A systematic review of the literature was performed to assess response rate, median progression-free survival, and adverse events associated with sorafenib therapy for metastatic thyroid cancers. The data show that treatment with sorafenib in patients with progressive differentiated or medullary thyroid cancer is a promising strategy, but the adverse event rate is high, leading to a high rate of dose reduction or discontinuation. Careful patient selection and side effect management are required.
Keywords: Tyrosine kinase inhibitors, Chemotherapy, Response rate, Adverse effects
Learning Objectives
Discuss the response rate, median PFS, and adverse events associated with sorafenib therapy for metastatic thyroid cancers.
Abstract
Background.
Sorafenib was recently approved by the U.S. Food and Drug Administration for radioiodine-resistant metastatic differentiated thyroid cancer (DTC). In addition, two drugs (vandetanib and cabozantinib) have received U.S. Food and Drug Administration approval for use in medullary thyroid cancer (MTC). Several published phase II trials have investigated the efficacy of sorafenib in thyroid cancers, but to date, results from those studies have not been compared.
Methods.
A systematic review of the literature was performed to assess response rate, median progression-free survival, and adverse events associated with sorafenib therapy for metastatic thyroid cancers.
Results.
This review included seven trials involving 219 patients: 159 with DTC (papillary, follicular, and poorly differentiated), 52 with MTC, and 8 with anaplastic thyroid cancer. No study reported complete responses to treatment. Overall partial response, stable disease, and progressive disease rates were 21%, 60%, and 20%, respectively. The median progression-free survival was 18 months for patients with all subtypes of thyroid cancer. Drug was discontinued in 16% of patients because of toxicities or intolerance, and the dose was reduced in a further 56%. Side effects with an incidence ≥50% were hand-foot syndrome (74%), diarrhea (70%), skin rash (67%), fatigue (61%), and weight loss (57%). Deaths not related to progressive disease occurred in nearly 4% of patients.
Conclusion.
Treatment with sorafenib in patients with progressive DTC and MTC is a promising strategy, but the adverse event rate is high, leading to a high rate of dose reduction or discontinuation. Consequently, sorafenib use in patients with metastatic thyroid cancer requires careful selection of patients and careful management of side effects.
Implications for Practice:
This meta-analysis of 219 patients treated with sorafenib for metastatic thyroid cancers demonstrated that 81% of patients had either partial response or stable disease, and none had a complete response. The partial response rate was best for medullary thyroid cancer, followed by differentiated thyroid cancer. Responses in anaplastic thyroid cancer were low. The overall median progression-free survival was 18 months for all histologies. There were significant dose reductions and discontinuations as a result of toxicities, which need to be considered when treating patients who may otherwise be asymptomatic and have reasonable overall survival.
Introduction
In general, the prognosis of differentiated thyroid cancer (DTC; papillary, follicular, and poorly differentiated) and medullary thyroid cancer (MTC) is quite good, owing to these cancers’ indolent course and the efficacy of standard treatment. However, patients who present with metastatic MTC have a median overall survival (OS) of only 3 years [1], and nearly 50% of patients with metastatic radioactive iodine (RAI)-refractory DTCs will die of the disease. The 10-year median OS of patients with distant metastasis is reported to be 25%–42% [2, 3]. Overall, 9% of all thyroid cancer patients will succumb to their disease [4]. Response rates to cytotoxic chemotherapeutic drugs are poor and short lived and, thus, are no longer the standard of care [5]. Sorafenib was recently approved for patients with RAI-resistant distant metastses and are part of the National Comprehensive Cancer Network and the American Thyroid Association guidelines [2, 6]. Recently, two drugs—vandetanib and cabozantinib—have been approved by the FDA for metastatic or unresectable progressive MTC. Vandetanib is a RET, EGFR, and VEGFR inhibitor that was approved in 2011. Cabozantinib is a RET, VEGFR, and MET inhibitor that was approved in 2012.
Several mutations or gene rearrangements have been detected in DTC and MTC and lead to activation of the MAPK and PI3K pathways. Germline mutations in RET are seen in virtually all patients with hereditary MTC, whereas somatic mutations in RET are seen in approximately 50% of patients with sporadic MTC [7]. RET gene rearrangements also occur in 5%–30% of papillary thyroid cancers (PTCs) [8–10]. RAS mutations are seen in approximately 40% of follicular thyroid cancers and 15% of PTCs and most recently have been identified in patients with wild-type RET sporadic MTC [11–14]. VEGFR and platelet-derived growth factor receptor overexpression also have been identified in these cancers [15].
Sorafenib (Nexavar, BAY 43-9006; Bayer, Leverkusen, Germany, http://www.bayer.com) is an inhibitor of RET, VEGFR1, VEGFR2, VEGFR3, Flt3, c-KIT, and wild type and mutant (V600E) BRAF [16]. Several published phase II trials and open-label studies have examined the efficacy of sorafenib in metastatic thyroid cancer [16–23]. The studies published thus far have small sample sizes and grouped different histologies together. We undertook this review of the published literature on this topic to assess the efficacy of sorafenib and possibly to identify a subset of patients that might benefit from this therapy. Consequently, the aims of this study were to systematically review the existing literature on this topic and perform a meta-analysis of the response rates and median progression-free survival (PFS) of patients treated with sorafenib for metastatic thyroid cancer and to assess the incidence of various adverse events attributed to sorafenib in this setting.
Materials and Methods
In December 2012, we performed an electronic search of the PubMed, Embase, and Medline databases using the search terms “thyroid cancer” and “sorafenib” to identify relevant literature for the period. Further manual searching of bibliographies from included studies was also done. The search was restricted to English-language literature, and attempts were made to contact the authors for details, clarifications, and any updates to the status of the studies. Inclusion criteria were studies of adult patients that included (a) response rates for which individual patient response with histology could be identified; (b) treatment initiation with single-agent sorafenib at 400 mg twice daily; and (c) standard reporting criteria for response and adverse events. Exclusion criteria were studies reporting on multiple drugs and case reports, review articles, phase I trials, and trials involving nonthyroid cancers.
Statistical Analysis
The overall results in the meta-analysis were weighted averages of study-specific results, with each study weighted by sample size. The overall rates for responses, adverse events, median time of PFS, and 95% confidence intervals (CIs) were estimated. The forest plot was used to present median PFS and 95% CI. All analyses were conducted using SAS version 9.2 (SAS Institute, Inc., Cary, NC, http://www.sas.com) and S-plus (TIBCO Software Inc., Palo Alto, CA, http://www.tibco.com) statistical software.
Results
The electronic search returned nine studies, of which eight met our inclusion criteria. One study (by Chen et al.) was excluded because of different drug dosing [24]. All papers used Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0 for assessing tumor response, and all except one mention using Common Terminology Criteria for Adverse Events (CTCAE) version 3 for reporting adverse effects. The study by Schneider et al. [19] published longer-term data on the study by Hoftijzer et al. [16], so data were combined from both papers and analyzed as one study. All studies were phase II trials except for those by Cabanillas et al. [21] and Capdevila et al. [23], which were retrospective studies. In the study by Cabanillas et al., data for two patients who had received sunitinib were removed and results were recalculated. No specific quality assessment tools were used to grade the quality of included papers. Three authors (L.T., S.Y.L., M.E.C.) independently tabulated data from the studies on an Excel spreadsheet (Microsoft, Redmond, WA, http://www.microsoft.com) and verified accuracy.
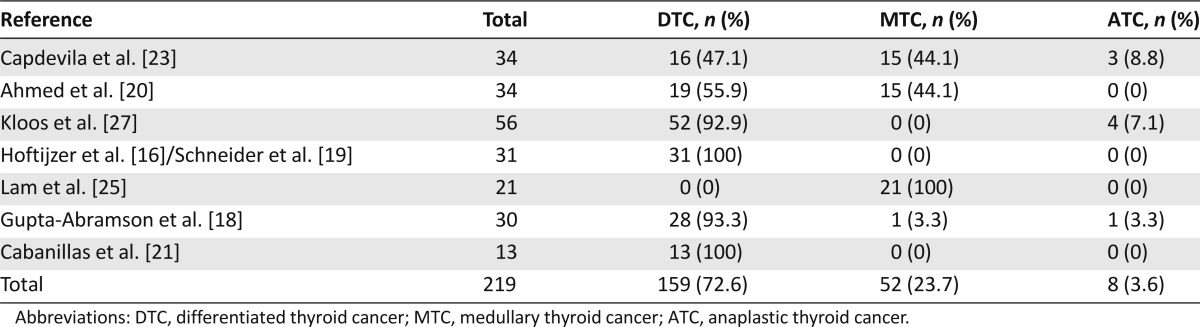
A total of 219 patients were included in this review. The distribution of histologies was 159 DTCs (PTC, follicular thyroid cancers, and poorly differentiated cancers), 52 MTCs, and 8 anaplastic thyroid cancers (ATCs). The histology of the tumors included in each study is presented in Table 1.
Table 1.
Descriptive summary of the number of patients and the distribution of tumor histology for each included sorafenib study
All of the studies, except the phase II trial by Kloos et al. [17], enrolled only patients with progressive disease (PD) in the preceding months, either by radiological or biochemical criteria. In the study by Kloos et al., eight patients had stable disease (SD) prior to enrollment, and the disease status was unknown in three patients. The remaining 45 patients had PD at time of enrollment.
Response Rate
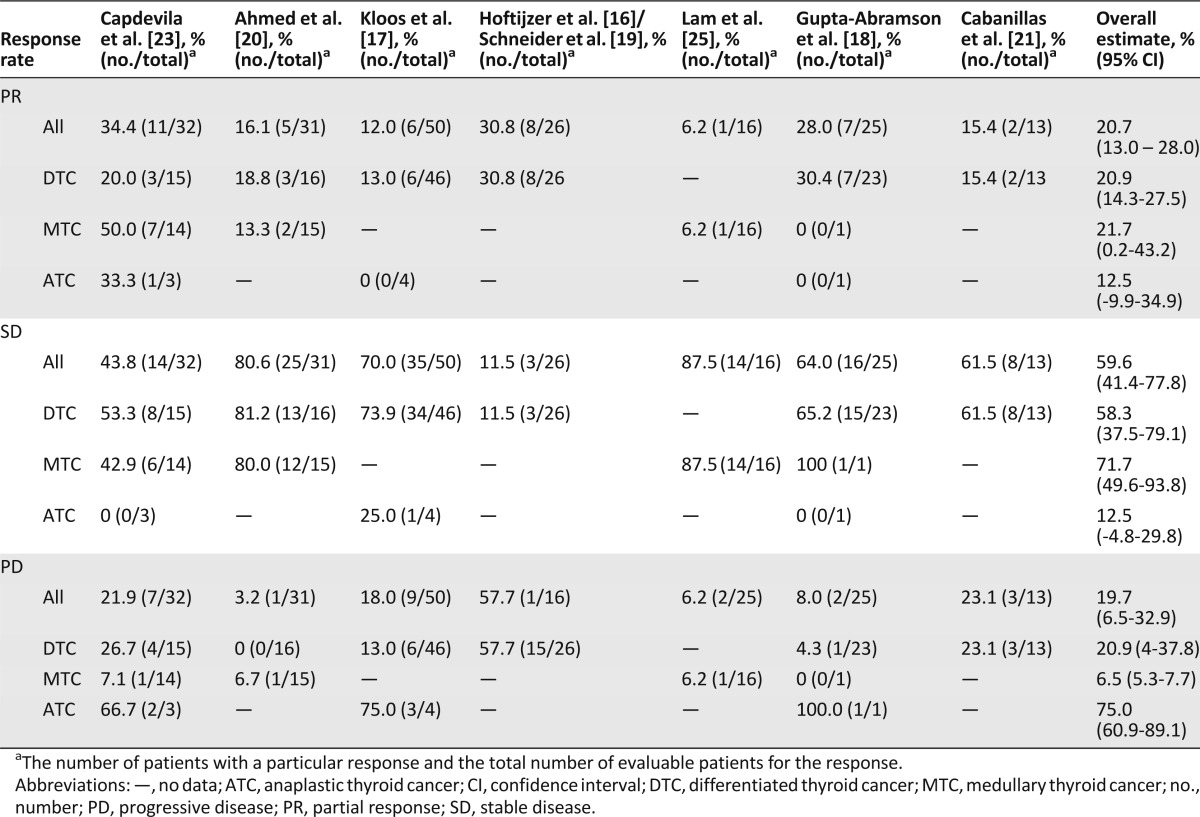
Three studies evaluated response at 6 months from start of therapy [16, 17, 20]. No study reported complete responses. The overall partial response (PR) rate was 21% for DTC, 22% for MTC, and 13% for ATC. The overall PR rate for all histologies combined was 21%. Table 2 shows PR, SD, and PD for each study and the overall estimate for all studies. Overall, clinical benefit (PR and SD responses) was 93% for MTC and about 79% for DTC. Three studies [16, 18, 21] mentioned good response in pulmonary metastases. Three studies mentioned lack of response of bony metastases to therapy [16, 21, 25], and Hoftijzer et al. [16] noted that patients with bony metastases tended to have worse outcomes than those without.
Table 2.
Meta-analysis of response rates to sorafenib for all types of thyroid carcinoma and each type of thyroid carcinoma
Biochemical Response
The results for biochemical response could not be analyzed in this review because raw data were not available. Five studies noted that biochemical response (i.e., reduction in serum thyroglobulin for DTC; calcitonin and carcinoembryonic antigen levels for MTC) correlated with radiological response [16, 18, 20, 21, 23], whereas others did not find any correlation [17, 25]. Even in studies that indicated a correlation, authors noted a rise in tumor marker level after an initial decrease, despite persisting radiological response [16, 20, 25]. Gupta-Abramson et al. [18] mentioned that serum thyroglobulin started falling before objective tumor response, whereas Cabanillas et al. [21] identified that response significantly correlated with the log of thyroglobulin levels.
Median PFS
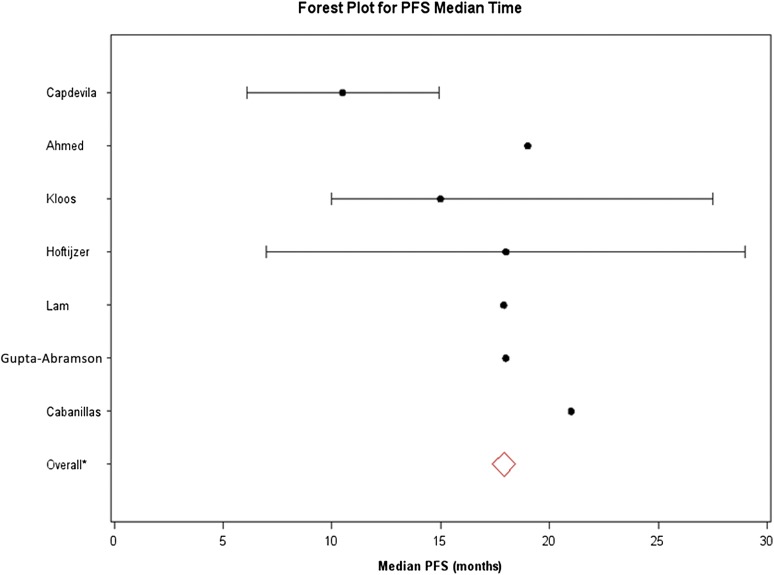
Median PFS could not be calculated for individual histologies because the data could not be teased out from the included studies. The recalculated median PFS (excluding sunitinib patients) was 21 months in the study by Cabanillas et al. [21]. The overall median PFS for all of the included studies was 18 months. The forest plot of median PFS is shown in Figure 1. Among the seven studies, 95% CIs were unavailable from the papers by Ahmed et al. [20] and Gupta-Abramson et al. [18]. For the studies by Lam et al. [25] and Cabanillas et al. [21], the upper limits of 95% CIs for median PFS time were not reached. Consequently, the CIs were presented only for the remaining three studies (Capdevila et al. [23], Kloos et al. [17], and the combined studies by Hoftijzer et al. [16] and Schneider et al. [19]). Three studies enrolled ATC patients [17, 18, 23]. Capdevila et al. [23] reported a median PFS of 4.4 months in the three ATC patients who were enrolled. The single ATC patient enrolled in the study by Gupta-Abramson et al. [18] had clinical progression in 4 days. The third study that enrolled ATC did not report PFS or OS in these patients [17].
Figure 1.
Forest plot of median progression-free survival for the seven included sorafenib clinical trials (all histologies). The solid lines represent the 95% confidence intervals (CIs). The 95% CIs were unavailable from the papers by Ahmed et al. [20] and Gupta-Abramson et al. [18]. For the studies by Lam et al. [25] and Cabanillas et al [21], the upper limits of 95% CIs for median PFS time were not reached. Consequently, CIs were presented only for the remaining three studies. The overall median PFS (red diamond) for all included trials was 17.9 months (95% CI: 17.9–18).
Abbreviation: PFS, progression-free survival.
OS
OS was not reported in four of the papers. The recalculated median OS was 23 months for Cabanillas et al. [21]. The OS for the study by Ahmed et al. [20] was 100% at 2 years, and Capdevila et al. [23] reported 23.6 months as the median OS. Kloos et al. [17] also noted a median OS of at least 23 months, with PTC patients who had prior chemotherapy having OS of 37.5 months. The best median OS for ATC was 5 months, as reported by Capdevila et al. [23].
Adverse Reactions
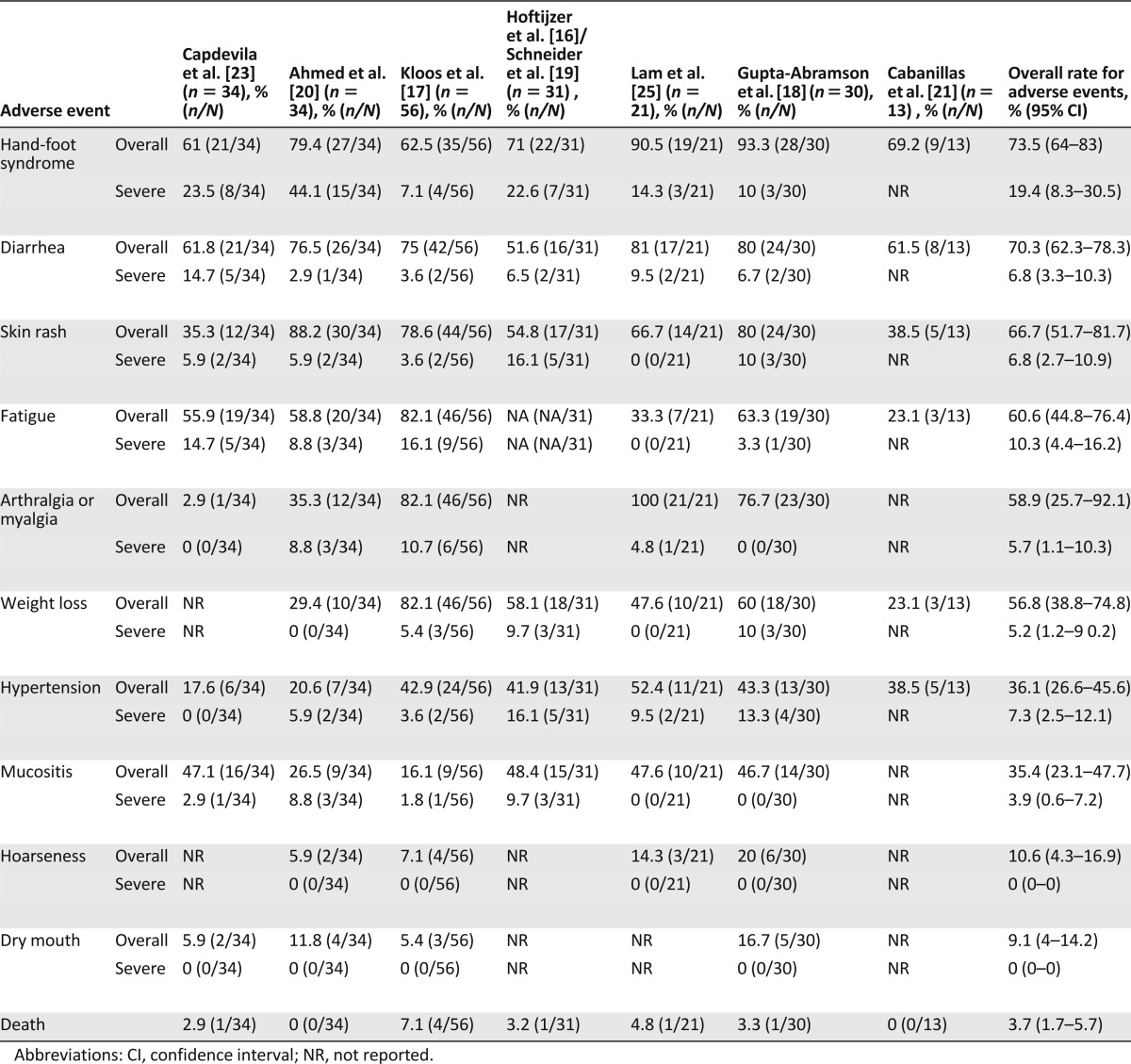
Incidence of adverse drug reactions is given in Table 3. More than 70% of patients suffered from hand-foot syndrome and diarrhea, and more than 50% of patients experienced skin rash, fatigue, and weight loss. Because sorafenib is a VEGF inhibitor, cardiovascular adverse effects were present, especially hypertension, which occurred in 36% of patients. Bleeding at any site occurred in 28 patients (13.6%). There were three reported acute myocardial infarctions (3.8%) and three instances of congestive heart failure (2.2%). Other rare but serious adverse events included one event of bowel perforation and ruptured aortic graft and two events of retinal vein occlusion. It is important to note that severe hypocalcemia (grade 3 or higher) occurred in three patients (2.5%) because thyroid cancer patients often have hypoparathyroidism as a result of previous neck dissections. Six patients (8.7%) developed cutaneous squamous cell cancer (four were keratoacanthoma) [17, 21]. Hoftijzer et al. [16] mentioned two nondermatologic cancers in their cohort of patients, one with small cell cancer of the lung within a month of starting therapy (most likely pre-existing) and one with oral tongue squamous cell cancer 46 months after initiation of therapy. Regarding thyroxine dose adjustments, two studies [17, 23] mentioned that patients did not need thyroxine dose adjustments, whereas a total of 27 patients (15%) needed either dose reductions or escalations of thyroid hormone [16, 18, 20].
Table 3.
Meta-analysis of adverse event rates associated with sorafenib therapy
There were 8 reported deaths among 219 patients (3.7%) that were not attributed to disease progression. These deaths were individually due to intracranial bleed, myocardial infarction, ischemic necrosis of colon and consequent septicemia, sudden death, Aspergillus pneumonia, acute myeloid leukemia, hip fracture from trauma, and liver failure. The patient with Aspergillus pneumonia had been treated with steroids for 3 years, and the patient with leukemia had a history of receiving 523 mCi of iodine 131 and external-beam radiation to the neck. Ahmed et al. [20] and Cabanillas et al. [21] did not report deaths in their studies.
Approximately 16% (95% CI: 8.6%–23.4%) of patients discontinued medication because of toxicity and 56% (95% CI: 43.4%–69.3%) had dose reductions for toxicity. Overall, 72% of patients could not tolerate the initial planned dosage of 400 mg twice daily. The most common dose reduction was to 400 mg once daily. Some patients needed drug withdrawals or holidays and were subsequently restarted at a lower dose; however, this information was not included in much detail in the papers included in this study.
Discussion
Sorafenib was recently approved by the FDA for metastatic, differentiated thyroid cancer in the United States; however, results of the phase III trial are not yet published. Although several phase II and retrospective studies have been published on sorafenib for the treatment of metastatic thyroid cancers, it is difficult to draw meaningful conclusions regarding efficacy because of small samples of individual types of thyroid cancers in each study. This meta-analysis is significant because it analyzes multiple studies, with a total of 219 patients with a variety of tumor histologies. Most patients have been on phase II trials with fairly uniform reporting standards. All of the studies used a starting dose of 400 mg b.i.d., and all used RECIST version 1.0 criteria for assessing response. All but one study used standardized criteria for adverse reactions (Common Terminology Criteria for Adverse Events version 3.0). Given the uniformity of the studies, a meta-analysis of these data was quite feasible.
Our overall analysis demonstrated PR in 22% of MTC patients and 21% of DTC patients, although PR in ATC reached only 13%. The majority of DTC and MTC patients showed clinical benefit, with PD noted in only 6.5% of MTC patients and 21% of DTC patients. The median PFS is within a narrow range (15–21 months) in all of the studies except Capdevila et al. (10.5 months) [23]. OS was not reported in most studies, but three of the studies included in the analysis reported median OS of around 23 months.
Our overall analysis demonstrated PR in 22% of MTC patients and 21% of DTC patients, although PR in ATC reached only 13%. The majority of DTC and MTC patients showed clinical benefit, with PD noted in only 6.5% of MTC patients and 21% of DTC patients.
In DTC patients, the response rate shown in this paper is higher than recently reported at the American Society of Clinical Oncology by Brose et al. in their phase III placebo-controlled trial of sorafenib in treatment-naïve patients with DTC, which showed a PR rate of 12% in the sorafenib arm versus 0.5% with placebo [26]. Of note, 73% of patients had tumor-size reduction, although they did not qualify for PR. The best response for most patients in this study was SD, and PFS in the sorafenib arm was 10.8 months versus 5.8 months with placebo. Although this trial shows a statistically significant improvement in PFS (hazard ratio [HR]: 0.587, p < .0001) with sorafenib, it may not necessarily answer the question of OS because of patient crossover. The differences between our meta-analysis results and this phase III trial could be explained by the study design and the challenges that arise from using RECIST criteria. In progressive, RAI-refractory DTC, several phase II trials testing other tyrosine kinase inhibitors (TKIs) with similar mechanism of action have been reported. Some showed higher response rates than sorafenib but similar PFS duration. These studies had different entry criteria and, therefore, are difficult to compare [27–33]. Motesanib is not commercially available but is one of the first TKIs to show promising results in thyroid cancer and open this field for future research [32]. Of the 93 DTC patients enrolled in the phase II trial with motesanib, 14% achieved PR and 67% achieved SD. The median PFS was 10 months. In a phase II sunitinib trial, 8 of 29 patients with RAI-refractory DTC achieved a response (response rate 28% for DTC and 50% for MTC) [27]. For all patients, the median time to progression was 12.8 months. Pazopanib showed promising results as well. In patients with RAI-refractory DTC, PR was seen in 18 of 37 patients (49%) included in a phase II trial, and the median duration of PFS was 11.7 months. A phase II trial studied the effect of lenvatinib (E7080) in 58 patients with DTC [33]. PRs were observed in 50% of patients, and median PFS was 12.6 months. Because of encouraging results in a phase I trial (53% PR) [30], cabozantinib is currently being studied in a phase II study (ClinicalTrials.gov identifier NCT01811212). Drugs with different mechanisms of action such as vemurafenib [34] and dabrafenib (BRAF inhibitors) (ClinicalTrials.gov identifier NCT01723202), selumetinib (MEK inhibitor) [35, 36], and everolimus (mammalian target of rapamycin inhibitor) [37] have also been tested or are currently in phase II trials in DTC and represent novel promising strategies.
In MTC, a phase III trial with sorafenib has not yet been performed, although several phase III trials have investigated the use of other treatments. A double-blind, placebo-controlled, phase III study with vandetanib demonstrated a significant prolongation of PFS (HR: 0.46) [38]. The median PFS in the placebo arm was 19.3 months and had not been reached in the vandetanib arm (predicted median PFS was 30.5 months). The objective response rate in the vandetanib arm was 45% (all PRs). A randomized placebo-controlled phase III trial of cabozantinib in progressive MTC demonstrated a median PFS of 11 months in the treatment group versus 4 months with placebo (HR: 0.28), with an overall response rate of 28% in the treatment arm and 0% in the placebo arm [39]. This response rate is similar to our own findings with sorafenib. Overall, the response rates with sorafenib are lower than observed with vandetanib. It is important to note that disease progression was not required for enrollment in the vandetanib trial; therefore, comparing results from these studies may be difficult. Furthermore, both vandetanib and cabozantinib target RET, whereas sorafenib does not. Both vandetanib and cabozantinib are approved by the FDA for progressive MTC.
As expected in ATC, the PR rate with sorafenib was observed in only 13% of patients, and the few responses observed were very short lived. Since this analysis was performed, a phase II trial of sorafenib in ATC that included 20 patients was published [40]. The PR rate was 10%, and 25% had SD. The overall median PFS was only 1.9 months, and median OS was 3.9 months. The authors of that study concluded that sorafenib had limited efficacy in ATC; however, a subgroup of patients with transformation from a more well-differentiated histology may respond to this agent. Given the limitations of existing treatments for ATC, new therapies are urgently needed.
The targeted therapy agents are associated with significant incidence of adverse events and a small risk of death. The most common drug toxicities with sorafenib found in this analysis were dermatologic (hand-foot skin reaction and rash) and gastrointestinal in nature. Weight loss, fatigue, hypertension, and arthralgias were also common side effects. BRAF inhibitors are known to cause squamous cell carcinomas and keratoacanthomas of the skin because of the paradoxical increase in signaling via the MAPK pathway in RAS-mutated tissues. The development of noncutaneous squamous cell carcinoma while on sorafenib, notably in the lung and tongue, is particularly concerning. One case of lung squamous cell carcinoma has been reported in the literature with a selective BRAF inhibitor [34]. This case was believed to be a dedifferentiated thyroid cancer rather than primary lung cancer. Nonetheless, squamous cell carcinomas of the skin are a known adverse event associated with sorafenib (and other BRAF inhibitors), thus careful attention should be given to the skin and oral mucosa when conducting physical examinations in patients receiving these drugs.
Squamous cell carcinomas of the skin are a known adverse event associated with sorafenib (and other BRAF inhibitors), thus careful attention should be given to the skin and oral mucosa when conducting physical examinations in patients receiving these drugs.
High numbers of dose reductions (56%) and significant incidence of drug discontinuation (16%) because of toxicity were reported with sorafenib at 400 mg b.i.d. These findings were consistent with the phase III DTC trial by Brose et al. [26]. In this trial, dose reductions were required in 64% of patients receiving sorafenib, and discontinuation because of adverse events was required for nearly 19% of patients in the sorafenib treatment arm [26]. It is interesting to note that Chen et al. [24] (not included in this meta-analysis) used half the dose of other studies (200 mg b.i.d. instead of 400 mg b.i.d.) and did not have any dose reductions or discontinuation while maintaining a similar response rate; however, it is important to point out that this was a very small study with only nine DTC patients. Future trials to evaluate different starting doses of sorafenib with larger sample sizes may be informative.
Although there is evidence of efficacy with TKIs, these drugs may diminish quality of life because of significant toxicities; therefore, it is important to assess the need for treatment. Most patients with metastatic disease do not require systemic therapy. Progressive disease within 1 year, symptomatic disease, and RAI-refractory disease (in the case of DTC) are indications for systemic therapy. Future studies should focus on salvage therapy after sorafenib failure, continuation of drug beyond progression, management of bony metastatic disease, symptom control, and quality of life measures to fully understand the role of sorafenib and other biochemotherapy agents.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgment
We thank Jaume Capdevila for contributing updated data.
Footnotes
For Further Reading: Taofeek K. Owonikoko, Rajasree P. Chowdry, Zhengjia Chen et al. Clinical Efficacy of Targeted Biologic Agents as Second-Line Therapy of Advanced Thyroid Cancer. The Oncologist 2013;18:1262–1269.
Implications for Practice: Significant benefit can be achieved in patients with iodine-refractory thyroid cancer treated with targeted agents in the first-line setting. It is currently unknown whether additional benefit would be obtained with the use of different biologic agents to treat patients after failing first-line therapy. This article documents the authors' experience using biologic agents as second-line treatment for advanced thyroid cancer and shows that patients derived additional benefit, albeit modest, in comparison to the front-line treatment. These findings are relevant for the clinical management of patients and for future studies of second-line targeted therapy of thyroid cancer.
Author Contributions
Conception/Design: Maria E. Cabanillas, Ligy Thomas, Rachel M. Regone
Provision of study material or patients: Maria E. Cabanillas
Collection and/or assembly of data: Maria E. Cabanillas, Ligy Thomas, Stephen Y. Lai, Ramona Dadu, Rachel M. Regone
Data analysis and interpretation: Maria E. Cabanillas, Wenli Dong, Lei Feng, Ramona Dadu
Manuscript writing: Maria E. Cabanillas, Ligy Thomas, Stephen Y. Lai, Ramona Dadu
Final approval of manuscript: Maria E. Cabanillas, Ligy Thomas, Stephen Y. Lai, Wenli Dong, Lei Feng, Ramona Dadu
Disclosures
Maria E. Cabanillas: Roche, Exelixis, Eisai (RF); Exelixis, Eisai (H); Stephen Y. Lai: GlaxoSmithKline (RF). The other authors indicated no financial relationships.
Section Editors: Herbert Chen: None; Stan Sidhu: None Reviewer “A”: None Reviewer “B”: None
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Roman S, Lin R, Sosa JA. Prognosis of medullary thyroid carcinoma: Demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer. 2006;107:2134–2142. doi: 10.1002/cncr.22244. [DOI] [PubMed] [Google Scholar]
- 2.Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 3.Pacini F, Schlumberger M, Dralle H, et al. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 2006;154:787–803. doi: 10.1530/eje.1.02158. [DOI] [PubMed] [Google Scholar]
- 4.Mazzaferri EL. An overview of the management of papillary and follicular thyroid carcinoma. Thyroid. 1999;9:421–427. doi: 10.1089/thy.1999.9.421. [DOI] [PubMed] [Google Scholar]
- 5.Schlumberger MJ. Diagnostic follow-up of well-differentiated thyroid carcinoma: Historical perspective and current status. J Endocrinol Invest. 1999;22(Suppl):3–7. [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network NCCN clinical practice guidelines in oncology (NCCN Guidelines): Thyroid carcinoma, version 2.2013. http://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf Available at.
- 7.Elisei R, Cosci B, Romei C, et al. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: A 10-year follow-up study. J Clin Endocrinol Metab. 2008;93:682–687. doi: 10.1210/jc.2007-1714. [DOI] [PubMed] [Google Scholar]
- 8.Kebebew E, Weng J, Bauer J, et al. The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann Surg. 2007;246:466–470; discussion 470–471. doi: 10.1097/SLA.0b013e318148563d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riesco-Eizaguirre G, Gutiérrez-Martínez P, García-Cabezas MA, et al. The oncogene BRAF V600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na+/I- targeting to the membrane. Endocr Relat Cancer. 2006;13:257–269. doi: 10.1677/erc.1.01119. [DOI] [PubMed] [Google Scholar]
- 10.Kimura ET, Nikiforova MN, Zhu Z, et al. High prevalence of BRAF mutations in thyroid cancer: Genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63:1454–1457. [PubMed] [Google Scholar]
- 11.Bhaijee F, Nikiforov YE. Molecular analysis of thyroid tumors. Endocr Pathol. 2011;22:126–133. doi: 10.1007/s12022-011-9170-y. [DOI] [PubMed] [Google Scholar]
- 12.Schulten HJ, Al-Maghrabi J, Al-Ghamdi K, et al. Mutational screening of RET, HRAS, KRAS, NRAS, BRAF, AKT1, and CTNNB1 in medullary thyroid carcinoma. Anticancer Res. 2011;31:4179–4183. [PubMed] [Google Scholar]
- 13.Boichard A, Croux L, Al Ghuzlan A, et al. Somatic RAS mutations occur in a large proportion of sporadic RET-negative medullary thyroid carcinomas and extend to a previously unidentified exon. J Clin Endocrinol Metab. 2012;97:E2031–E2035. doi: 10.1210/jc.2012-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moura MM, Cavaco BM, Pinto AE, et al. High prevalence of RAS mutations in RET-negative sporadic medullary thyroid carcinomas. J Clin Endocrinol Metab. 2011;96:E863–E868. doi: 10.1210/jc.2010-1921. [DOI] [PubMed] [Google Scholar]
- 15.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 16.Hoftijzer H, Heemstra KA, Morreau H, et al. Beneficial effects of sorafenib on tumor progression, but not on radioiodine uptake, in patients with differentiated thyroid carcinoma. Eur J Endocrinol. 2009;161:923–931. doi: 10.1530/EJE-09-0702. [DOI] [PubMed] [Google Scholar]
- 17.Kloos RT, Ringel MD, Knopp MV, et al. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol. 2009;27:1675–1684. doi: 10.1200/JCO.2008.18.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta-Abramson V, Troxel AB, Nellore A, et al. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol. 2008;26:4714–4719. doi: 10.1200/JCO.2008.16.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider TC, Abdulrahman RM, Corssmit EP, et al. Long-term analysis of the efficacy and tolerability of sorafenib in advanced radio-iodine refractory differentiated thyroid carcinoma: Final results of a phase II trial. Eur J Endocrinol. 2012;167:643–650. doi: 10.1530/EJE-12-0405. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed M, Barbachano Y, Riddell A, et al. Analysis of the efficacy and toxicity of sorafenib in thyroid cancer: A phase II study in a UK based population. Eur J Endocrinol. 2011;165:315–322. doi: 10.1530/EJE-11-0129. [DOI] [PubMed] [Google Scholar]
- 21.Cabanillas ME, Waguespack SG, Bronstein Y, et al. Treatment with tyrosine kinase inhibitors for patients with differentiated thyroid cancer: The M. D. Anderson experience. J Clin Endocrinol Metab. 2010;95:2588–2595. doi: 10.1210/jc.2009-1923. [DOI] [PubMed] [Google Scholar]
- 22.Hong DS, Cabanillas ME, Wheler J, et al. Inhibition of the Ras/Raf/MEK/ERK and RET kinase pathways with the combination of the multikinase inhibitor sorafenib and the farnesyltransferase inhibitor tipifarnib in medullary and differentiated thyroid malignancies. J Clin Endocrinol Metab. 2011;96:997–1005. doi: 10.1210/jc.2010-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Capdevila J, Iglesias L, Halperin I, et al. Sorafenib in metastatic thyroid cancer. Endocr Relat Cancer. 2012;19:209–216. doi: 10.1530/ERC-11-0351. [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Shen Y, Luo Q, et al. Response to sorafenib at a low dose in patients with radioiodine-refractory pulmonary metastases from papillary thyroid carcinoma. Thyroid. 2011;21:119–124. doi: 10.1089/thy.2010.0199. [DOI] [PubMed] [Google Scholar]
- 25.Lam ET, Ringel MD, Kloos RT, et al. Phase II clinical trial of sorafenib in metastatic medullary thyroid cancer. J Clin Oncol. 2010;28:2323–2330. doi: 10.1200/JCO.2009.25.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in locally advanced or metastatic patients with radioactive iodine-refractory differentiated thyroid cancer: The phase III DECISION trial. J Clin Oncol. 2013;31(suppl):4a. doi: 10.3978/j.issn.2304-3865.2014.01.02. [DOI] [PubMed] [Google Scholar]
- 27.Carr LL, Mankoff DA, Goulart BH, et al. Phase II study of daily sunitinib in FDG-PET-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin Cancer Res. 2010;16:5260–5268. doi: 10.1158/1078-0432.CCR-10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bible KC, Suman VJ, Molina JR, et al. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: Results of a phase 2 consortium study. Lancet Oncol. 2010;11:962–972. doi: 10.1016/S1470-2045(10)70203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen EE, Rosen LS, Vokes EE, et al. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: Results from a phase II study. J Clin Oncol. 2008;26:4708–4713. doi: 10.1200/JCO.2007.15.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cabanillas ME, Brose MS, Lee Y, et al. Antitumor activity of cabozantinib (XL184) in a cohort of patients (pts) with differentiated thyroid cancer (DTC) J Clin Oncol. 2012;30:5547a. [Google Scholar]
- 31.Schlumberger MJ, Elisei R, Bastholt L, et al. Phase II study of safety and efficacy of motesanib in patients with progressive or symptomatic, advanced or metastatic medullary thyroid cancer. J Clin Oncol. 2009;27:3794–3801. doi: 10.1200/JCO.2008.18.7815. [DOI] [PubMed] [Google Scholar]
- 32.Sherman SI, Wirth LJ, Droz JP, et al. Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med. 2008;359:31–42. doi: 10.1056/NEJMoa075853. [DOI] [PubMed] [Google Scholar]
- 33.Sherman SI, Jarzab B, Cabanillas ME, et al. A phase II trial of the multitargeted kinase inhibitor E7080 in advanced radioiodine (RAI)-refractory differentiated thyroid cancer (DTC) J Clin Oncol. 2011;29:5503a. [Google Scholar]
- 34.Kim KB, Cabanillas ME, Lazar AJ, et al. Clinical responses to vemurafenib in patients with metastatic papillary thyroid cancer harboring BRAF(V600E) mutation. Thyroid. 2013;23:1277–1283. doi: 10.1089/thy.2013.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayes DN, Lucas AS, Tanvetyanon T, et al. Phase II efficacy and pharmacogenomic study of selumetinib (AZD6244; ARRY-142886) in iodine-131 refractory papillary thyroid carcinoma with or without follicular elements. Clin Cancer Res. 2012;18:2056–2065. doi: 10.1158/1078-0432.CCR-11-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho AL, Grewal RK, Leboeuf R, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 2013;368:623–632. doi: 10.1056/NEJMoa1209288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim SM, Chang H, Yoon MJ, et al. A multicenter, phase II trial of everolimus in locally advanced or metastatic thyroid cancer of all histologic subtypes. Ann Oncol. 2013;24:3089–3094. doi: 10.1093/annonc/mdt379. [DOI] [PubMed] [Google Scholar]
- 38.Wells SA, Jr, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: A randomized, double-blind phase III trial. J Clin Oncol. 2012;30:134–141. doi: 10.1200/JCO.2011.35.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elisei R, Schlumberger MJ, Müller SP, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31:3639–3646. doi: 10.1200/JCO.2012.48.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savvides P, Nagaiah G, Lavertu P, et al. Phase II trial of sorafenib in patients with advanced anaplastic carcinoma of the thyroid. Thyroid. 2013;23:600–604. doi: 10.1089/thy.2012.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]