Miao ZF, Chang EE, Tsai FY, Yeh SC, Wu CF, et al. (2009) Increased aquaglyceroporin 9 expression disrupts arsenic resistance in human lung cancer cells. Toxicol In Vitro 23: 209–216. [DOI] [PubMed] [Google Scholar]
- 32. Onuki N, Wistuba Travis WD II, Virmani AK, Yashima K, et al. (1999) Genetic changes in the spectrum of neuroendocrine lung tumors. Cancer 85: 600–607. [DOI] [PubMed] [Google Scholar]
- 33. Hida T, Yatabe Y, Achiwa H, Muramatsu H, Kozaki K, et al. (1998) Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res 58: 3761–3764. [PubMed] [Google Scholar]
- 34. Eymin B, Gazzeri S, Brambilla C, Brambilla E (2001) Distinct pattern of E2F1 expression in human lung tumours: E2F1 is upregulated in small cell lung carcinoma. Oncogene 20: 1678–1687. [DOI] [PubMed] [Google Scholar]
- 35. Eymin B, Leduc C, Coll JL, Brambilla E, Gazzeri S (2003) p14ARF induces G2 arrest and apoptosis independently of p53 leading to regression of tumours established in nude mice. Oncogene 22: 1822–1835. [DOI] [PubMed] [Google Scholar]
- 36. Kishore BK, Krane CM, Di Iulio D, Menon AG, Cacini W (2000) Expression of renal aquaporins 1, 2, and 3 in a rat model of cisplatin-induced polyuria. Kidney Int 58: 701–711. [DOI] [PubMed] [Google Scholar]
- 37. Langer CJ, Manola J, Bernardo P, Kugler JW, Bonomi P, et al. (2002) Cisplatin-based therapy for elderly patients with advanced non-small-cell lung cancer: implications of Eastern Cooperative Oncology Group 5592, a randomized trial. J Natl Cancer Inst 94: 173–181. [DOI] [PubMed] [Google Scholar]
- 38. Rowe JM, Andersen JW, Mazza JJ, Bennett JM, Paietta E, et al. (1995) A randomized placebo-controlled phase III study of granulocyte-macrophage colony-stimulating factor in adult patients (>55 to 70 years of age) with acute myelogenous leukemia: a study of the Eastern Cooperative Oncology Group (E1490). Blood 86: 457–462. [PubMed] [Google Scholar]
- 39. Lilenbaum RC, Herndon JE 2nd, List MA, Desch C, Watson DM, et al. (2005) Single-agent versus combination chemotherapy in advanced non-small-cell lung cancer: the cancer and leukemia group B (study 9730). J Clin Oncol 23: 190–196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers of all the selected SNPs.
(DOCX)
Association of the other single nucleotide polymorphisms and platinum-based chemotherapy response in all lung cancer patients.
(DOCX)
Stratification analyses of the associations of the other polymorphisms and chemotherapy efficacy in genotypic, dominant, recessive models.
(DOCX)