This study assessed the feasibility of solid cancer treatment in older patients. Feasibility rates were considerably lower for chemotherapy than for surgery, radiotherapy, and hormonal therapy. Utilization of geriatric oncology resources may be optimized by referral of elderly cancer patients initially considered for chemotherapy to geriatric oncology clinics.
Keywords: Feasibility studies, Elderly, Cancer, Treatment, Chemotherapy
Abstract
Purpose.
To assess solid cancer treatment feasibility in older patients
Methods.
Between 2007 and 2010, 385 consecutive elderly patients (mean age: 78.9 ± 5.4 years; 47.8% males) with solid malignancies referred to two geriatric oncology clinics were included prospectively. We recorded feasibility of first-line chemotherapy (planned number of cycles in patients without metastases and three to six cycles depending on tumor site in patients with metastases), surgery (patient alive 30 days after successfully performed planned surgical procedure), radiotherapy (planned dose delivered), and hormonal therapy (planned drug dose given), and we recorded overall 1-year survival.
Results.
Main tumor sites were colorectal (28.6%), breast (23.1%), and prostate (10.9%), and 47% of patients had metastases. Planned cancer treatment was feasible in 65.7% of patients with metastases; this proportion was 59.0% for chemotherapy, 82.6% for surgery, 100% for radiotherapy, and 85.2% for hormonal therapy. In the group without metastases, feasibility proportions were 86.8% overall, 72.4% for chemotherapy, 95.7% for surgery, 96.4% for radiotherapy, and 97.9% for hormonal therapy. Factors independently associated with chemotherapy feasibility were good functional status defined as Eastern Cooperative Oncology Group performance status <2 (p < .0001) or activities of daily living >5 (p = .01), normal mobility defined as no difficulty walking (p = .01) or no fall risk (p = .007), and higher creatinine clearance (p = .04).
Conclusion.
Feasibility rates were considerably lower for chemotherapy than for surgery, radiotherapy, and hormonal therapy. Therefore, utilization of limited geriatric oncology resources may be optimized by preferential referral of elderly cancer patients initially considered for chemotherapy to geriatric oncology clinics.
Implications for Practice:
Among cancer treatment modalities, surgery, hormonal therapy, and radiotherapy were usually feasible in older patients seen in everyday practice. Feasibility was lowest for chemotherapy (72.4% and 59%, respectively, in the groups without and with metastasis). Functional status, mobility, metastasis status, and creatinine clearance were independently associated with chemotherapy feasibility, whereas age was not. Given the growing number of older patients with cancer, the limited number of geriatricians, and the time-consuming nature of the Comprehensive Geriatric Assessment, those older cancer patients considered for chemotherapy may benefit the most from referral to a geriatric oncology clinic before the final treatment decision.
Introduction
Cancer is among the leading causes of death in industrialized countries [1]. The incidence of cancer rises with age, with an 11-fold increase after age 65 compared with younger age groups [1], and more than 30% of all cancers are diagnosed in patients aged 75 years or older [2]. Cancer treatment strategies involve variable combinations of surgery, chemotherapy, targeted therapies, hormonal therapy, and radiotherapy in older patients. Although the treatment strategy is chiefly dictated by histology, tumor site, and tumor stage [3], individually tailored strategies are increasingly being used, most notably in older patients. Data from pivotal randomized controlled trials suggest similar feasibility and survival benefits of cancer treatment in younger and older patients [4–9]; however, the older patients included in these trials were usually selected based on good general health [10] with few comorbidities and good performance status [11]. Consequently, the general applicability of the trial findings to the overall population of older patients with cancer is in doubt. Several observational studies [12–16] showed that older patients with cancer were less often treated than their younger counterparts, the main reason being concern about side effects of treatment [16–18]. Furthermore, a few studies indicated that premature cancer treatment discontinuation was more common among older than middle-aged cancer patients and seemed associated with poorer outcomes [19, 20]. Accurately assessing discontinuation rates for each treatment modality would help to identify patient groups at high risk for early discontinuation. Several studies focused on the feasibility of chemotherapy, but only one concomitantly assessed the feasibility of the various treatment modalities in the same population [21]. In studies involving a Comprehensive Geriatric Assessment (CGA) at baseline, malnutrition, cognitive impairment [22], and comorbidities were independently associated with chemotherapy feasibility [23]. To our knowledge, a single study in elderly cancer patients (who had metastatic colorectal cancer) simultaneously evaluated associations linking oncologic and geriatric parameters to chemotherapy feasibility [24].
The objective of this study in older patients with solid cancer was to assess the frequency of treatment feasibility and to identify CGA factors associated with treatment feasibility.
Patients and Methods
Study Design
The Elderly Cancer Patient (ELCAPA) study is a prospective open-cohort survey of consecutive patients aged 70 years or older with histologically confirmed cancer who are referred by an oncologist, a radiotherapist, a surgeon, or other specialist to one of two geriatric oncology clinics in teaching hospitals in the Paris urban area of France. For the present analysis, ELCAPA-03, we included all ELCAPA patients with solid cancer for whom a treatment strategy was established during a multidisciplinary meeting between January 2007 and December 2010. Patients scheduled for supportive care only were not included. The study inclusion date was the date of the first geriatric oncology visit.
This observational study is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology statement [25].
Informed consent was obtained from all study patients prior to inclusion. The protocol was approved by the local ethics committee (Comité de Protection des Personnes Ile-de-France I, Paris, France).
Data Collection
The data were collected prospectively. At baseline, age, gender, tumor characteristics (site, metastatic status, and number and location of metastases), and pretreatment laboratory data (creatinine, albumin, and C-reactive protein) were recorded. Renal dysfunction was defined as creatinine clearance (estimated using the Cockcroft algorithm) <60 mL/minute and severe renal dysfunction as creatinine clearance <30 mL/minute [26]. Tumor sites were classified as follows: colorectal, breast, upper gastrointestinal tract and liver, prostate, urinary tract, and other.
At baseline, each patient underwent a CGA, as described in detail previously [27]. Briefly, the CGA uses validated tests and scores to assess nine domains, according to international recommendations [28, 29]: functional status (activities of daily living score [ADL] [30] and Eastern Cooperative Oncology Group performance status [ECOG-PS] [31]), mobility (timed get-up-and-go test and walking impairments [32]), nutritional status (Mini-Nutritional Assessment [MNA] [33]), cognitive status (Mini-Mental State Examination [MMSE]) [34], mood (Mini-Geriatric Depression Scale [mini-GDS] [35]), comorbidities (Cumulative Illness Rating Scale for Geriatrics [CIRS-G] [36]), polypharmacy (number of medications), social environment, and urinary and/or fecal incontinence. The social environment was evaluated based on marital status, use of homemaker services, and whether the patient lived alone. Polypharmacy was defined as the intake of five or more medications per day. After the geriatric oncology visit, a multidisciplinary meeting was held for each patient to plan the cancer treatment strategy.
Outcomes
The primary endpoint was feasibility of the planned cancer treatment, which was assessed prospectively using three separate data sources, namely, the paper and hospital electronic medical charts (MediWeb version 4.0, DIH-CHU Henri-Mondor, Créteil, France) and the hospital chemotherapy database (CHIMIO version 2.0). Each chemotherapy drug given during each cycle (name and dose) is recorded routinely and validated by the pharmacist in charge at the time of chemotherapy delivery in the oncology department. Reasons for nonfeasibility were recorded based on the medical charts. Chemotherapy toxicities were classified using Common Terminology Criteria for Adverse Events version 4.0 [37].
The cancer treatment plan was defined as the full treatment strategy chosen for each individual patient during the multidisciplinary meeting, based on tumor site and metastatic status. This strategy included one or more of the following modalities: chemotherapy, surgery, radiotherapy, and hormonal therapy. We assessed feasibility of the overall cancer treatment plan and each of its components.
Feasibility of surgery was defined as successful performance of the planned surgical procedure without mortality within the next 30 days [38]. For radiotherapy and hormonal therapy, feasibility was delivery of the initially planned dose selected during the multidisciplinary meeting. Chemotherapy feasibility was delivery of the planned number of cycles determined based on tumor site and metastatic status. The optimal duration of chemotherapy remains unclear [39, 40]. During the multidisciplinary meetings, the number of cycles selected was the number of cycles in standard chemotherapy regimens used in clinical practice in accordance with French guidelines [41]: at least 12 cycles for colorectal cancer [19] and at least 4 cycles for other tumor sites [22] in patients without metastases and at least 6 cycles for colorectal cancer and 3 cycles for other tumor sites in patients with metastases. For each patient, treatment feasibility was adjudicated by a panel of experts (two oncologists [S.C., C.T.], one radiation therapist [J.L.L.], one geriatrician [E.P.], and one pharmacist [M.C.V.]).
Overall 1-year mortality was recorded based on both the hospital medical charts and the public records office of each patient’s place of birth.
Statistical Analysis
Patient characteristics are described as number (percentage) for qualitative data and mean (±1 SD) or median (25th–75th percentile) for quantitative data. Baseline characteristics of study patients and patients lost to follow-up before the feasibility assessment were compared using the chi-square test, Fisher’s exact test, or nonparametric Mann-Whitney test, as appropriate.
Rate of Treatment Feasibility
Treatment feasibility rates were calculated with their 95% confidence intervals (CIs) estimated using normal or binomial distributions, as appropriate. The objective of cancer treatment could differ between patients with and without metastases; therefore, we evaluated these two groups separately.
In the subgroup of patients with metastases who were scheduled for chemotherapy, we conducted a sensitivity analysis by excluding patients with disease progression or refusal to continue chemotherapy [42].
CGA-Associated Factors
We conducted univariate and multivariate analyses to identify factors associated with treatment feasibility. Univariate analyses relied on the chi-square test or Fisher’s exact test for qualitative variables and the t test or nonparametric Wilcoxon-Mann-Whitney test for quantitative variables. Variables yielding p values <.15 by univariate analysis were entered into a multivariate logistic regression model. Adjusted odds ratios (ORs) with their 95% CIs were estimated. To ensure consistency with the descriptive analysis performed separately in the groups with and without metastases, we added an interaction term between metastatic status and variables reflecting general health (ECOG-PS, ADL, and mobility).
We did not perform multiple imputations for missing data. All tests were two-tailed. The p values <.05 were considered statistically significant. The data were analyzed using Stata statistical software version 11.0 (StataCorp, College Station, TX, http://www.stata.com).
Results
Data on treatment feasibility were available for 385 of the 421 eligible patients (Fig. 1). Compared with the study patients, patients lost to follow-up for the feasibility assessment (n = 36, 8.6%) were older and had worse functional status (mean age: 82.5 ± 4.9 years vs. 78.9 ± 5.4 years, p < .001; median ADL: 5.7 [range: 3.5–6] vs. 6 [range: 5.5–6], p < .001).
Figure 1.
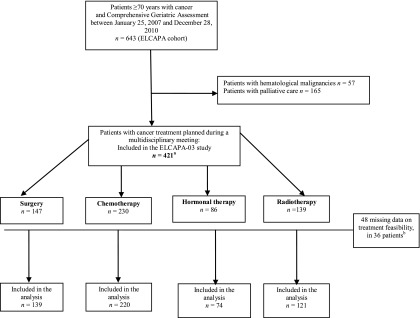
Patient flowchart for the ELCAPA-03 study. Data on treatment feasibility were available for 385 of the 421 patients eligible for recruitment into the ELCAPA cohort.
aSome patients received two or more treatment modalities and the sum of the patients in the four modality groups is therefore greater than 421.
bSome patients had missing data for two or more treatment feasibility.
Table 1 lists the main baseline characteristics of the 385 patients, most of whom had no functional impairments (79% with ADL >5) or cognitive impairments (87.7% with MMSE ≥24 of 30). The most common comorbidities were renal dysfunction (n = 208, 54.0%), which was severe in 28 patients (13.5%); hypertension (n = 240, 62.3%); and diabetes mellitus (n = 83, 21.6%). A total of 15 different cancer treatment plans were identified (Table 2). Chemotherapy was scheduled for 220 patients, alone in 143 (65%) and combined with another treatment in 77 (35%). Among the 220 patients given chemotherapy, 9 received targeted therapies, which were used alone in 5 patients and combined in 4 patients. Median follow-up was 13.4 months (range: 5.3–24.6). Overall 1-year mortality was 11.3% (23 patients) in the group without metastases and 42.0% (76 patients) in the group with metastases.
Table 1.
Demographic and clinical characteristics of the 385 ELCAPA-03 patients included in the treatment feasibility assessment
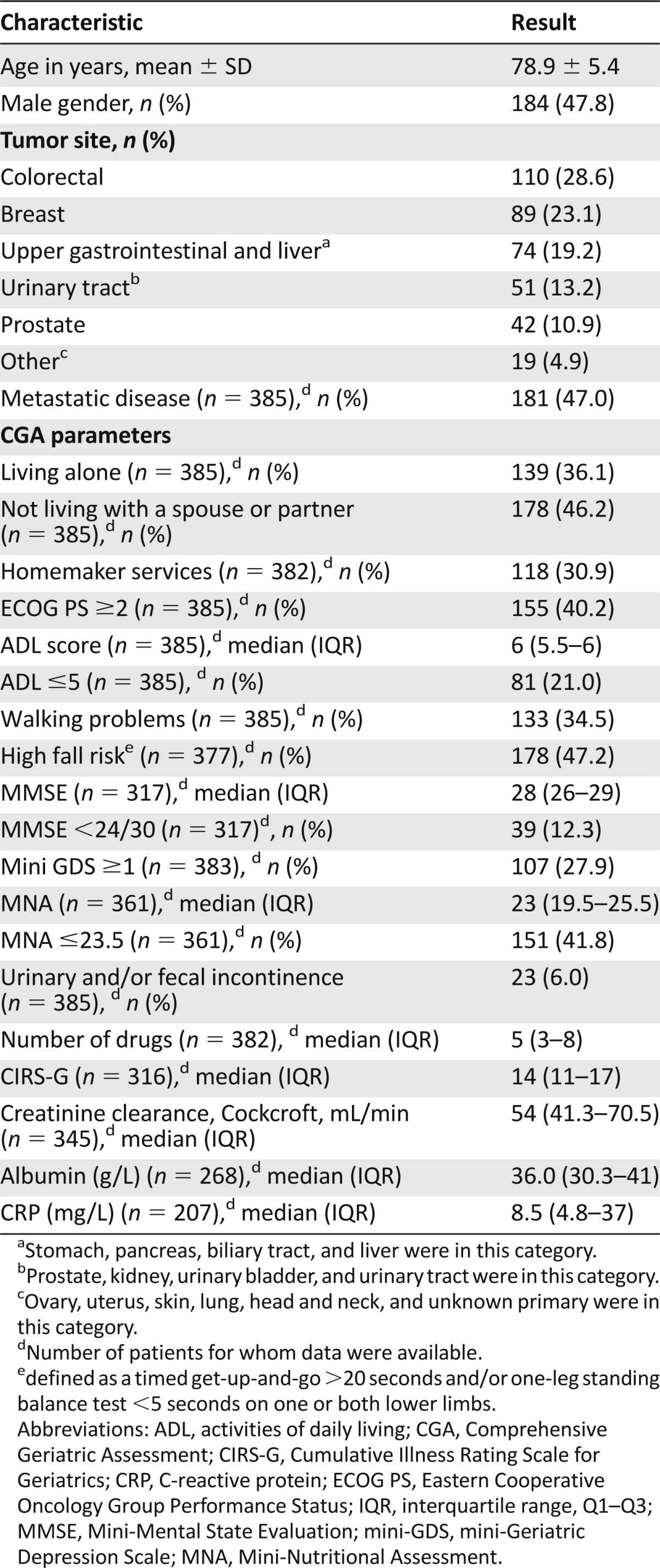
Table 2.
Cancer treatment plans
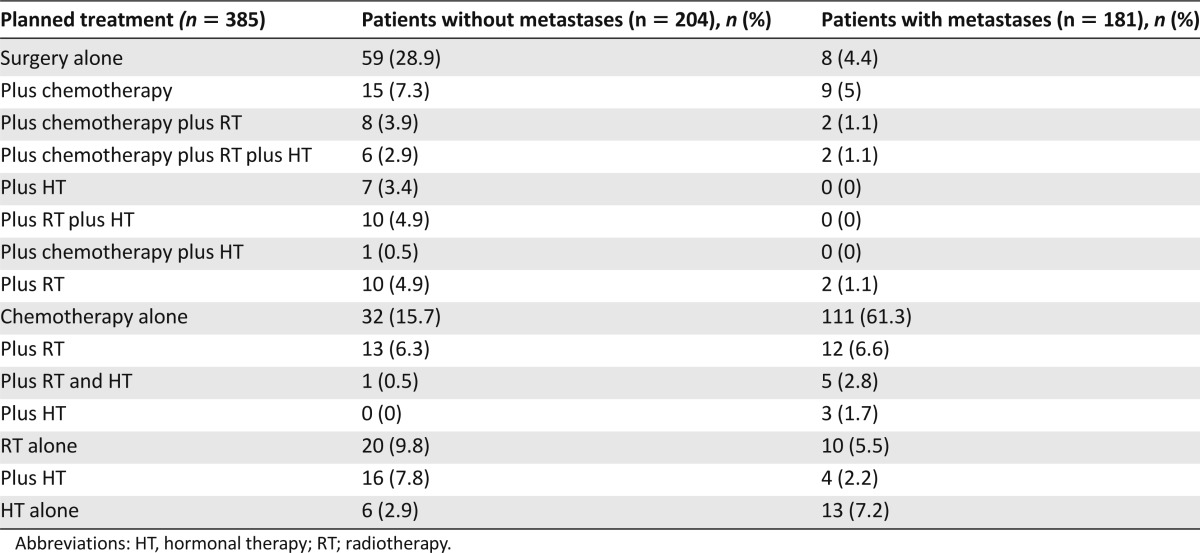
The overall feasibility rate was 86.8% (95% CI: 82.1–91.5) in the group without metastases and 65.7% (95% CI: 58.8–72.7) in the group with metastases. The majority of patients with either failure to start or early discontinuation of cancer treatment had chemotherapy scheduled: 21 in the group without metastases (alone in 7 and combined in 14 patients) and 58 in the group with metastases (alone in 47 and combined in 1). Table 3 reports the feasibility rates for each treatment modality, and Table 4 reports the reasons for failure to start or early discontinuation of cancer treatment. Among the nine patients who died before beginning chemotherapy, all had metastases and seven were scheduled to receive chemotherapy alone; five had pancreatic cancer with intestinal obstruction and scheduled gemcitabine chemotherapy but experienced a fast decline in general health; three (colon, prostate, and stomach cancer, respectively) had major functional impairment at baseline (ECOG-PS 3 or 4); and one died of complications after surgery for colorectal cancer.
Table 3.
Rates of cancer treatment feasibility in the ELCAPA-03 study
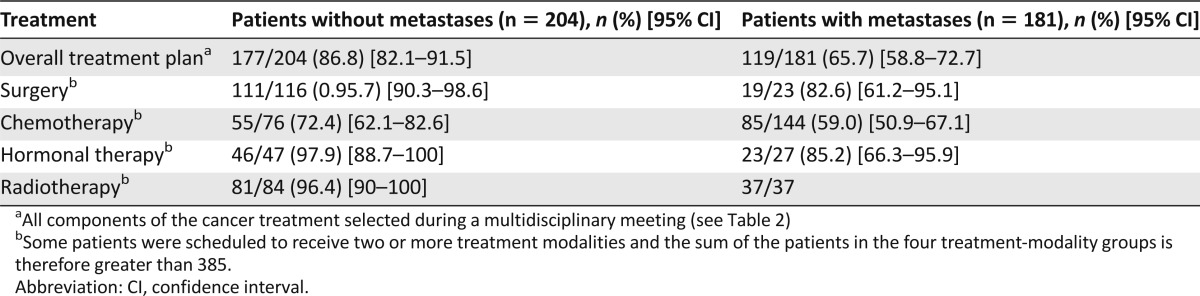
Table 4.
Reasons for either failure to start or early discontinuation of cancer treatment in the ELCAPA-03 patients

Few patients failed to receive scheduled surgery, radiotherapy, and/or hormonal therapy. Consequently, we report the results of the univariate and multivariate analyses of associations linking CGA parameters to chemotherapy feasibility. By univariate analysis, factors associated with chemotherapy feasibility at p < .15 were younger age (mean: 77.2 ± 4.4 years when feasible vs. 78.6 ± 4.5 years when not feasible; p = .04), female gender (52.8% vs. 42.5%; p = .14), nonmetastatic disease (39.3% vs. 26.2%; p = .05), living with a partner (61.1% vs. 47.5% p = .05), ECOG-PS <2 (66.4% vs. 36.2%; p < .0001) or ADL >5 (87.1% vs. 67.5%; p < .001), absence of difficulty walking (76.4% vs. 51.9%; p < .0001) or absence of fall risk (64.8% vs. 41%; p = .001), absence of depressive mood defined as mini-GDS <1 (75.6% vs. 63.9%; p = .08), higher MMSE (median: 28 [range: 27–30] vs. 27 [range: 26–29]; p = .05), higher MNA (median: 22.7 [range: 19.5–25] vs. 20. 5 [range: 15.5–24.5]; p = .001), lower comorbidity index as assessed by the CIRS-G (median: 13 [range: 11–16] vs. 15 [range: 13–17]; p = .05), higher creatinine clearance (median: 59.5 mL/minute [range: 45.7–78.1 mL/minute] vs. 52.5 mL/min [range: 41.3–66.8 mL/minute]; p = .001), and higher serum albumin (35.4 g/L [range: 30.7–40.2 g/L] vs. 32.7 g/L [range: 27.6–37 g/L]; p = .03). The concomitant use of surgery (20.7% when chemotherapy was feasible vs. 17.5% when not feasible; p = .56) or hormonal therapy (8.7% vs. 7.5%; p = .78) or radiotherapy (20.7% vs. 25.6%; p = .4) was not associated with chemotherapy feasibility.
Albumin was not entered into the multivariate analysis because of the large number of missing data (79 patients [35.9%]). Colinearity precluded simultaneous entry into the same multivariate model of both measures of functional status (ECOG-PS and ADL) or mobility (walking difficulties and fall risk). By multivariate analysis, factors independently associated with chemotherapy feasibility were ECOG-PS <2 (adjusted OR [aOR]: 4.0; 95% CI: 1.87–8.7; p < .0001) or ADL >5 (aOR: 3.01; 95% CI: 1.28–7.09; p = .01) or absence of walking difficulties (aOR: 2.7; 95% CI: 1.25–5.85; p = .01) or absence of fall risk (aOR: 2.85; 95% CI: 1.33–6.08; p = .007) and higher creatinine clearance (aOR1 mL/minute: 1.3; 95% CI: 1.01–1.7; p = .04). Nonmetastatic disease and higher MNA showed associations of borderline significance with chemotherapy feasibility (nonmetastatic vs. metastatic [aOR: 2.45; 95% CI: 0.88–6.8; p = .08] and [aOR1 point increase: 1.07; 95% CI: 0.99–1.15; p = .08], respectively). Associations with age, CIRS-G, mini-GDS, and MMSE were no longer significant after adjustment for measures of functional status or mobility limitations.
In the patients with metastases, 1-year mortality was 27.7% in the group with overall feasibility and 56.4% in the group with overall treatment failure (p < .0001). In the patients without metastases, corresponding 1-year mortality rates were 10.2% and 14.8%, respectively (p = .47).
Sensitivity Analysis
When patients with disease progression or refusal to continue chemotherapy were excluded from the subgroup with metastatic disease, the chemotherapy feasibility rate increased slightly to 61.1% (95% CI: 52.9%–69.3%).
Discussion
Planned cancer treatment was feasible in 86.8% of patients without metastases and 65.8% of those with metastases. The feasibility rate was lowest in the patients scheduled for chemotherapy. The main reasons for either failure to start chemotherapy or early discontinuation of chemotherapy were toxicities, an early decline in general health, and death. Conversely, surgery, hormonal therapy, and radiotherapy were usually feasible in everyday practice in older cancer patients: Feasibility rates for these modalities were more than 95% in the group without metastases and ranged from 82.6% to 100% in the group with metastases. Functional status (ECOG-PS or ADL), mobility (walking difficulties or fall risk), and renal function were independently associated with chemotherapy feasibility. Metastasis and nutritional status were of borderline significance.
At baseline, age and geriatric syndromes (most notably functional and cognitive impairments) in our study patients were comparable to those reported in other surveys of older cancer patients using CGA [43, 44]. Chemotherapy feasibility rates were consistent with previous studies, which ranged from 49.1% [19, 20, 23, 43, 45–48] to 80% [49]. The highest feasibility rates were found in studies [19, 20, 49] that included only patients without metastases, whose functional status was probably better than in our population.
Whether feasibility is more common in younger versus older cancer patients seen in everyday practice remains unclear. Experimental data suggest similar feasibility rates across age groups [4–9]. In one study, feasibility rates in middle-aged patients with colorectal cancer were similar to those in our study [48], whereas two observational studies found lower feasibility rates in older cancer patients than in middle-aged cancer patients [20, 23]. Importantly, most of these studies failed to correct for confounding factors such as functional status and competing comorbidities. We found that functional impairment (ECOG-PS or ADL alterations) or mobility limitations (walking difficulties or fall risk) were confounding factors for the association between age and chemotherapy feasibility. In two studies of functional status in patients with ovarian carcinoma [50] or with breast, lung, or colorectal cancer [43], functional status was associated with chemotherapy feasibility. To our knowledge, the association between renal dysfunction and chemotherapy feasibility has not been reported previously.
Only two studies concomitantly assessed the feasibility rates of chemotherapy and radiotherapy [21, 51]. Both were small sample studies (n = 41 and n = 36, respectively) in patients with rectal cancer and found very high radiotherapy feasibility rates, contrasting with a 77% rate of chemotherapy feasibility [21].
Definitions of feasibility vary widely, particularly for surgery and chemotherapy [19, 20, 23, 24, 43, 45–48]. Surgery feasibility has been defined as successful completion of the planned surgical procedure, tumor-free resection margins, or absence of postoperative complications with survival on day 30. In one study, surgery failure, defined as death by day 30, was only 3.5% [38]. The chemotherapy feasibility criteria used to date include various measures: dosage reduction [24, 52], cycle number reduction, wider spacing of cycles [52], or a combined endpoint [43] including all-cause mortality and/or grade III or IV toxicity [24, 43] and/or treatment interruption and/or admission during chemotherapy [24].
This study has a number of strengths. To the best of our knowledge, it reports the first data on feasibility of each individual treatment modality in older cancer patients. Patient recruitment, follow-up, and data collection were conducted prospectively using a standardized case-report form and database. Very few patients were lost to follow-up. The feasibility measures were relevant to tumor site and metastases and were assessed using objective quantitative criteria (number of chemotherapy cycles, radiation dose, or hormonal therapy dose). In addition, data on feasibility were obtained from three sources (paper and electronic hospital medical charts and hospital chemotherapy database) to limit misclassification bias. In a previous study, sensitivity and specificity of Medicare claims for identifying chemotherapy cycles were lower than those of medical record review [53].
Our study has several limitations. First, patient recruitment at two teaching hospitals led to selection bias. In a study of patients with ovarian cancer, a higher proportion of patients received the full chemotherapy course in a teaching hospital than in a nonteaching hospital [54]. Second, approximately 60% of older cancer patients in the study teaching hospitals were referred to the geriatric oncology clinics [27]. Consequently, our results may not apply to the overall population of older patients with solid cancer. Our population included patients with a wide variety of tumor sites. Finally, the number of patients was too small for a robust analysis of factors associated with chemotherapy feasibility in the subgroups with and without metastasis; however, the interaction term between metastasis and functional status enabled us to assess these factors and to take into account the differences between the groups with and without metastasis.
Clinical Implications
Given the growing number of older patients with cancer, the limited number of geriatricians, and the time-consuming nature of the CGA (1–2 hours per patient), our results suggest that, among older cancer patients, those initially considered for chemotherapy by the oncologist or other specialist may benefit the most from referral to a geriatric oncology clinic before the final treatment decision.
Recent studies in patients with advanced colorectal cancer (stage III or IV) indicated that cognitive and functional impairments, depressive mood, and hypoalbuminemia were independently associated with treatment failure [24, 55, 56]. Further studies are needed to confirm the independent association of these factors with chemotherapy failure in patients with tumors at other sites and to determine whether geriatric follow-up targeting these factors improves treatment feasibility, overall survival, and quality of life.
Conclusion
Our data from everyday practice showed that planned cancer treatment was feasible in 86.8% of older cancer patients without metastases and 65.7% of those with metastases. Feasibility rates were considerably lower for chemotherapy than for surgery, radiotherapy, and hormonal therapy. Consequently, utilization of limited geriatric oncology resources may be optimized by the preferential referral of older cancer patients initially considered for chemotherapy to geriatric oncology clinics [57].
Acknowledgments
We thank Elias Assaf, M.D., Isabelle Baumgaertner, M.D., Yazid Belkacemi, M.D., Ph.D., Francesco Brunetti, M.D., Marie Chaubet, M.D., Stéphane Culine, M.D., Ph.D., Carolina Saldana, M.D., Laurent Salomon, M.D., Ph.D., Iradj Sobhani, M.D., Ph.D., Alexandre de la Taille, M.D., Ph.D., Hélène Vincent, M.D., Dimitri Vordos, M.D., for recruiting patients included in the ELCAPA cohort. We thank Antoinette Wolfe, M.D., for editing the manuscript. Funding was provided by a National Cancer Institute grant to the Geriatric Oncologic Unit. Findings were presented at the 12th International Society of Geriatric Oncology meeting (October 2012, Manchester, U.K.). The ELCAPA Study Group was composed of two oncologists (S.C., C.T.), one radiotherapist (J.L.L.), five geriatricians (P.C., M.L., E.L., E.P., H.V.), three epidemiologists (F.C.P., S.B.G., E.A.), one pharmacist (M.C.V.), one biostatistician (A.L.T.), one clinical-research medical doctor (N.R.), and two clinical research assistants (A.R., J.F.). Marie Laurent and Elena Paillaud contributed equally to the study.
Author Contributions
Conception/Design: Florence Canouï-Poitrine, Marie Laurent, Elena Paillaud, Philippe Caillet, Aurélie Le Thuaut, Sylvie Bastuji-Garin
Provision of study material or patients: Florence Canouï-Poitrine, Marie Laurent, Elena Paillaud, Christophe Tournigand, Philippe Caillet, Jean-Léon Lagrange, Stéphane Culine, Hélène Vincent, Muriel Carvalho-Verlinde
Collection and/or assembly of data: Florence Canouï-Poitrine, Marie Laurent, Elena Paillaud, Philippe Caillet, Aurélie Le Thuaut, Sylvie Bastuji-Garin, Muriel Carvalho-Verlinde
Data analysis and interpretation: Florence Canouï-Poitrine, Marie Laurent, Elena Paillaud, Aurélie Le Thuaut, Sylvie Bastuji-Garin
Manuscript writing: Florence Canouï-Poitrine, Marie Laurent, Elena Paillaud, Christophe Tournigand, Philippe Caillet, Aurélie Le Thuaut, Jean-Léon Lagrange, Olivier Beauchet, Hèléne Vincent, Muriel Carvalho-Verlinde, Stéphane Culine, Sylvie Bastuji-Garin
Final approval of manuscript: Florence Canouï-Poitrine, Marie Laurent, Elena Paillaud, Christophe Tournigand, Philippe Caillet, Aurélie Le Thuaut, Jean-Léon Lagrange, Olivier Beauchet, Hélène Vincent, Muriel Carvalho-Verlinde, Stéphane Culine, Sylvie Bastuji-Garin
Disclosures
Jean-Léon Lagrange: INSERM (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Smith BD, Smith GL, Hurria A, et al. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Jonker DJ, Spithoff K, Maroun J. Adjuvant systemic chemotherapy for stage II and III colon cancer after complete resection: An updated practice guideline. Clin Oncol (R Coll Radiol) 2011;23:314–322. doi: 10.1016/j.clon.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg RM, Tabah-Fisch I, Bleiberg H, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol. 2006;24:4085–4091. doi: 10.1200/JCO.2006.06.9039. [DOI] [PubMed] [Google Scholar]
- 5.Muss HB, Berry DA, Cirrincione C, et al. Toxicity of older and younger patients treated with adjuvant chemotherapy for node-positive breast cancer: The Cancer and Leukemia Group B experience. J Clin Oncol. 2007;25:3699–3704. doi: 10.1200/JCO.2007.10.9710. [DOI] [PubMed] [Google Scholar]
- 6.Pavlidis TE, Marakis G, Ballas K, et al. Safety of bowel resection for colorectal surgical emergency in the elderly. Colorectal Dis. 2006;8:657–662. doi: 10.1111/j.1463-1318.2006.00993.x. [DOI] [PubMed] [Google Scholar]
- 7.Spivak H, Maele DV, Friedman I, et al. Colorectal surgery in octogenarians. J Am Coll Surg. 1996;183:46–50. [PubMed] [Google Scholar]
- 8.Folprecht G, Seymour MT, Saltz L, et al. Irinotecan/fluorouracil combination in first-line therapy of older and younger patients with metastatic colorectal cancer: Combined analysis of 2,691 patients in randomized controlled trials. J Clin Oncol. 2008;26:1443–1451. doi: 10.1200/JCO.2007.14.0509. [DOI] [PubMed] [Google Scholar]
- 9.Cassidy J, Saltz LB, Giantonio BJ, et al. Effect of bevacizumab in older patients with metastatic colorectal cancer: Pooled analysis of four randomized studies. J Cancer Res Clin Oncol. 2010;136:737–743. doi: 10.1007/s00432-009-0712-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Spall HG, Toren A, Kiss A, et al. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: A systematic sampling review. JAMA. 2007;297:1233–1240. doi: 10.1001/jama.297.11.1233. [DOI] [PubMed] [Google Scholar]
- 11.Köhne CH, Folprecht G, Goldberg RM, et al. Chemotherapy in elderly patients with colorectal cancer. The Oncologist. 2008;13:390–402. doi: 10.1634/theoncologist.2007-0043. [DOI] [PubMed] [Google Scholar]
- 12.Aparicio T, Navazesh A, Boutron I, et al. Half of elderly patients routinely treated for colorectal cancer receive a sub-standard treatment. Crit Rev Oncol Hematol. 2009;71:249–257. doi: 10.1016/j.critrevonc.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J Clin Oncol. 2011;29:3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Audisio RA, Bozzetti F, Gennari R, et al. The surgical management of elderly cancer patients; recommendations of the SIOG surgical task force. Eur J Cancer. 2004;40:926–938. doi: 10.1016/j.ejca.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Carreca I, Balducci L, Extermann M. Cancer in the older person. Cancer Treat Rev. 2005;31:380–402. doi: 10.1016/j.ctrv.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Soubeyran P, Henriques de Figueiredo B, Soubeyran I, et al. Therapeutic strategies in elderly and very elderly patients. Best Pract Res Clin Haematol. 2012;25:91–100. doi: 10.1016/j.beha.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Townsley C, Pond GR, Peloza B, et al. Analysis of treatment practices for elderly cancer patients in Ontario, Canada. J Clin Oncol. 2005;23:3802–3810. doi: 10.1200/JCO.2005.06.742. [DOI] [PubMed] [Google Scholar]
- 18.Mitry E, Rollot F, Jooste V, et al. Improvement in survival of metastatic colorectal cancer: Are the benefits of clinical trials reproduced in population-based studies? Eur J Cancer. 2013;49:2919–2925. doi: 10.1016/j.ejca.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Dobie SA, Baldwin LM, Dominitz JA, et al. Completion of therapy by Medicare patients with stage III colon cancer. J Natl Cancer Inst. 2006;98:610–619. doi: 10.1093/jnci/djj159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed S, Ahmad I, Zhu T, et al. Early discontinuation but not the timing of adjuvant therapy affects survival of patients with high-risk colorectal cancer: A population-based study. Dis Colon Rectum. 2010;53:1432–1438. doi: 10.1007/DCR.0b013e3181e78815. [DOI] [PubMed] [Google Scholar]
- 21.Margalit DN, Mamon HJ, Ancukiewicz M, et al. Tolerability of combined modality therapy for rectal cancer in elderly patients aged 75 years and older. Int J Radiat Oncol Biol Phys. 2011;81:e735–e741. doi: 10.1016/j.ijrobp.2010.12.056. [DOI] [PubMed] [Google Scholar]
- 22.Aaldriks AA, Maartense E, le Cessie S, et al. Predictive value of geriatric assessment for patients older than 70 years, treated with chemotherapy. Crit Rev Oncol Hematol. 2011;79:205–212. doi: 10.1016/j.critrevonc.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Neugut AI, Matasar M, Wang X, et al. Duration of adjuvant chemotherapy for colon cancer and survival among the elderly. J Clin Oncol. 2006;24:2368–2375. doi: 10.1200/JCO.2005.04.5005. [DOI] [PubMed] [Google Scholar]
- 24.Aparicio T, Jouve JL, Teillet L, et al. Geriatric factors predict chemotherapy feasibility: Ancillary results of FFCD 2001-02 phase III study in first-line chemotherapy for metastatic colorectal cancer in elderly patients. J Clin Oncol. 2013;31:1464–1470. doi: 10.1200/JCO.2012.42.9894. [DOI] [PubMed] [Google Scholar]
- 25.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 26.Agence Nationale d’Accréditation et d’Evaluation en Santé Diagnostic de l’insuffisance rénale chronique chez l’adulte. Synthèse des recommandations, Septembre 2002 Available at http://www.has-sante.fr/portail/upload/docs/application/pdf/irc_chez_ladulte_2002_-_synth_350se.pdf Accessed September 15, 2013.
- 27.Caillet P, Canoui-Poitrine F, Vouriot J, et al. Comprehensive geriatric assessment in the decision-making process in elderly patients with cancer: ELCAPA study. J Clin Oncol. 2011;29:3636–3642. doi: 10.1200/JCO.2010.31.0664. [DOI] [PubMed] [Google Scholar]
- 28.Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007;25:1824–1831. doi: 10.1200/JCO.2007.10.6559. [DOI] [PubMed] [Google Scholar]
- 29.Extermann M, Aapro M, Bernabei R, et al. Use of comprehensive geriatric assessment in older cancer patients: Recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG) Crit Rev Oncol Hematol. 2005;55:241–252. doi: 10.1016/j.critrevonc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 31.Orr ST, Aisner J. Performance status assessment among oncology patients: A review. Cancer Treat Rep. 1986;70:1423–1429. [PubMed] [Google Scholar]
- 32.Podsiadlo D, Richardson S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 33.Guigoz Y, Vellas B. The Mini Nutritional Assessment (MNA) for grading the nutritional state of elderly patients: Presentation of the MNA, history and validation. Nestle Nutr Workshop Ser Clin Perform Programme. 1999;1:3–11. doi: 10.1159/000062967. ; discussion 11–12. [DOI] [PubMed] [Google Scholar]
- 34.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 35.Clément JP, Darthout N, Nubukpo P. Life events, personality and dementia. [in French] Psychol Neuropsychiatr Vieil. 2003;1:129–138. [PubMed] [Google Scholar]
- 36.Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16:622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 37.Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. http://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf Available at. Accessed May 2013.
- 38.Audisio RA, Pope D, Ramesh HS, et al. Shall we operate? Preoperative assessment in elderly cancer patients (PACE) can help. A SIOG surgical task force prospective study. Crit Rev Oncol Hematol. 2008;65:156–163. doi: 10.1016/j.critrevonc.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Des Guetz GUB, Morere JF, Perret G, et al. Duration of adjuvant chemotherapy for patients with non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2010:CD007046. doi: 10.1002/14651858.CD007046.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Best LSP, Baughan C, Buchanan R, et al. Palliative chemotherapy for advanced or metastatic colorectal cancer. Cochrane Database Syst Rev. 2000:CD001545. doi: 10.1002/14651858.CD001545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haute Autorité de Santé Available at http://www.has-sante.fr/portail/jcms/c_5073/professionels-de-sante?cid=c_5073 Accessed September 15, 2013.
- 42.Freyer G, Geay JF, Touzet S, et al. Comprehensive geriatric assessment predicts tolerance to chemotherapy and survival in elderly patients with advanced ovarian carcinoma: A GINECO study. Ann Oncol. 2005;16:1795–1800. doi: 10.1093/annonc/mdi368. [DOI] [PubMed] [Google Scholar]
- 43.Marinello R, Marenco D, Roglia D, et al. Predictors of treatment failures during chemotherapy: A prospective study on 110 older cancer patients. Arch Gerontol Geriatr. 2009;48:222–226. doi: 10.1016/j.archger.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 44.Soubeyran P, Fonck M, Blanc-Bisson C, et al. Predictors of early death risk in older patients treated with first-line chemotherapy for cancer. J Clin Oncol. 2012;30:1829–1834. doi: 10.1200/JCO.2011.35.7442. [DOI] [PubMed] [Google Scholar]
- 45.Chrischilles EA, Link BK, Scott SD, et al. Factors associated with early termination of CHOP therapy and the impact on survival among patients with chemosensitive intermediate-grade non-Hodgkin’s lymphoma. Cancer Contr. 2003;10:396–403. doi: 10.1177/107327480301000507. [DOI] [PubMed] [Google Scholar]
- 46.Fairfield KM, Murray K, Lucas FL, et al. Completion of adjuvant chemotherapy and use of health services for older women with epithelial ovarian cancer. J Clin Oncol. 2011;29:3921–3926. doi: 10.1200/JCO.2010.34.1552. [DOI] [PubMed] [Google Scholar]
- 47.Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012;118:3377–3386. doi: 10.1002/cncr.26646. [DOI] [PubMed] [Google Scholar]
- 48.André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 49.O’Connor TL, Edge S, Kossoff EB, et al. Factors affecting the delivery of adjuvant/neoadjuvant chemotherapy in older women with breast cancer. J Geriatr Oncol. 2012;3:320–328. [Google Scholar]
- 50.Gronlund B, Høgdall C, Hansen HH, et al. Performance status rather than age is the key prognostic factor in second-line treatment of elderly patients with epithelial ovarian carcinoma. Cancer. 2002;94:1961–1967. doi: 10.1002/cncr.10385. [DOI] [PubMed] [Google Scholar]
- 51.Fiorica F, Cartei F, Carau B, et al. Adjuvant radiotherapy on older and oldest elderly rectal cancer patients. Arch Gerontol Geriatr. 2009;49:54–59. doi: 10.1016/j.archger.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 52.Janssen-Heijnen ML, Maas HA, van de Schans SA, et al. Chemotherapy in elderly small-cell lung cancer patients: Yes we can, but should we do it? Ann Oncol. 2011;22:821–826. doi: 10.1093/annonc/mdq448. [DOI] [PubMed] [Google Scholar]
- 53.Lund JL, Sturmer T, Harlan LC, et al. Identifying specific chemotherapeutic agents in Medicare data: A validation study. Med Care. 2013;51:e27–e34. doi: 10.1097/MLR.0b013e31823ab60f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paulsen T, Kjaerheim K, Kaern J, et al. Improved short-term survival for advanced ovarian, tubal, and peritoneal cancer patients operated at teaching hospitals. Int J Gynecol Cancer. 2006;16(suppl 1):11–17. doi: 10.1111/j.1525-1438.2006.00319.x. [DOI] [PubMed] [Google Scholar]
- 55.Carola E. Predictive factors for chemotherapy feasibility in elderly patients with solid tumor: Results of GERCOR old prospective multicenter study. Presentation at: American Society of Clinical Oncology annual meeting; May 31–June 4, 2013; Chicago, IL. [Google Scholar]
- 56.Groupe Cooperateur Multidisciplinaire en Oncologie Combination chemotherapy in treating older patients with metastatic breast, colorectal, or ovarian cancer that cannot be removed by surgery [identifier NCT00664911]. Available at http://clinicaltrials.gov/ct2/show/NCT00664911?term=NCT00664911&rank=1 Accessed September 15, 2013.
- 57.Decoster L, Kenis C, Van Puyvelde K, et al. The influence of clinical assessment (including age) and geriatric assessment on treatment decisions in older patients with cancer. J Geriatr Oncol. 2013;4:235–241. doi: 10.1016/j.jgo.2013.04.010. [DOI] [PubMed] [Google Scholar]


