The FOLFIRINOX regimen (5-fluorouracil, oxaliplatin, irinotecan, and leucovorin) followed by chemoradiation as neoadjuvant therapy for borderline resectable pancreatic adenocarcinoma is safe, and an initial experience suggests favorable resection rates compared with previous reports in this high-risk patient population.
Keywords: Neoadjuvant therapy, FOLFIRINOX, Pancreatic adenocarcinoma, Borderline resectable pancreas cancer
Abstract
Background.
Borderline resectable pancreatic cancer is best treated by multimodality therapy. FOLFIRINOX (5-fluorouracil, oxaliplatin, irinotecan, and leucovorin) tripled the response rate and significantly increased median survival for patients with advanced pancreatic cancer and shows promise for neoadjuvant use. Toxicity concerns prompted a careful analysis of our initial FOLFIRINOX experience.
Methods.
All patients diagnosed with borderline resectable, biopsy-proven pancreatic adenocarcinoma treated with neoadjuvant FOLFIRINOX between July 2010 and December 2012 were reviewed. Primary outcome was surgical resectability. Secondary outcomes were treatment-related toxicities and survival.
Results.
FOLFIRINOX followed by gemcitabine- or capecitabine-based chemoradiation was initiated in 18 patients. The most common grade 3 or 4 toxicities during chemotherapy were gastrointestinal, including nausea/emesis (n = 5), weight loss (n = 3) and diarrhea (n = 2), and hematologic (n = 2; neutropenia); five patients (36%) required a total of six admissions. Neoadjuvant therapy was completed in 15 of 18 patients (83%), and 12 (67%) underwent pancreatectomy (10 Whipple, 2 total pancreatectomy) including portal vein resection/reconstruction in 10 (83%). Disease progression precluded surgery in 6 of the 18 patients (33%). All 12 resected patients had negative (R0) margins. Only 2 of 12 (17%) were node positive (median node count: 26.5 [range: 15–39]). There were no in-hospital or 30-day mortalities and no clinical pancreatic leaks or reoperations. Of the 12 patients who completed all intended therapy, 7 (58.3%) are alive, including 5 who have no evidence of disease (median months from diagnosis: 22 months [range: 18–35 months). The six patients who did not complete all planned therapy are deceased (months from diagnosis: 6.9–17.5 months).
Conclusion.
FOLFIRINOX followed by chemoradiation as neoadjuvant therapy for borderline resectable pancreatic adenocarcinoma is safe, and our initial experience suggests favorable resection rates compared with previous reports in this high-risk patient population.
Implications for Practice:
FOLFIRINOX, the combination chemotherapy consisting of fluorouracil, oxaliplatin, irinotecan, and leucovorin, was previously used in the setting of metastatic pancreatic cancer, resulting in a tripling of response rates and a significant increase in median survival. The authors report their initial experience with neoadjuvant FOLFIRINOX given to borderline resectable pancreas cancer patients. In this neoadjuvant setting, FOLFIRINOX was found to be safe and did not adversely affect completion of all intended therapy, including chemoradiation and surgery. In addition, resection rates were favorable, and final pathology results were encouraging in this high-risk population.
Introduction
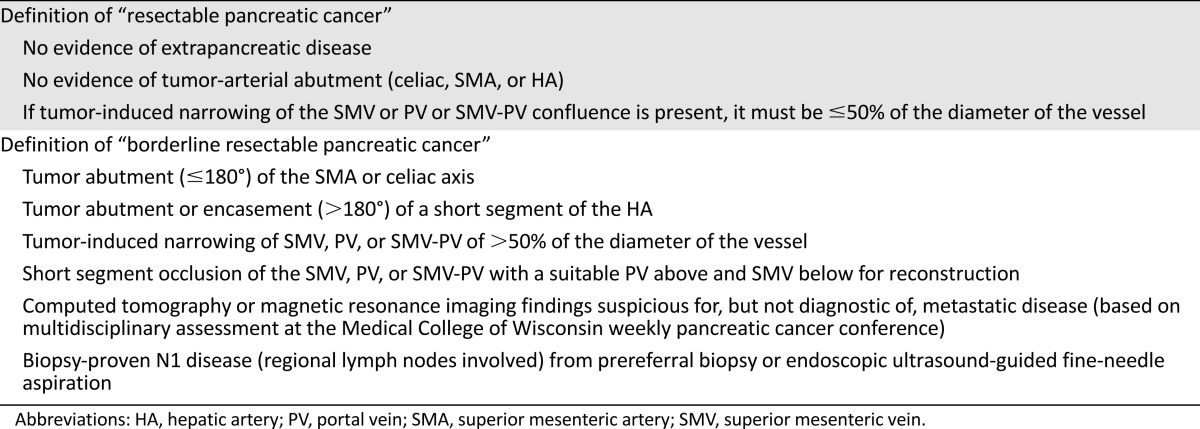
Patients with pancreatic cancer, similar to patients with other more common solid tumors, require accurate pretreatment staging based on reproducible anatomic criteria (arterial and venous relationships to the tumor) to allow clinicians to develop optimal stage-specific treatment sequencing [1]. Although there remain subtle differences in how experts in the field define “borderline resectable” pancreatic cancer, there is general consensus that borderline resectable tumors include those with tumor abutment (≤180°) of the superior mesenteric artery (SMA) and/or celiac axis or short segment encasement of the hepatic artery amenable to resection/reconstruction. The definition of “borderline resectable” with respect to the tumor superior mesenteric vein-portal vein (SMV-PV) relationship varies by the guideline referenced [2–5]. The University of Texas MD Anderson Cancer Center definition of “borderline resectable” allows for short segment occlusion of the SMV-PV, provided that a suitable vein for reconstruction exists above and below the area involved by tumor [2]. That center also incorporates patients into the “borderline resectable” category who have technically resectable tumors but radiographic findings that are indeterminate for metastatic disease and/or marginal or poor performance status with the potential for improvement. National Comprehensive Cancer Network and American Hepato-Pancreato-Biliary Association/Society of Surgical Oncology guidelines take a more conservative view of the tumor-vein relationship and suggest that even impingement or narrowing of the SMV-PV should be considered borderline resectable [3–5]. Most important, in contrast to treatment sequencing for patients with resectable pancreatic cancer (stages I and II), which remains controversial (surgery first vs. neoadjuvant therapy), there is national consensus that patients with borderline resectable disease—inclusive of all published definitions—are at the highest possible risk for harboring radiographically occult metastatic disease and for undergoing a margin-positive resection; therefore, they should not be taken directly to surgery in the absence of induction therapy [3]. However, as the toxicity of induction therapy increases, such toxicity may affect surgery-related morbidity and mortality. To the extent that surgery remains necessary, although not sufficient for long-term survival, induction therapy that prevents surgery or increases surgery-related complications poses an obvious concern.
The results of the ACCORD4/Partenariat de Recherche en Oncologie Digestive (PRODIGE) 11 trial have had a profound impact on the management of patients with metastatic pancreatic cancer [6]. Combination therapy with FOLFIRINOX (5-flourouracil, leucovorin, irinotecan, oxaliplatin) tripled the response rate and significantly increased the median overall survival (OS; 11.1 months vs. 6.8 months; hazard ratio [HR]: 0.57; 95% confidence interval [CI]: 0.45–0.73; p < .001) and progression-free survival (PFS; 6.4 months vs. 3.3 months; HR: 0.47; 95% CI: 0.37–0.59; p < .001) for patients with metastatic pancreatic cancer compared with those treated with gemcitabine. To the extent that response to systemic therapy is related to patient performance status and tumor burden, we hypothesized an even greater benefit for FOLFIRINOX in patients with less advanced disease (resectable or borderline resectable disease). However, in the same trial, the safety profile of FOLFIRINOX was less favorable than gemcitabine as a result of neutropenia, febrile neutropenia, thrombocytopenia, diarrhea, and sensory neuropathy. Toxicity, which could delay or compromise surgical resection and potentially increase perioperative complications, is the obvious concern with the use of this regimen in the neoadjuvant setting.
The Medical College of Wisconsin (MCW) Pancreatic Cancer Program first applied neoadjuvant FOLFIRINOX to those patients with borderline resectable pancreatic cancer after a small experience operating on patients (referred from other institutions) who had received this regimen as part of a multimodality program for presumed locally advanced pancreatic cancer. This positive anecdotal experience, combined with the published results for patients with metastatic pancreatic cancer, supported the use of FOLFIRINOX in patients with borderline resectable disease for whom there was consensus that both systemic therapy and chemoradiation should be delivered before surgery [5]. Such patients have a very high risk of harboring occult metastatic disease, require more complex surgery to remove the primary tumor, and are at risk for a margin-positive resection if surgery is attempted. The program reported in this paper included a minimum of 2 months of systemic chemotherapy followed by 5.6 weeks of chemoradiation prior to surgical resection. Our hypothesis was that more effective systemic therapy would increase the potential of patients to complete all intended therapy, including surgery, by effectively treating both the local tumor and radiographically occult micrometastases. Stated another way, disease progression at the time of restaging or surgery would be less common than historically reported in the MD Anderson experience in which less than 50% of patients with borderline resectable disease completed all therapy including successful pancreatectomy [2]. More effective induction therapy including FOLFIRINOX represents a potential solution to the problem of borderline resectable pancreas cancer as long as chemotherapy-related toxicity does not preclude completion of all planned treatment including surgery.
The purpose of this report is to review our initial experience with neoadjuvant FOLFIRINOX as induction therapy for patients with borderline resectable pancreas cancer. All patients were treated in the setting of a robust multidisciplinary working group with experience in multimodality management of such complex patients.
Methods
All patients diagnosed with pancreatic adenocarcinoma at MCW between July 1, 2010, and December 3, 2012, were identified from available institutional databases. Confirmation of clinical staging was performed at a weekly multidisciplinary pancreatic cancer conference and was based on a staging system using multidetector computed tomography (CT); the MCW staging system differed slightly from previously published staging systems and current National Comprehensive Cancer Network guidelines (Table 1) [3, 7]. We reviewed all patients with borderline resectable pancreatic cancer who received induction FOLFIRINOX followed by chemoradiation. Restaging evaluation (patient examination and assessment of performance status, cross-sectional imaging, and tumor marker profile) was performed at 2-month intervals including before the start of chemoradiation and again before surgery. Local disease/tumor progression was defined as the development of (progression to) locally advanced disease, as defined in Table 1; changes in tumor size on CT images in the absence of a change in tumor-vessel relationships (which speaks to stage of disease) is of uncertain significance likely because of the dense stroma/microenvironment in the primary tumor and its unpredictable response to induction therapy. Standard demographic and clinicopathologic data were collected including treatment delays, grade 3 or 4 toxicities, chemotherapy dose reductions, and hospitalizations. Chemotherapy toxicity was graded according to the Common Terminology Criteria for Adverse Events, version 4.0. Cancer antigen 19-9 (CA19-9) levels reported in this paper were all recorded in the setting of a serum bilirubin within the normal range. Perioperative complications were recorded including reoperation, pancreatic fistula, and hospital readmission. Operative mortality was defined as death during the same hospitalization or within 30 days of surgery. Time to last follow-up, recurrence, PFS, and OS were calculated from the date of tissue diagnosis. This analysis was approved by the institutional review board of MCW.
Table 1.
Medical College of Wisconsin clinical/radiographic staging system for pancreatic cancer
Neoadjuvant Therapy
Neoadjuvant therapy consisted of induction chemotherapy with FOLFIRINOX followed by chemoradiation. The FOLFIRINOX regimen consisted of oxaliplatin at a dose of 85 mg/m2, administered as a 2-hour intravenous infusion, immediately followed by leucovorin at a dose of 400 mg/m2, given as a 2-hour intravenous infusion, with the addition, after 30 minutes, of irinotecan at a dose of 180 mg/m2, given as a 90-minute intravenous infusion. This treatment was followed by fluorouracil (5-FU) dosed at 400 mg/m2, administered as an intravenous bolus, followed by a continuous intravenous infusion of 2,400 mg/m2 over a 46-hour period. Treatment was administered every 2 weeks. Dose adjustments, administration of growth factor support (pegylated filgrastim), as well as supportive care, consisting of hydration and intravenous antiemetics between cycles, were at the discretion of the treating medical oncologist.
Chemoradiation was administered with radiosensitizing gemcitabine (300–400 mg/m2 at a fixed dose rate, infused weekly for 6 weeks during radiation therapy), or capecitabine (825 mg/m2 orally twice daily on radiation days) at the discretion of the treating physicians. The prescription dose for external beam radiation therapy was 50.4 Gy at 1.8 Gy per fraction using intensity-modulated radiation therapy. For head and uncinate lesions, the clinical target volume included the pancreatic head, SMA origin, SMA and SMV adjacent to the pancreatic head, enlarged lymph nodes, with or without the celiac axis, and with a 1-cm expansion to planning target volume. For lesions in the tail, the clinical target volume included the primary mass and celiac axis with an expansion. All patients were treated with daily image guidance, and respiratory gating was used if superior-inferior motion was more than 1.0 cm. Patients were treated at the 40%–60% respiratory phase. An internal target volume of the primary mass was created if motion was greater than 1.0 cm using the three-dimensional, 0, and 50% respiratory phases of respiration. As stated, restaging scans were performed after completion of every four cycles of FOLFIRINOX and again after chemoradiation, prior to surgery. In the absence of disease progression, patients were taken to the operating room for planned pancreatectomy.
Surgery and Pathologic Evaluation of the Surgical Specimens
Pancreaticoduodenectomy (PD) and total pancreatectomy were completed as previously described by Evans et al. [8]. Laparoscopy was routinely performed prior to laparotomy at the same anesthetic induction unless prevented by adhesions from prior abdominal surgery. PD was divided into six discrete steps, with the final step being of the greatest oncologic importance, namely, the dissection of the SMA. Vascular resection and reconstruction, when necessary, was a planned event based on preoperative imaging and used techniques we have previously described in detail [8, 9]. Frozen-section evaluation of PD specimens was limited to the pancreatic and common bile/hepatic duct transection margins. Positive bile duct or pancreatic resection margins were re-resected until clear margins were achieved. The SMA margin was inked and then sectioned perpendicular to the inked margin for histologic evaluation, according to the American Joint Committee on Cancer guidelines [10]. The distance from tumor to the closest inked margin was microscopically assessed and recorded in millimeters. Histologic assessment of the extent of treatment response was performed by a single senior pathologist (H.W.) using the grading system reported by Evans et al. [11].
Statistical Analyses
Variables of interest were compared using the Student’s t test, Pearson’s chi-square test, or Wilcoxon rank sum, as appropriate. Cutoff values for continuous variables were obtained using receiver operating characteristic curves. Overall survival time was estimated using the nonparametric product limit method [12] from the time of diagnosis to the date of death or censoring. Similarly, progression-free survival was estimated from the date of diagnosis to date of progression or censoring. Differences in survival were examined using the log-rank test. A p value <.05 was considered significant. All statistical analyses were performed using Stata version 11.0 (StataCorp, College Station, TX, http://www.stata.com).
Results
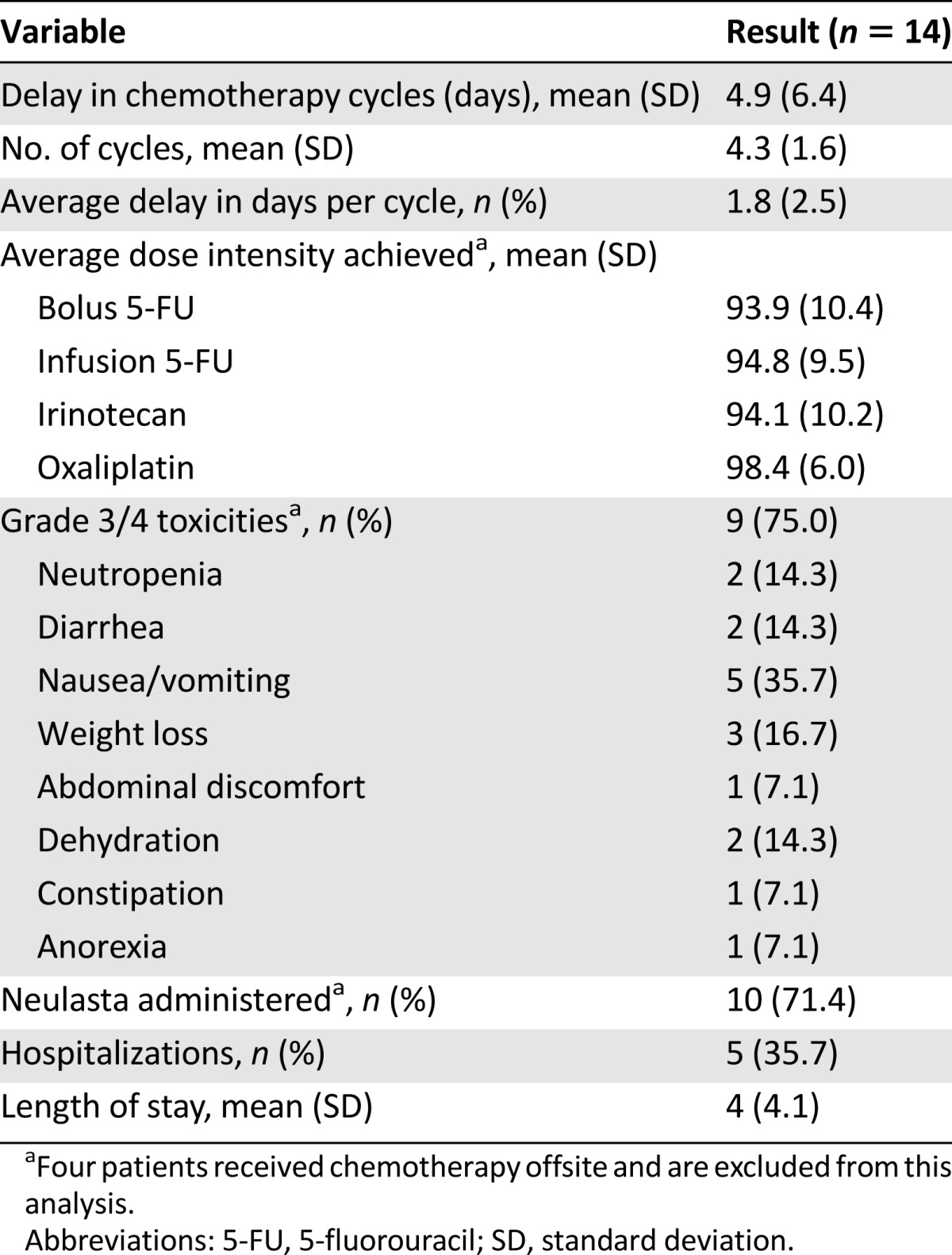
Neoadjuvant FOLIRINOX was administered to 18 patients with biopsy-proven pancreatic adenocarcinoma staged as borderline resectable (Table 2). During this same period of time, we saw 85 additional patients staged as having borderline resectable pancreatic cancer; most of these patients were from outside of our geographic region, were referred for an opinion regarding surgery, and had received systemic therapy and/or external beam radiation therapy prior to consultation with our program. All 18 study patients were previously untreated and received FOLFIRINOX as initial therapy (median number of cycles: 4 [range: 3–8]). All 18 then received gemcitabine-based chemoradiation (n = 9) or capecitabine-based chemoradiation (n = 9). Systemic toxicities associated with FOLFIRINOX are summarized in Table 3. Four patients received their chemotherapy offsite and were excluded from the toxicity analysis. The planned chemotherapy schedule was delayed by an average of 4.9 days (SD: 6.4 days), and the average delay per cycle was 1.8 days. The most common grade 3 or 4 toxicities during chemotherapy were gastrointestinal, including nausea/emesis (n = 5), weight loss (n = 3), and diarrhea (n = 2), and hematologic (n = 2; neutropenia); five patients (36%) required a total of six admissions.
Table 2.
Comparison of patients completing all therapy versus those receiving neoadjuvant therapy only
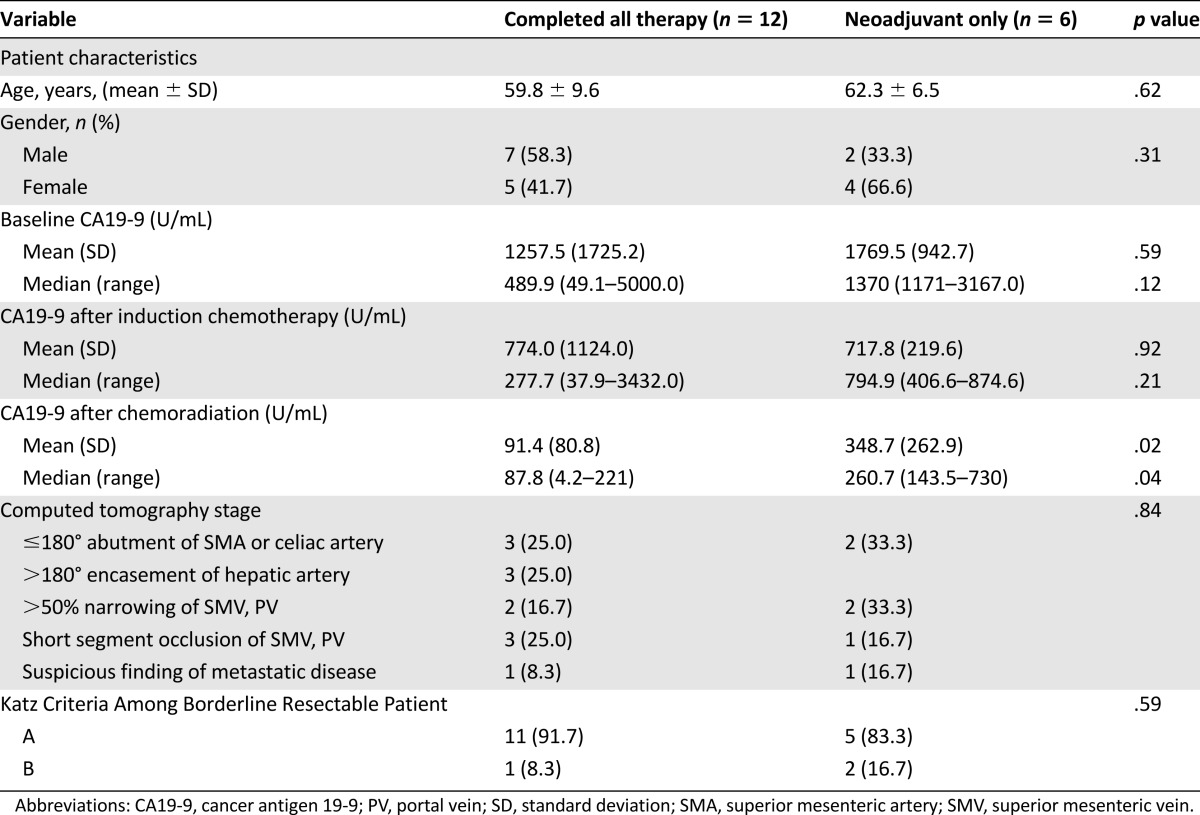
Table 3.
FOLFIRINOX-associated toxicities
Restaging After Neoadjuvant Therapy
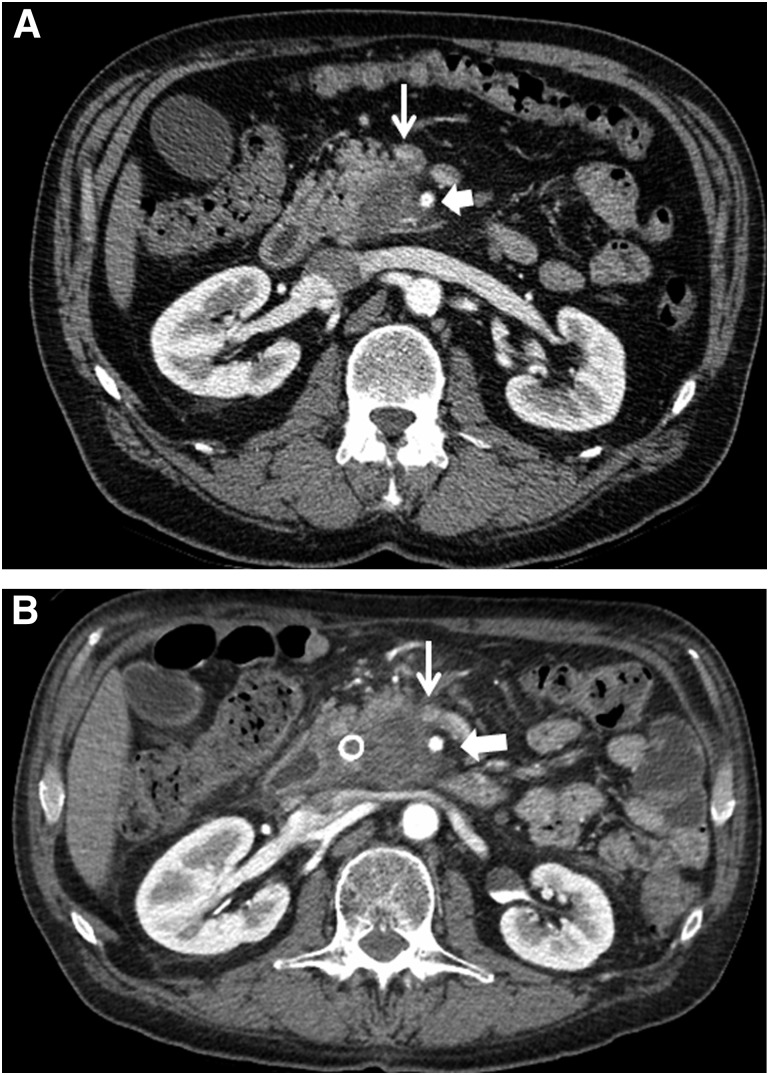
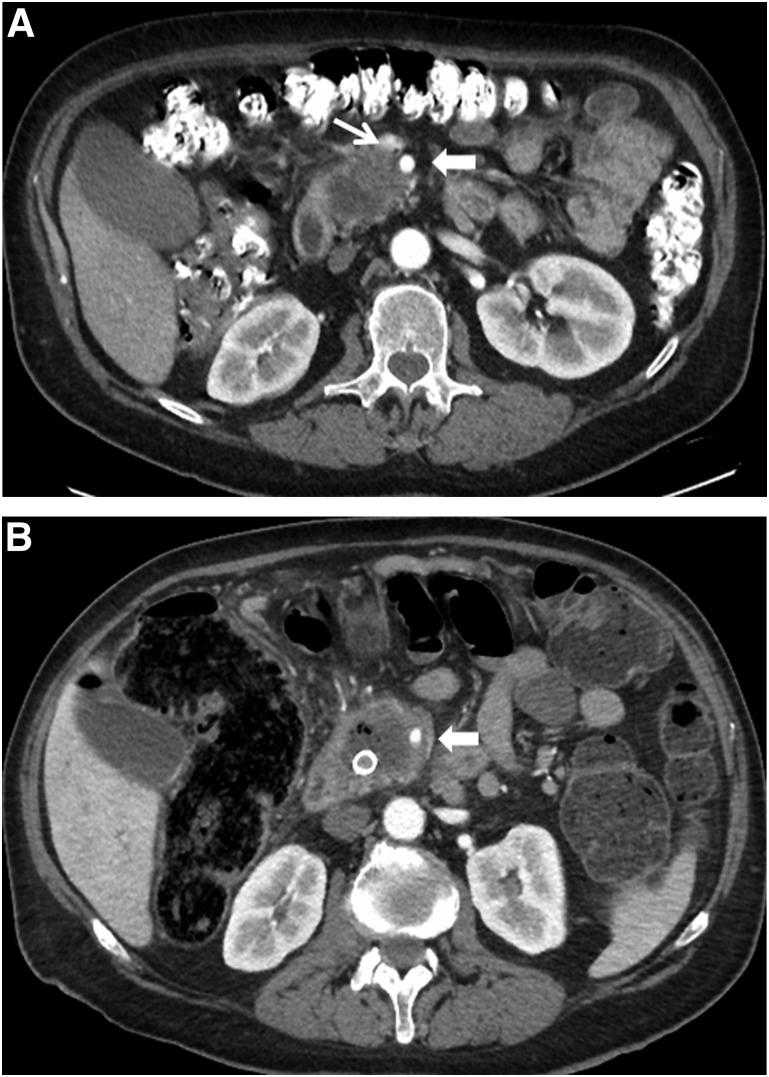
At the time of initial restaging, after FOLFIRINOX and prior to chemoradiation, none of the 18 patients were found to have disease progression (at either local or distant sites) and all 18 patients went on to receive chemoradiation. At the time of postchemoradiation preoperative restaging, three patients were not considered for surgery because of local disease progression. Patient 1 had local tumor anatomy consistent with SMA abutment (180° tumor-vessel interface), but after induction FOLFIRINOX and chemoradiation, the tumor-vessel interface progressed to encasement (>180°) despite a small reduction in overall tumor size on axial CT images. Most important, this patient also experienced a declining performance status, suggesting more advanced disease than was apparent on imaging studies. Serum levels of CA19-9 were never elevated in this patient. This patient refused further therapy and died of disease progression 2.5 months after chemoradiation (9.2-month overall survival from diagnosis). Patient 2 experienced a mixed response to induction FOLFIRINOX (slight decline in serum CA19-9) in the setting of a complicated series of medical comorbidities that delayed chemoradiation by approximately 6 weeks. When restaged after chemoradiation, there was subtle increased tumor abutment of the SMA (approximately 3 months after the completion of FOLFIRINOX) (Fig. 1A, 1B). Surgery was not performed in this patient for two reasons. Similar to patient 1, his poor performance status suggested more extensive disease than was apparent on imaging studies, and the technical aspects of tumor removal placed his operation at the highest level of risk and complexity—an operation not appropriate in a patient with marginal performance status. Patient 2 ultimately received second-line chemotherapy but developed clinically and radiographically evident bone metastases 3 months after chemoradiation and died of disease 9.3 months after diagnosis (7.3 months after completing FOLFIRINOX). The tumor of patient 3 abutted the SMA for 180° and narrowed the SMV at or just below the splenic vein confluence (Fig. 2A). He received four cycles of FOLFIRINOX and went on to receive capecitabine-based chemoradiation, and at final preoperative restaging, he also had a mixed response. He reported increased abdominal and back pain, yet his CA19-9 fell from a pretreatment value of 1,171 U/mL down to 117 U/mL, and CT imaging showed an increase in size of the primary tumor with worsening of the SMV anatomy such that a distal target for reconstruction was no longer available (Fig. 2B). He was scheduled to receive further chemotherapy locally but died 6.9 months after diagnosis of progressive disease (local and liver).
Figure 1.
Axial CT imaging of patient 2. (A): Image in the arterial phase demonstrating a low-density tumor that abuts the SMA for approximately 180°. Thin arrow identifies the SMV; wide arrow identifies the SMA. (B): Image in the arterial phase at the time of restaging after neoadjuvant FOLFIRINOX and chemoradiation that now demonstrates subtle increased abutment/encasement of the SMA (and SMV). Thin arrow identifies SMV; wide arrow identifies the SMA.
Figure 2.
Axial CT imaging of patient 3. (A): Image in the arterial phase demonstrating a low-density tumor that abuts the SMA for 180° and narrows the SMV; the extent of venous involvement is not well seen on this arterial phase image. Thin arrow identifies the SMV, wide arrow identifies the SMA. (B): Image at restaging after neoadjuvant FOLFIRINOX and chemoradiation now demonstrates encasement of the SMA and the SMV is now occluded with no distal target for resection/reconstruction (the latter is not visible in this arterial phase). Wide arrow identifies the SMA.
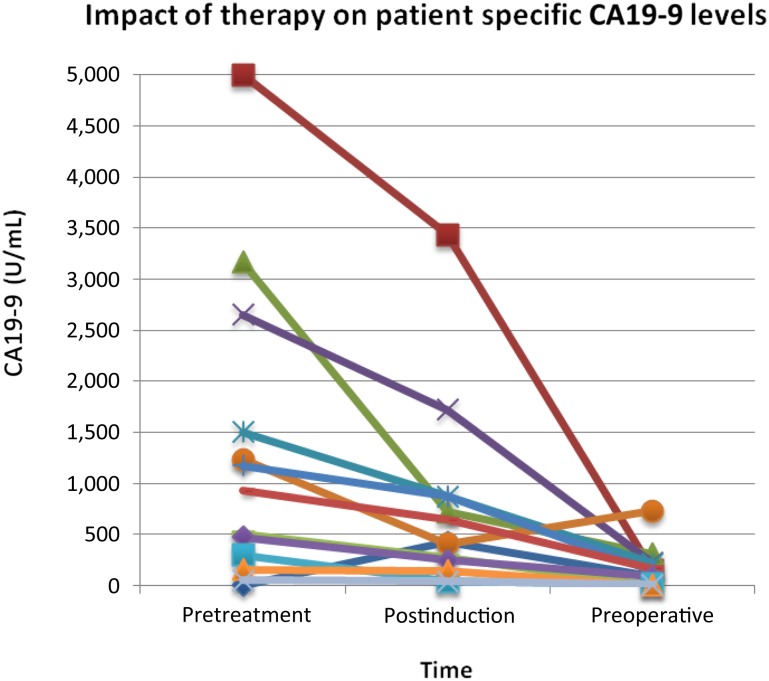
As shown in Table 2 and Figure 3, median CA19-9 levels before treatment and after each stage of therapy in those patients who had elevated pretreatment levels showed a steady decline (respective medians: 489.9 U/mL, 277.7 U/mL, 87.8 U/mL). When comparing patients who received only neoadjuvant chemotherapy and chemoradiation with those patients who completed all intended therapy including surgical resection, the decline in CA19-9 after chemoradiation was significantly greater in those who underwent successful surgery.
Figure 3.
CA19-9 response to chemotherapy and chemoradiation therapy.
Abbreviation: CA19-9, cancer antigen 19-9.
Surgical Outcomes
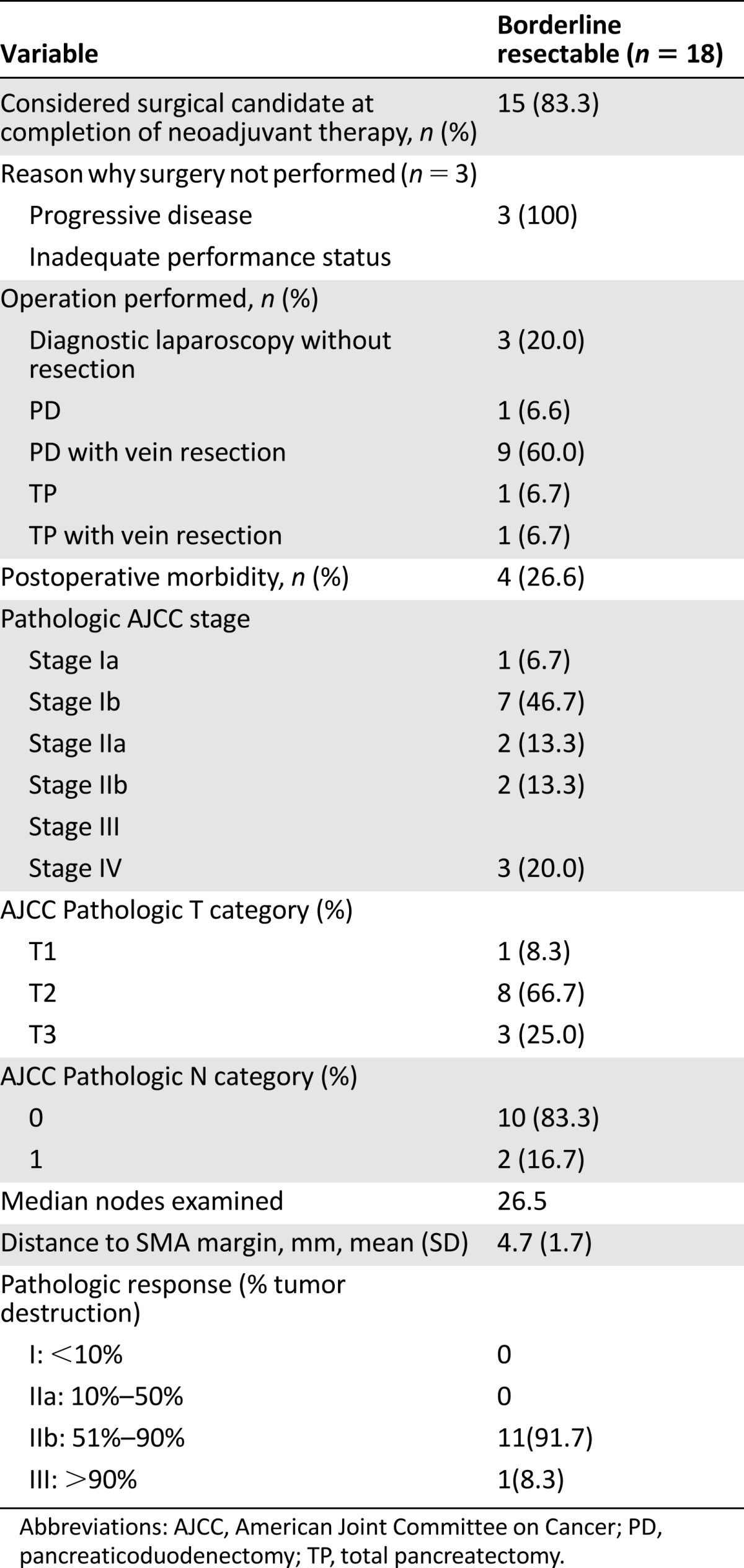
Fifteen of the 18 patients (83%) were taken to the operating room, 1 for diagnostic thoracoscopy related to indeterminate pulmonary nodules and 17 for a potentially curative pancreatectomy. Three patients were found to have biopsy-proven metastatic disease at laparoscopy (n = 2) or thoracoscopy (n = 1), and 12 (80%) underwent a complete resection of the primary tumor (Table 3). All patients who underwent an open laparotomy had the primary tumor successfully removed with a complete gross resection, including 10 PDs and two total pancreatectomies. Vascular resection and reconstruction at the time of pancreatectomy was required in 10 of the 12 patients (83.3%). Pathologic evaluation of resected specimens appears in Table 4 [11]. There were no complete pathologic responses in the primary tumor; however, after very meticulous examination of the resected specimen, only 2 of 12 patients were found to have positive regional lymph nodes (median number of lymph nodes evaluated: 27 [range: 15–39]).
Table 4.
Surgical outcomes after neoadjuvant FOLFIRINOX and chemoradiotherapy
There were no perioperative or in-hospital deaths related to the surgical procedure. Of the 12 patients who underwent complete resection of the pancreatic tumor, there were no pancreatic leaks and no reoperations. The median length of hospital stay was 9.5 days (range: 7–21 days). Two patients were readmitted for nausea and either vomiting or diarrhea. One patient’s symptoms resolved in 2 days with medical management, and one of the total pancreatectomy patients required a 15-day stay for nausea, vomiting, and failure to thrive.
Survival
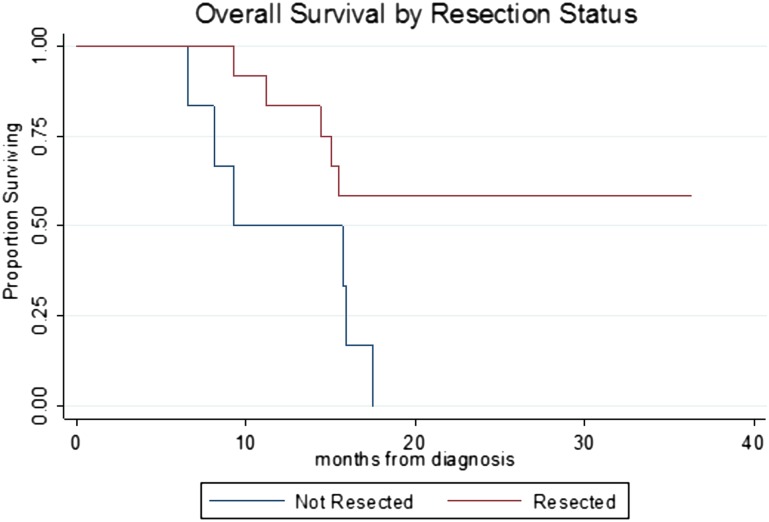
Overall survival is seen in Figure 4. Of the six patients who did not undergo pancreatectomy as a result of disease progression, all six are deceased (median survival from the date of diagnosis: 12.5 months [range: 6.9–17.5 months]). Of the 12 patients who completed all intended preoperative therapy and pancreatectomy, only one patient (treated most recently) received postoperative adjuvant therapy. Additional adjuvant or back-end systemic therapy may become more common because oncologists are considering a minimum of 6 months of nonsurgical therapy to be the evolving standard for this disease (among others). At a median of 22 months from diagnosis (range: 18–35 months), 7 of 12 (58.3%) are alive, including 5 who have no evidence of disease and 2 who are alive with disease (23 months and 29 months), and 5 have died; 4 died of disease at a median of 14.7 months from diagnosis. The first site of treatment failure in these four patients included liver in three patients and periaortic lymph nodes in one patient. One patient died at 9.3 months from diagnosis and had no detectable cancer at autopsy but experienced a progressive decline in performance status with ongoing nausea, diarrhea, and failure to thrive that did not respond to medical intervention including hyperalimentation.
Figure 4.
Overall survival by resection status. Log-rank, p = .02. Median survival: not resected, 9.3 months; resected, not reached.
Since this report was submitted, we have treated an additional 12 patients with borderline resectable disease (excluding those enrolled in an investigator-initiated clinical trial at our institution) with FOLFIRINOX followed by chemoradiation and pancreatectomy. Ten of these 12 completed all intended therapy, including surgical resection of the pancreatic tumor. The cumulative median survival of these 10 patients plus the 12 patients reported herein (22 patients who completed neoadjuvant FOLFIRINOX/chemoradiation and pancreatectomy) has not been reached, with a minimum survival for all living patients of 11.2 months.
Discussion
There is now consensus that patients with borderline resectable pancreatic adenocarcinoma, defined by established definitions of local tumor anatomy, should undergo neoadjuvant therapy and should not be taken directly to the operating room [2, 13]. Accurate pretreatment staging allows physicians to prescribe stage-specific therapy with defined endpoints, thereby clarifying the goals of therapy for both physicians and patients. To be included in the “borderline resectable” category, surgery must be technically possible at the time of initial staging and a commitment must be made to proceed with pancreatectomy if there is no evidence of disease progression following induction therapy. The goal of induction chemotherapy is to treat radiographically occult micrometastatic disease at distant sites. Neoadjuvant chemoradiation following induction chemotherapy is then used in an attempt to sterilize the periphery of the primary tumor, allowing for a complete gross resection of the tumor and increased rates of R0 resection. Even if a microscopically positive (R1) SMA margin is found, in the setting of induction chemoradiation and a meticulous approach to surgical technique, recent data suggest that its impact on survival duration may be minimal [14]. Most important, the impact of a positive margin is likely not the same in the absence of induction therapy [14].
It is reasonable to assume that patients with radiographically occult micrometastatic disease and excellent performance status should have a distinct biologic advantage over those patients with grossly visible metastatic disease and declining performance status. Thus, the natural progression of clinical trial development is to build on the favorable results achieved (with more intensive therapy) in the metastatic setting and to extrapolate those results to the treatment of patients with earlier stage disease. Consequently, we have explored the use of FOLFIRINOX as neoadjuvant therapy for patients with borderline resectable pancreatic cancer. One theoretical concern with using FOLFIRINOX followed by chemoradiation and surgery was the potential for cumulative toxicity. In this initial experience, chemotherapy toxicity was quite manageable with aggressive antiemetic support, planned hydration, routine use of pegylated filgrastim, and dose reductions when necessary. Importantly, we did not try to treat patients with ongoing evidence of biliary obstruction but rather placed metal stents endoscopically in those patients with biliary obstruction prior to initiation of chemotherapy. All patients could aliment themselves, had no evidence of mechanical gastric outlet obstruction, were independent with self-care, and had an Eastern Cooperative Oncology Group performance status of 2 or lower at the time of diagnosis (after maximizing diabetes management, nutritional support, and pain management).
Although appropriate selection of patients for aggressive multimodality therapy is critically important and requires multidisciplinary consensus, our results also reflect the importance of supportive care during the administration of FOLFIRINOX including multiagent antiemetic therapy with aprepitant (antagonist of human substance P/neurokinin 1 receptors) as well as standard antiemetics, near universal use of growth factor support to avoid treatment delays, and preemptive hydration to prevent complications related to gastrointestinal toxicity and consequent dehydration. Furthermore, the overall incidence of grade 3 or 4 toxicities was sufficiently low to justify the presumed benefits of this regimen in the neoadjuvant setting. Importantly, we could not appreciate that FOLFIRINOX-related toxicities had any impact on the delivery of chemoradiation, and they did not compromise surgical resection or increase perioperative morbidity or mortality. We assume that narrow fields with intensity-modulated radiation therapy delivered by experienced radiation oncologists contributed to the excellent tolerance of chemoradiation. The apparent safety of FOLFIRINOX followed by chemoradiation in this report may not be transferable to centers less experienced in the coordinated multidisciplinary management of patients with pancreatic cancer or in those centers with an inherent bias against the use of more durable metal stents for biliary decompression. MCW’s Pancreatic Cancer Program has a full-time dietician and a full-time nurse practitioner with specific expertise in diabetes management, in addition to physicians and nurse practitioners who are disease focused. Despite such a robust support system, which we would argue is necessary to safely treat patients with resectable and borderline resectable pancreatic cancer, there likely are some patients of advanced age or with significant medical comorbidities who may be unable to tolerate multiple sequential treatment modalities, especially when surgery follows induction chemotherapy and chemoradiation. Such patients cannot always be defined by a specific age or performance status at diagnosis; however, as suggested in this report, patients who experience a decline in performance status during neoadjuvant therapy (that is not clearly reversible in a short period of time, such as a few weeks) usually have progressive pancreatic cancer, even if it is hard to prove radiographically. As aggressive therapies are extended to older and perhaps less robust individuals, this observation may not be true, and modifications in the chemotherapy program may be very important in such individuals.
We have demonstrated that surgery after induction therapy with FOLFIRINOX can be safely performed. This information is very important for the recently opened Alliance A021101 trial as well as for the development of future clinical trials in patients with resectable disease for which systemic therapy is an accepted part of the treatment program for all patients, understanding that the sequencing of therapy and the role of chemoradiation remain controversial. Although this paper represents an initial report of a small sample, we had no surgery-related deaths and complications were minimal. There were no pancreatic leaks, no reoperations, and just two readmissions, one of which was only 2 days in duration. These results were noted despite the need for vascular resection and reconstruction in 10 of the 12 patients. Importantly, all vascular resections performed at the time of pancreatectomy were planned events based on careful review of detailed preoperative imaging. When vascular resection at the time of pancreatectomy is an unplanned event secondary to an operative misadventure, similar results are unlikely to be realized. Three patients with borderline resectable disease reported in this paper experienced local disease progression defined by an increase in tumor size on CT imaging. Although uncommon, this likely reflects lack of response (at local and distant sites) to both systemic therapy and chemoradiation. All three patients experienced a rapid global decline reflecting systemic disease progression that would not have been altered by a local therapy such as surgery. Although anecdotal, our experience with these three patients suggests that local disease progression after induction therapy, including chemoradiation, predicts short survival and a survival duration that could not have been altered by a surgical procedure. In an effort to better match the patient/tumor biology with the planned therapy, current clinical trials at our institution aim to characterize tumors based on molecular analysis of pretreatment tumor biopsies as a guide to the optimal selection of systemic therapies for individual patients.
Although treatment response was not the focus of this report, it is of interest that all 12 resected specimens demonstrated histologic evidence of more than 50% tumor cell destruction (>50% nonviable tumor; pathologic partial response); 10 of the 12 patients who completed all therapy had negative regional lymph nodes, and all 12 had negative margins of resection. The profound response to induction therapy in regional lymph nodes is not a new clinical observation but perhaps is even more impressive following FOLFIRINOX. The extent to which radiographically occult distant metastases may experience a similar response to induction therapy is impossible to evaluate; however, these results are very encouraging if one considers occult disease in liver, lung, or bone as perhaps more similar to disease in regional lymph nodes (absent the complex stroma present in the pancreatic primary). Such disease may be even more sensitive to systemic therapies with increased efficacy such as FOLFIRINOX.
Conclusion
Induction chemotherapy using neoadjuvant FOLFIRINOX followed by chemoradiation for borderline resectable pancreatic adenocarcinoma is safe, and our initial experience suggests that resection rates are favorable compared with previous reports in this high-risk patient population.
Footnotes
For Further Reading: Jason E. Faris, Lawrence S. Blaszkowsky, Shaunagh McDermott et al. FOLFIRINOX in Locally Advanced Pancreatic Cancer: The Massachusetts General Hospital Cancer Center Experience. The Oncologist 2013;18:543-548.
Implications for Practice: The prognosis for patients with locally advanced pancreatic cancer, who constitute almost a third of patients presenting with a new diagnosis of pancreatic cancer, is quite poor, with a median survival of approximately 1 year. The ideal treatment paradigm for these patients is unclear, but based on the experience with FOLFIRINOX in the metastatic setting, multiple institutions have begun to treat with FOLFIRINOX for patients with locally advanced disease. In this paper, the authors describe their institutional experience with FOLFIRINOX followed by chemoradiation in patients with locally advanced pancreatic cancer. They provide evidence for substantial activity, with conversion to surgical resectability in more than 20% of patients. Further study is warranted on this promising treatment approach for patients with locally advanced pancreatic cancer.
Author Contributions
Conception/Design: Kathleen K. Christians, Paul Ritch, Douglas B. Evans, Ben George
Provision of study material or patients: Kathleen K. Christians, Beth Erickson, Douglas B. Evans
Collection and/or assembly of data: Kathleen K. Christians, Susan Tsai, Anna Mahmoud, Ben George
Data analysis and interpretation: Kathleen K. Christians, Susan Tsai, Huamin Wang, Ben George
Manuscript writing: Kathleen K. Christians, Paul Ritch, James P. Thomas, Huamin Wang, Douglas B. Evans, Ben George
Final approval of manuscript: Kathleen K. Christians, Susan Tsai, Paul Ritch, James P. Thomas, Lauren Wiebe, Tracy Kelly, Beth Erickson, Huamin Wang, Douglas B. Evans, Ben George
Disclosures
The authors indicated no financial relationships.
References
- 1.Evans DB, Erickson BA, Ritch P. Borderline resectable pancreatic cancer: Definitions and the importance of multimodality therapy. Ann Surg Oncol. 2010;17:2803–2805. doi: 10.1245/s10434-010-1285-8. [DOI] [PubMed] [Google Scholar]
- 2.Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer: The importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833–846. doi: 10.1016/j.jamcollsurg.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. NCCN practice guidelines for pancreatic cancer, version 2. Available at http://www.nccn.org/professionals/physician_gls/recently_updated.asp
- 4.Abrams RA, Lowy AM, O’Reilly EM, et al. Combined modality treatment of resectable and borderline resectable pancreas cancer: Expert consensus statement. Ann Surg Oncol. 2009;16:1751–1756. doi: 10.1245/s10434-009-0413-9. [DOI] [PubMed] [Google Scholar]
- 5.Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: Expert consensus statement. Ann Surg Oncol. 2009;16:1727–1733. doi: 10.1245/s10434-009-0408-6. [DOI] [PubMed] [Google Scholar]
- 6.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 7.Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: Definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035–1046. doi: 10.1245/ASO.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Evans DB, Christians KC, Foley WD. Pancreaticoduodenectomy (Whipple operation) and total pancreatectomy for cancer. In: Fischer JL, editor. Mastery of Surgery. 6th ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins; 2012. pp. 1445–1466. [Google Scholar]
- 9.Christians K, Evans DB. Pancreaticoduodenectomy and vascular resection: Persistent controversy and current recommendations. Ann Surg Oncol. 2009;16:789–791. doi: 10.1245/s10434-009-0322-y. [DOI] [PubMed] [Google Scholar]
- 10.Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010. [Google Scholar]
- 11.Evans DB, Rich TA, Byrd DR, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg. 1992;127:1335–1339. doi: 10.1001/archsurg.1992.01420110083017. [DOI] [PubMed] [Google Scholar]
- 12.Dawson B, Trapp RG. Basic and Clinical Biostatistics. New York, NY: Lange Medical Books/McGraw-Hill; 2001. pp. 211–232. [Google Scholar]
- 13.Evans DB, Crane CH, Charnsangavej C, et al. The added value of multidisciplinary care for patients with pancreatic cancer. Ann Surg Oncol. 2008;15:2078–2080. doi: 10.1245/s10434-008-9972-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raut CP, Tseng JF, Sun CC, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246:52–60. doi: 10.1097/01.sla.0000259391.84304.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]