Abstract
Anti-EGFR therapy appears to be a potential treatment option for squamous cell anal carcinoma (SCAC). KRAS mutation is a rare event in SCAC, indicating the absence of the principal mechanism of resistance to this type of therapy. However, no information is available from the literature regarding the status of BRAF or PIK3CA in this cancer type. We analysed KRAS, BRAF and PIK3CA status in SCAC patients in relation to the clinical-pathological characteristics of patients and to the presence of the human papilloma virus (HPV). One hundred and three patients were treated with the Nigro scheme for anal cancer from March 2001 to August 2012. Fifty patients were considered for the study as there was insufficient paraffin-embedded tumour tissue to perform molecular analysis the remaining 53. DNA was extracted from paraffin-embedded sections. KRAS, BRAF and PIK3CA gene status and HPV genotype were evaluated by pyrosequencing. KRAS and BRAF genes were wild-type in all cases. Conversely, PIK3CA gene was found to be mutated in 11 (22%) cases. In particular, 8 mutations occurred in exon 9 and 3 in exon 20 of the PIK3CA gene. These findings suggest that SCAC could potentially respond to an anti-EGFR drug. PIK3CA mutation may be involved in the process of carcinogenesis in some cases of SCAC.
Introduction
Although anal carcinoma is not a common tumour, its incidence has increased progressively in parallel with transmitted viral infections. Infection from human papilloma virus (HPV) is the main etiologic factor for anal cancer and a high percentage of patients are HPV-positive [1]. Up until 20 or 30 years ago, surgery was the standard treatment for this tumour, consisting in abdominoperineal resection or Miles Operation. The overall 5-year survival is around 50–70%.
In recent years, the therapeutic approach to anal cancer has changed dramatically from demolitive surgery to conservative treatment with radiochemotherapy. Today surgery is mainly used for diagnostic purposes and/or as salvage treatment in locoregional failure after radiochemotherapy.
The first description of the use of radiochemotherapy goes back to the 1970s when the Nigro regimen showed a high rate of complete responses in patients undergoing surgery after preoperative treatment with low doses of radiation (30 Gy) administered in combination with 5-fluorouracil and mitomycin C. Treatment has changed very little since then. Recently, a clinical response to anti-EGFR drugs was observed in single patients [2], [3] and in small case series of patients [4], suggesting their potential effectiveness in this type of cancer.
EGFR expression in anal carcinoma is observed in approximately 80–90% of cases [5]–[7]. Moreover, some studies have demonstrated that KRAS mutations, the principal mechanism of resistance to anti-EGFR therapy, are virtually absent in this tumour [5]–[7]. Such information could represent an important prerequisite for the use of anti-EGFR strategies. However, the incidence of other gene alterations, e.g. BRAF and PIK3CA mutations, involved in the response to anti-EGFR therapies in colorectal cancer [8] has not been studied in anal carcinoma.
In the present study we set out to verify the incidence of KRAS, BRAF and PIK3CA mutations in a series of patients with anal carcinoma and analysed the association between these alterations and the clinical-pathological characteristics of patients.
Patients and Methods
Patient Population
We retrospectively analysed 103 patients with SCAC consecutively treated with chemotherapy and radiotherapy at Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori in Meldola (Italy) and the Medical Oncology Units of Faenza, Ravenna and Macerata Hospitals (Italy) from March 2001 to August 2012. There was insufficient paraffin-embedded tumour tissue to perform molecular analysis in 53 cases. Our study thus comprised 50 patients with stage I to IV invasive SCAC treated with the Nigro regimen (radiation therapy with concurrent 5-fluorouracil and mitomycin C) for whom paraffin-embedded tumour tissue was available.
According to TNM classification [9], 29 patients had early stage SCAC (stage 1 or 2), 19 patients had stage 3 disease and 2 patients had metastatic cancer. Thirty-four patients had a grade 1 or 2 tumour, and 16 had a grade 3 tumour. Forty-four patients were HIV-negative and 6 were HIV-positive. Thirteen patients had recurrent SCAC.
The study protocol was reviewed and approved by the Medical Scientific Committee of IRST IRCCS, and written informed consent was obtained from patients or from their next of kin for the use of biological samples for research purposes.
Mutation Analysis
Formalin-fixed paraffin-embedded (FFPE) tumour blocks were reviewed for quality and tumour content. For each case, an area containing at least 50% of tumour cells was selected in hematoxylin and eosin-stained sections, and corresponding 5-μM sections were macrodissected and collected in specific tubes for DNA extraction. Tumour cells were lysed in 50 mM of KCl, 10 mM of Tris-HCl pH 8.0, 2.5 mM of MgCl2 and Tween-20 (0.45%) in the presence of 1.25 mg/ml of proteinase K, overnight at 56°C. Proteinase K was inactivated at 95°C for 10 minutes and the samples were then centrifuged twice at 6000 rpm to eliminate debris. DNA was purified using QIAamp DNA Micro kit (Qiagen, Hilden, Germany) in accordance with the “Genomic DNA clean-up” protocol. DNA quantity and quality were assessed by Nanodrop (Celbio, Milan, Italy).
KRAS (exons 2 and 3), BRAF (exons 11 and 15) and PIK3CA (exons 9 and 20) genes were analysed by pyrosequencing using 3 different kits: anti-EGFR MoAb response (KRAS status), anti-EGFR MoAb response (BRAF status) and anti-EGFR MoAb response (PIK3CA status) (Diatech Pharmacogenetics, Jesi, Italy), according to the manufacturer's instructions. Reactions were run on a PyroMark Q96 ID system (Qiagen).
HPV detection
HPV DNA was detected in genomic tumour DNA using the HPV sign kit (Diatech Pharmacogenetics), which allows for the amplification and sequencing of a hypervariable region of a highly conserved region of the HPV gene extracted from tumour tissue. Specific genotypes were assigned by pyrosequencing using the Pyromark Q96 ID system (Qiagen) which analyses and aligns the determined sequence with the sequencing library supplied in the kit.
Statistical Analyses
Progression-free survival (PFS) was calculated from the first day of treatment to the date of the first observation of disease progression or to the date of the last follow-up. Overall survival (OS) was calculated from the first day of treatment to the date of death due to any cause or to the date of the last follow-up. PFS, OS and their 95% confidence intervals (95% CI) were estimated using the Kaplan-Meier life-table method [10] and survival curves were compared by the logrank test [11]. The association between KRAS, BRAF and PIK3CA alteration/mutation and the clinical-pathological characteristics of the patients was analysed by the chi-square test.
Statistical significance was assumed for P<0.05 [12]. Statistical analyses were carried out with SAS Statistical software (version 9.3, SAS Institute, Cary, NC).
Results
Fifty patients (19 males, 31 females) were evaluated. Median age was 62 years (range, 37–87 years). Nineteen (38%) patients had regional nodal involvement, 18 (36.0%) had stage 3–4 disease and 2 (4%) had distant metastases.
All patients had wild type KRAS (codons 12, 13, 61 and 146) and BRAF (exons 11 and 15), whereas 11 (22%) had a mutation in the PIK3CA gene. In particular, 8 had a PIK3CA mutation in exon 9 and 3 had a mutation in exon 20 (Table 1). The clinical characteristics of patients are reported in Table 2. All patients were HPV-positive; 46 (92%) had HPV type 16, one had an HPV-16/18 co-infection and the other 3 patients had HPV type 31, 35 or 6.
Table 1. Results of PIK3CA mutations.
| PIK3CA mutations | No. mutated cases | % Total cases (n = 50) |
| All | 11 | 22 |
| Exon 9 | 8 | 16 |
| E545K | 5 | 10 |
| E542K | 2 | 4 |
| Q546E | 1 | 2 |
| Exon 20 | 3 | 6 |
| H1047L | 2 | 4 |
| H1047R | 1 | 2 |
Table 2. Clinical characteristics of the patients with PIK3CA mutation.
| Exon 9 | Exon 20 | |
| All patients | 8 | 3 |
| Gender | ||
| Male | 2 | 1 |
| Female | 6 | 2 |
| Age, years | ||
| <60 | 3 | 2 |
| ≥60 | 5 | 1 |
| Histological grade | ||
| Grade 1+/Grade 2 | 3 | 3 |
| Grade 3 | 5 | 0 |
| Tumour classification | ||
| 1–2 | 5 | 2 |
| 3–4 | 3 | 1 |
| Lymph node status | ||
| Negative | 5 | 2 |
| Positive | 3 | 1 |
| Stage | ||
| 1 | 2 | 0 |
| 2 | 3 | 2 |
| 3 | 4 | 1 |
| 4 | 0 | 0 |
| HIV status | ||
| Negative | 5 | 3 |
| Positive | 3 | 0 |
| HPV status | ||
| HPV-16 positive | 8 | 3 |
| HPV-16 negative | 0 | 0 |
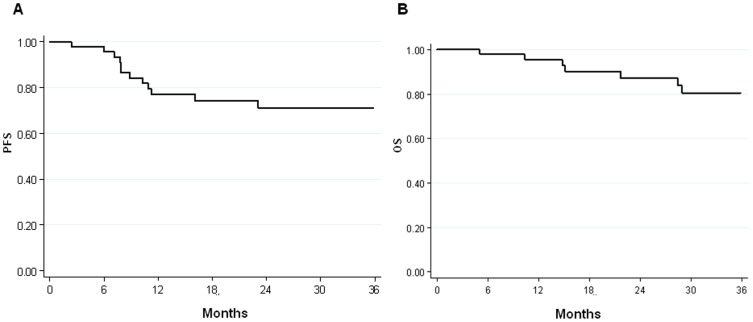
At a median follow-up of 38 months (range, 2–138 months), 12 patients had died and 13 patients were in progression. Of these, 7 patients had locoregional failure and 6 had systemic failure. PFS was 77% (95% CI 64–90) at one year and 72% (95% CI 57–85) at 3 years. The overall survival rate was 95% (95% CI 89–100) at 1 year and 81% (95% CI 67–94) at 3 years (Fig. 1). OS and PFS in relation to the clinical-pathological characteristics of patients are summarised in Tables 3 and 4, respectively.
Figure 1. Kaplan-Meier survival curves of (A) progression-free survival (PFS) and (B) overall survival (OS).
Table 3. Progression-free survival in relation to clinical-pathological characteristics of patients.
| PFS | No. of patients | No. of events | % 1-year PFS (95% CI) | % 3-year PFS (95% CI) | P |
| All patients | 50 | 13 | 77 (64–90) | 72 (57–85) | - |
| Type of recurrence | |||||
| Local | 7 | 7 | 0 | 0 | - |
| Distant | 5 | 5 | 40 (0–83) | 20 (0–55) | 0.042 |
| Histological grade | |||||
| Grade 1+/Grade 2 | 34 | 5 | 86 (74–99) | 82 (68–97) | - |
| Grade 3 | 16 | 8 | 59 (33–84) | 49 (21–76) | 0.013 |
| Tumour classification | |||||
| 1–2 | 27 | 3 | 87 (73–100) | 87 (73–100) | - |
| 3–4 | 18 | 7 | 68 (45–91) | 61 (36–86) | 0.026 |
| 1–2–3 | 37 | 6 | 84 (71–97) | 80 (66–94) | - |
| 4 | 8 | 4 | 58 (22–95) | 58 (22–95) | 0.031 |
| Lymph node status | |||||
| Negative | 31 | 6 | 81 (67–96) | 77 (60–93) | - |
| Positive | 19 | 7 | 69 (46–92) | 61 (37–86) | 0.068 |
| Stage | |||||
| 1 | 8 | 0 | 100 | 100 | - |
| 2 | 21 | 4 | 79 (61–97) | 79 (61–97) | - |
| 3 | 19 | 7 | 74 (53–96) | 57 (31–84) | - |
| 4 | 2 | 2 | 0 | 0 | <0.0001 |
| HIV status | |||||
| Negative | 44 | 12 | 74 (60–88) | 71 (56–86) | - |
| Positive | 6 | 1 | 100 | 50 (0–100) | 0.787 |
| PIK3CA mutation | |||||
| No | 39 | 10 | 77 (63–91) | 73 (57–88) | - |
| Yes | 11 | 3 | 78 (41–100) | 65 (32–97) | 0.889 |
| HPV status | |||||
| HPV-16 positive | 45 | 10 | 82 (69–94) | 75 (61–89) | - |
| HPV-16 negative | 5 | 3 | 40 (0–83) | 40 (0–83) | 0.061 |
Table 4. Overall survival in relation to clinical-pathological characteristics of patients.
| No. of patients | No. of events | % 1-year OS (95% CI) | % 3-year OS (95% CI) | P | |
| All patients | 50 | 12 | 95 (89–100) | 81 (67–94) | - |
| Type of recurrence | |||||
| Local | 7 | 7 | 71 (38–100) | 18 (0–49) | - |
| Distant | 5 | 5 | 100 | 40 (0–83) | 0.286 |
| Histological grade | |||||
| Grade 1 +/Grade 2 | 34 | 5 | 97 (91–100) | 88 (74–100) | - |
| Grade 3 | 16 | 7 | 93 (79–100) | 64 (35–93) | 0.014 |
| Tumour classification | |||||
| 1–2 | 27 | 3 | 95 (87–100) | 89 (75–100) | - |
| 3–4 | 18 | 6 | 94 (83–100) | 70 (45–95) | 0.054 |
| 1–2–3 | 37 | 6 | 97 (90–100) | 84 (70–99) | - |
| 4 | 8 | 3 | 87 (65–100) | 66 (25–100) | 0.231 |
| Lymphnode status | |||||
| Negative | 31 | 6 | 100 | 87 (73–100) | - |
| Positive | 19 | 6 | 87 (71–100) | 70 (44–95) | 0.075 |
| Stage | |||||
| 1 | 8 | 0 | 100 | 100 | - |
| 2 | 21 | 4 | 100 | 81 (61–100) | - |
| 3 | 19 | 7 | 93 (81–100) | 78 (55–100) | - |
| 4 | 2 | 1 | 50 (0–100) | 50 (0–100) | 0.0002 |
| HIV status | |||||
| Negative | 44 | 11 | 95 (88–100) | 79 (65–93) | - |
| Positive | 6 | 1 | 100 | 100 | 0.700 |
| PIK3CA mutation | |||||
| No | 39 | 9 | 94 (87–100) | 81 (66–97) | - |
| Yes | 11 | 3 | 100 | 75 (45–100) | 0.706 |
| HPV status | |||||
| HPV-16 positive | 45 | 10 | 91 (88–100) | 82 (68–95) | - |
| HPV-16 negative | 5 | 2 | 100 | 75 (33–100) | 0.538 |
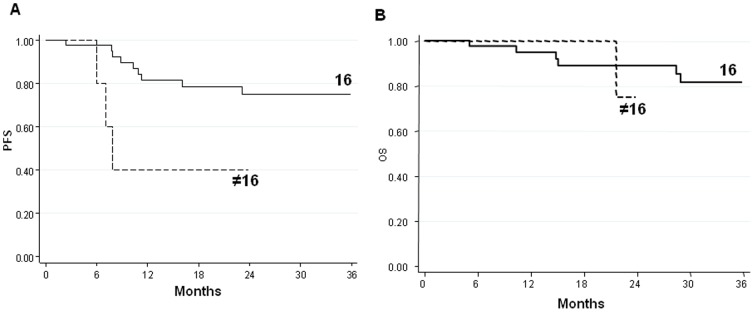
No association was found between PIK3CA mutations and clinical characteristics of patients. Moreover, no correlation was found between PFS and OS (data not shown). Conversely, HPV-16 was associated with a significantly longer PFS and a higher, albeit not statistically significant, OS compared to the other HPV genotypes (Fig. 2).
Figure 2. Kaplan-Meier survival curves of (A) progression-free survival (PFS) and (B) overall survival (OS) in relation to HPV-16 status.
Discussion
Although anal cancer is considered a curable disease, around 20% of patients relapse and a further 30% undergo colostomy for treatment-related toxicities. Treatment has essentially remained the same for 30 years. In our study, PFS and OS were influenced by tumour grade, primary tumour dimension and lymph node status (Fig. 2), in agreement with data presented in the literature [13]. Yhim et al. [14] analysed the correlation between HPV infection and PFS and OS in 48 patients with anal carcinoma. In the HPV-16-positive group, 4-year PFS and OS rates were 63.1% (95% CI, 53.1–73.1) and 84.6% (95% CI, 76.2–93.0), respectively, while 4-year PFS and OS rates in the HPV-16-negative group were 15.6% (95% CI, 5.6–25.7) and 39.8% (95% CI, 26.1–53.4), respectively. HPV-16-positive patients had significantly longer PFS and OS than HPV-16-negative cases (PFS, P<0.001; OS, P = 0.008). In our study, HPV-16 positive patients had a longer PFS than those with other HPV genotypes, showing a trend towards statistical significance (P = 0.061). Such a trend was not observed for OS (P = 0.747), even though HPV-16 positive patients showed a longer OS than negative cases.
We did not observe mutations of the KRAS gene, in agreement with the findings of other studies [5]–[7]. Moreover, no mutations of the BRAF gene were detected. Conversely, 22% of patients were found to have PIK3CA exon 9 and exon 20 mutations. No association was found between PIK3CA mutations and prognosis. Cetuximab (moAb anti-EGFR) represents a promising treatment choice for anal carcinoma. Several studies on colorectal cancer (CRC) have shown that KRAS gene mutations impair response to the drug [15]. It has also been demonstrated that BRAF and PIK3CA gene mutations in CRC may be associated with a worse response to treatment and poorer prognosis [16]. Our study shows that KRAS and BRAF are not mutated in anal carcinoma, whereas PIK3CA, mutated in about 20% of cases, may represent a mechanism of resistance to anti-EGFR treatment, such as cetuximab. KRAS mutation appears to be a rare event in other squamous cell tumours, such as head and neck cancer [17] and cervical cancer [18]. However, the few studies published on BRAF mutations in squamous cell carcinomas would seem to indicate a low mutation rate [17]. Recently, some studies reported an incidence of PIK3CA mutations in other squamous cell cancers similar to that observed in our study. In particular, Lui and co-workers found a PIK3CA mutation in 30.5% of head and neck cancer patients who were sensitive to anti-mTOR treatment [19]. In a study by McIntyre and co-workers, about 20% of squamous cervical cancer patients showed a PIK3CA mutation and these patients had a better prognosis than those with wild-type tumours [20]. These results, together with our findings, suggest that the PIK3CA pathway could play an important role in the carcinogenesis of squamous cell carcinoma, especially those related to HPV infection. A more complete understanding of this pathway could facilitate the design of targeted therapy for these types of malignancies.
Two interesting reports were recently published on the use of cetuximab in anal cancer. The first was a phase I study of cetuximab in combination with 5-FU, cisplatin and radiotherapy for locally-advanced anal cancer. Of the 10 patients enrolled, all completed full-dose radiotherapy, 9 were evaluable and 7 (78%) achieved a complete response [21]. The second report focused on the phase II ACCORD 16 study of cetuximab in combination with 5-FU, cisplatin and radiotherapy [22]. Of the 16 patients included in this study, only 8 received the full planned treatment (6 cetuximab administrations and 2 5FU-cisplatin cycles. Accrual was suspended after 10 serious adverse events (6 of which were SUSARs) occurred in 7 of the first 10 patients enrolled. The protocol was amended by decreasing the 5FU and cisplatin dose was decreased and introducing mandatory intensity-modulated radiation therapy. After the accrual of 6 additional patients, a further 6 SAEs occurred in 5 patients. It was thus decided to close the trial due to the high rate of toxicity. Further studies are needed in the neoadjuvant setting to identify acceptable doses of chemotherapy and radiotherapy and thus reduce the risk of severe side-effects. Although it has also been hypothesized that cetuximab could benefit patients with recurrent disease after radiochemotherapy, few studies have been carried out in this area.
In conclusion, despite the relatively small sample size of our study, we believe it is sufficient to demonstrate the presence or absence of the mutations in question. Our results also suggest that the use of the anti-EGFR drug could be used in anal carcinoma, but further studies analysing the effect of this drug in relation to molecular alterations are needed.
Acknowledgments
The authors would like to thank Ursula Elbling for editing the manuscript.
Funding Statement
These authors have no support or funding to report.
References
- 1. Škamperle M, Kocjan BJ, Maver PJ, Seme K, Poljak M (2013) Human papillomavirus (HPV) prevalence and HPV type distribution in cervical, vulvar, and anal cancers in Central and Eastern Europe. Acta Dermatovenerol Alp Panonica Adriat 22: 1–5. [PubMed] [Google Scholar]
- 2. Barmettler H, Komminoth P, Schmid M, Duerr D (2012) Efficacy of cetuximab in combination with FOLFIRI in a patient with KRAS wild-type metastatic anal cancer. Case Rep Oncol 5: 428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bamba T, Suda T, Nakano M, Terashima T, Umezu H (2012) Pathologically complete response for unresectable stage IV rectal cancer using systemic chemotherapy with panitumumab - a case report. Gan To Kagaku Ryoho 39: 311–315. [PubMed] [Google Scholar]
- 4. Lukan N, Ströbel P, Willer A, Kripp M, Dinter D, et al. (2009) Cetuximab-based treatment of metastatic anal cancer: correlation of response with KRAS mutational status. Oncology 77: 293–299. [DOI] [PubMed] [Google Scholar]
- 5. Paliga A, Onerheim R, Gologan A, Chong G, Spatz A, et al. (2012) EGFR and K-ras gene mutation status in squamous cell anal carcinoma: a role for concurrent radiation and EGFR inhibitors? Br J cancer 107: 1864–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zampino MG, Magni E, Sonzogni A, Renne G (2009) K-ras status in squamous cell anal carcinoma (SCC): it's time for target-oriented treatment? Cancer Chemother Pharmacol 65: 197–199. [DOI] [PubMed] [Google Scholar]
- 7. Van Damme N, Deron P, Van Roy N, Demetter P, Bols A, et al. (2010) Epidermal Growth Factor Receptor and K-RAS status in two cohorts of squamous cell carcinomas. BMC Cancer 10: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ulivi P, Capelli L, Valgiusti M, Zoli W, Scarpi E, et al. (2012) Predictive role of multiple gene alterations in response to cetuximab in metastatic colorectal cancer: a single center study. J Transl Med 10: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, et al.. (2010) AJCC Cancer Staging Manual (7th Edition). New York: Springer.
- 10. Kaplan EL, Meier P (2005) Non-parametric estimation from incomplete observation. J Am Stat Assoc 24: 341–358. [Google Scholar]
- 11.Lawkess JS (1982) Statistical models and methods for life-time data. New York: John Wiley and Sons 10: : 316–317. [Google Scholar]
- 12. Cox DR (1972) Regression models and life tables. J Royal Stat Soc 34: 187–220. [Google Scholar]
- 13. Das P, Bhatia S, Eng C, Ajani JA, Skibber JM, et al. (2007) Predictors and patterns of recurrence after definitive chemoradiation for anal cancer. Int J Radiat Oncol Biol Phys 68: 794–800. [DOI] [PubMed] [Google Scholar]
- 14. Yhim HY, Lee NR, Song EK, Kwak JY, Lee ST, et al. (2011) The prognostic significance of tumour human papillomavirus status for patients with anal squamous cell carcinoma treated with combined chemoradiotherapy. Int J Cancer 129: 1752–1760. [DOI] [PubMed] [Google Scholar]
- 15. Lièvre A, Bachet JB, Le Corre D, Boige V, Landi B, et al. (2006) KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 66: 3992–3995. [DOI] [PubMed] [Google Scholar]
- 16. Sartore-Bianchi A, Di Nicolantonio F, Nichelatti M, Molinari F, De Dosso S, et al. (2009) Multi-determinants analysis of molecular alterations for predicting clinical benefit to EGFR-targeted monoclonal antibodies in colorectal cancer. PLoS One 4: e7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bruckman KC, Schönleben F, Qiu W, Woo VL, Su GH (2010) Mutational analyses of the BRAF, KRAS, and PIK3CA genes in oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 110: 632–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pochylski T, Kwaśniewska A (2003) Absence of point mutation in codons 12 and 13 of K-RAS oncogene in HPV-associated high grade dysplasia and squamous cell cervical carcinoma. Eur J Obstet Gynecol Reprod Biol 111: 68–73. [DOI] [PubMed] [Google Scholar]
- 19. Lui VW, Hedberg ML, Li H, Vangara BS, Pendleton K, et al. (2013) Frequent Mutation of the PI3K Pathway in Head and Neck Cancer Defines Predictive Biomarkers. Cancer Discov 3: 761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McIntyre JB, Wu JS, Craighead PS, Phan T, Köbel M, et al. (2013) PIK3CA mutational status and overall survival in patients with cervical cancer treated with radical chemoradiotherapy. Gynecol Oncol 128: 409–414. [DOI] [PubMed] [Google Scholar]
- 21.Olivatto LO, Meton F, Bezerra M, Cardoso A, Araujo CM, et al.. (2006) Phase I study of cetuximab (CET) in combination with 5-fluorouracil (5-FU), cisplatin (CP), and radiotherapy (RT) in patients with locally advanced squamous cell anal carcinoma (LAAC). J Clin Oncol (suppl. 243) (abstr. 4609).
- 22. Deutsch E, Lemanski C, Pignon JP, Levy A, Delarochefordiere A, et al. (2013) Unexpected toxicity of cetuximab combined with conventional chemoradiotherapy in patients with locally advanced anal cancer: results of the UNICANCER ACCORD 16 phase II trial. Ann Oncol 2013 24: 2834–2838. [DOI] [PubMed] [Google Scholar]