The primary objective of this study was to describe the incidence, risk factors, and management of infusion-related reactions (IRRs) during the first 12 weeks of trastuzumab therapy in a general population of breast cancer patients. Results support that the vast majority of IRRs occur with the first infusion and are mild in severity. Previously unknown risk factors were identified that may help to identify a population of patients at increased risk of IRRs with trastuzumab.
Keywords: Trastuzumab, Infusion-related reactions, Hypersensitivity, Monoclonal antibodies, Breast cancer
Learning Objectives
Identify risk factors associated with trastuzumab-associated infusion-related reactions.
Describe the impact of premedications on the incidence and/or severity of trastuzumab-associated infusion-related reactions.
Abstract
Background.
Trastuzumab has become a mainstay of therapy for human epidermal growth factor receptor-2 overexpressed breast cancer in nearly all stages of the disease. Like many monoclonal antibodies, trastuzumab is associated with infusion-related reactions (IRRs) that are not well described, and incidence varies widely between reports (0.7%–40% of patients).
Materials and Methods.
A retrospective chart review of breast cancer patients who received trastuzumab was conducted. The primary objective was to describe the incidence, risk factors, and management of IRRs during the first 12 weeks of trastuzumab therapy in a general population of breast cancer patients.
Results.
A total of 197 patients who received trastuzumab (1,788 doses) were evaluated. Thirty-three IRRs were identified in 32 patients, resulting in an incidence of 16.2% of patients and 1.8% of doses. All IRRs were mild or moderate in severity and were successfully managed with supportive medications and/or by temporarily stopping the infusion. All patients received subsequent cycles of trastuzumab, with only one patient experiencing a subsequent reaction. Body mass index, stage of disease, and use of premedications were significantly associated with IRRs by multivariate logistic regression analysis.
Conclusion.
Overall, these results support that the vast majority of IRRs occur with the first infusion, are mild in severity, and are easily managed. In addition, risk factors were identified that may help to identify a population of patients at increased risk of IRRs who may benefit from premedication.
Implications for Practice:
Trastuzumab is associated with infusion-related reactions (IRRs) that are not well described with incidence varying widely between reports (0.7% to 40% of patients). In addition, risk factors associated with trastuzumab-associated IRRs are unknown. This manuscript presents data from a large cancer center describing the incidence, risk factors, and management of IRRs with trastuzumab in clinical practice. A more complete understanding of the incidence and severity of IRRs related to trastuzumab as well as the characterization of risk factors could be beneficial to clinicians when educating patients about the toxicities associated with trastuzumab.
Introduction
Trastuzumab, a humanized monoclonal antibody targeted to the extracellular domain of the human epidermal growth factor receptor-2 (HER2) protein, has become a mainstay of therapy for HER2-overexpressed breast cancer in nearly all stages of the disease [1]. Like many monoclonal antibodies, trastuzumab is associated with infusion-related reactions (IRRs), also referred to as hypersensitivity reactions. The mechanism of IRRs associated with monoclonal antibodies is unclear. The degree of antibody humanization influences the frequency of monoclonal antibody-associated IRRs, with mouse and chimeric antibodies eliciting the highest frequency of immunogenic responses and fully human or humanized monoclonal antibodies, such as trastuzumab, having a relatively low immunogenicity by comparison [2]. Monoclonal antibodies may lead to cytokine release through interactions with their molecular targets on tumor or other circulating cells [3]. These cytokines have been associated with a wide range of symptoms, including fever, chills, rigors, hypotension, and dyspnea. The most well-studied example of this biologic consequence is described with rituximab and its molecular target, CD20. This mechanism may also be relevant for trastuzumab, resulting in symptoms such as fever and chills.
The definition of trastuzumab-related IRRs has evolved over time, which has created a challenge for clinicians when comparing trials and understanding the true incidence of reactions and/or the need for premedications. The pivotal registration trials reported individual symptoms (fever, chills, rigors, etc.) temporally associated with trastuzumab infusions. These registration trials reported the incidence of “chills and fever” as high as 40% with the first dose, “infusional toxicity” as high as 35% with subsequent doses, and “severe reactions” in up to 9% of patients [4]. Postmarketing surveillance data from the manufacturer later identified “pulmonary toxicity” and “anaphylaxis” as rare but potentially life-threatening reactions associated with less than 1% of trastuzumab infusions [4–6]. Thus, the current package insert for trastuzumab describes three categories of reactions that may be associated with trastuzumab infusions: generalized IRRs, pulmonary toxicity, and anaphylaxis [4]. Although these three categories are described separately, there is overlap between definitions. The type of data collection used in the registration trials influenced the definition of IRRs and contributed to this variability.
An additional factor contributing to the inconsistency in reported rates of IRRs may reflect differences in dosing and administration of trastuzumab. Clearly, the initial dose of a monoclonal antibody is more likely to be associated with IRRs. Rates of IRRs with subsequent maintenance doses are typically much lower. Infusion duration and/or peak serum concentrations of the monoclonal antibody may also influence the incidence of IRRs. Vogel et al. [7] compared two weekly trastuzumab dosing schedules, an 8 mg/kg loading dose followed by a 4 mg/kg maintenance dose and a 4 mg/kg loading dose followed by a 2 mg/kg maintenance dose, with the first infusion administered for 90 minutes and subsequent infusions for 30 minutes for both dosing schedules. A numerical increase in fever (45% vs. 36%), chills (40% vs. 22%), and dyspnea (25% vs. 15%) was reported with the higher dose. Subsequently, Baselga et al. [8] investigated a once every 3-week dosing strategy (8 mg/kg load, 6 mg/kg maintenance), with all doses administered for 90 minutes. Fifty-four percent of patients had at least one symptom associated with the first infusion, which primarily included rigors (18%) or pyrexia (15%). Importantly, these reactions could have occurred on the same day as the trastuzumab infusion or the day after. To assess the safety of shortening the trastuzumab infusion, Chan et al. [9] conducted a retrospective study utilizing an institutional adverse drug reaction (ADR) database to capture the incidence of IRRs before and after the implementation of 30-minute maintenance infusions regardless of dose. The incidence of IRRs before and after the implementation of 30-minute infusions of 2, 4, or 6 mg/kg maintenance doses was similar (0.11% before 30-minute infusion implementation; 0.04% after 30-minute infusion implementation), and the authors concluded that doses up to 6 mg/kg can be safely administered for 30 minutes. Lastly, in a letter to the editor, Ring et al. [10] reported that an every 3-week dosing regimen with 30-minute maintenance infusions was well tolerated (IRR incidence: 3.5% of patients, 1.5% of doses).
Although these reactions are not typically life-threatening, they may impart discomfort and inconvenience for patients and caregivers. Although rare, serious IRRs may result in hospitalization and/or death as a result of anaphylaxis and/or pulmonary toxicity. In addition, IRRs can pose problems for infusion centers, potentially increasing chair time, costs, and personnel required to manage these reactions. Risk factors associated with trastuzumab-related IRRs are unknown, and further characterization of IRRs related to trastuzumab and analysis of risk factors that may predispose patients to these reactions could prove to be important in prevention and management. Therefore, a retrospective chart review was conducted to describe the incidence, risk factors, and management of IRRs during the first 12 weeks of trastuzumab therapy in a general population of breast cancer patients.
Materials and Methods
Because ADR databases may underemphasize the incidence of mild-to-moderate expected adverse reactions, a detailed chart review was performed. The institutional pharmacy database was queried to identify all patients who received a dose of trastuzumab between May 1, 2010 and July 31, 2010 and then retrospectively evaluated to identify the date of their first dose of trastuzumab. With such a broad range of reported incidence (range 0.7%–40% of patients), this time frame was chosen to provide a sufficient patient population to capture reactions occurring at a moderate rate (midrange of reported incidence) and to detect differences in risk factors among patients experiencing reactions. Patients were included if they had a breast cancer diagnosis and received all evaluable doses at the institution’s main campus. Patients under 18 years of age were excluded. Patients were excluded if their medical information was not easily retrievable through the institution’s electronic medical record (EMR). The primary objective was to describe the incidence of IRRs during the first 12 weeks of trastuzumab therapy as ordered per departmental standards. Secondary objectives were to describe the influence of trastuzumab dose, duration of infusion, and premedication on the incidence of trastuzumab-related IRRs and evaluate other patient and disease characteristics that may be associated with an increased risk of IRRs.
The Department of Breast Medical Oncology at University of Texas MD Anderson Cancer Center utilizes established standards for trastuzumab dosing and administration that include the following: (a) all trastuzumab loading doses are administered for 90 minutes; (b) if the previous infusion was well tolerated, all maintenance doses are administered for 30 minutes; (c) the schedule of trastuzumab administration is generally based on the schedule of the concomitant anticancer therapy (e.g., weekly trastuzumab with weekly chemotherapy or every 3-week trastuzumab with every 3-week chemotherapy); (d) trastuzumab is routinely the first medication administered in a treatment regimen; (e) trastuzumab premedications are not routinely ordered; (f) concomitant chemotherapy premedications are routinely ordered to start 30 minutes before chemotherapy administration.
Patients’ demographic and tumor characteristics were collected from the EMR and the Breast Cancer Management System. A focused chart review of medication orders and nursing documentation was conducted, including medication administration records and vital signs. Reactions occurring in patients receiving concurrent anticancer medications (e.g., chemotherapy) were evaluated on a case-by-case basis to determine the causative agent. If the reaction was not clearly related to another drug, then the reaction was considered to be related to the trastuzumab infusion and included in the analysis.
Infusion-related reactions were defined and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 [11]. Data were analyzed using descriptive statistics. Univariate and multivariate logistic regression analyses were used to examine the relationships between the occurrence of trastuzumab-related IRRs and factors that may affect the incidence of reactions (age, race, stage of disease, body mass index, hormone receptor status, HER2 fluorescence in situ hybridization [FISH] copy number, and use of premedications). These analyses were limited to the 30 reactions that occurred during the first dose. This study protocol was approved by University of Texas MD Anderson Cancer Center’s Institutional Review Board, and a waiver of informed consent was obtained.
Results
A total of 327 patients were identified during the 3-month study period (May 1, 2010 through July 31, 2010). One hundred and ninety-seven patients met the inclusion criteria and received 1,788 evaluable doses. Patients were excluded for the following reasons: (a) first dose of trastuzumab was administered before May 1, 2009 (n = 68); (b) received doses outside the institution’s main campus (n = 48); and (c) did not have a breast cancer diagnosis (n = 14). Table 1 summarizes the baseline patient and tumor characteristics for this cohort; treatment characteristics are described in Table 2. The final population (n = 197) had a median age of 51 (range 28–92) years, and the majority of patients had nonmetastatic disease (stages I–III, 87%).
Table 1.
Patient characteristics
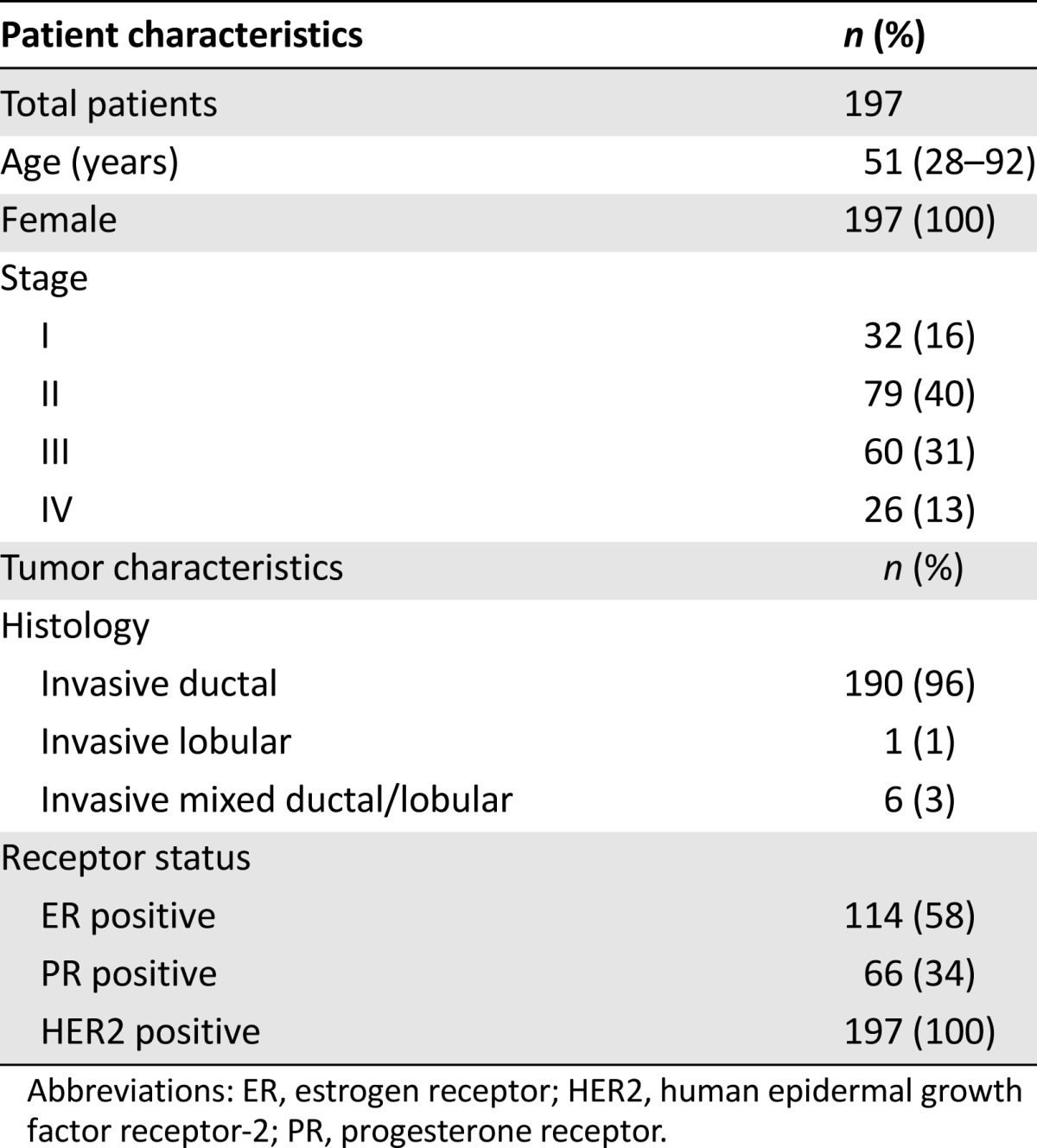
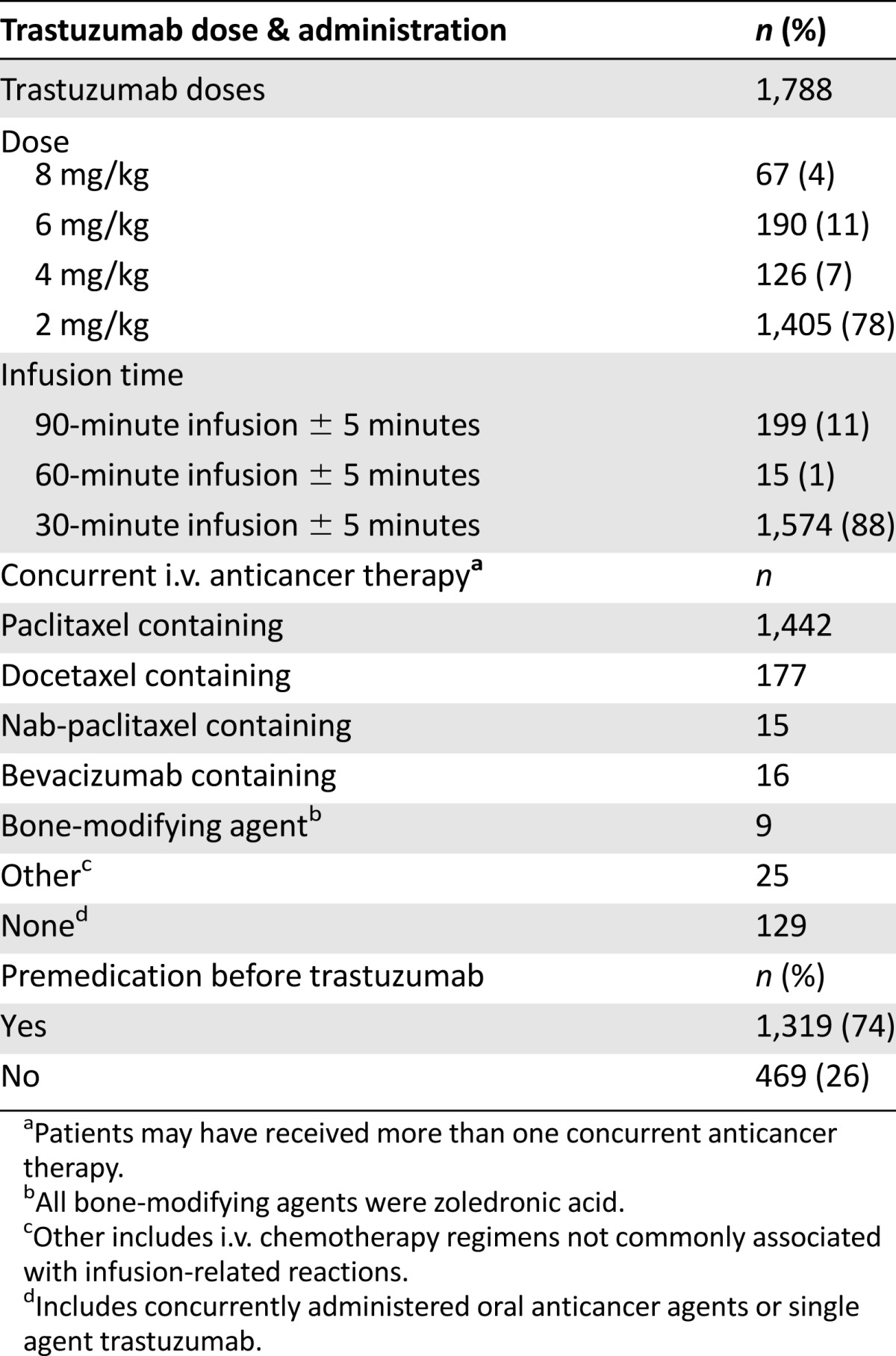
Table 2.
Treatment characteristics
Of the 197 patients evaluated, 33 IRRs were documented in 32 patients and 1,788 doses (16.2% of patients and 1.8% of doses). The institutional ADR database was queried for trastuzumab-related IRRs reported during the dates of the trial, and only 1 was found. This patient did not meet the inclusion criteria of this study and therefore was not included in our data.
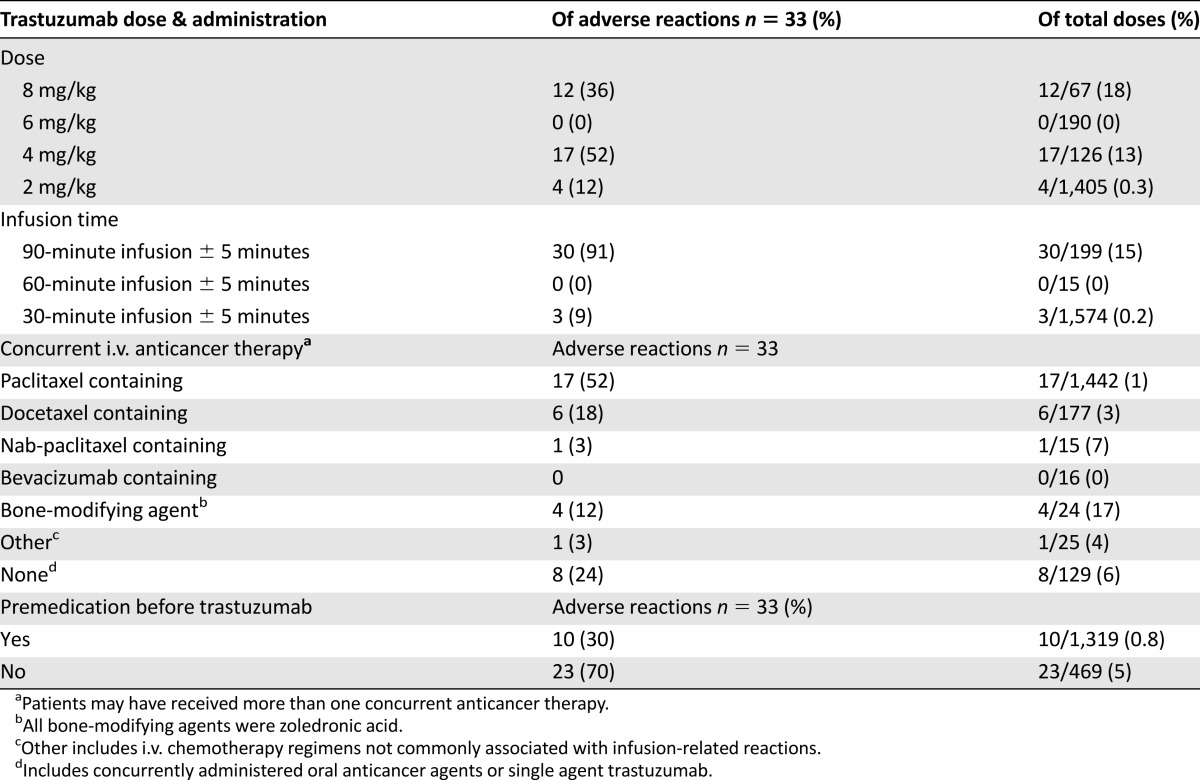
Information related to IRRs is listed in Table 3. The majority of reactions occurred during the first dose (91%) and one each with the second, fifth, and seventh doses. One patient had a reaction on dose 2 in addition to the first dose. Symptoms included chills (n = 19), pain (n = 9), rigors (n = 8), nausea (n = 6), headache (n = 5), shortness of breath (n = 5), vomiting (n = 2), numbness (n = 1), and fever (n = 1). Overall, the majority of reactions were grade 2 (97%), with one grade 1 reaction. None of the patients experienced a grade 3 or 4 reaction. There were no cases of anaphylaxis and/or pulmonary toxicity.
Table 3.
Infusion-related reaction information
Trastuzumab-associated IRRs were effectively managed by temporarily discontinuing the infusion and/or administering supportive medications (Table 4). Trastuzumab infusions were held in 26 of the 33 reactions (79%) and occurred an average of 65 (range 5–92) minutes into the infusion. Infusions were held for an average of 54 (range 10–100) minutes, which resulted in patients spending additional time in the infusion center. The majority of patients with an IRR (88%) required supportive medications, and all patients had resolution of symptoms. The most common supportive medications administered to manage trastuzumab IRRs were diphenhydramine, meperidine, and hydrocortisone.
Table 4.
Management of infusion-related reactions and subsequent therapy
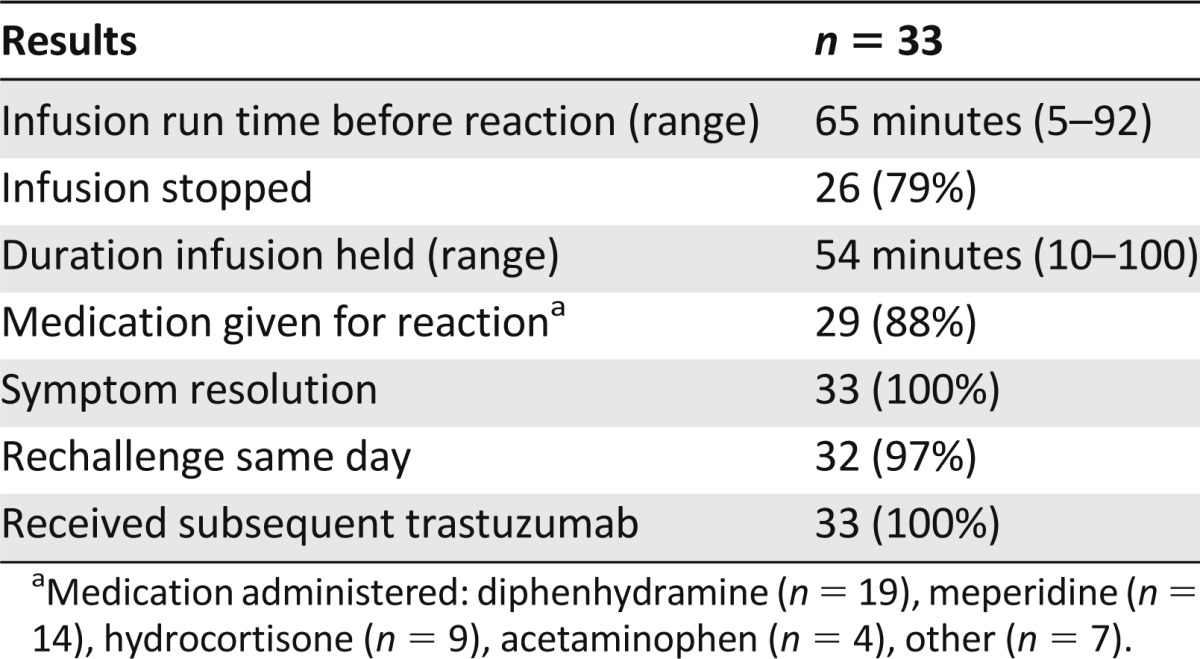
Only one patient was not rechallenged with trastuzumab on the same day, and all patients received subsequent doses of trastuzumab therapy. Twenty of the 32 patients (63%) received premedications before their subsequent trastuzumab dose. However, administration of these premedications was coincidental in all but two patients. Only one patient had an IRR to a subsequent trastuzumab dose, and this patient did not receive premedication before this infusion.
The majority of patients received weekly trastuzumab at 4 mg/kg for 90 min, followed by 2 mg/kg for 30 min (Table 2). Additionally, all loading doses were administered for 90 minutes (n = 197, 100%), and the vast majority of maintenance doses were administered for 30 minutes (n = 1,573, 98%). Twelve IRRs occurred during an 8 mg/kg loading dose administered for 90 minutes (12 of 67, 18%), and 17 IRRs occurred during a 4 mg/kg loading dose administered for 90 minutes (17 of 126, 13%) (Table 3). Four reactions occurred during maintenance infusions of 2 mg/kg administered for 30 minutes (4 of 1,405, 0.3%), and no IRRs were documented with the 6 mg/kg maintenance dose (n = 190) in this cohort of patients.
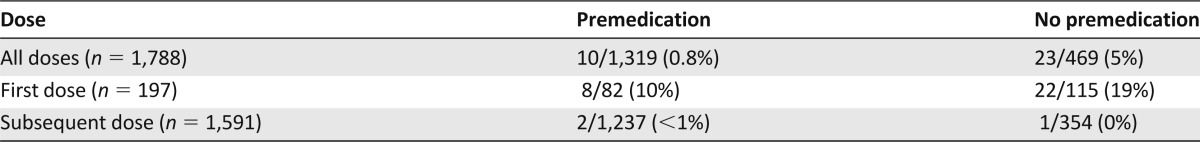
Although no orders were written for premedications to be administered before trastuzumab, 1,319 doses (74%) of trastuzumab were premedicated with drugs intended for prevention of adverse events as a result of the concurrent chemotherapy (e.g., taxane premedications administered before trastuzumab). Table 5 describes the relationship of IRRs with the administration of premedications. Of the doses that were premedicated, 10 IRRs were reported (0.8%, 10 of 1,319). In contrast, 23 IRRs were reported in the doses that were not premedicated (5%, 23 of 469). When evaluating the first dose of trastuzumab, IRRs occurred more commonly when patients did not receive a premedication compared with those who did (19% vs 10%, p = .065).
Table 5.
Infusion-related reactions with or without premedication
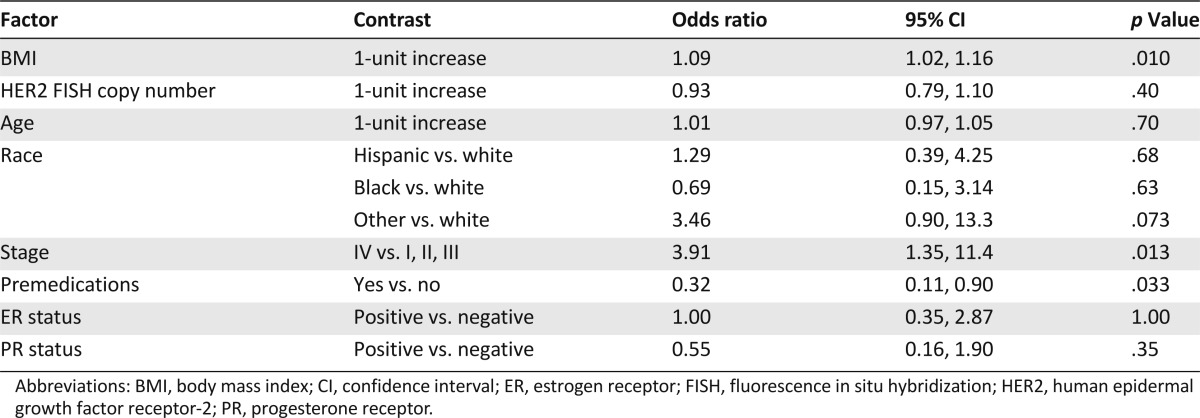
By univariate analysis, body mass index (BMI) (odds ratio per 1 unit increase = 1.05, 95% confidence interval [CI] 1.00, 1.11, p = .046) and stage of disease (stage IV vs other, odds ratio = 3.8, 95% CI 1.5, 9.6, p = .0074) were significantly associated with an increased risk of IRRs. When patients were separated into categories of BMI, patients who were obese (BMI ≥ 30, n = 82) had an increased risk of IRRs compared with patients in normal (18.5–24.9, n = 60) and overweight (25.0–29.9, n = 55) BMI groups (24%, 10%, and 7%, respectively, p = .0096). The univariate odds ratio for BMI > 29.9 compared with ≤29.9 was 3.4 (95% CI 1.5, 7.7, p = .0026). The odds ratio for BMI > 29.9 compared with ≤29.9 was 4.6 (95% CI 1.8, 12.3, p = .0024) after adjustment for the other study factors. Of all the factors evaluated, BMI, stage of disease, and use of premedications were significantly associated with IRRs by multivariate logistic regression analysis (Table 6).
Table 6.
Multivariate analysis of study factors and their relationship with infusion reactions
Discussion
In this retrospective analysis of breast cancer patients receiving trastuzumab, the overall incidence of trastuzumab-related IRRs was 16.2% of patients and 1.8% of doses, which appeared to be isolated to the first dose in all but three cases. Only one patient who had a trastuzumab-related IRR with the first infusion experienced a subsequent reaction. Reactions were mild to moderate in severity, and in this limited sample size there were no cases of anaphylaxis and/or pulmonary toxicity, emphasizing the rarity of such events. All patients had resolution of symptoms. Body mass index, stage, and use of premedications were significantly associated with IRRs by multivariate logistic regression analysis. To our knowledge, these associations have not been previously reported. The coincidental administration of premedications before trastuzumab loading doses (primarily intended for subsequent chemotherapy) decreased the first-dose IRRs from 19% to 10% (p = .065). Although this was not statistically significant by univariate analysis, it was significant by multivariate analysis after adjusting for confounding factors (p = .033) and is likely to be clinically relevant, although this requires prospective confirmation. Additionally, IRRs with maintenance doses did not appear to be dose-related and were rare; thus, the impact of premedications on maintenance doses could not be ascertained.
Regarding the influence of trastuzumab dose and duration of infusion on the incidence of trastuzumab-related IRRs, this retrospective study demonstrated slightly higher rates of IRRs with the 8 mg/kg loading dose compared with the 4 mg/kg loading dose, demonstrating a slight dose response similar to the previously mentioned results from Vogel et al. [7]. It should be noted that the number of patients who received the 8 mg/kg loading dose in our analysis was small. Of note, the administration of higher maintenance doses (e.g., 6 mg/kg) for 30 minutes did not appear to be associated with increased rates of IRRs, consistent with the Chan and Ring data [9, 10] discussed previously.
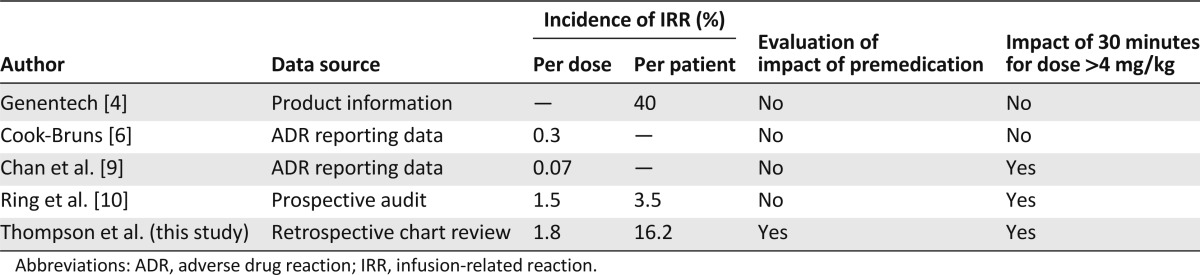
In reviewing the available literature, the methodology used to study the incidence of IRRs appears to impact reported rates of reactions. The overall incidence of IRRs in this retrospective study is lower than that reported in the trastuzumab product information, which is based on prospective clinical trial data [4]. However, it is higher than that reported via ADR databases (Table 7) [6, 9]. This phenomenon was also noted when our institutional ADR database was queried and represents a well-known reporting bias that underemphasizes the potential impact of mild-to-moderate expected adverse reactions on patient outcomes.
Table 7.
Comparison with published literature
Although not life-threatening, mild-to-moderate reactions negatively impact patients’ quality of life. Patients who experience IRRs associated with any therapy will experience adverse outcomes related to both the reaction itself and the medications used to manage the reaction. In our study, approximately 17% of patients experienced an IRR, and 88% of those patients required supportive medications. These management strategies could lead to additional adverse effects (e.g., sedation from antihistamines and/or meperidine, flushing from high-dose corticosteroids). Although we were unable to retrospectively assess patient outcomes related to these reactions, it is expected that patients who experience an IRR have some reduction in quality of life.
Although we did not capture patients’ actual length of stay in the infusion center, patients who had IRRs required dose interruptions that averaged 54 minutes (range 10–100 minutes), extending their chair time in the infusion center and potentially delaying other patients from receiving their scheduled treatments. These interruptions also impact chair time and the ability to move patients through the center, often requiring additional medications, supplies, and/or clinical staff time that ultimately create a financial burden on the health care system.
Premedications are commonly administered before many chemotherapy regimens to reduce IRRs but also to prevent other adverse events (e.g., nausea/vomiting, fluid retention, etc.). One interesting finding of this study was the influence of these chemotherapy premedications on the incidence of trastuzumab-associated IRRs. Because premedications required for taxanes are commonly administered 30 minutes before chemotherapy, these same premedications have the potential to be administered before a maintenance dose of trastuzumab. In the interest of patient convenience, this practice was commonly, yet inconsistently, applied in this analysis. To date, few published trials addressed the administration of premedications or their impact on outcomes related to trastuzumab. Although it is not standard practice to premedicate trastuzumab, coincidental administration of chemotherapy premedications before trastuzumab was observed (n = 1,319, 74%). This occurred more frequently with maintenance doses (n = 1,237, 78%) administered for 30 minutes. Premedication was less common with loading doses (n = 82, 42%), possibly because of longer trastuzumab infusion times (90 minutes), which prolong the time between premedications and chemotherapy administration. Nonetheless, the administration of premedications before trastuzumab maintenance doses appears to reduce the rates of IRRs and may impact overall patient outcomes in this small, retrospective analysis. Although the majority of these reactions are mild and short-lived, routine premedication of trastuzumab in a large, high-volume, infusion center may also indirectly reduce overall chair time, improving infusion center efficiency and throughput.
Although these results appear to support the hypothesis that manipulating the timing of ordered premedications may be beneficial, little is known regarding the addition of premedications to regimens that do not require them, such as trastuzumab monotherapy. Adding unnecessary premedications may add side effects, such as sedation from antihistamines, which can also adversely affect length of stay in the infusion center and patients’ quality of life. Lastly, the impact of administering the chemotherapy (primarily taxane) premedications before the trastuzumab infusion should be evaluated further to ensure adequate protection from taxane-associated IRRs. Therefore, this finding of a decreased incidence of IRRs with premedication should be considered hypothesis generating, and prospective validation is necessary before routine use of premedications with trastuzumab.
The finding that patients with an increased BMI experienced increased IRRs is also intriguing. Clinical outcomes related to obesity in patients with HER2-positive breast cancer receiving trastuzumab are limited, and no study to date has evaluated the effect of BMI on the rate of trastuzumab IRRs [12, 13]. Whether the increased incidence of IRRs in patients with increased BMI in our study is related to a larger dose of trastuzumab because of increased weight or other underlying mechanisms is unclear and warrants further investigation.
Compared with patients with early stage or locally advanced breast cancer (stages I–III), patients with metastatic disease (stage IV) experienced a significant increase in IRRs. The cause for this association is unknown, but may be related to the increased tumor burden and resulting increased number of HER2 binding sites in metastatic patients. It could be postulated that the greater the number of HER2-positive tumor cells, the more cytokine release and/or recruitment of immune effector cells, which may lead to higher rates of IRRs. The study by Baselga et al. [8] discussed above found an inverse relationship between trastuzumab serum concentrations and the number of metastatic sites of disease, which may suggest more trastuzumab is bound to its therapeutic target in patients with a higher tumor burden. Other data supporting this hypothesis, which may be extrapolated from use of other monoclonal antibodies, include the observation that increased circulating lymphocytes have been correlated with increased severity of IRRs with rituximab in patients with B cell chronic lymphocytic leukemia [3]. The association of disease stage and trastuzumab-related IRRs appears to be multifactorial and warrants further investigation.
The therapeutic target of trastuzumab is the extracellular domain of the HER2 protein that can be measured through immunohistochemistry (IHC) and is subjective. The FISH analysis of gene copy number has been more closely correlated with response to trastuzumab compared with IHC [14]. In this analysis, the HER2 FISH copy number was not correlated with the incidence of IRRs. This finding may indicate that the HER2 FISH copy number is not always reflective of extracellular protein expression. Unfortunately, measurement of the HER2 extracellular domain (ECD) in circulation was not available in this retrospective analysis. It would be interesting to determine whether the correlation seen with circulating lymphocytes and rituximab may also be exhibited with circulating HER2 ECD and trastuzumab.
The risk factors for IRRs listed in Table 6 may provide a framework to identify patients at a sufficiently high risk to require prophylaxis. For example, 55% of patients who were obese and had metastatic disease (n = 11) experienced an IRR compared with 7% of patients who were not obese and were treated for an early stage or locally advanced breast cancer (n = 100). Patients with obesity but not advanced stage (n = 71) and those with advanced stage but not obesity (n = 15) had an intermediate risk of IRRs (20% for both groups). Prospective evaluation is needed to determine whether prophylaxis would be beneficial in patients at high risk for IRRs.
A potential confounder in this study is the inability to discriminate between trastuzumab IRRs and IRRs from other chemotherapies (e.g., taxanes, carboplatin). This was minimized by only capturing reactions that occurred during the trastuzumab infusion or immediately after the infusion. Additionally, reactions to other chemotherapy agents generally occur within minutes of infusion initiation, whereas most trastuzumab IRRs occur during or within 2 hours of trastuzumab administration [3]. Another potential limitation of this study was that patients were not followed for the entire course of trastuzumab therapy, which can be lifelong. IRRs are rarely associated with infusions other than the loading dose, a fact confirmed with our data. Furthermore, because of the retrospective nature of this study, reactions that occurred after the patients left the infusion center were not captured, limiting the ability to evaluate rare, delayed pulmonary toxicity that may have occurred.
Conclusion
In summary, this retrospective review found the incidence of mild-to-moderate IRRs with trastuzumab to be lower than what is reported in the product information, but higher than what is reported utilizing ADR database queries. This study also further supports the safety of 30-minute maintenance trastuzumab infusions regardless of dose and the potential benefit of premedicating trastuzumab. We have also identified previously unknown risk factors associated with the development of trastuzumab-associated IRRs, specifically obesity and advanced stage of breast cancer, which may allow for identification of patients at high risk for IRRs who could potentially benefit from premedications, although prospective validation of this hypothesis is needed. Whereas the mechanisms behind the associations among BMI, stage, and rate of IRRs are unknown, these findings contribute to the existing body of literature with regard to defining the incidence, risk factors, and management of IRRs. Further evaluation is warranted with regard to the safety and efficacy of utilizing premedications before all trastuzumab loading doses and utilization of risk stratification to identify patients at the highest risk for IRRs.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
We acknowledge Erika Brown, Pharm.D., for assistance with data collection.
This study was previously presented in part at the Hematology Oncology Pharmacy Association 7th Annual Conference, Salt Lake City, UT, March 2011.
Author Contributions
Conception/Design: Lisa M. Thompson, Laura B. Michaud, Francisco J. Esteva, Chad M. Barnett
Provision of study material or patients: Lisa M. Thompson, Laura B. Michaud, Francisco J. Esteva, Chad M. Barnett
Collection and/or assembly of data: Lisa M. Thompson, Karen Eckmann, Bonnie L. Boster, Laura B. Michaud, Chad M. Barnett
Data analysis and interpretation: Lisa M. Thompson, Karen Eckmann, Bonnie L. Boster, Kenneth R. Hess, Laura B. Michaud, Francisco J. Esteva, Chad M. Barnett
Manuscript writing: Lisa M. Thompson, Gabriel N. Hortobágyi, Laura B. Michaud, Chad M. Barnett
Final approval of manuscript: Lisa M. Thompson, Gabriel N. Hortobágyi, Karen Eckmann, Bonnie L. Boster, Kenneth R. Hess, Laura B. Michaud, Francisco J. Esteva, Chad M. Barnett
Disclosures
Gabriel N. Hortobágyi: Amgen, Antigen Express, Galena, Genentech, Novartis (C/A); Novartis (R/F). The other authors indicated no financial relationships.
Section Editors: Kathleen Pritchard: Novartis, Roche, AstraZeneca, Pfizer, Boehringer-Ingelheim, GlaxoSmithKline, Sanofi, Ortho-Biotech, Amgen, Bristol-Myers Squibb (C/A); (H) Reviewer “A”: Genentech (C/A); (RF) Reviewer \x{201c}B\x{201d}: None Reviewer “C”: Genentech (C/A)
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Ross JS, Slodkowska EA, Symmans WF, et al. The HER-2 receptor and breast cancer: Ten years of targeted anti-HER-2 therapy and personalized medicine. The Oncologist. 2009;14:320–368. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 2.Pichler WJ. Adverse side-effects to biological agents. Allergy. 2006;61:912–920. doi: 10.1111/j.1398-9995.2006.01058.x. [DOI] [PubMed] [Google Scholar]
- 3.Chung CH. Managing premedications and the risk for reactions to infusional monoclonal antibody therapy. The Oncologist. 2008;13:725–732. doi: 10.1634/theoncologist.2008-0012. [DOI] [PubMed] [Google Scholar]
- 4.Herceptin [Package Insert]. South San Francisco, CA: Genentech, Inc.; October 2010. http://www.gene.com/download/pdf/herceptin_prescribing.pdf Accessed May 23, 2013.
- 5.Lenz HJ. Management and preparedness for infusion and hypersensitivity reactions. The Oncologist. 2007;12:601–609. doi: 10.1634/theoncologist.12-5-601. [DOI] [PubMed] [Google Scholar]
- 6.Cook-Bruns N. Retrospective analysis of the safety of Herceptin immunotherapy in metastatic breast cancer. Oncology. 2001;61(suppl 2):58–66. doi: 10.1159/000055403. [DOI] [PubMed] [Google Scholar]
- 7.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 8.Baselga J, Carbonell X, Castañeda-Soto NJ, et al. Phase II study of efficacy, safety, and pharmacokinetics of trastuzumab monotherapy administered on a 3-weekly schedule. J Clin Oncol. 2005;23:2162–2171. doi: 10.1200/JCO.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Chan AS, Freeswick S, Sklarin NT. Trastuzumab 6 mg/kg and 4 mg/kg can be infused safely over 30 minutes. Cancer Res 2009;69(2 Suppl):Abstract nr 3150.
- 10.Ring A, Simcock R, Mitra S, et al. Infusion of trastuzumab maintenance doses over 30 minutes. Ann Oncol. 2008;19:1509–1510. doi: 10.1093/annonc/mdn390. [DOI] [PubMed] [Google Scholar]
- 11.National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v 4.03. Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm Accessed September 8, 2010.
- 12.Parolin V, Fiorio E, Mercanti M et al. The prognostic and predictive impact of BMI on clinical outcome of HER2-positive breast cancer. J Clin Oncol (Meeting Abstracts) May 2010 vol. 28 no. 15_suppl 1130. [Google Scholar]
- 13.Crozier JA, Moreno-Aspitia A, Ballman KV, et al. Correlation between BMI and clinical outcome of patients with early stage HER2+ breast cancer from the N9831 clinical trial. Cancer. 2013 [E-pub ahead of print] [Google Scholar]
- 14.Sauter G, Lee J, Bartlett JM, et al. Guidelines for human epidermal growth factor receptor 2 testing: Biologic and methodologic considerations. J Clin Oncol. 2009;27:1323–1333. doi: 10.1200/JCO.2007.14.8197. [DOI] [PubMed] [Google Scholar]


