The main objective of this analysis was to examine the relationship between location of residence and mortality in patients with diffuse large B-cell lymphoma diagnosed and treated in British Columbia, Canada. Findings show that rural patients have similar survival to those in large urban areas, whereas patients living in small and medium urban areas experience worse outcomes.
Keywords: Lymphoma, Disparities, Cancer care quality, Access to care
Abstract
Background.
We examined the relationship between location of residence at the time of diagnosis of diffuse large B-cell lymphoma (DLBCL) and health outcomes in a geographically large Canadian province with publicly funded, universally available medical care.
Patients and Methods.
The British Columbia Cancer Registry was used to identify all patients 18–80 years of age diagnosed with DLBCL between January 2003 and December 2008. Home and treatment center postal codes were used to determine urban versus rural status and driving distance to access treatment.
Results.
We identified 1,357 patients. The median age was 64 years (range: 18–80 years), 59% were male, 50% were stage III/IV, 84% received chemotherapy with curative intent, and 32% received radiotherapy. There were 186 (14%) who resided in rural areas, 141 (10%) in small urban areas, 183 (14%) in medium urban areas, and 847 (62%) in large urban areas. Patient and treatment characteristics were similar regardless of location. Five-year overall survival (OS) was 62% for patients in rural areas, 44% in small urban areas, 53% in medium urban areas, and 60% in large urban areas (p = .018). In multivariate analysis, there was no difference in OS between rural and large urban area patients (hazard ratio [HR]: 1.0; 95% confidence interval [CI]: 0.7–1.4), although patients in small urban areas (HR: 1.4; 95% CI: 1.0–2.0) and medium urban areas (HR: 1.4; 95% CI: 1.0–1.9) had worse OS than those in large urban areas.
Conclusion.
Place of residence at diagnosis is associated with survival of patients with DLBCL in British Columbia, Canada. Rural patients have similar survival to those in large urban areas, whereas patients living in small and medium urban areas experience worse outcomes.
Implications for Practice:
Patients who reside in rural or distant communities may encounter difficulties accessing adequate cancer care. Those with potentially curable malignancies may experience worse outcomes if they are not diagnosed or treated in a timely fashion. This study cautions that health care disparities are difficult to foresee and abate, even in the setting of a public health care system. Health care providers should be sensitive to the various challenges that patients from small communities face during their cancer journeys and make all efforts possible to support and facilitate their care so that their outcomes are not compromised.
Introduction
The location of a person’s residence may influence their health outcomes by affecting access to certain diagnostic and/or treatment resources, traveling distance to health care, and logistical support. Several studies suggest that rural patients are diagnosed with solid tumors at more advanced stages [1–3], encounter greater challenges accessing specialized cancer care [1, 2, 4–10], and may experience worse survival than their urban counterparts [4–6, 11].
Limited data suggest that rural patients with different lymphoma subtypes may experience adverse outcomes [12–14]. Patients with curable aggressive lymphomas, such as diffuse large B-cell lymphoma (DLBCL), could be particularly vulnerable to the effect of place of residence because timely administration of therapy at appropriate dose intensity is considered vital for optimizing outcome.
In British Columbia (BC), Canada, patients with cancer benefit from publicly funded, universally available health care coverage as well as an extensive Community Oncology Network coordinated through the BC Cancer Agency (BCCA) and its main cancer centers [15]. It is unknown whether this system results in uniform care and outcomes for patients with lymphoma across the province. The main objective of this analysis was to examine the relationship between location of residence and mortality in patients with DLBCL diagnosed and treated in BC.
Patients and Methods
Patient Identification
The BC Cancer Registry (BCCR) was used to identify all patients 18–80 years old residing in BC at the time of diagnosis of DLBCL. This database collects demographic and baseline characteristics for every patient with a new cancer diagnosis in BC as well as mortality information through linkage with provincial vital statistics data [16].
Patients with DLBCL not otherwise specified and primary mediastinal large B-cell lymphoma diagnosed between January 1, 2003, and December 31, 2008, were included. Patients with primary central nervous system lymphoma were excluded.
Baseline characteristics including stage, performance status, and DLBCL subtype were available for the majority of patients in the BCCR. Data were supplemented by linking the dataset to the BCCA Lymphoid Cancer Database, which contains additional baseline, treatment, and follow-up data for most lymphoma patients in BC.
Location of Residence at Diagnosis
The postal code of each subject’s home address at the time of diagnosis was used to determine additional socioeconomic and demographic characteristics. The Statistics Canada Postal Code Conversion File, which links postal codes to 2006 census geographic area data, was used to determine whether each postal code corresponded to a rural or urban area [17]. An urban area was defined as having a population of at least 1,000 and a population density of ≥400 people per square kilometer. Urban areas were further subdivided into small (population of 1,000–29,999), medium (population of 30,000–99,999), and large (population ≥100,000). All areas with <1,000 people were considered rural. These cutoffs are based on existing conventions because they constitute the standard definitions used by Statistics Canada for census and population statistics purposes [18].
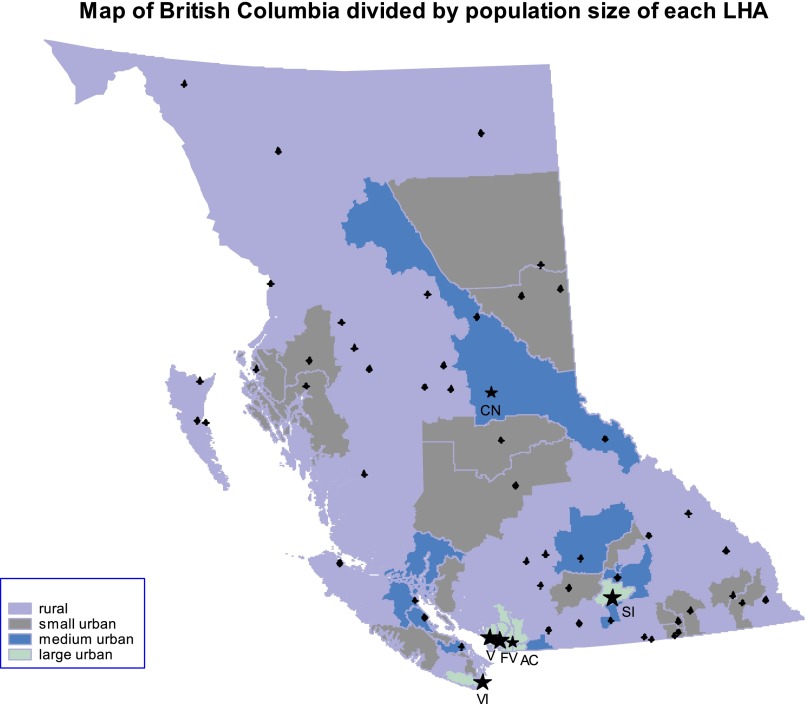
The geographic distribution of urban and rural areas across the province of BC is shown graphically in Figure 1 by mapping the coordinates of each patient’s postal code to its corresponding local health authority (LHA) code. During the study period, the province of BC was divided into 89 LHAs. Using the population size and density definitions described, each LHA was then categorized as rural, small urban, medium urban, or large urban. LHAs were used only to construct Figure 1 and do not constitute part of any other analysis.
Figure 1.
Map of British Columbia showing the geographic distribution of rural, small urban, medium urban, and large urban areas by local health authorities. The four BC Cancer Agency centers—Vancouver Island, Vancouver, Southern Interior, and Fraser Valley—are represented by asterisks, and cities are represented by circles on the map. Two additional BC Cancer Agency centers, Abbotsford and Center for the North, opened after the study period.
Abbreviations: AC, Abbotsford; CN, Center for the North; FV, Fraser Valley; SI, Southern Interior; V, Vancouver; VI, Vancouver Island.
Postal codes were also linked to neighborhood income data from the 2006 Canadian census to estimate median household income, as derived from income tax returns during that particular year [19].
Treatment Type, Location, and Distance
The following three treatments were evaluated for each patient: chemotherapy, radiotherapy, and stem cell transplantation (SCT). Chemotherapy utilization was obtained using the BCCA Provincial Pharmacy Database, a centralized database containing systemic therapy information for all cancer patients in BC. During the study period, 2003–2008, the standard chemotherapy regimen recommended for the treatment of patients with DLBCL was CHOP-R (cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab). The Cancer Agency Information System was used to identify patients who received radiotherapy or hematopoietic SCT at any point in their disease course.
The corresponding catchment chemotherapy and radiotherapy centers for a given postal code were identified in the BCCR. During the study period, there were 38 chemotherapy and four radiotherapy centers throughout the province [15]. All hematopoietic SCTs in BC were performed through the Leukemia/Bone Marrow Transplantation Program of BC at Vancouver General Hospital. The driving distances in kilometers between patients’ home postal codes and that of the catchment treatment centers for each modality were calculated using Google Maps or MapQuest (if the former was not informative).
Study Endpoints
The primary endpoint of the study was overall survival (OS), calculated from the date of diagnosis to the date of death from any cause. As a secondary endpoint, disease-specific survival (DSS) was calculated from the date of diagnosis to the date of death from lymphoma or treatment-related complications; unrelated deaths were censored. Because relapse data were not available for all patients, progression-free survival was not calculated.
Statistical Analysis
Baseline characteristics and type of location at diagnosis were assessed using contingency tables. Categorical variables were compared with Pearson’s chi-square test, and continuous variables were compared with the Kruskal-Wallis test. Continuous variables such as driving distance to the treatment centers were further grouped into binary variables using the Akaike information criterion test [20]. The following driving distance cutoffs were determined: 100 km, 200 km, and 200 km to chemotherapy, radiation therapy, and transplant center, respectively.
OS and DSS were estimated with the Kaplan-Meier method and compared across rural and urban areas using the log-rank test [21]. Multivariate Cox proportional hazards regression analysis was performed by including variables with p < .40 in univariate analyses into the model, followed by backward stepwise elimination. The assumption of proportionality was tested for the Cox models. Variables with p < .05 were considered statistically significant in the final models.
Statistical analysis was performed using SPSS 14.0 (IBM Corp., Armonk, NY, http://www-01.ibm.com/software/analytics/spss/), SAS 9.3 (SAS Institute, Inc., Cary, NC, http://www.sas.com), and R 2.15.0 (R Foundation for Statistical Computing, Vienna, Austria, http://www.r-project.org). The study was approved by the University of British Columbia/BCCA research ethics board.
Results
Patient and Disease Characteristics
A total of 1,440 patients diagnosed with DLBCL between 2003 and 2008 were identified in the BCCR, of whom 83 had primary central nervous system lymphoma and were excluded. The final cohort was composed of 1,357 patients, of whom 186 (14%) resided in a rural location at diagnosis and 1,171 (86%) resided in an urban setting: 141 (10%) in a small urban area, 183 (14%) in a medium urban area, and 847 (62%) in a large urban area. Figure 1 shows their geographic distribution across the province of BC.
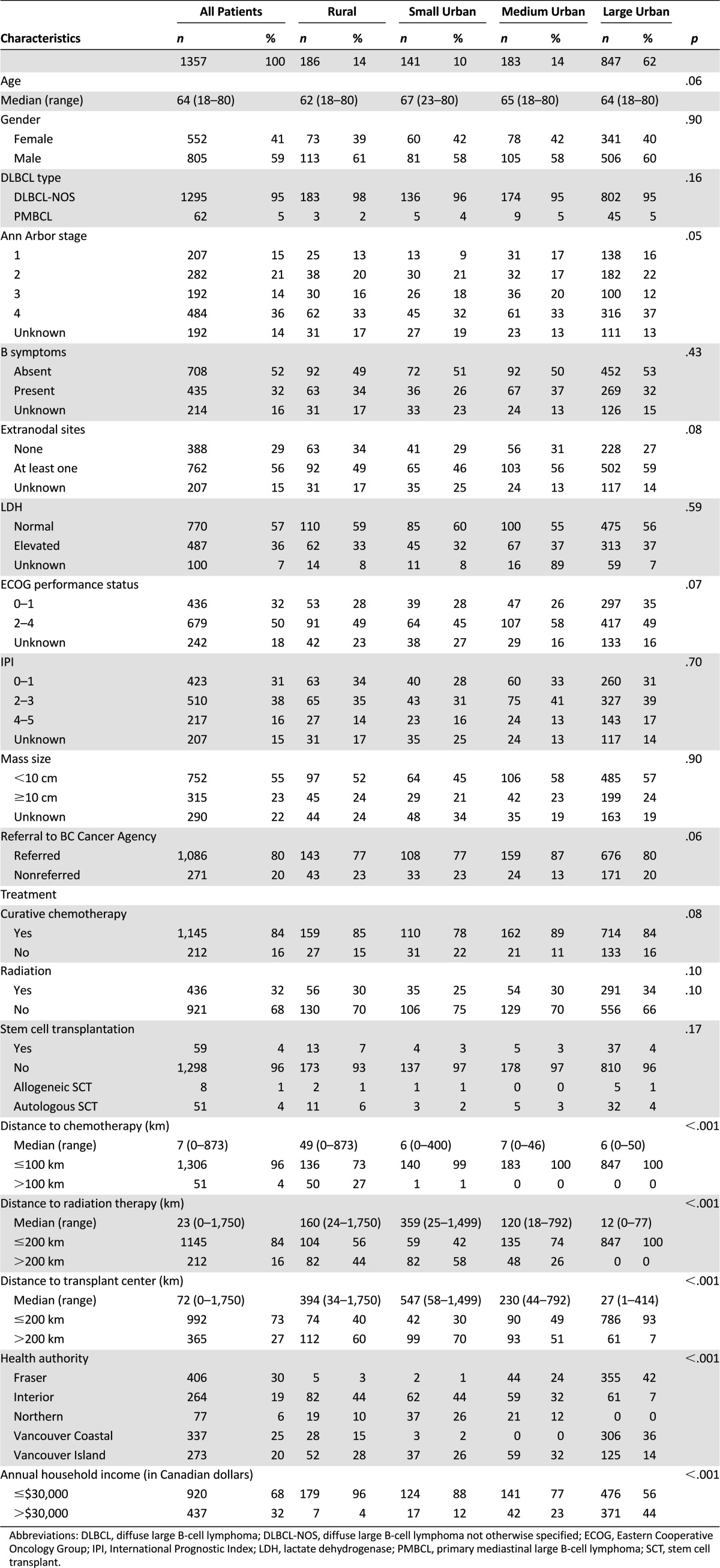
Table 1 shows that the age, gender, DLBCL subtype, stage, presence of B symptoms (weight loss, fever, night sweats), involvement of extranodal sites, lactate dehydrogenase (LDH), International Prognostic Index (IPI), and mass size distributions at diagnosis were similar across rural and urban areas, although prognostic factors were unknown for 15% of patients. Approximately 80% of patients were referred to the BCCA for management, although there was a trend toward lower referral rates for those in rural and small urban areas (p = .06).
Table 1.
Baseline characteristics
Treatment Characteristics
Table 1 shows that the majority of patients (84%) received combination chemotherapy with curative intent: 1,101 received CHOP-R, 43 received CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), and 1 received the Magrath protocol [22]. The majority of patients who received CHOP alone were treated in 2003, the year when rituximab was introduced in BC as an addition to CHOP as the standard of care for the curative treatment of patients with DLBCL. Eleven patients refused treatment, and 201 patients received palliative systemic or radiation therapy without combination chemotherapy.
There was a trend toward a difference in administration of curative chemotherapy across groups, with the lowest in small urban areas at 78% (p = .08). Radiotherapy was given to 32% of patients at any point in their treatment course, with no difference across urban and rural areas. A minority of patients (4%) received high-dose chemotherapy and SCT, of which the majority were autologous SCTs, with no significant difference across subgroups, although numbers are small (p = .17).
Distance to Treatment
Rural patients faced the longest driving distances to access treatment, including >100 km for chemotherapy in 27% patients, >200 km for radiotherapy in 44%, and >200 km for SCT in 60% of patients (Table 1). Patients in urban areas did not experience driving distances >100 km for chemotherapy with the exception of one patient. However, a significant proportion of patients in small and medium urban areas faced driving distances >200 km to access radiotherapy (58% small urban, 26% medium urban) and SCT (70% small urban, 51% medium urban). Patients in large urban areas did not experience long driving distances for radiotherapy, although 7% of patients in large urban areas outside of Vancouver faced a >200 km distance to access SCT.
Outcomes
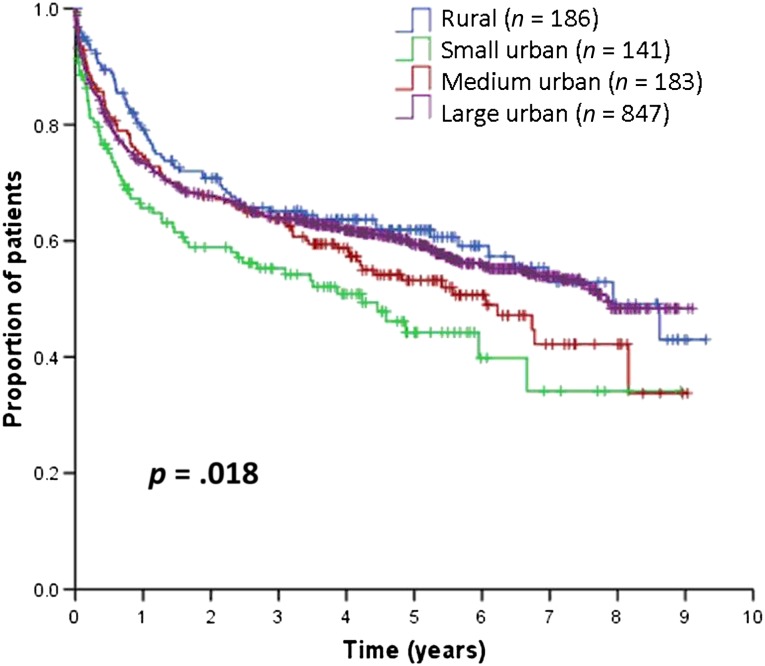
With a median follow-up of 4.9 years (range: 0–9.3 years) for living patients, the 5-year OS for the entire cohort was 60% (95% confidence interval [CI]: 56%–63%). Figure 2 and Table 2 show that 5-year OS was 62% for patients in rural areas, 44% in small urban areas, 53% in medium urban areas, and 60% in large urban areas (p = .018). The 5-year OS rate for all urban areas combined was 57%, which was not significantly different from that of rural areas (p = .208). Compared with patients in large urban areas, those in small urban areas experienced significantly worse OS, followed by patients in medium urban areas.
Figure 2.
Overall survival.
Table 2.
Five-year survival estimates
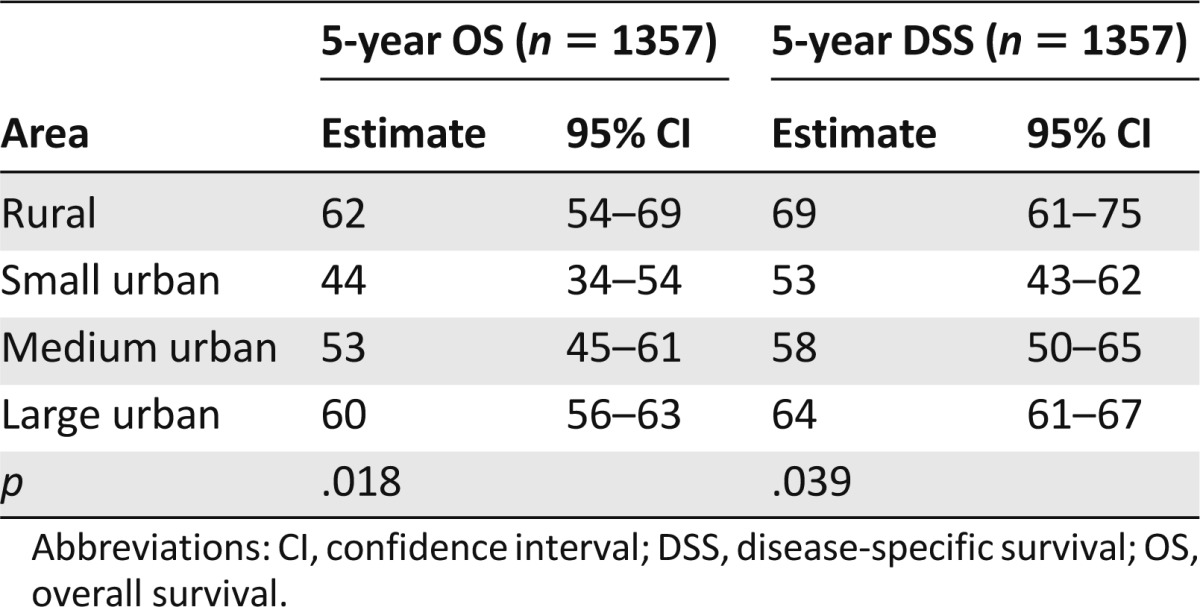
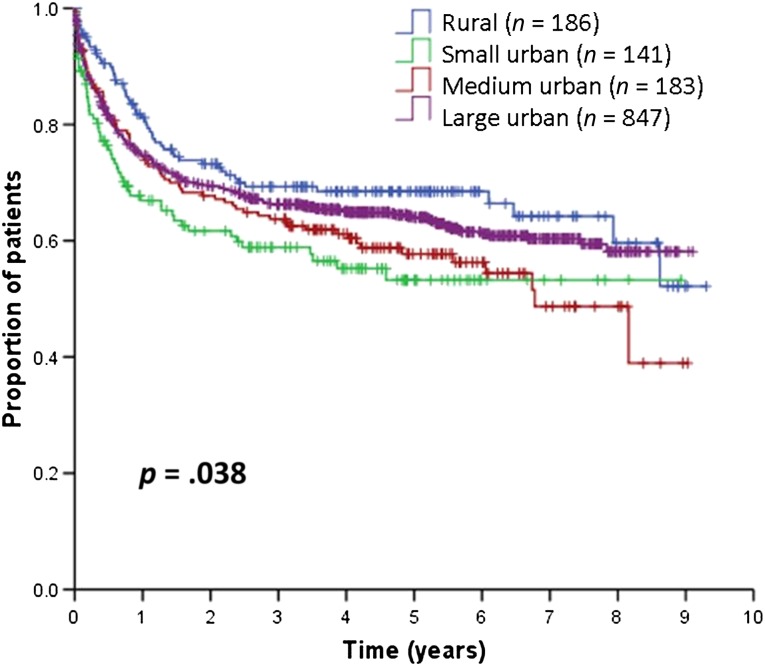
The 5-year DSS was 64% (95% CI: 61%–67%) for the entire cohort. Figure 3 and Table 2 show that the 5-year DSS was 69% for patients in rural areas, 53% in small urban areas, 58% in medium urban areas, and 64% in large urban areas (p = .039). The 5-year DSS of all urban areas combined was 62%, which was not significantly different from that of rural areas (p = .092). Again, patients in small urban areas experienced worse DSS, followed by those in medium urban areas.
Figure 3.
Disease-specific survival.
Causes of Death
The majority of patients died of lymphoma-related causes: 31% in rural areas, 41% in small urban areas, 45% in medium urban areas, and 40% in large urban areas. These included treatment-related mortality in 1% of rural patients, 4% of small urban area patients, 2% of medium urban area patients, and 2% of large urban area patients. Unrelated causes of death occurred in 8% of rural patients, 8% of small urban area patients, 5% of medium urban area patients, and 6% of large urban patients. Causes of death did not differ across subgroups (p = .26).
Multivariate Analysis
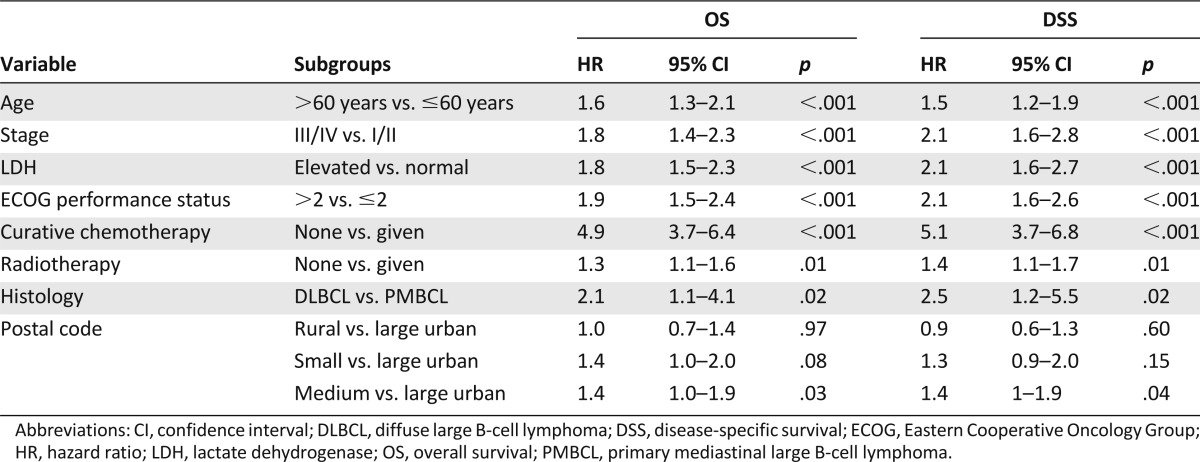
The following variables were selected for multivariate analysis: age, gender, stage, B symptoms, performance status, IPI group, presence of extranodal sites, elevated LDH, referral to BCCA, curative chemotherapy, radiotherapy, SCT, distance to treatments, and rural/urban area. Table 3 shows the effect of area of residence on OS and DSS, adjusted for prognostic factors in multivariate analysis. There was no difference in OS between rural and large urban area patients (hazard ratio [HR]: 1.0; 95% CI: 0.7–1.4, p = .97). Patients in small urban areas had borderline significantly worse OS compared with those in large urban areas (HR: 1.4; 95% CI: 1.0–2.0, p = .08). Patients in medium urban areas had worse OS compared with those in large urban areas (HR: 1.4; 95% CI: 1.0–1.9, p = .03). Effects were similar for DSS, although the difference between small and large urban areas was not significant (HR: 1.3; 95% CI: 0.9–2.0, p = .15). Distance to treatments, income, health authority, and BCCA referral did not have independent prognostic value in multivariate models.
Table 3.
Multivariate analyses
Discussion
In this population-based cohort of patients with DLBCL, the place of residence at diagnosis significantly affected health outcomes. Rural patients experienced similar OS and DSS as those in urban areas, particularly large urban areas; however, patients in small and medium urban areas experienced worse outcomes.
In a publicly funded health care system, it is reassuring that rural patients with DLBCL achieve outcomes similar to those in large urban areas. Rural patients do not appear to present with more advanced disease or adverse prognostic factors than their large urban counterparts, and both groups received similar rates of potentially curative therapy. In addition, referral rates to BCCA were similar between rural (77%) and large urban area (80%) patients. Following a diagnosis of aggressive lymphoma, patients in rural areas are often referred to their corresponding large urban catchment center, with subsequent prompt initiation of treatment. Moreover, access to professional expertise and treatment through the Communities Oncology Network may be particularly beneficial for patients living in rural areas. Although these hypotheses can be suggested, they cannot be verified with our data.
There are several possible reasons behind the observation that patients in small and medium urban areas experience worse outcomes. There were trends toward slightly older age, advanced stage, worse performance status, and lower administration of curative chemotherapy in patients from small and medium urban areas. Although these patients also faced long driving distances to access treatment and had lower income than patients in large urban areas, these factors did not have independent prognostic value in multivariate analysis. In addition, there was an imbalance of prognostic factors in the univariate and multivariate analyses because these data were not available for all patients and were particularly missing in rural and small urban areas, and in nonreferred patients. There may be other unmeasured factors accounting for these differences, such as wait times, individual physician or hospital performance, or other aspects of logistical support that may or may not be available to patients in smaller communities.
There are sparse data describing the effect of place of residence on the outcomes of patients with lymphoma. In the Canadian province of Manitoba, where a similar provincewide cancer control program is in place, a population-based study of all patients undergoing bone marrow transplantation for lymphoma failed to show a survival disadvantage for rural patients [23]. In a population-based, retrospective cohort of 2,330 patients with lymphoma from the U.S. state of Nebraska, patients from rural areas had inferior OS compared with those residing in urban areas [12]. In addition, rural patients treated in Nebraska were less likely to receive SCT as part of their treatment [13, 24]. Rural patients in the U.S. have been demonstrated to have increased mortality after autologous [14], but not necessarily following allogeneic, SCT [24].
The main strengths of the current study are the use of a population registry that captures all new cancer diagnoses across the province, entirely eliminating referral bias, and the equal access to sophisticated cancer care, regardless of socioeconomic status, available to patients in Canada. Importantly, treatment data are complete and accurate for all patients. We included patients treated between 2003 and 2008 because this era reflects the current treatment approach to DLBCL. By incorporating geographic and organizational aspects of the structure of the health care system, results can be generalized to our population.
This study has various limitations. First, the exposure (place of residence) was defined using a census definition of population areas with cutoffs that were not developed for this particular kind of study. Distances to treatment may have been a better way to define the exposure; however, they did not have independent prognostic value in multivariate Cox models when adjusting for clinical covariates. Second, some diagnoses of DLBCL may be inaccurate because the BCCR relies on local pathology reporting without central review, although misclassification for this histologic subtype is generally low (data not shown). Third, because of the retrospective study design, prognostic factor data were incomplete somewhat more frequently in rural and small urban areas, and in non-BCCA-referred patients.
The incompleteness of our prognostic factor data are strongly counterbalanced by the comprehensiveness of our case ascertainment and the completeness of our treatment data. Thus, we could validly demonstrate that small and medium urban area patients received less treatment. Patients from these two types of urban regions received both chemotherapy and radiation less frequently. The explanation for this discrepancy is not readily apparent, but this observation deserves further exploration.
Conclusion
Place of residence appears to influence health outcomes in patients with DLBCL across BC. This study identifies specific populations within the province that may benefit from further research and targeted resource investment.
Acknowledgments
This research project was made possible through dedicated funding to the first author (B.L.) through the 2012 American Society of Hematology Trainee Research Award and the 2012 BC Cancer Studentship. A preliminary analysis of the results reported in this paper was presented at the 12th International Conference on Malignant Lymphoma, June 19–22, 2013, Lugano, Switzerland.
Author Contributions
Conception/Design: Diego Villa, Joseph Connors
Provision of study material or patients: Diego Villa, Joseph Connors, Laurie Sehn, Kerry Savage, Tamara Shenkier, Richard Klasa
Collection and/or assembly of data: Diego Villa, Benny Lee, Kevin Hay
Data analysis and interpretation: Diego Villa, Benny Lee, Ozge Goktepe, Joseph Connors, Laurie Sehn, Kerry Savage, Tamara Shenkier, Alina Gerrie
Manuscript writing: Diego Villa, Benny Lee, Ozge Goktepe, Kevin Hay, Joseph Connors, Laurie Sehn, Kerry Savage, Tamara Shenkier, Richard Klasa, Alina Gerrie
Final approval of manuscript: Diego Villa, Benny Lee, Ozge Goktepe, Kevin Hay, Joseph Connors, Laurie Sehn, Kerry Savage, Tamara Shenkier, Richard Klasa, Alina Gerrie
Disclosures
Diego Villa: Roche Canada (RF); Alina Gerrie: Roche Canada (H); Roche Canada, Lundbeck Canada (RF); Kerry Savage: Roche (RF); Roche (Other); Kerry Savage: Roche (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Elliott TE, Elliott BA, Renier CM, et al. Rural-urban differences in cancer care: Results from the Lake Superior Rural Cancer Care Project. Minn Med. 2004;87:44–50. [PubMed] [Google Scholar]
- 2.Olson RA, Nichol A, Caron NR, et al. Effect of community population size on breast cancer screening, stage distribution, treatment use and outcomes. Can J Public Health. 2012;103:46–52. doi: 10.1007/BF03404068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parikh-Patel A, Bates JH, Campleman S. Colorectal cancer stage at diagnosis by socioeconomic and urban/rural status in California, 1988-2000. Cancer. 2006;107(Suppl):1189–1195. doi: 10.1002/cncr.22016. [DOI] [PubMed] [Google Scholar]
- 4.Baade PD, Youlden DR, Coory MD, et al. Urban-rural differences in prostate cancer outcomes in Australia: What has changed? Med J Aust. 2011;194:293–296. doi: 10.5694/j.1326-5377.2011.tb02977.x. [DOI] [PubMed] [Google Scholar]
- 5.Coory MD, Baade PD. Urban-rural differences in prostate cancer mortality, radical prostatectomy and prostate-specific antigen testing in Australia. Med J Aust. 2005;182:112–115. doi: 10.5694/j.1326-5377.2005.tb06609.x. [DOI] [PubMed] [Google Scholar]
- 6.Craft PS, Buckingham JM, Dahlstrom JE, et al. Variation in the management of early breast cancer in rural and metropolitan centres: Implications for the organisation of rural cancer services. Breast. 2010;19:396–401. doi: 10.1016/j.breast.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 7.Kok DL, Chang JH, Erbas B, et al. Urban-rural differences in the management of screen-detected invasive breast cancer and ductal carcinoma in situ in victoria. ANZ J Surg. 2006;76:996–1001. doi: 10.1111/j.1445-2197.2006.03917.x. [DOI] [PubMed] [Google Scholar]
- 8.Markossian TW, Hines RB. Disparities in late stage diagnosis, treatment, and breast cancer-related death by race, age, and rural residence among women in Georgia. Women Health. 2012;52:317–335. doi: 10.1080/03630242.2012.674091. [DOI] [PubMed] [Google Scholar]
- 9.Onega T, Duell EJ, Shi X, et al. Geographic access to cancer care in the U.S. Cancer. 2008;112:909–918. doi: 10.1002/cncr.23229. [DOI] [PubMed] [Google Scholar]
- 10.Tyldesley S, McGahan C. Utilisation of radiotherapy in rural and urban areas in British Columbia compared with evidence-based estimates of radiotherapy needs for patients with breast, prostate and lung cancer. Clin Oncol (R Coll Radiol) 2010;22:526–532. doi: 10.1016/j.clon.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Hines RB, Markossian TW. Differences in late-stage diagnosis, treatment, and colorectal cancer-related death between rural and urban African Americans and whites in Georgia. J Rural Health. 2012;28:296–305. doi: 10.1111/j.1748-0361.2011.00390.x. [DOI] [PubMed] [Google Scholar]
- 12.Loberiza FR, Jr, Cannon AJ, Weisenburger DD, et al. Survival disparities in patients with lymphoma according to place of residence and treatment provider: A population-based study. J Clin Oncol. 2009;27:5376–5382. doi: 10.1200/JCO.2009.22.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loberiza FR, Jr, Zhang MJ, Lee SJ, et al. Association of transplant center and physician factors on mortality after hematopoietic stem cell transplantation in the United States. Blood. 2005;105:2979–2987. doi: 10.1182/blood-2004-10-3863. [DOI] [PubMed] [Google Scholar]
- 14.Rao K, Darrington DL, Schumacher JJ, et al. Disparity in survival outcome after hematopoietic stem cell transplantation for hematologic malignancies according to area of primary residence. Biol Blood Marrow Transplant. 2007;13:1508–1514. doi: 10.1016/j.bbmt.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 15.BC Cancer Agency. Communities Oncology Network. Available at http://www.bccancer.bc.ca/RS/CommunitiesOncologyNetwork/default.htm Accessed March 20, 2013.
- 16.BC Cancer Agency. BC cancer statistics. Available at http://www.bccancer.bc.ca/HPI/CancerStatistics/default.htm#bccanreg Accessed March 20, 2013.
- 17.Statistics Canada. Postal code conversion file (PCCF). Available at http://www5.statcan.gc.ca/bsolc/olc-cel/olc-cel?catno=92F0153XCE&lang=eng Accessed November 7, 2013.
- 18.Statistics Canada. Definitions of “rural.” Available at http://www.statcan.gc.ca/pub/21-601-m/21-601-m2002061-eng.htm Accessed November 7, 2013.
- 19.BCStats. Income and taxation: Neighbourhood income and demographics, 2006. Available at http://www.bcstats.gov.bc.ca/statisticsbysubject/LabourIncome/OtherData/IncomeTaxation.aspx Accessed November 7, 2013.
- 20.Akaike H. Likelihood and the Bayes procedure. Trabajos de Estadistica Y de Investigacion Operativa. 1980;31:143–166. [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 22.Magrath I, Adde M, Shad A, et al. Adults and children with small non-cleaved-cell lymphoma have a similar excellent outcome when treated with the same chemotherapy regimen. J Clin Oncol. 1996;14:925–934. doi: 10.1200/JCO.1996.14.3.925. [DOI] [PubMed] [Google Scholar]
- 23.Paulson K, Lambert P, Bredeson C, et al. Does location matter? Rural vs urban outcomes after blood and marrow transplantation in a population-based Canadian cohort. Bone Marrow Transplant. 2010;45:1167–1173. doi: 10.1038/bmt.2009.332. [DOI] [PubMed] [Google Scholar]
- 24.Loberiza FR, Jr, Lee SJ, Klein JP, et al. Outcomes of hematologic malignancies after unrelated donor hematopoietic cell transplantation according to place of residence. Biol Blood Marrow Transplant. 2010;16:368–375. doi: 10.1016/j.bbmt.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]