Learning Objectives
Compare outcomes in patients with diffuse large B-cell lymphoma of the bone treated with different modalities.
Compare relapse rates and relapse sites in patients with diffuse large B-cell lymphoma of the bone treated with different modalities.
Keywords: Diffuse large B-cell lymphoma, Bone lymphoma, Radiotherapy, Osteolymphoma, Central nervous system prophylaxis
The authors reviewed the clinical features, management, and prognosis of 161 patients with stage I–II diffuse large B-cell lymphoma of the bone. Patients managed with primary chemotherapy had a significantly better outcome than patients treated with primary radiotherapy. The addition of consolidative radiotherapy after primary chemotherapy was not associated with improved outcome.
Abstract
Introduction.
The clinical features, management, and prognosis of stage I–II diffuse large B-cell lymphoma of the bone (PB-DLBCL) included in an international database of 499 lymphoma patients with skeletal involvement were reviewed.
Methods.
HIV-negative patients (n = 161) with diffuse large B-cell lymphoma of the bone (PB-DLBCL) after complete staging workup were considered. The primary objective of this study was to identify the most effective treatment modality; the secondary objectives were to define the contribution of irradiation fields and doses and the pattern of relapse.
Results.
Median age was 55 years (range, 18–99 years), with a male/female ratio of 1:2; 141 (87%) patients had stage I, 14 (9%) had B symptoms, 37 (23%) had bulky lesion, 54 (33%) showed elevated lactate dehydrogenase serum levels, and 25 (15%) had fracture. Thirteen (8%) patients received chemotherapy alone, 23 (14%) received radiotherapy alone, and 125 (78%) received both treatments. The response to the first-line treatment was complete in 131 of 152 assessed patients (complete response rate, 86%; 95% confidence interval [CI], 81%–91%) and partial in 7, with an overall response rate of 91% (95% CI, 87%–95%). At a median follow-up of 54 months (range, 3–218), 107 (67%) patients remained relapse-free, with a 5-year progression-free survival of 68% (SE: 4). Four (2.5%) patients had meningeal relapse; 119 patients were alive (113 disease-free), with a 5-year overall survival of 75% (SE: 4). Patients managed with primary chemotherapy, whether followed by radiotherapy or not, had a significantly better outcome than patients treated with primary radiotherapy, whether followed by chemotherapy or not. The addition of consolidative radiotherapy after primary chemotherapy was not associated with improved outcome; doses >36 Gy and the irradiation of the whole affected bone were not associated with better outcome.
Conclusion.
Patients with PB-DLBCL exhibit a favorable prognosis when treated with primary anthracycline-based chemotherapy whether followed by radiotherapy or not. In patients treated with chemoradiotherapy, the use of larger radiation fields and doses is not associated with better outcome. Central nervous system dissemination is a rare event in PB-DLBCL patients.
Implications for Practice:
Patients with limited-stage diffuse large B-cell lymphoma of the bone exhibit a favorable prognosis when treated with primary anthracycline-based chemotherapy whether followed by radiotherapy or not. In patients treated with chemoradiotherapy, the use of larger radiation fields and doses are not associated with better outcome. Central nervous system dissemination is a rare event in these patients, suggesting that specific prophylaxis is superfluous.
Introduction
Primary bone lymphoma (PBL) is a rare disease, comprising approximately 7% of all malignant bone tumors, 4%–5% of all extranodal non-Hodgkin lymphoma, and less than 1% of all malignant lymphomas [1, 2]. Diffuse large B-cell lymphoma (DLBCL) is the most common histological type among all PBL, and most patients have limited-stage disease (stage IE-IIE) at presentation [3–5]. The available literature on diffuse large B-cell lymphoma of the bone (PB-DLBCL) consists of mostly small, monoinstitutional series, resulting in a vague description of clinical features, management, and prognosis. In the past 2 decades, the use of primary anthracycline-based chemotherapy followed by consolidative irradiation has been the most commonly used strategy for patients with PB-DLBCL [6–9], with good overall disease control and survival rates. However, the natural clinical behavior as well as some diagnostic and management issues have yet to be addressed. In particular, the role of different chemotherapeutic combinations, the impact on local disease control of radiation therapy, the contribution of irradiation fields and doses, the pattern of relapse, and the management of pathological fractures and long-term sequelae remain to be defined in an adequate number of patients.
Against this background and under the sponsorship of the International Extranodal Lymphoma Study Group (IELSG),we analyzed presentation, management, clinical behavior, and outcome of 161 cases of PB-DLBCL (stage IE-IIE DLBCL of the bone) included in a multicenter database of 499 lymphoma patients with skeletal involvement.
Materials and Methods
Study Population
The members of the IELSG were invited to participate in a retrospective study focused on PBL (the IELSG-14 study). A questionnaire requesting information about patient characteristics, clinical presentation, diagnosis, staging, International Prognostic Index (IPI) score, treatment, objective response, site and date of relapse, second-line treatment, long-term sequelae, and survival of patients with a histopathological diagnosis of non-Hodgkin lymphoma and bone involvement at presentation was sent to referring centers from November 1980 to January 2005.
In order to investigate clinical and therapeutic features of PB-DLBCL, we reviewed records of patients fulfilling the following criteria: (a) age ≥18 years old; (b) histological diagnosis of DLBCL; (c) stage IE and IIE disease according to the Ann Arbor staging system, that is a single bone lesion with (stage IIE) or without (stage IE) regional lymphadenopathies; (d) complete staging workup with at least enhanced total-body computed tomography scan and bone marrow biopsy; and (e) no evidence of HIV-1 infection (negative serologic tests; absence of epidemiological risk, opportunistic infections, or lymphocytopenia for patients diagnosed in the early 1980s) or other immunodeficiencies. The study conformed to the tenets of the Declaration of Helsinki and was approved by the institutional review boards of the participating centers.
Response Definition
Response to treatment was recorded as follows: a complete response (CR) was defined as the complete disappearance of all evidence of lymphoma, partial response (PR) was defined as ≥50% decrease in tumor size, progressive disease (PD) was defined as ≥25% increase in tumor size or the appearance of any new tumor lesion, and stable disease (SD) was defined when these criteria were not met [10].
Statistical Considerations
The primary objective of this study was to identify the most effective treatment modality; the secondary objectives were to define the contribution of irradiation fields and doses and the pattern of relapse. Clinical characteristics and response rates of the treatment subgroups were compared using the chi-square test or Fisher’s exact test for categorical variables, according to the sample size. Survival curves were generated by the Kaplan-Meier method. Overall survival (OS) was calculated from the date of pathologic diagnosis to death or to the last date of follow-up, and progression-free survival (PFS) was calculated from the first day of treatment to relapse, progression, death, or to the last date of follow-up. Patients who did not progress or die at the last date of follow-up were censored, respectively, for PFS and OS analyses. Survival rates were reported as 5-year OS/PFS ± SE. Impact on survival of clinical and therapeutic variables was evaluated by comparing the survival curves by means of the log-rank test. Each patient was assigned to a therapeutic group according to the planned first-line treatment. The independent prognostic value of variables was analyzed using the Cox proportional hazard model. All the probability values were two-sided. Analyses were carried out using the SPSS 13.0 statistical package for Windows (LEAD Technologies, Inc., 2004; http://www.leadtools.com).
Results
Patient Characteristics
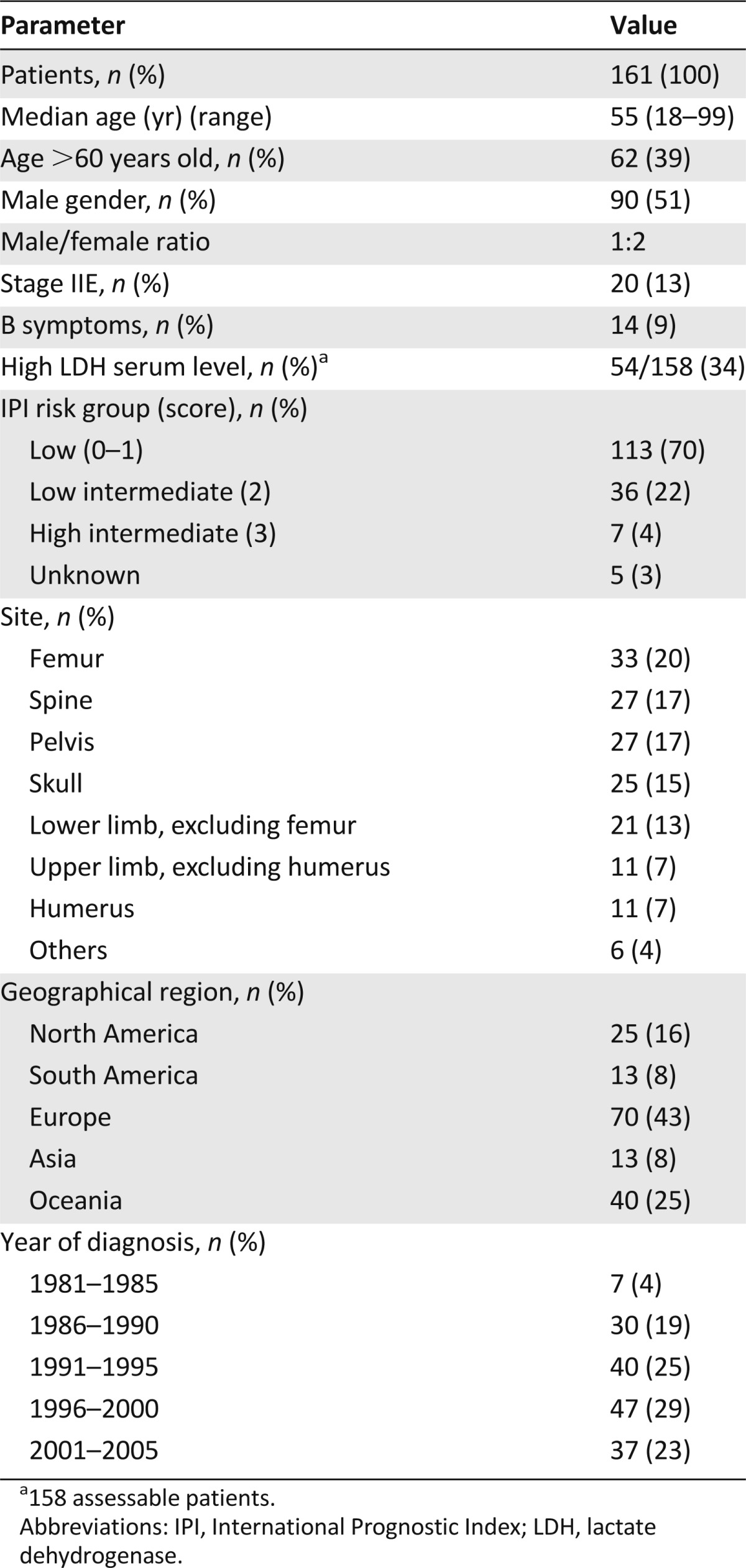
The database of the IELSG-14 study included 499 patients from 32 institutions in 14 countries (the list of participating centers with the number of registered patients/center appears at the end of the text). We excluded from analysis 110 patients with lymphoma categories other than DLBCL, 165 patients with advanced-stage DLBCL, 54 patients with incomplete staging workup, and 9 patients with unavailable therapeutic data. The remaining 161 patients with PB-DLBCL are the study population of the present study (Table 1). The median age of study population was 55 years (range, 18–99 years), with a male/female ratio of 1:2. At presentation, 141 (87%) patients had stage I and 20 (13%) patients had stage II, 14 (9%) patients had B symptoms, 37 (23%) had bulky lesion (>10 cm), 54 (33%) showed elevated lactate dehydrogenase (LDH) serum levels, 132 (82%) patients complained of pain as their main symptom, and 25 (15%) had fracture. The femur was the most commonly involved bone (Table 1); 145 (90%) patients had a solitary lesion, and 14 (9%) had multifocal lesions in a single bone. IPI score was 0–1 in 113 patients (70%), 2 in 36 (22%), and 3 in 7 (4%); it was unknown in 5 (3%).
Table 1.
Patient characteristics
Treatments
Patients were treated according to institutional guidelines, including chemotherapy alone (n = 13; 8%), radiotherapy alone (n = 23; 14%), or both (n = 125; 78%) (Tables 2 and 3). Of the 138 patients treated with chemotherapy, 118 (85%) received a doxorubicin-containing regimen, cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) being the most commonly used (n = 103; 87%). No patient received rituximab as part of first-line treatment. Six patients received some central nervous system (CNS) prophylaxis with intrathecal chemotherapy or intravenous high doses of methotrexate (dose ≥1 g/m2) and/or cytarabine (dose ≥2 g/m2). Among the 148 patients treated with radiotherapy, the irradiated volume included the whole of the affected bone in 97 (65%) patients and only a part of the affected bone in 36 (24%); data on the irradiated volume were not available in 15 cases. The median dose was 40 Gy (range, 12–56 Gy), in 2.0-Gy fractions. Patients treated with radiotherapy alone had a higher median age, with a higher proportion of patients older than 60 years, and were more commonly diagnosed before 1996 (Table 2).
Table 2.
Patient characteristics according to treatment
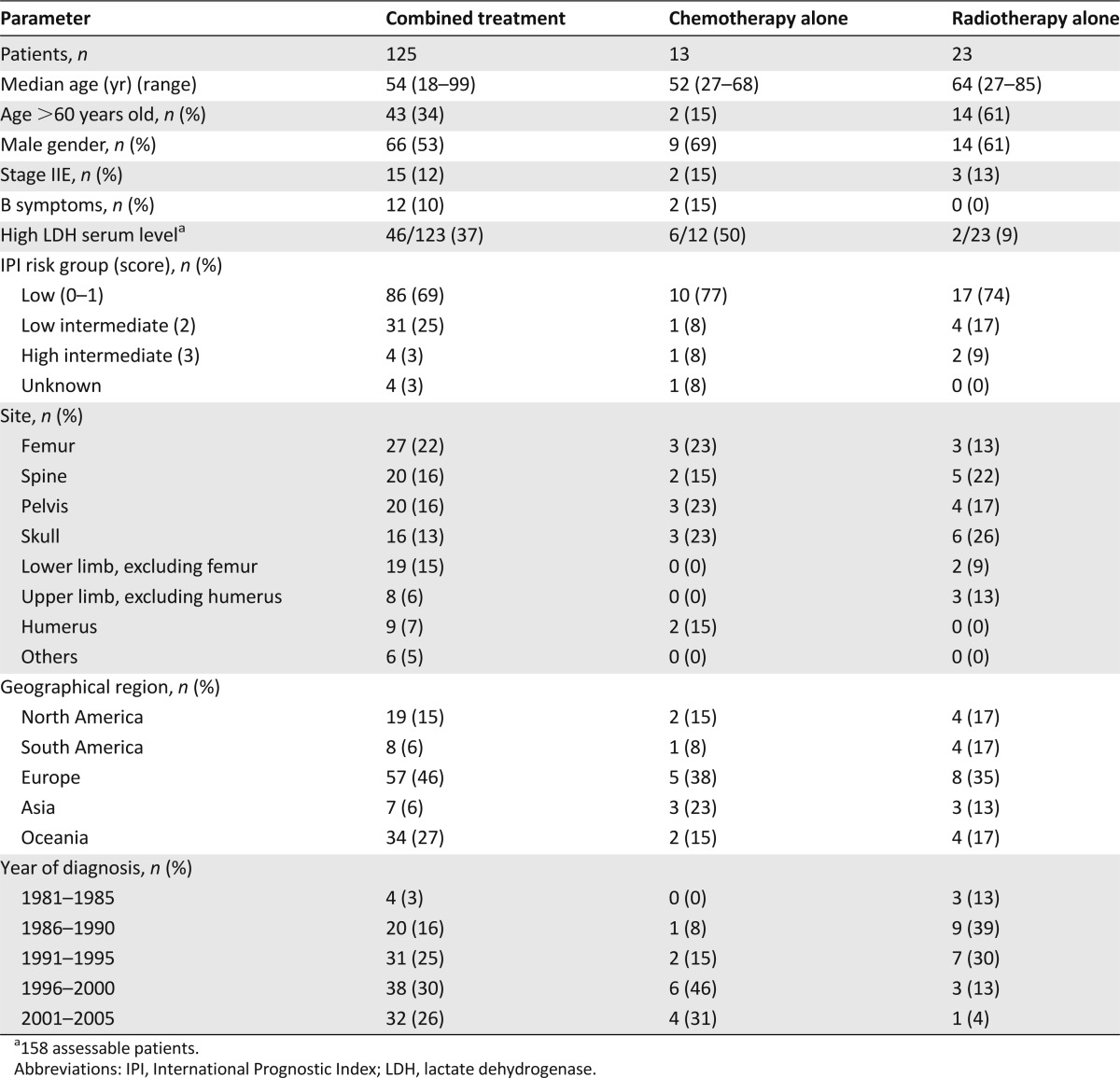
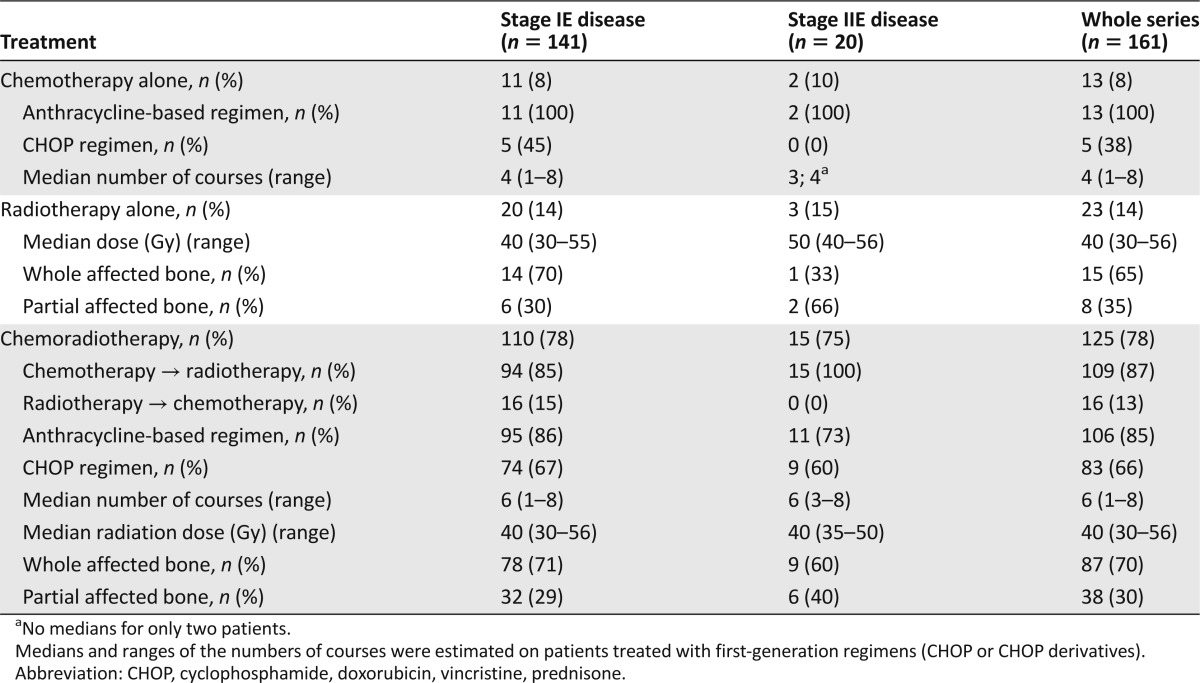
Table 3.
Treatment according to stage of disease
Response
The response after the first-line treatment was assessed in 152 patients: 131 patients achieved a CR (complete response rate [CRR] = 86%; 95% confidence interval [CI], 81%–91%) and 7 a PR, with an overall response rate (ORR) of 91% (95% CI, 87%–95%); 1 (0.6%) patient had SD and 13 (9%) patients experienced PD.
The response was assessed in 123 of the 125 patients treated with chemotherapy and radiotherapy: 107 patients achieved a CR (CRR= 87%; 95% CI, 81%–93%) and 6 a PR, with an ORR of 92% (95% CI, 87%–97%); 1 (0.8%) patient had SD and 9 (7%) experienced PD.
The response was assessed in 20 of the 23 patients treated with radiotherapy alone: 16 patients achieved a CR (CRR = 80%; 95% CI, 53%–97%) and 1 a PR, with an ORR of 90% (95% CI, 77%–100%); 3 patients experienced PD (15%).
The response was assessed in 9 of the 13 patients treated with chemotherapy alone: 8 patients achieved a CR (CRR = 89%; 95% CI, 69%–100%) and 1 patient experienced PD (11%).
Progression-Free Survival and Relapse Patterns
At a median follow-up of 54 months (range, 3–218 months), 107 (67%) patients remained relapse-free, and 54 (34%) patients experienced relapse, with a median PFS of 35+ months and a 5-year PFS of 68% ± 4% (Fig. 1). Failure (relapse/PD) involved the primary site of disease in 9 (17%) patients, and involved other bones in 11 patients (21%), regional lymph nodes in 1 (2%), other lymph nodes in 10 (19%), multiple sites in 7 (13%), meninges in 4 (8%), and other sites in 6 (11%). The relapse site was unknown in 5 (9%) patients. Forty-nine of 148 irradiated patients experienced relapse, which was inside the irradiated volume in 8 cases, invariably as exclusive site of relapse. The 4 patients with meningeal relapse originally had osteolytic lesions in the pelvic bones and did not receive CNS prophylaxis; all of them died of PD.
Figure 1.
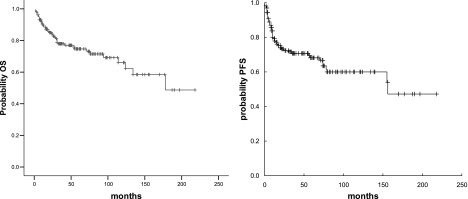
OS (left) and PFS (right) curves for the whole series.
Abbreviations: OS, overall survival; PFS, progression-free survival.
Forty-eight (34%) of the 141 patients with stage I disease and 6 (30%) of the 20 patients with stage II disease experienced failure, with a 5-year PFS of 68% ± 4% and 72% ± 11%, respectively.
Patients managed with primary chemotherapy, whether followed by radiotherapy or not, had a significantly better PFS (5 year: 73% ± 4% vs. 52% ± 8%, p = .0001) than patients treated with primary radiotherapy, whether followed by chemotherapy or not. Overall, 35 (28%) of the 125 patients treated with chemotherapy and radiotherapy, 4 (31%) of the 13 patients treated with chemotherapy alone, and 14 (61%) of the 23 patients treated with radiotherapy alone experienced relapse, with a 5-year PFS of 72% ± 4%, 67% ± 14%, and 51% ± 10%, respectively (Fig. 2).
Figure 2.
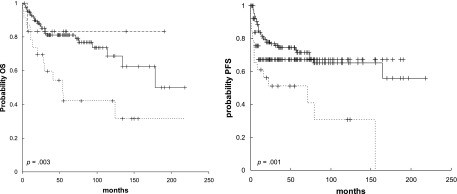
OS (left) and PFS (right) curves of the whole series by treatment: chemotherapy followed by radiotherapy (solid lines), chemotherapy alone (long dashed lines), and radiotherapy alone (short dashed lines).
Abbreviations: OS, overall survival; PFS, progression-free survival.
The impact of consolidative radiotherapy was assessed by comparing patients managed with chemotherapy followed by radiotherapy with patients treated with chemotherapy alone (5-year PFS: 74% ± 5% vs. 67% ± 14%, p = .47).
The effect of radiation dose on disease control was analyzed in the 101 patients managed with primary chemotherapy followed by irradiation. There was no significant difference in PFS between the 47 patients irradiated with a dose ≤36 Gy and the 58 patients irradiated with >36 Gy (5 year: 72% ± 7% vs. 75% ± 7%, p = .57). Radiation volume was irrelevant to disease control, with a similar PFS for patients with the whole of the affected bone irradiated compared with those with a part of a bone irradiated (5-year PFS: 76% ± 5% vs. 64% ± 9%, p = .31).
Survival
One hundred nineteen patients were alive (113 disease-free), with a 5-year OS of 75% ± 4% (Fig. 1). Forty-two patients died: 34 patients died of lymphoma, 1 died of toxicity, and 7 died of unrelated causes. The 5-year OS was 75% ± 4% for patients with stage I disease and 76% ± 10% for patients with stage II disease (p = .96).
Overall, patients managed with primary chemotherapy, whether followed by radiotherapy or not, had a significantly better OS compared with patients treated with primary radiotherapy, whether followed by chemotherapy or not (5 year: 84% ± 3% vs. 48% ± 9%, p < .0001). Patients treated with combined treatment showed a significantly longer OS than patients treated with radiotherapy alone (5 year: 81% ± 4% vs. 42% ± 11%, p = .003) (Fig. 2). In the subgroup of patients treated with combined modality, primary chemotherapy followed by radiotherapy was associated with a significantly better OS than the inverse sequence (5 year: 85% ± 4% vs. 58% ± 13%, p = .001).
In the subgroup of patients treated with primary chemotherapy, the addition of consolidation radiotherapy was not associated with better OS (5 year: 85% ± 4% vs. 83% ± 10%, p = .88) (Fig. 2); in these patients, the use of a radiation dose >36 Gy (5-year OS: 88% ± 5% vs. 82% ± 6%, p = .11) and the irradiation of the whole affected bone (5-year OS: 84% ± 4% vs. 82% ± 7%, p = .51) were not associated with better outcome.
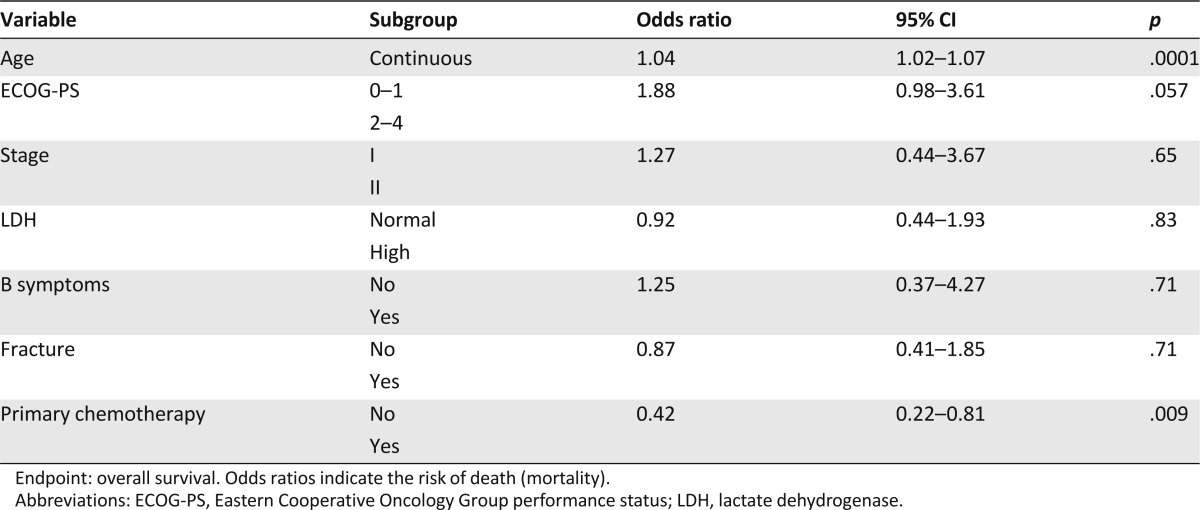
Multivariate analysis on the whole series showed that age and primary chemotherapy were independently associated with OS (Table 4).
Table 4.
Multivariate analysis
Discussion
This retrospective study, focused on the largest series of limited-stage DLBCL of the bone, shows that patients affected by these lymphomas exhibit a favorable prognosis when treated with primary anthracycline-based chemotherapy whether followed by radiotherapy or not, with an ORR >90% and a 5-year OS of 84%. This study demonstrates that this strategy produces significantly better results than primary radiotherapy whether followed by chemotherapy or not. Most patients had favorable prognostic factors at diagnosis, with increased age and poor performance status associated with a high risk of relapse and death. As expected for a good-risk cohort, CNS dissemination was recorded in 2.5% of patients, which is a lower rate than those reported in other DLBCL series in the pre-rituximab era.
The main limitations of this study were its retrospective nature, the lack of a formal central pathology review, and the fact that most patients were treated in a pre-rituximab and pre-positron emission tomography (PET) era. Despite a recent retrospective study suggesting that the addition of rituximab does not provide a clear survival advantage in patients affected by localized extranodal lymphomas [11], it is reasonable to expect a positive effect on disease control with the addition of this antibody, as reported for low-risk patients with DLBCL enrolled in the MInT prospective randomized trial [12]. The absence of a central pathology review poses a risk that misclassification has occurred and may be associated with bias. However, selected cases were reviewed at participating institutions, which are specialized centers with high levels of expertise in the management of lymphomas. Treatment was given per physician’s discretion over a broad time period when patterns of practice may have evolved. Moreover, small numbers in some comparisons may be underpowered, and results should be interpreted with caution.
DLBCL constitutes the vast majority of PBL, ranging from 68% in Japan [13] to 80% in Western countries [8, 14]. Nevertheless, PB-DLBCL remains a rare malignancy, whose natural history has been poorly established and therapeutic guidelines remain to be defined. The few published studies of PBL are limited by the absence of a uniformly accepted definition of PBL [15, 16]. Moreover, some authors considered together cases of primary and secondary bone lymphomas [17, 18], which further complicated the analysis and the reliability of the conclusions. Recently, the need to differentiate between limited-stage PBL and systemic disease with skeletal involvement was emphasized [19]. The current study contributes to our knowledge on the clinical presentation and natural behavior of PB-DLBCL in the largest reported, unselected series. Thus, PB-DLBCL can arise at any age, with equal distribution between genders, and it usually presents with favorable features, including the absence of B symptoms (91% of cases), normal LDH serum level (65%), and low or low-intermediate IPI (90%). Stage II disease is uncommon (13%), but this rate could be underestimated because of the lack of PET scanning in the staging workup. In any case, in the present study, local lymph node involvement was not associated with poorer outcome, suggesting that patients with either stage I or stage II disease should be treated with a similar strategy.
In the current study, patients with limited-stage DLBCL of the bone managed with chemotherapy, followed or not by radiotherapy, had 5-year PFS and OS of 73% and 85%, respectively. These figures compare favorably with outcomes of patients with limited-stage DLBCL not otherwise specified treated with the same approach in previous trials, which have been 66% and 82% in the MInT trial [20]. Importantly, the MInT trial exclusively enrolled younger patients with IPI scores of 0–1, whereas, in the present series, 39% of patients were older than 60 years, and 26% of patients had IPI scores of 2–3, which suggests a negative selection. In line with previous studies [16, 21, 22], reported in the pre-rituximab era, patients treated with combined modality treatment had a significantly better response and survival rates than patients treated with radiotherapy alone. Among patients treated with combined modality, primary chemotherapy followed by radiotherapy was associated with significantly better outcome than patients treated with the reverse sequence. Interestingly, the addition of involved-field radiotherapy in patients treated with primary chemotherapy was not associated with an improved outcome, but this observation should be regarded with caution considering the retrospective nature of the study and the small number of patients managed with chemotherapy alone. However, our data confirm that primary anthracycline-based chemotherapy, whether followed by radiotherapy or not, is the first therapeutic choice for patients with PB-DLBCL.
The role of consolidation radiotherapy in limited-stage DLCBL remains to be defined, with a few randomized trials suggesting that it is superfluous in patients who achieve a CR after primary chemotherapy [23]. In some pre-rituximab trials, postchemotherapy consolidation with low-dose radiotherapy resulted in prolonged disease-free survival, but no OS benefit was observed [24]. In the rituximab era, the addition of consolidation radiotherapy significantly improved outcome in a large retrospective series treated with rituximab-CHOP combination [25]. Overall, the use of intensive immunochemotherapy without radiation therapy requires formal testing and validation in a randomized clinical trial before it can be used as an alternative treatment regimen for early-stage DLBCL. No studies addressing this issue in a large PBL series exist. As mentioned above, the present study suggests that postchemotherapy bone irradiation did not improve the outcome in PB-DLBCL. No differences were seen in relapse and survival rates between patients receiving radiotherapy to the whole bone and those patients with only part of the affected bone irradiated. In patients managed with chemoradiotherapy, radiation doses >36 Gy were not associated with better PFS than doses ≤36 Gy. Thus, if radiotherapy is to be given to the whole length of an affected bone, the dose should be limited to 36 Gy to avoid toxicity.
CNS relapse is an early and fatal event in DLBCL management, and it has been reported in approximately 5% of patients with DLBCL treated in pre-rituximab era [26]. Specific prophylaxis with systemic and/or intrathecal chemotherapy could prevent this severe event [27]. Skeletal involvement has been reported to be associated with a high risk of CNS dissemination in patients with DLBCL, mostly with advanced disease [28]. The present study demonstrates that limited-stage DLBCL of the bone is not associated with a high risk of CNS dissemination; this event was reported in 2.5% of cases, which is only half of the widely recognized risk of CNS dissemination in DLBCL patients [29–31]. CNS relapse consisted of meningeal lymphomatosis and was invariably fatal. These patients had bulky lesions in the pelvic bones and had at least one unfavorable indicator among increased LDH serum level, high IPI, bulky disease, and B symptoms, but the small number of events hampered the identification of reliable predictors. Nevertheless, the present study clearly suggests that CNS prophylaxis is unnecessary. Probably, this rate would be even lower with the addition of rituximab because CNS dissemination was halved in DLBCL patients treated with this antibody [32].
In conclusion, this study demonstrates that PB-DLBCL has specific features that define it as a separate entity, usually with favorable clinical features and good prognosis. These patients should be treated with primary anthracycline-based chemotherapy, whether followed by consolidative radiotherapy or not. In patients treated with chemoradiotherapy, the irradiation of the whole affected bone and the use of a radiation dose >36 Gy are not associated with better outcome. CNS dissemination is a rare event in these patients.
List of Participating Centers (Number of Registered Patients)
Princess Margaret Hospital, Ontario Cancer Institute, Toronto, Ontario, Canada (n = 97); San Raffaele Scientific Institute, Milan, Italy (n = 37); Auckland Hospital, Auckland, New Zealand (n = 30); Christie Hospital NHS Trust, Manchester, United Kingdom (n = 30); Korea Cancer Center Hospital, Seoul, Korea (n = 28); National University Hospital, Seoul, Korea (n = 24); MD Anderson Cancer Center, Houston, Texas, USA (n = 19); Istituto Europeo di Oncologia, Milan, Italy (n = 19); Westmead Hospital, Westmead, Australia (n = 19); Wesley Cancer Care Centre, Brisbane, Queensland, Australia (n = 18); Northern Centre for Cancer Treatment, Newcastle, United Kingdom (n = 16); Queensland Radium Institute, Brisbane, Queensland, Australia (n = 14); Istituto Oncologico della Svizzera Italiana, Bellinzona, Switzerland (n = 13); National Cancer Institute, Bratislava, Slovakia (n = 13); North Shore Hospital, Sydney, Australia (n = 13); Royal Prince Alfred Hospital, Sydney, Australia (n = 13); Ospedale Policlinico Di Borgo Roma, Verona, Italy (n = 13); Victoria Geelong Hospital, Geelong, Australia (n = 10); Università Degli Studi La Sapienza, Roma, Italy (n = 8); Ospedale di Circolo Fondazione Macchi, Varese, Italy (n = 8); Fundacion Argentina Contra la Leucemia, Buenos Aires, Argentina (n = 7); Blokhin Cancer Research Center, Moscow, Russia (n = 7); Instituto de Enfermedades Neoplásicas, Surquillo, Lima, Perú (n = 6); East Coast Cancer Center, Tugun, Queensland, Australia (n = 6); Peter MacCallum Cancer Institute, Melbourne, Australia (n = 5); Prince of Wales Hospital, Sydney, Australia (n = 5); The James Cook University Hospital, Cleveland, United Kingdom (n = 4); Royal South Hants Hospital, Southampton, United Kingdom (n = 4); Woden Valley, Canberra, Australia (n = 2); National Institute of Oncology and Radiobiology, Havana, Cuba (n = 2); Ospedale Umberto I, Mestre, Italy (n = 2); Victoria Border Medical Oncology, Wodonga, Australia (n = 2); Royal Adelaide Hospital, Adelaide, Australia (n = 1); Saint Alphonsus Cancer Treatment Center, Boise, Idaho, USA (n = 1); Hospital Israelita, Buenos Aires, Argentina (n = 1); Azienda Ospedaliera “Maggiore della Carità,” Novara, Italy (n = 1); Rabin Medical Center, Petah-Tiqwa, Israel (n = 1).
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
Marta Bruno Ventre and Andrés J.M. Ferreri contributed equally to this article. Emanuele Zucca and David Christie shared senior authorship.
Author Contributions
Conception/design: Andrés J. Ferreri, Emanuele Zucca, David Christie
Provision of study material or patients: Mary Gospodarowicz, Andrés J. Ferreri, Silvia Govi, Carlo Messina, David Porter, John Radford, Dae Seog Heo, Yeon Park, Giovanni Martinelli, Emma Taylor, Helen Lucraft, Angela Hong
Collection and/or assembly of data: Mary Gospodarowicz, Andrés J. Ferreri, Silvia Govi, Carlo Messina, Lydia Scarfò
Data analysis and interpretation: Andrés J. Ferreri, Silvia Govi, Emanuele Zucca, David Christie
Manuscript writing: Marta Bruno Ventre, Andrés J. Ferreri
Final approval of manuscript: Andrés J. Ferreri, Emanuele Zucca, David Christie
Disclosures
The authors indicated no financial relationships.
Section Editor: George P. Canellos: Celgene Business Advisory Board (C/A) Reviewer “A”: None Reviewer “B”: None (C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/ inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Fletcher CD. The evolving classification of soft tissue tumours: An update based on the new WHO classification. Histopathology. 2006;48:3–12. doi: 10.1111/j.1365-2559.2005.02284.x. [DOI] [PubMed] [Google Scholar]
- 2.Isaacson P, Wright DH. Extranodal malignant lymphoma arising from mucosa-associated lymphoid tissue. Cancer. 1984;53:2515–2524. doi: 10.1002/1097-0142(19840601)53:11<2515::aid-cncr2820531125>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 3.Dubey P, Ha CS, Besa PC, et al. Localized primary malignant lymphoma of bone. Int J Radiat Oncol Biol Phys. 1997;37:1087–1093. doi: 10.1016/s0360-3016(97)00106-5. [DOI] [PubMed] [Google Scholar]
- 4.Heyning FH, Hogendoorn PC, Kramer MH, et al. Primary non-Hodgkin’s lymphoma of bone: A clinicopathological investigation of 60 cases. Leukemia. 1999;13:2094–2098. doi: 10.1038/sj.leu.2401582. [DOI] [PubMed] [Google Scholar]
- 5.Mulligan ME, McRae GA, Murphey MD. Imaging features of primary lymphoma of bone. AJR Am J Roentgenol. 1999;173:1691–1697. doi: 10.2214/ajr.173.6.10584821. [DOI] [PubMed] [Google Scholar]
- 6.Pellegrini C, Gandolfi L, Quirini F, et al. Primary bone lymphoma: Evaluation of chemoimmunotherapy as front-line treatment in 21 patients. Clin Lymphoma Myeloma Leuk. 2011;11:321–325. doi: 10.1016/j.clml.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Cai L, Stauder MC, Zhang YJ, et al. Early-stage primary bone lymphoma: A retrospective, multicenter Rare Cancer Network (RCN) Study. Int J Radiat Oncol Biol Phys. 2012;83:284–291. doi: 10.1016/j.ijrobp.2011.06.1976. [DOI] [PubMed] [Google Scholar]
- 8.Ramadan KM, Shenkier T, Sehn LH, et al. A clinicopathological retrospective study of 131 patients with primary bone lymphoma: A population-based study of successively treated cohorts from the British Columbia Cancer Agency. Ann Oncol. 2007;18:129–135. doi: 10.1093/annonc/mdl329. [DOI] [PubMed] [Google Scholar]
- 9.Ng AK, Mauch PM. Role of radiation therapy in localized aggressive lymphoma. J Clin Oncol. 2007;25:757–759. doi: 10.1200/JCO.2006.09.5562. [DOI] [PubMed] [Google Scholar]
- 10.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 11.Gutiérrez-García G, Colomo L, Villamor N, et al. Clinico-biological characterization and outcome of primary nodal and extranodal diffuse large B-cell lymphoma in the rituximab era. Leuk Lymphoma. 2010;51:1225–1232. doi: 10.3109/10428194.2010.483301. [DOI] [PubMed] [Google Scholar]
- 12.Rieger M, Osterborg A, Pettengell R, et al. Primary mediastinal B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: Results of the Mabthera International Trial Group study. Ann Oncol. 2011;22:664–670. doi: 10.1093/annonc/mdq418. [DOI] [PubMed] [Google Scholar]
- 13.Maruyama D, Watanabe T, Beppu Y, et al. Primary bone lymphoma: A new and detailed characterization of 28 patients in a single-institution study. Jpn J Clin Oncol. 2007;37:216–223. doi: 10.1093/jjco/hym007. [DOI] [PubMed] [Google Scholar]
- 14.Rathmell AJ, Gospodarowicz MK, Sutcliffe SB, et al. Localised lymphoma of bone: Prognostic factors and treatment recommendations. Br J Cancer. 1992;66:603–606. doi: 10.1038/bjc.1992.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbieri E, Cammelli S, Mauro F, et al. Primary non-Hodgkin’s lymphoma of the bone: Treatment and analysis of prognostic factors for stage I and stage II. Int J Radiat Oncol Biol Phys. 2004;59:760–764. doi: 10.1016/j.ijrobp.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 16.Fidias P, Spiro I, Sobczak ML, et al. Long-term results of combined modality therapy in primary bone lymphomas. Int J Radiat Oncol Biol Phys. 1999;45:1213–1218. doi: 10.1016/s0360-3016(99)00305-3. [DOI] [PubMed] [Google Scholar]
- 17.Lewis VO, Primus G, Anastasi J, et al. Oncologic outcomes of primary lymphoma of bone in adults. Clin Orthop Relat Res. 2003;(415):90–97. doi: 10.1097/01.blo.0000093901.12372.ad. [DOI] [PubMed] [Google Scholar]
- 18.Horsman JM, Thomas J, Hough R, et al. Primary bone lymphoma: A retrospective analysis. Int J Oncol. 2006;28:1571–1575. doi: 10.3892/ijo.28.6.1571. [DOI] [PubMed] [Google Scholar]
- 19.Jawad MU, Schneiderbauer MM, Min ES, et al. Primary lymphoma of bone in adult patients. Cancer. 2010;116:871–879. doi: 10.1002/cncr.24828. [DOI] [PubMed] [Google Scholar]
- 20.Pfreundschuh M, Kuhnt E, Trümper L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12:1013–1022. doi: 10.1016/S1470-2045(11)70235-2. [DOI] [PubMed] [Google Scholar]
- 21.Beal K, Allen L, Yahalom J. Primary bone lymphoma: Treatment results and prognostic factors with long-term follow-up of 82 patients. Cancer. 2006;106:2652–2656. doi: 10.1002/cncr.21930. [DOI] [PubMed] [Google Scholar]
- 22.Zinzani PL, Carrillo G, Ascani S, et al. Primary bone lymphoma: Experience with 52 patients. Haematologica. 2003;88:280–285. [PubMed] [Google Scholar]
- 23.Reyes F, Lepage E, Ganem G, et al. ACVBP versus CHOP plus radiotherapy for localized aggressive lymphoma. N Engl J Med. 2005;352:1197–1205. doi: 10.1056/NEJMoa042040. [DOI] [PubMed] [Google Scholar]
- 24.Horning SJ, Weller E, Kim K, et al. Chemotherapy with or without radiotherapy in limited-stage diffuse aggressive non-Hodgkin’s lymphoma: Eastern Cooperative Oncology Group study 1484. J Clin Oncol. 2004;22:3032–3038. doi: 10.1200/JCO.2004.06.088. [DOI] [PubMed] [Google Scholar]
- 25.Phan J, Mazloom A, Medeiros LJ, et al. Benefit of consolidative radiation therapy in patients with diffuse large B-cell lymphoma treated with R-CHOP chemotherapy. J Clin Oncol. 2010;28:4170–4176. doi: 10.1200/JCO.2009.27.3441. [DOI] [PubMed] [Google Scholar]
- 26.Ferreri AJ, Reni M, Ceresoli GL, et al. Therapeutic management with adriamycin-containing chemotherapy and radiotherapy of monostotic and polyostotic primary non-Hodgkin’s lymphoma of bone in adults. Cancer Invest. 1998;16:554–561. doi: 10.3109/07357909809032885. [DOI] [PubMed] [Google Scholar]
- 27.Abramson JS, Hellmann M, Barnes JA, et al. Intravenous methotrexate as central nervous system (CNS) prophylaxis is associated with a low risk of CNS recurrence in high-risk patients with diffuse large B-cell lymphoma. Cancer. 2010;116:4283–4290. doi: 10.1002/cncr.25278. [DOI] [PubMed] [Google Scholar]
- 28.Tomita N, Yokoyama M, Yamamoto W, et al. Central nervous system event in patients with diffuse large B-cell lymphoma in the rituximab era. Cancer Sci. 2012;103:245–251. doi: 10.1111/j.1349-7006.2011.02139.x. [DOI] [PubMed] [Google Scholar]
- 29.Villa D, Connors JM, Shenkier TN, et al. Incidence and risk factors for central nervous system relapse in patients with diffuse large B-cell lymphoma: The impact of the addition of rituximab to CHOP chemotherapy. Ann Oncol. 2010;21:1046–1052. doi: 10.1093/annonc/mdp432. [DOI] [PubMed] [Google Scholar]
- 30.Boehme V, Schmitz N, Zeynalova S, et al. CNS events in elderly patients with aggressive lymphoma treated with modern chemotherapy (CHOP-14) with or without rituximab: An analysis of patients treated in the RICOVER-60 trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL) Blood. 2009;113:3896–3902. doi: 10.1182/blood-2008-10-182253. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Chen B, Xu X. Impact of rituximab on incidence of and risk factors for central nervous system relapse in patients with diffuse large B-cell lymphoma: A systematic review and meta-analysis. Leuk Lymphoma. 2013 [Epub ahead of print] doi: 10.3109/10428194.2013.811239. [DOI] [PubMed] [Google Scholar]
- 32.Guirguis HR, Cheung MC, Mahrous M, et al. Impact of central nervous system (CNS) prophylaxis on the incidence and risk factors for CNS relapse in patients with diffuse large B-cell lymphoma treated in the rituximab era: A single centre experience and review of the literature. Br J Haematol. 2012;159:39–49. doi: 10.1111/j.1365-2141.2012.09247.x. [DOI] [PubMed] [Google Scholar]


