Abstract
In contrast with the wealth of data involving bHLH and homeodomain transcription factors in retinal cell type determination, the molecular bases underlying neurotransmitter subtype specification is far less understood. Using both gain and loss of function analyses in Xenopus, we investigated the putative implication of the bHLH factor Ascl1 in this process. We found that in addition to its previously characterized proneural function, Ascl1 also contributes to the specification of the GABAergic phenotype. We showed that it is necessary for retinal GABAergic cell genesis and sufficient in overexpression experiments to bias a subset of retinal precursor cells towards a GABAergic fate. We also analysed the relationships between Ascl1 and a set of other bHLH factors using an in vivo ectopic neurogenic assay. We demonstrated that Ascl1 has unique features as a GABAergic inducer and is epistatic over factors endowed with glutamatergic potentialities such as Neurog2, NeuroD1 or Atoh7. This functional specificity is conferred by the basic DNA binding domain of Ascl1 and involves a specific genetic network, distinct from that underlying its previously demonstrated effects on catecholaminergic differentiation. Our data show that GABAergic inducing activity of Ascl1 requires the direct transcriptional regulation of Ptf1a, providing therefore a new piece of the network governing neurotransmitter subtype specification during retinogenesis.
Introduction
During development, neural specification leads to the emergence of a large diversity of neuronal subtypes that will serve distinct functions in the adult nervous system. A key issue concerns the nature and action of the molecular cues underlying the acquisition of both generic and specific characteristics of neurons. A small number of basic helix-loop-helix (bHLH) transcription factors are necessary and sufficient for progenitor cell commitment towards a neuronal lineage at the expense of a glial fate and have consequently been qualified as “proneural genes” [1]. Beside their generic function, several studies have shown that proneural genes also display context-dependent effects contributing to the differentiation of particular neuronal subtypes [1], [2]. This is the case for the atonal-related neurogenin genes (Neurog1 and Neurog2) and the achaete scute gene Ascl1 (achaete-scute complex homolog 1; also called Mash1 in mouse or Xash1 in Xenopus). These genes are mostly expressed in complementary patterns in the murine central and peripheral nervous system where they drive the production of distinct neuronal populations [3], [4], [5], [6]; reviewed in [1], [7], [8]. In particular, Neurog2 and Ascl1 appear respectively as key regulators of glutamatergic (excitatory) versus GABAergic (inhibitory) neuronal fates during telencephalic development [4], [6], [9], [10], [11], [12]. The implication and requirement of Ascl1 in GABAergic cell specification has however proved to be highly time and space dependent [10], [13], [14]. In addition, Ascl1 is also involved in the development of other neuronal subtypes such as hindbrain serotonergic neurons [15], [16], central and peripheral noradrenergic neurons [17], [18], [19] or mesencephalic dopaminergic neurons [20]. Exploring its involvement in other nervous system regions, such as the retina, and unravelling how it integrates within distinct genetic networks are now required to better understand its divergent properties.
In the retina, beside its proneural function [21], [22], [23], Ascl1 may also have a more specialized role in the commitment of particular cell subtypes since its expression is restricted to subsets of neuronal progenitors, mostly distinct here again from the Neurog2-expressing ones [3], [24], [25], [26]. If numerous studies have addressed the intrinsic mechanisms responsible for the generation of the diverse classes of retinal neurons such as photoreceptor or ganglion cells [27], [28], [29], studies reporting on the molecular cues governing the specification of retinal subtypes with a specific neurotransmitter phenotype remain sparse [29], [30], [31], [32], [33], [34]. As in the brain, GABAergic and glutamatergic neurons represent the most abundant inhibitory and excitatory neurons of the retina, respectively. They are distributed among the 6 major classes of retinal neurons: photoreceptor, bipolar and ganglion cells are mainly glutamatergic, whereas most GABAergic retinal neurons are found within amacrine and horizontal cells. Only a few factors, such as the bHLH transcription factor BHLHB5 [35], the cofactor LMO4 [36], the orphan nuclear receptor Nr4a2 [37] or the PACAP receptor PAC1 [38] have so far been involved in GABAergic amacrine cell production in the retina.
Our previous studies and others highlighted that the bHLH gene Ptf1a (pancreas transcription factor 1a), drives inhibitory neuron commitment in the retina, at the expense of a glutamatergic destiny [39], [40], [41], [42], [43]. However, upstream regulators, partners and targets of Ptf1a within the retina remain to be investigated. Considering the GABAergic instructive function of Ascl1 in various regions of the brain, and the fact that Ptf1a expression is decreased in the dorsal spinal cord and retina of Ascl1−/− mice [14], [21], we asked whether it could be required for retinal GABAergic cell specification and whether it could take part to the Ptf1a transcriptional network.
To address these issues, we first analysed Ascl1 function during Xenopus retinogenesis. We found that Ascl1 is required for GABAergic retinal neuron genesis and sufficient to bias a subset of retinal progenitors towards a GABAergic destiny. Then, we took advantage of the Xenopus ectopic neurogenesis induction paradigm to investigate which genetic interactions contribute to Ascl1 activity as an inducer of neurotransmitter phenotypes. We showed that distinct Ascl1-dependent transcriptional networks sustain the production of GABAergic and catecholaminergic neurons. Together, our data suggest that the GABAergic activity of Ascl1 requires Ptf1a, this latter being a direct transcriptional target of Ascl1.
Materials and Methods
Constructs
pCS2-Ascl1 (also called Xash1 [44]), pCS2-Ptf1a, pCS2-Ptf1a-GR [45], pCS2-XNeurog2 (also called XNgnr-1), pCS2-Neurog2-GR [46], pCS2-Atoh7 (also called Xath5 [47]), pCS2-NeuroD1 (also called NeuroD [48]), pCS2-GFP [49] and all glucocorticoid-inducible chimeric Ascl1:Neurog2 constructs [50] have previously been described. pCS2-flag-Ascl1-GR was generated by subcloning the Ascl1 coding sequence from pCS2-NLSMT plasmid into EcoRI and XhoI sites of pCS2-flag-GR [51]. Protein activity of GR constructs was induced by addition of 4 μg/ml dexamethasone (dex, Sigma).
Embryos, in vitro RNA synthesis and microinjection
Xenopus laevis embryos (up to stage 41) were obtained by conventional procedures of in vitro fertilization and staged according to Nieuwkoop and Faber [52]. At the desired stage, embryos were fixed in 4% paraformaldehyde. Capped sense mRNAs were prepared from CS2 plasmids after Not1 digestion and transcribed using the mMessage mMachine SP6 kit (Ambion). mRNAs were then purified with Sephadex Column (Roche). 100–150 pg of mRNAs were injected into one or two blastomeres at the two- or four-cell stage. GFP mRNA was co-injected as a tracer. Loss of function experiments were performed using already described and validated antisense oligonucleotides morpholinos: Ptf1a-Mo [39], Ascl1-Mo (a mix of two morpholinos, Ascl1-Mo1 and Ascl1-Mo2, that target the two Xenopus laevis Ascl1 alloalleles; [53]), Phox2a-Mo and Hand2-Mo [53]. As a control, standard morpholinos purchased from GeneTools (LLC) were used. 8 ng of morpholinos were injected into one or two blastomeres at the two-cell stage. All embryos were co-injected with GFP mRNA as a tracer and only embryos exhibiting GFP fluorescence in the eye were selected for further analysis.
In vivo lipofection
pCS2-Ascl1, pCS2-Neurog2, pCS2-Atoh7, pCS2-NeuroD1 were transfected in stage 18 neurula into the presumptive region of the retina as previously described [54], [55]. pCS2-GFP was co-lipofected and used as a tracer to follow transfected cells. Of note, co-lipofection efficiency was previously shown to be extremely high (85%–100%) [54]. Embryos were fixed at stage 41 in 4% paraformaldehyde plus 0,3% glutaraldehyde and cryostat sectioned (12 μm). Transfected cells were counted and cell types were identified based upon their laminar position and morphology.
Immunohistochemistry
Immunohistochemistry was performed using rabbit polyclonal anti-GABA (1/1000; ImmunoStar), mouse monoclonal anti-GFP (1/200; Molecular Probes) and anti-mouse or anti-rabbit fluorescent secondary antibodies (1/1000; Alexa, Molecular Probes). Cell nuclei were counterstained with Hoechst (Sigma).
In situ hybridization
Digoxigenin-labeled antisens RNA probes for gad1, VGlut1 [56], TH [53], tubb2b [48], Bru [57], dpysl3 [58], Ptf1a [45] and Ascl1 [50] were generated according to the manufacturer’s instruction (Roche). Whole mount in situ hybridization [59] and double fluorescent in situ hybridizations on cryosections [60] were carried out as previously described.
RT-qPCR
Stage 19 embryos were treated with cycloheximide (CHX, 10 μg/ml) during 2.5 hours. Total RNA from 8 embryos was then isolated using the Nucleospin RNA XS kit (Macherey Nagel). Reverse transcription was performed using IscriptcDNA Synthesis Kit (Biorad). RNA quality was evaluated using Experion (BioRad). qPCR reactions were performed in triplicate using SsoFast Eva green Supermix (Biorad) on a C1000 Thermal Cycler (CFX96 Real-Time System, Biorad). All values were normalized to the level of the reference gene ornithine decarboxylase (ODC) using Biorad CFX Manager Software. Primers sequences are: tubb2b forward 5′cccgtgccatccttgtggatttt3′, tubb2b reverse 5′gcccagttattgccagcaccactt3′, Ptf1a forward 5′gccgctcaggaaccccaaca3′, Ptf1a reverse 5′ggcagcccgtagtctgggtca3′, ODC forward 5′catggcattctccctgaagtacaagaa3′ and ODC reverse 5′ggacagtggtaggggcaagctca3′.
Western-blot analyses
Total protein lysates were prepared from stage 14 embryos and submitted to western blot analysis using an anti-Myc antibody (Sigma) as previously described [61].
Image analysis and quantification
Shown in figures are representative data from one experiment that has been performed at least in duplicate. Fluorescent staining was visualized with a M2 Zeiss microscope. Images were captured with a digital camera AxioCam MRc and AxioVision Rel 7.8 software. The quantification of in situ hybridization signal intensity in the eyes of whole embryos was quantified using Adobe Photoshop CS4.
Ethics statement
All animal procedures were conducted under the supervision of several licensed personnel, including the director of research of the CNRS and professor at the university Paris-Sud, with licenses to perform Xenopus experimentation (authorization 91-29 and 91-28), in accordance with French government policies. The study was conducted under an institutional license (number B 91-471-102 up to 2012 and C 91-471-102 since 2013). The study protocol was approved by the institutional animal care committee, the Direction Départementale de la Protection des Populations (license B/C 91-471-102).
Results
Ascl1 knockdown impairs retinal GABAergic neuron genesis
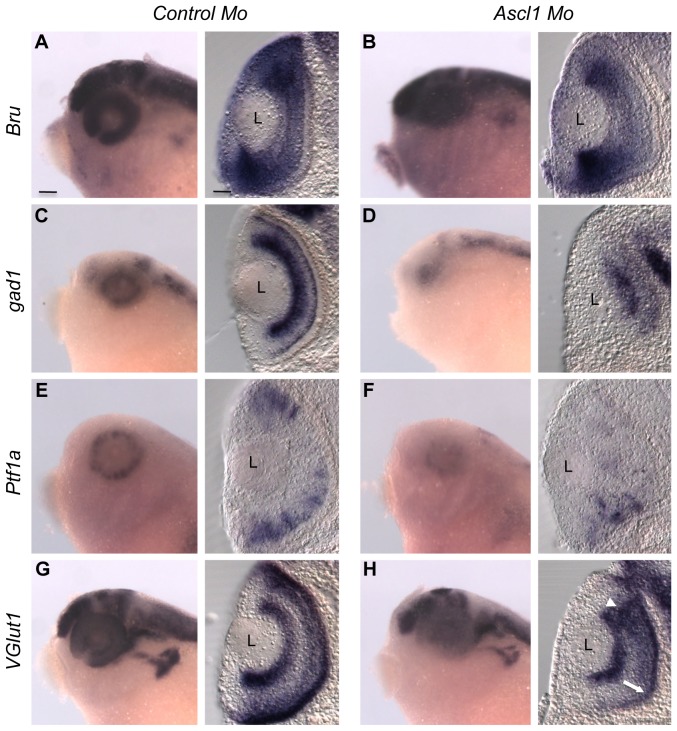
To address the potential requirement of Ascl1 in GABAergic and glutamatergic phenotype acquisition, we performed morpholino (Mo) injections in two cell stage embryos. We examined the effect of Ascl1 loss-of-function by analysing the expression of gad1 (glutamic acid decarboxylase) and VGlut1 (vesicular glutamate transporter 1), which respectively encode the rate-limiting enzyme for GABA biosynthesis and a glutamate transporter expressed in neurons. The expression of a retinal pan-neuronal marker Bru [57] remained largely unaffected in Ascl1-Mo injected retinas compared to control ones (80% unaffected, n = 10 embryos; Fig. 1A, B), suggesting that Ascl1 knockdown does not significantly impair neuronal commitment within the retina, as shown in the mouse [23]. Ascl1 inhibition however caused a marked reduction of gad1 retinal expression (100% reduced, n = 10 embryos; Fig. 1C, D), indicative of an impaired production of GABAergic neurons. Importantly, this was accompanied by a significant decrease in the expression of Ptf1a (90% reduced, n = 10 embryos; Fig. 1E, F), a crucial determining factor of GABAergic fate in the retina [39], [40], [43]. Then, we observed in Ascl1-Mo injected retinas that the different glutamatergic neuronal populations were unevenly affected, with VGlut1 expression being largely unchanged in ganglion cells, reduced in the photoreceptor layer, while appearing expanded in the inner nuclear layer (INL) (Fig. 1G, H). Thus, in contrast to the Ptf1a knockdown phenotype [39], Ascl1 morphant retinas do not exhibit an overall increase of VGlut1 staining. As a whole, blocking Ascl1 mainly precludes commitment of precursor cells towards a GABAergic fate. However, the overall observed defects, in particular the loss of some glutamatergic cells in the outer nuclear layer, cannot be simply explained by a switch from GABAergic to glutamatergic cell types. A plausible explanation would be that part of the Ascl1 knockdown phenotype primarily results from a loss of its proneural activity.
Figure 1. Ascl1 is required for retinal GABAergic cell genesis.
Whole mount in situ hybridization on stage 35 embryos following Ascl1 or control morpholino (Mo) injection in one blastomere at the two-cell stage. Shown for each indicated probe are representative pictures of the observed labelling in the head region (lateral views, anterior on the left) and on retinal cross sections (dorsal side up). The expression of the pan-neuronal marker Bru (A, B) is not affected by Ascl1 knockdown while gad1 and Ptf1a stainings are severely reduced (C-F). VGlut1 expression (G, H) is unequally affected in the different cell layers: decreased in the photoreceptor layer (white arrow) and extended in the inner nuclear layer (white arrowhead). L: Lens. Scale bar represents 300 μm (heads) or 50 μm (sections).
Ascl1 overexpression in the retina biases a subset of precursor cells towards a GABAergic destiny
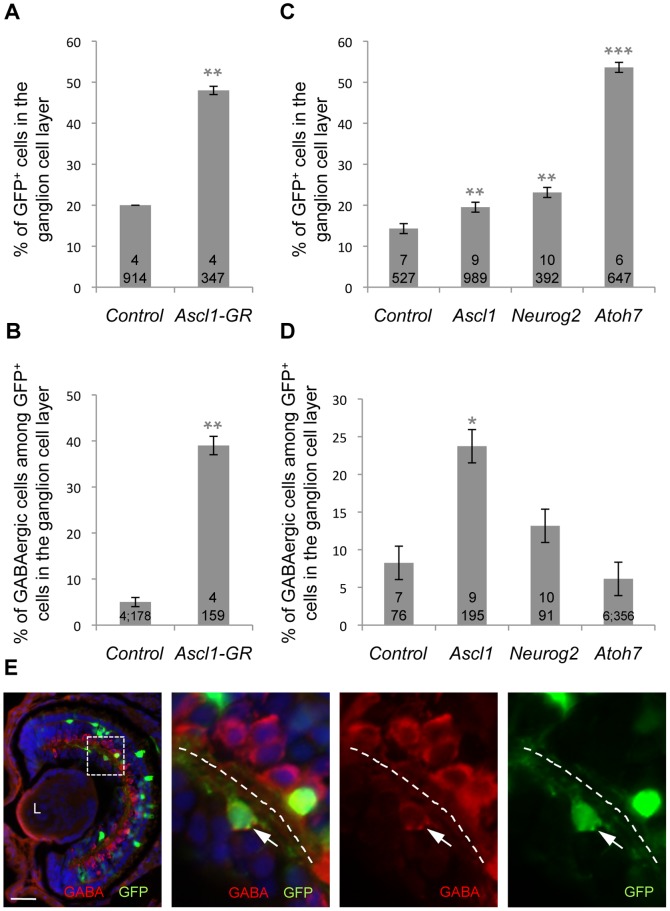
To investigate in more detail Ascl1 involvement in the specification of neurotransmitter phenotypes, we turned to a gain of function strategy using a glucocorticoid-inducible Ascl1 construct (Ascl1-GR), which avoids affecting early nervous system development. We analysed the fate of Ascl1-overexpressing cells at stage 38. Contrasting with the Ptf1a gain of function, which results in a dramatic overproduction of amacrine and horizontal cells [39], Ascl1 overexpression led to a typical neurogenic phenotype, with early born neurons being increased (ganglion cells, Fig. 2A) at the expense of late born cells (bipolar and Müller glial cells, data not shown). However, similarly to the Ptf1a phenotype, quantification of GABA-positive neurons among Ascl1-overexpressing cells revealed an enhanced GABAergic neuronal yield compared to the control situation (Fig. 2B). These supernumerary inhibitory neurons were found within the ganglion cell layer and thus probably correspond to displaced amacrine cells [62]. Importantly, similar results were obtained in a clonal analysis after in vivo lipofection (Fig. 2C-E). This rules out the possibility that the Ascl1 phenotype observed in mRNA injection experiments may be secondary to early morphogenetic defects of the retina and confirms that Ascl1 exerts a cell-autonomous function in GABAergic cell type determination.
Figure 2. Ascl1 overexpression in the retina favours GABAergic cell genesis.
Cell fate analysis following overexpression of the indicated construct by either mRNA injection in one blastomere at the four-cell stage and dex treatment at stage 16 (A, B; analysis at stage 38) or in vivo lipofection at the neurula stage (C, D; analysis at stage 41). In both cases, GFP was used as a tracer to visualize injected/transfected cells. Note that Ascl1, Neurog2 and Atoh7 lipofections all result in an increased percentage of cells in the ganglion cell layer but only Ascl1-overexpressing cells are biased towards a GABAergic destiny. Total number of analyzed retinas and counted cells per condition is indicated in each bar. Values are given as mean +/– s.e.m. p<0,001 (***), p<0,01 (**), p<0,05 (*) (Student’s t-test). (E) shows a typical section of stage 41 retinas lipofected with GFP plus Ascl1 and immunostained with anti-GABA (red) and anti-GFP (green) antibodies. Panels on the right are higher magnifications of the dotted square delineated region. The dotted line indicates the inner plexiform layer, with the ganglion cell layer on the left and the inner nuclear layer on the right. White arrow points to a transfected GABA-positive cell within the ganglion cell layer. L: Lens. Scale bar represents 300 μm (heads) or 50 μm (sections).
In order to figure out whether this GABAergic inducing activity is specific to Ascl1 compared to other bHLH proteins endowed as well with neurogenic properties, we performed similar in vivo lipofection experiments with the atonal-related genes Neurog2 and Atoh7. As expected from previous studies [46], [47], [63], overexpression of these genes, as for Ascl1, resulted in an imbalanced production of early- versus late-born cell types (Fig. 2C and data not shown). However, in contrast to the Ascl1 gain of function phenotype, the ratio of GABAergic neurons among Neurog2 or Atoh7 transfected cells in the ganglion cell layer did not differ from the control situation (Fig. 2D).
Altogether, our data highlight that, although Ascl1 shares with Neurog2 or Atoh7 a neurogenic activity impacting on retinal cell type distribution, it has the specific ability to bias a subset of progenitors towards a GABAergic fate.
Ascl1 has a specific GABAergic inducing activity and is epistatic to glutamatergic factors
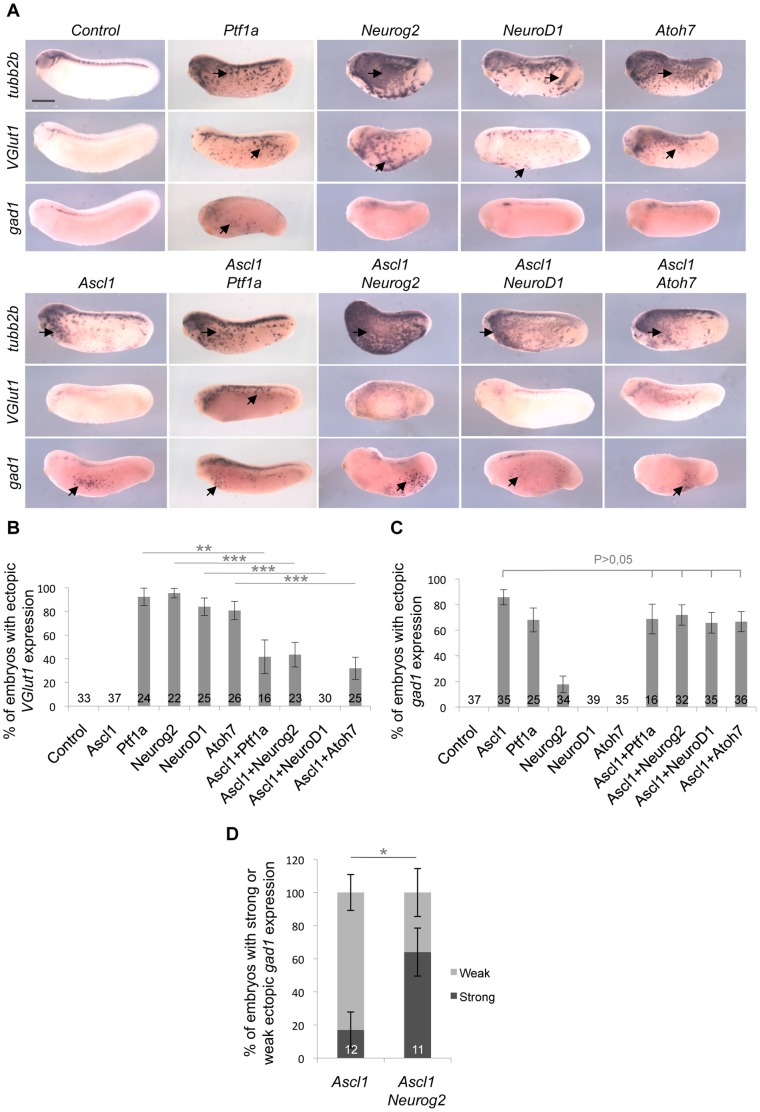
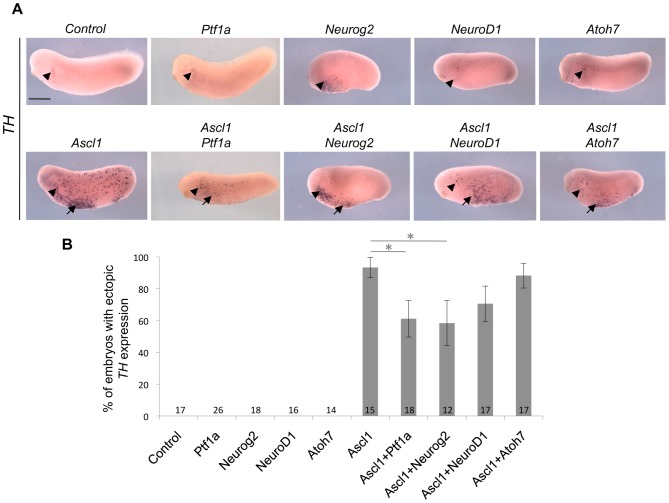
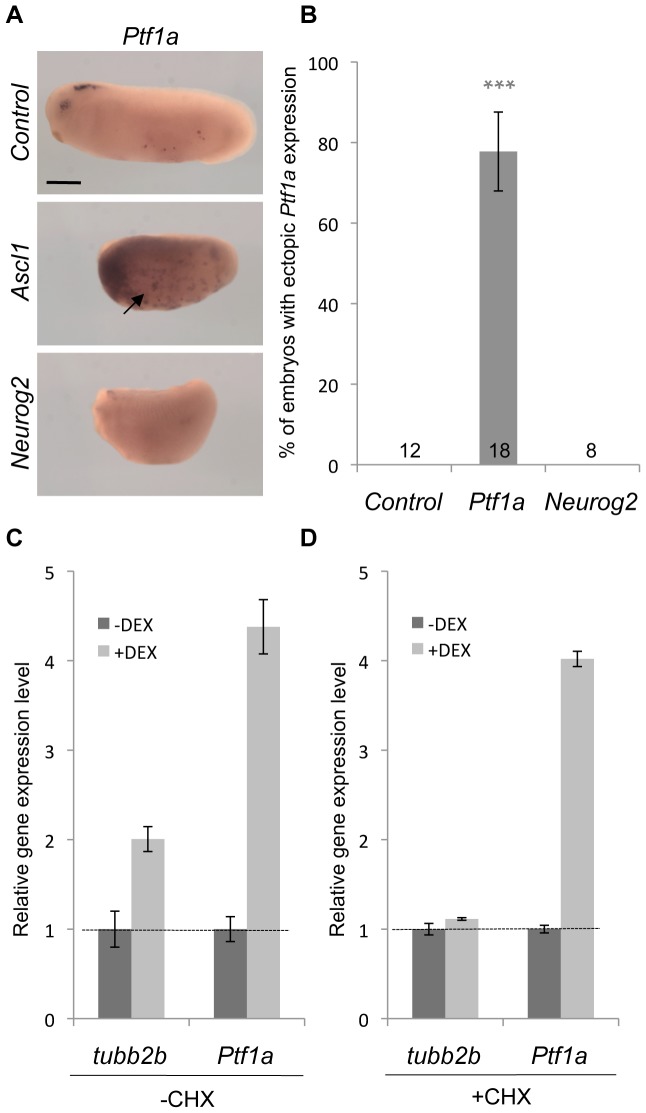
It has previously been shown that Ascl1, Neurog2, NeuroD1, Atoh7 and Ptf1a are all sufficient to induce ectopic neurogenesis when overexpressed in the epidermis of Xenopus embryos [47], [50], [64], [65], [66], [67]. To further assess the functional specificity of Ascl1 as a GABAergic inducing factor, we injected the corresponding mRNA as well as those encoding the aforementioned bHLH factors and compared the neurotransmitter phenotypes they induced in ectopic neurons formed within the epidermis. As expected, each of them induced ectopic tubb2b (previously called N-tubulin) expression in stage 24 embryos (Fig. 3A). Except for Ascl1, this was associated with a robust VGlut1 staining (Fig. 3A, B). Only Ptf1a and Ascl1 were able to promote ectopic gad1 expression (Fig. 3A, C). We therefore conclude that Ascl1 has the unique property among these factors, to convert presumptive epidermal cells into GABAergic neurons without simultaneously inducing glutamatergic ones.
Figure 3. Comparison of GABAergic and glutamatergic inducing activities of five different bHLH factors.
Whole mount in situ hybridization analyses of tubb2b, VGlut1 or gad1 expression on stage 24 embryos injected with the indicated mRNAs in one blastomere at the two-cell stage. (A) Arrows indicate ectopic expression in the epidermis (anterior on the left, dorsal side up). All the tested bHLH factors exhibit neurogenic activity, as inferred from tubb2b ectopic expression. However, only Ascl1 specifically induces gad1 + neurons without inducing VGlut1 + ones. (B, C) Quantification of embryos displaying VGlut1 (B) or gad1 (C) ectopic expression. Ascl1 interferes with Ptf1a-, Neurog2-, NeuroD1- and Atoh7-dependent production of VGlut1 + neurons, while none of these factors affect Ascl1 GABAergic inducing activity. (D) Quantification of embryos with weak or strong ectopic gad1 staining following mRNA injection of Ascl1 alone or together with Neurog2. Note that Neurog2 enhances Ascl1 GABAergic inducing activity. Total number of analyzed embryos per condition is indicated in each bar. Error bars represent 95% confidence intervals. p<0,001 (***), p<0,01 (**), p<0,05 (*) (binomial test). Scale bar represents 500 μm.
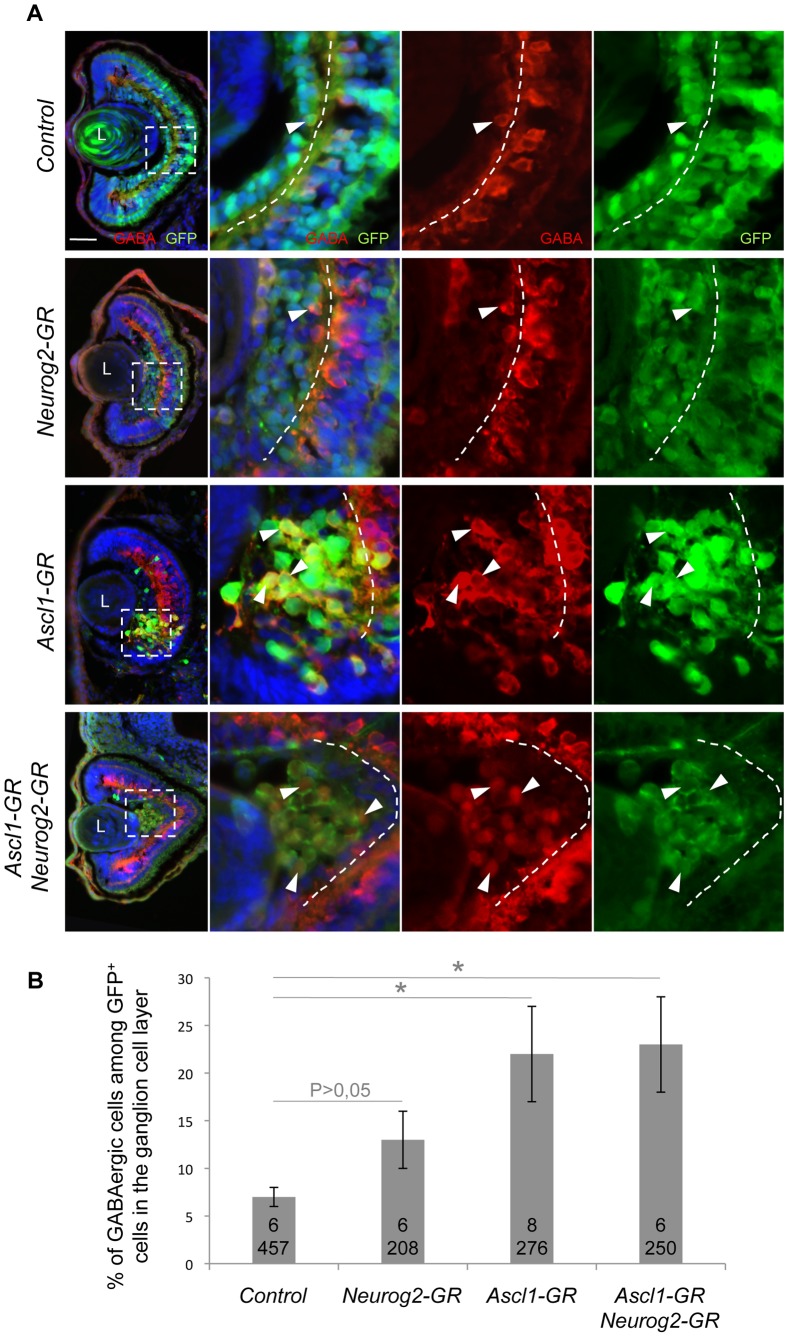
We next wondered whether Ascl1 might act as an inhibitor of glutamatergic neuron genesis and thus investigated the genetic relationships between Ascl1 and the other bHLH genes, in co-injection experiments. The glutamatergic inducing activities of Neurog2, NeuroD1, Atoh7 and Ptf1a were all dramatically reduced upon Ascl1 misexpression (Fig. 3A, B), indicating that Ascl1 indeed actively represses the glutamatergic fate of ectopically produced neurons. In contrast, neither Neurog2, NeuroD1 or Atoh7 were able to interfere with the Ascl1-dependent induction of GABAergic neurons (Fig. 3A, C). Surprisingly, we found that embryos overexpressing both Ascl1 and Neurog2 displayed more ectopic gad1-positive cells compared to embryos injected with Ascl1 alone (Fig. 3D), suggesting an unexpected synergistic effect. Altogether, these data demonstrate that, in this ectopic context, Ascl1 is epitastic to these various bHLH genes. In the retina as well, overexpression of Ascl1 together with Neurog2 still resulted in an increased production of GABAergic cells within the ganglion cell layer (Fig. 4A, B). However, no synergy was observed in this context.
Figure 4. Ascl1 overexpression together with Neurog2 still promotes production of GABAergic cells in the retina.
(A) Stage 41 retinal sections immunostained with anti-GABA (red) and anti-GFP (green) antibodies. Embryos were injected with the indicated mRNAs in one blastomere at the four-cell stage and treated with dexamethasone at stage 16. Panels on the right are higher magnifications of the dotted square delineated region. The dotted line indicates the inner plexiform layer. Arrowheads point to GFP/GABA-positive cells within the ganglion cell layer. (B) Quantification of GABAergic cell proportion among GFP+ cells in the ganglion cell layer, showing that Ascl1 GABAergic inducing activity is not affected by Neurog2 misexpression. Total number of analyzed retinas and counted cells per condition is indicated in each bar. p<0,05 (*) (Student’s t-test). Values are given +/– s.e.m. L: Lens. Scale bar represents 50 μm.
Ascl1 catecholaminergic inducing activity is impaired by glutamatergic factors
As recently shown, Ascl1 misexpression in the epidermis also leads to the ectopic generation of catecholaminergic neurons [53], authenticated by the expression of tyrosine hydroxylase (TH). We thus decided to pursue our comparative study by monitoring TH labelling after Ascl1, Neurog2, NeuroD1, Atoh7 or Ptf1a mRNA injection. In contrast to Ascl1, none of these genes were able to promote the production of TH-positive neurons, emphasizing again the specific role of Ascl1 in neuronal subtype determination (Fig. 5A). Epistatic analyses revealed that the proportion of embryos displaying ectopic TH expression was slightly decreased upon co-injection of Ascl1 with either Atoh7 or NeuroD1, although this was not statistically significant. It was however significantly reduced when Ascl1 was misexpressed together with Neurog2 or Ptf1a, suggesting that these two genes could interfere with the Ascl1 catecholaminergic inducing activity (Fig. 5A, B). Such a result contrasts with the interaction previously demonstrated for the induction of GABAergic neurons and reflects that the same set of transcription factors establishes variable genetic interactions within distinct differentiation pathways.
Figure 5. Comparison of the catecholaminergic inducing activities of five different bHLH factors.
(A) Whole mount in situ hybridization analysis of Tyrosine Hydroxylase (TH) expression on stage 24 embryos injected with the indicated mRNAs in one blastomere at the two-cell stage (anterior on the left, dorsal side up). Arrowheads indicate the position of previously described TH-positive antero-ventral neurons [53], while arrows point to ectopic TH staining. Note that, among all tested bHLH factors, only Ascl1 induces ectopic TH expression. Sibling embryos were also hybridized with the tubb2b probe as a positive control (see Figure 3A). (B) Quantification of embryos displaying ectopic TH+ neurons, showing that Neurog2 and Ptf1a significantly reduce the catecholaminergic inducing activity of Ascl1. Total number of analyzed embryos per condition is indicated in each bar. Error bars represent 95% confidence intervals. p<0,05 (*) (binomial test). Scale bar represents 500 μm.
The GABAergic and catecholaminergic inducing activities of Ascl1 are conferred by its basic domain
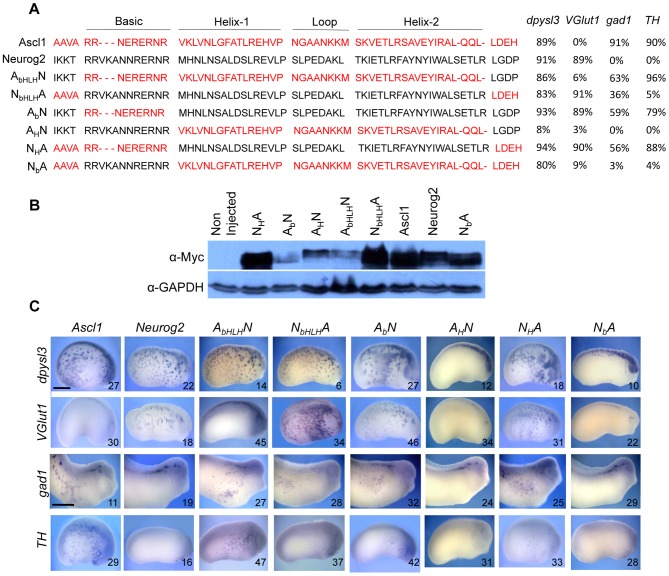
We next examined which Ascl1 domains account for the protein activity in neuronal subtype specification. We used a series of chimeric Ascl1:Neurog2 constructs (Fig. 6A) with interchanged basic and/or HLH domains (DNA binding and dimerization domain, respectively) and tested their ability to induce TH, gad1 and VGlut1 ectopic expression. Importantly, the chimeric proteins were properly expressed following mRNA injection (Fig. 6B) and all but one (AHN) retained a neurogenic activity, as inferred by their ability to promote the expression of the pan-neuronal marker dpysl3 (previously called CRMP4; Fig. 6A, C). Noticeably, swapping the entire bHLH domains of Ascl1 and Neurog2 was sufficient to invert their respective properties in neurotransmitter phenotype induction. The Neurog2 protein containing the Ascl1 bHLH indeed converted into a GABAergic and catecholaminergic neuron inducer, while the Ascl1 protein with the Neurog2 bHLH turned into a glutamatergic one (Fig. 6A, C; compare AbHLHN to Ascl1 and NbHLHA to Neurog2). This demonstrates that the functional specificity of these two proteins resides in their respective bHLH domains. In addition, we found that the presence of the Ascl1 basic domain in chimeric constructs was necessary and sufficient to trigger TH and gad1 ectopic expression (Fig. 6A, C; compare AbN and NHA to NbA for gad1 and TH staining). Conversely, only the chimeric proteins containing the Neurog2 HLH domain had the ability to induce glutamatergic neurons (Fig. 6A, C; see VGlut1 labelling for AbN and NHA versus NbA). In line with this observation, the two chimeric proteins containing both the basic domain of Ascl1 and the HLH of Neurog2 (AbN and NHA proteins) were able to simultaneously promote gad1, TH and VGlut1 expression. Altogether, these results suggest that the functional specificities of Ascl1 and Neurog2 in neurotransmitter subtype specification do not reside in the same protein domain. GABAergic and catecholaminergic inducing activity of Ascl1 primarily relies on its basic domain while glutamatergic inducing activity of Neurog2 is imparted by its HLH domain.
Figure 6. Mapping of Ascl1 and Neurog2 domains accounting for their functional specificity in neuronal subtype specification.
(A) bHLH protein sequences of the Myc-tagged inducible Ascl1:Neurog2 chimeric constructs used in B and C. Ascl1 and Neurog2 sequences are depicted in red and black, respectively. Shown on the right and illustrated in (C) is the ability of the different proteins to induce dpysl3, VGlut1, gad1 or TH. Percentages of embryos with ectopic expression are indicated. (B, C) Embryos were injected in one blastomere at the four-cell stage with the indicated wild-type or chimeric mRNA and dexamethasone was added at stage 13. (B) Western-blot using an anti-Myc antibody showing that each chimeric protein has been produced. Detection of GAPDH serves as a loading control. (C) Whole mount in situ hybridization analyses of dpysl3, VGlut1, gad1 or TH expression on stage 22 or 28 injected embryos (anterior on the right, dorsal side up). Altogether, these domain-swapping experiments show that the GABAergic inducing activity of Ascl1 resides in its basic domain, while the glutamatergic inducing activity of Neurog2 is imparted by its HLH domain. In each panel the number of analysed embryos is indicated. Scale bar represents 250 μm.
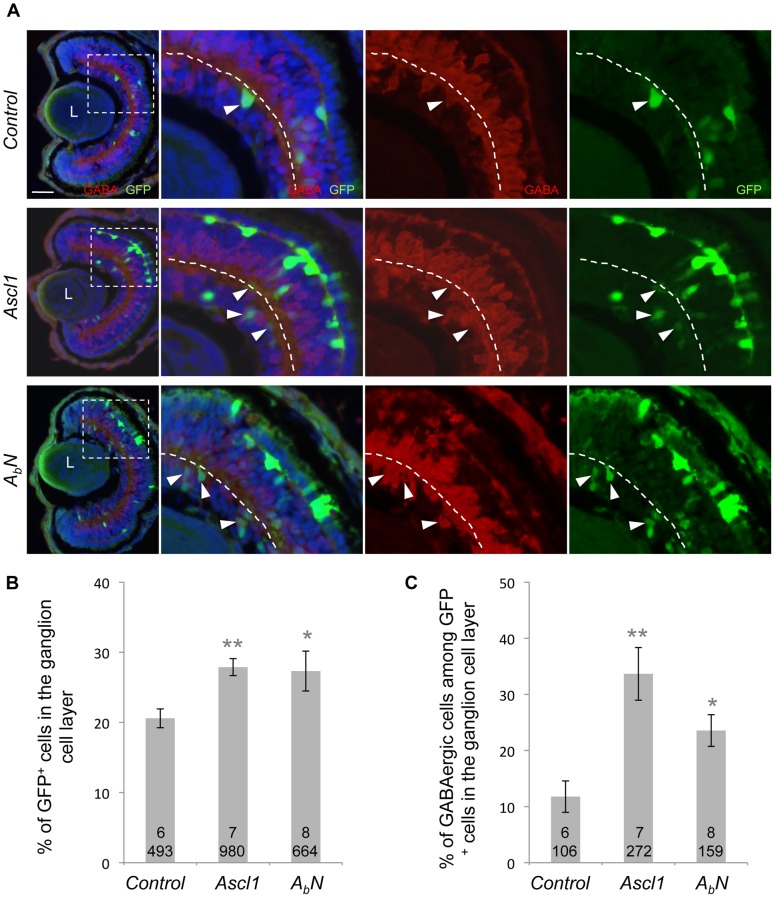
To assess whether this also holds true within the retina, we overexpressed the chimeric construct AbN by in vivo lipofection (Fig. 7A). Similarly to wild-type Ascl1, AbN transfection increased the proportion of cells within the ganglion cell layer (Fig. 7B) and biased these additional neurons towards a GABAergic destiny (Fig. 7C). This indicates that Ascl1 basic domain is sufficient to drive a subset of retinal progenitor cells towards a GABAergic fate in the retina.
Figure 7. Retinal GABAergic inducing activity of Ascl1 resides in its basic domain.
(A) Stage 41 retinal sections immunostained with anti-GABA (red) and anti-GFP (green) antibodies. Embryos were lipofected with Ascl1 or the inducible ABN chimeric construct (encoding a Neurog2 protein with the basic domain of AsclI). Dexamethasone was added immediately after lipofection at stage 18. Panels on the right are higher magnifications of the dotted square delineated region. The dotted line indicates the inner plexiform layer. Arrowheads indicate GABA+/GFP+ cells within the ganglion cell layer. (B-C) Quantification of GFP+ cells and GABA+/GFP+ in the ganglion cell layer showing that similarly to Ascl1, ABN increases the percentage of cells in the ganglion cell layer but also biases these cells towards a GABAergic destiny. Total number of analyzed retinas and counted cells per condition is indicated in each bar. Values are given as mean +/– s.e.m. p<0,01 (**), p<0,05 (*) (Student’s t-test). L: Lens. Scale bar represents 50 μm.
Phox2a and Hand2 are essential downstream components of Ascl1 catecholaminergic pathway but are dispensable for its GABAergic inducing activity
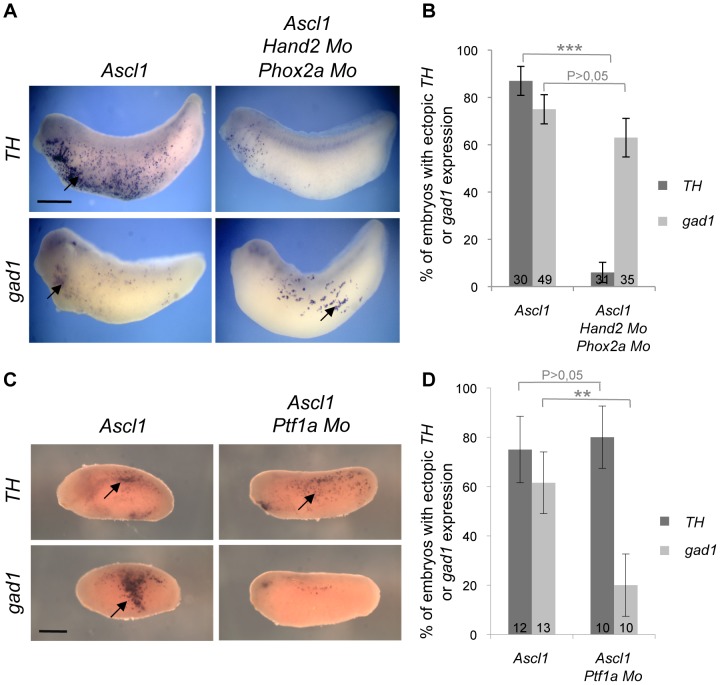
The DNA binding basic domain of Ascl1 being central to its activity, we next sought to identify potential transcriptional targets involved in its GABAergic versus catecholaminergic inducing activity. The homeodomain Phox2a and bHLH Hand2 proteins act downstream Ascl1 for the determination and differentiation of noradrenergic neurons [17], [19], [68], [69]. To examine whether these genes may also be involved in Ascl1 GABAergic inducing activity, we simultaneously misexpressed Ascl1 while knocking down both Phox2a and Hand2 using specific morpholinos. As previously described, this resulted in a significantly decreased proportion of embryos exhibiting ectopic TH staining. In contrast, the ability of Ascl1 to induce gad1-positive neurons appeared largely unaffected (Fig. 8A, B). This demonstrates that Phox2a and Hand2, although required for Ascl1-dependent production of catecholaminergic neurons, are dispensable for its GABAergic inducing activity.
Figure 8. Distinct transcriptional networks sustain Ascl1 GABAergic and catecholaminergic inducing activities.
Whole mount in situ hybridization analyses of TH or gad1 expression on stage 24 embryos following injection of mRNA and/or morpholinos (Mo), as indicated, in one blastomere at the two-cell stage. (A, C) Arrows point to ectopic TH or gad1 expression (anterior on the left, dorsal side up). Sibling embryos were also hybridized with the tubb2b probe as a positive control (data not shown). (B, D) Quantification of embryos displaying ectopic TH or gad1 expression. Note that Phox2a and Hand2 knockdown significantly reduces Ascl1 ability to induce TH expression but does not impair its GABAergic inducing activity (B), which specifically depends on Ptf1a (D). Total number of analyzed embryos per condition is indicated in each bar. Error bars represent 95% confidence intervals. P<0,001 (***), p<0,01 (**) (binomial test). Scale bar represents 500 μm.
Ptf1a is required for Ascl1 GABAergic inducing activity as a direct transcriptional target
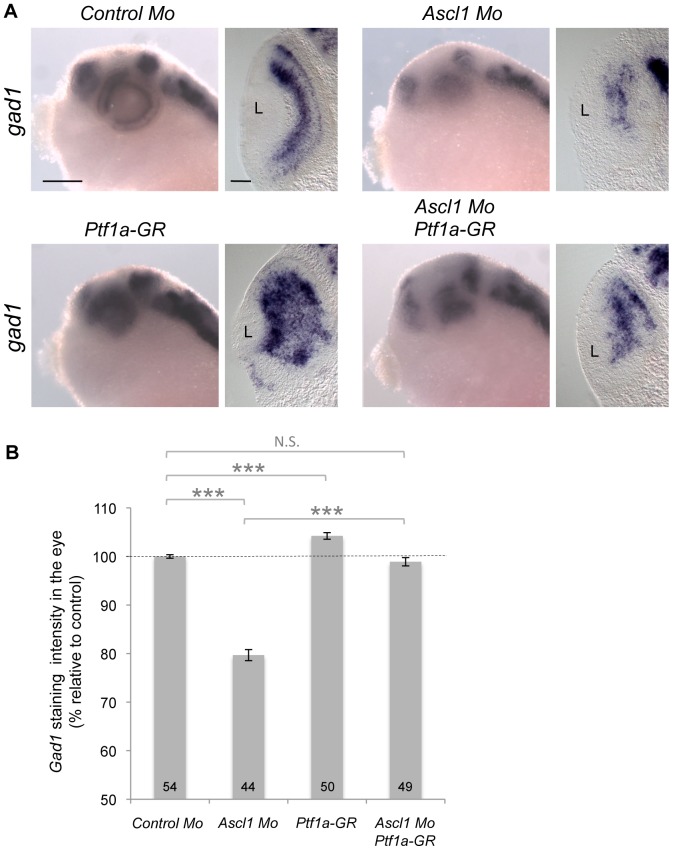
Since both Ascl1 and Ptf1a share the ability to induce ectopic GABAergic neurons, in contrast to the other tested bHLH genes (Fig. 3), we next investigated whether they could act in the same transcriptional network. We first examined Ptf1a requirement for Ascl1 GABAergic and catecholaminergic inducing activities by simultaneously overexpressing Ascl1 and knocking down Ptf1a (Fig. 8C, D). The Ascl1-dependent ectopic TH expression was unaffected by Ptf1a inhibition, showing that this gene is dispensable for Ascl1 catecholaminergic inducing activity. In contrast, the percentage of embryos displaying ectopic gad1 staining was significantly decreased by the concomitant blockade of Ptf1a. These results indicate that Ptf1a acts downstream Ascl1 for the generation of ectopic GABAergic neurons.
We then tested whether this Ptf1a dependency might rely on a transcriptional interaction. We found that Ascl1 mRNA injection promoted Ptf1a ectopic expression in the epidermis (Fig. 9A, B). In contrast, Neurog2 misexpression was unable to do so. Together, these data suggest the existence of a positive transcriptional regulation exerted by Ascl1 on Ptf1a. We thus investigated whether Ptf1a could constitute a direct target of Ascl1. In this purpose, we performed gene induction assays on Ascl1-GR injected embryos in the absence of protein synthesis, using the translation-blocking drug cycloheximide (CHX). In the absence of CHX, Ascl1 overexpression lead to a strong increase of both tubb2b and Ptf1a expression level, as assayed by RT-qPCR analysis (Fig. 9C). Only Ptf1a up-regulation persisted upon CHX treatment, consistent with Ptf1a being a direct Ascl1 target gene (Fig. 9D).
Figure 9. Ptf1a is a direct transcriptional target of Ascl1.
(A) Whole mount in situ hybridization analyses of Ptf1a expression on stage 22 embryos injected with Ascl1 or Neurog2 mRNA in one blastomere at the two-cell stage. Note that Ascl1 is able to activate Ptf1a expression in the epidermis (arrow). Sibling embryos were also hybridized with the tubb2b probe as a positive control (data not shown). (B) Quantification of embryos displaying Ptf1a ectopic expression. Total number of analysed embryos per condition is indicated in each bar. Error bars represent 95% confidence intervals. P<0,001 (***) (binomial test). (C, D) RT-qPCR analyses of tubb2b and Ptf1a expression in embryos injected with Ascl1-GR at the two-cell stage and treated with dexamethasone (dex) and/or cycloheximide (CHX) from stage 19 to stage 21. Note that Ptf1a up-regulation upon dex treatment is maintained in the presence of CHX. Scale bar represents 500 μm.
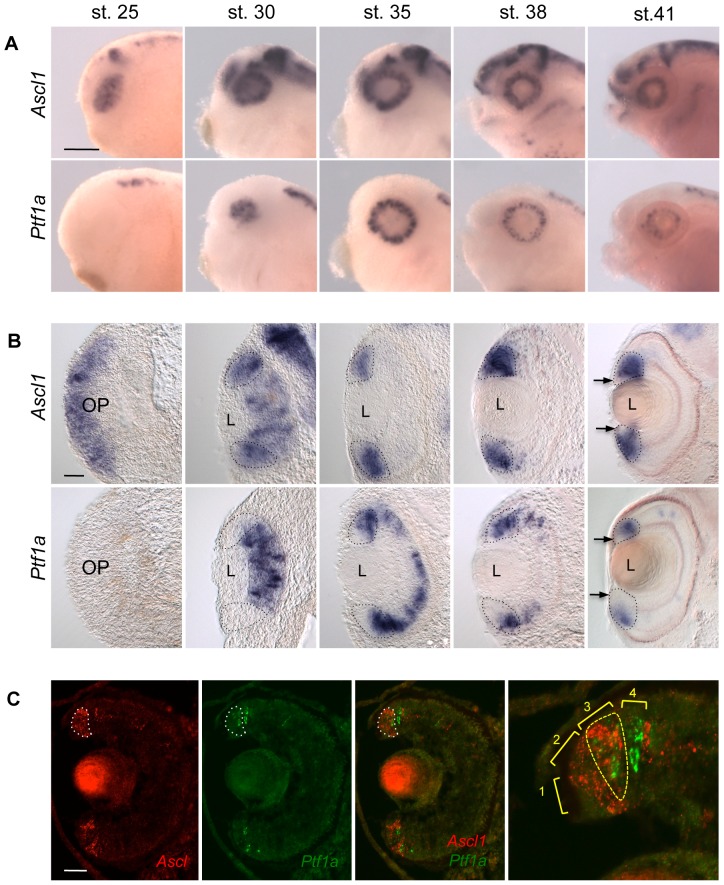
In order to know whether such transcriptional interaction could also hold true in the retina, we first carefully compared their expression pattern during retinogenesis (Fig. 10). Consistent with a Ptf1a-independent proneural function, we found that Ascl1 was broadly expressed in early retinal precursors prior to the onset of Ptf1a expression. However, from stage 30 onwards, regions of overlapping expression were clearly observed in the neural retina of the optic cup and then within the neurogenic zone of the mature retina called the ciliary marginal zone (CMZ) [70]. We next asked whether Ptf1a overexpression could rescue Ascl1 knockdown. We found indeed that gad1 expression was restored to a control level in the retina of Ascl1 morphant embryos overexpressing Ptf1a (Fig. 11). Altogether, these results are consistent with Ascl1 acting upstream Ptf1a in the transcriptional network controlling GABAergic neuron genesis in the retina.
Figure 10. Comparison of Ascl1 and Ptf1a expression patterns during retinogenesis.
(A, B) Whole mount in situ hybridization analysis of Ascl1 and Ptf1a expression during retinogenesis. Shown are representative pictures of the observed labelling in the head region (A; lateral views, anterior on the left) and on retinal cross sections (B; dorsal side up). Ascl1 and Ptf1a are expressed in overlapping domains of the neural retina at stage 30 and in the ciliary marginal zone (CMZ, delineated by doted line) from stage 35 onwards. (C) Double fluorescent in situ hybridization against Ptf1a (green) and Ascl1 (red) performed on stage 40 retinal sections. Ascl1 staining in the CMZ is delineated by a white doted line. Panel on the right shows a magnification of the dorsal CMZ. Neither Ascl1 nor Ptf1a are detected in zone 1 (stem cell compartment). In zone 2 (early progenitors), only Ascl1 is expressed. The expression patterns of both genes overlap in zone 3 (late progenitors; yellow doted line). In zone 4 (postmitotic retinoblasts), Ascl1 expression vanishes while that of Ptf1a persists. OP: Optic vesicle, L: Lens. Scale bar represents 300 μm (A) or 50 μm (B, C).
Figure 11. Ptf1a overexpression rescues Ascl1 knockdown in the retina.
(A) Whole mount in situ hybridization analysis of gad 1 expression at stage 37. Embryos were injected with the indicated mRNAs and/or morpholinos (Mo) in two blastomeres at the two-cell stage and treated with dexamethasone at stage 22. Shown are representative pictures of the observed labelling in the head region (lateral views, anterior on the left) and on retinal sections (dorsal side up). (B) Quantification of gad1 staining intensity in the eye of injected embryos. Results are presented as percentage increase/decrease relative to the average intensity found in controls. Total number of analyzed embryos per condition is indicated in each bar. Values are given as mean +/– s.e.m. p<0,001 (***) (Student’s t-test); N.S. : Non significant; L: Lens. Scale bar represents 300 μm (heads) or 50 μm (sections).
Discussion
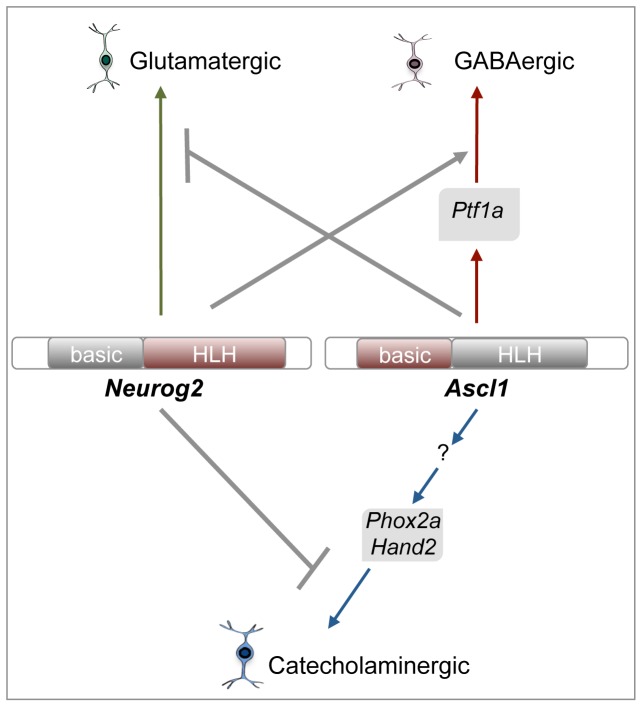
The molecular bases underlying neurotransmitter subtype determination in the retina remain largely unexplored. As previously shown in the brain and peripheral nervous system [6], [15], [18], [68], our data suggest that during retinogenesis, Ascl1 does not simply act as a proneural factor committing progenitor cells to a generic neuronal fate, but also contributes to the specification of their neurotransmitter identity. We showed indeed that Ascl1 is required for retinal GABAergic cell type genesis and sufficient to redirect a subpopulation of progenitors towards this inhibitory neuronal destiny. Besides, taking advantage of an in vivo neurogenic assay in the Xenopus epidermis, we found that Ascl1 GABAergic determining activity is instructive, as inferred by its ability to counteract glutamatergic differentiation programs. Finally, we gained insights into downstream genetic networks underlying Ascl1 functions in neurotransmitter subtype specification by showing that its GABAergic activity involves the direct transcriptional regulation of Ptf1a (Fig. 12).
Figure 12. Summary of Ascl1 genetic network in neuronal subtype specification.
Green, red and blue arrows point to neuronal subtypes induced by Neurog2 and Ascl1. Identified downstream components required for their respective activities are indicated. Grey arrows illustrate epistatic relationships between Neurog2 and Ascl1, as suggested by our results. Protein domains in red confer to Ascl1 and Neurog2 their specific GABAergic/catecholaminergic and glutamatergic activities, respectively (see text for more details).
Ascl1 GABAergic determining activity in the retina
Most Ascl1 gain and loss of function analyses in the retina have so far uncovered phenotypes predominantly related to its proneural activity. Ascl1 has indeed been shown to be required for neuronal versus glial fate decisions during late neurogenesis [21], [22], [23], [71] and to participate, with other proneural genes, to the spatiotemporal progression of the neurogenic wave in the retina [72]. In line with this, recent lineage experiments in mouse revealed that Ascl1-expressing progenitors contribute to all major cell types of the retina with the exception of ganglion cells [3]. Such a primary function in determining a generic neuronal program has thus hindered the identification of its potential specific activity in retinal cell subtype specification. In line with this, overexpression of Ascl1 or other bHLH genes such as Neurog2 or Atoh7 results in apparently similar cell type distribution defects within the Xenopus retina [46], [47], [55], [63]. In the present manuscript, we revealed that Ascl1 is actually endowed with a GABAergic determining function that superimposes to its proneural activity. Of note, neither Neurog2 nor Atoh7 exhibit this property. Importantly, our results are consistent with a previous study in chick reporting that, unlike Neurog2, NeuroD1 or Atoh7, Ascl1 misexpression promotes an overproduction of amacrine cells, the major retinal GABAergic cell type [73]. Therefore, we propose that Ascl1 not only promotes neurogenesis but also biases, in combination with other bHLH factors (see below), a subset of precursor cells towards particular neurotransmitter subtypes, thereby contributing to neuronal diversity during retinogenesis.
Epistatic relationships of bHLH genes in neuronal subtype specification
The strong context-dependency of Ascl1 function in neurotransmitter subtype specification relies on the presence of other regionally expressed transcription factors including bHLH ones [2]. In line with this, our data show that Ascl1 ability to induce ectopic catecholaminergic neurons is impaired in the presence of Neurog2, NeuroD1 or Atoh7. In contrast, its GABAergic inducing activity is not counteracted by these factors, both ectopically and in retinal progenitors. Thus Ascl1 likely exerts an instructive role in promoting GABAergic cell fate as previously proposed in the ventral telencephalon [4], [6].
Unexpectedly, Ascl1-dependent ectopic GABAergic neuron production was found to be enhanced by Neurog2 co-expression. This suggests that, in the few locations where both factors are co-expressed in the same cells [3], [74], [75], [76], they might act synergistically or at least cooperate within neuronal subtype specification programs. This could be the case in the retina where, although the Ascl1 and Neurog2 lineages are largely distinct, such progenitors expressing both genes have recently been described [3]. Interestingly, Neurog2 was identified as a direct transcriptional target of Ptf1a in the chick dorsal spinal cord and cerebellum [77], two regions where Ptf1a, Ascl1 and Neurog2 expressions partially overlap [78]. Neurog2 might thus participate within these domains in the Ascl1/Ptf1a-dependent GABAergic program. Additionally, Ascl1 and Neurog2 were shown to heterodimerize and function in common transcriptional complexes [77], [79], [80]. Investigating Ascl1 interactions with other expressed bHLH genes in the retina will surely help understand the combinatorial codes governing neurotransmitter subtype determination.
Functional domain and transcriptional network of Ascl1 involved in GABAergic cell determination
Previous reports proposed that the HLH domain of murine Ascl1 contains the information underlying its specific activity in neuronal subtype specification [20], [81]. In particular, a study using chimeric and mutated constructs concluded that Ascl1-mediated acquisition of GABAergic identity in forebrain precursor cultures does not occur through DNA binding, but rather through HLH domain protein-protein interactions [10]. The authors however raised the possibility that, in their assays, alterations of endogenous Ascl1 expression may have interfered with proper GABAergic differentiation. Our domain swapping experiments led to an opposite conclusion, highlighting the essential role of Ascl1 basic domain in both its catecholaminergic and GABAergic inducing activities. Interestingly, the same was not true for Neurog2, whose ability to induce glutamatergic neurons was found to be imparted by its HLH domain. Although we cannot exclude that Ascl1 or Neurog2 might act through different molecular mechanisms depending on the cellular context, our data suggest that distinct domains within these bHLH factors mediate their functional specificities in neurotransmitter phenotype specification.
We showed that Ascl1 exerts its GABAergic and catecholaminergic determining activities by controlling distinct genetic cascades involving Ptf1a and Phox2a/Hand2, respectively. We found in particular that Ascl1 ability to promote gad1-positive neurons requires Ptf1a. Additionally, Ascl1 was able to activate Ptf1a transcription and to be necessary for its retinal expression. In line with such a positive transcriptional regulation, previous data showed that Ascl1 is required for the induction and/or maintenance of Ptf1a during the late phase of dorsal sensory interneuron development [14], [82]. Moreover, we provide some evidence that Ptf1a might constitute a direct transcriptional target of Ascl1. Consensus binding sites for Ascl1 have previously been described in two conserved Ptf1a enhancer regions but have not proved to be functional [82]. Additional Ascl1 binding sites may however lie in other Ptf1a regulatory regions.
Altogether, our work demonstrated for the first time a role for Ascl1 in the generation of GABAergic interneurons in the retina and provided insights into the regulatory circuits responsible for its activity in neurotransmitter subtype specification.
Acknowledgments
We thank M. Saha, K. Zimmerman and J.E. Lee for sending plasmids. We are grateful to C. de Meideros for animal care.
Funding Statement
NM is a Neuropôle de Recherche Francilien fellow; DP is a Belgian Fonds pour la formation à la Recherche dans l'Industrie et dans l'Agriculture fellow. This research was supported by grants to MP from the Agence Nationale de la Recherche (056054), Centre national de la recherche scientifique, University Paris-Sud, Retina France, ARC (1108), IFR144 and to EB from the Belgian Fonds de la Recherche Scientifique (FRFC 3.4635.06), the Belgian Queen Elisabeth Medical Foundation, the Fédération Wallonie-Bruxelles (Action de Recherche Concertée) and the Walloon Region Excellence Programme (“CIBLES”). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bertrand N, Castro DS, Guillemot F (2002) Proneural genes and the specification of neural cell types. Nat Rev Neurosci 3: 517–530. [DOI] [PubMed] [Google Scholar]
- 2. Powell LM, Jarman AP (2008) Context dependence of proneural bHLH proteins. Curr Opin Genet Dev 18: 411–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brzezinski JAt, Kim EJ, Johnson JE, Reh TA (2011) Ascl1 expression defines a subpopulation of lineage-restricted progenitors in the mammalian retina. Development 138: 3519–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fode C, Ma Q, Casarosa S, Ang SL, Anderson DJ, et al. (2000) A role for neural determination genes in specifying the dorsoventral identity of telencephalic neurons. Genes Dev 14: 67–80. [PMC free article] [PubMed] [Google Scholar]
- 5. Osorio J, Mueller T, Retaux S, Vernier P, Wullimann MF (2010) Phylotypic expression of the bHLH genes Neurogenin2, Neurod, and Mash1 in the mouse embryonic forebrain. J Comp Neurol 518: 851–871. [DOI] [PubMed] [Google Scholar]
- 6. Parras CM, Schuurmans C, Scardigli R, Kim J, Anderson DJ, et al. (2002) Divergent functions of the proneural genes Mash1 and Ngn2 in the specification of neuronal subtype identity. Genes Dev 16: 324–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schuurmans C, Guillemot F (2002) Molecular mechanisms underlying cell fate specification in the developing telencephalon. Curr Opin Neurobiol 12: 26–34. [DOI] [PubMed] [Google Scholar]
- 8. Wilkinson G, Dennis D, Schuurmans C (2013) Proneural genes in neocortical development. Neuroscience 253: 256–273. [DOI] [PubMed] [Google Scholar]
- 9. Casarosa S, Fode C, Guillemot F (1999) Mash1 regulates neurogenesis in the ventral telencephalon. Development 126: 525–534. [DOI] [PubMed] [Google Scholar]
- 10. Jo AY, Park CH, Aizawa S, Lee SH (2007) Contrasting and brain region-specific roles of neurogenin2 and mash1 in GABAergic neuron differentiation in vitro. Exp Cell Res 313: 4066–4081. [DOI] [PubMed] [Google Scholar]
- 11. Schuurmans C, Armant O, Nieto M, Stenman JM, Britz O, et al. (2004) Sequential phases of cortical specification involve Neurogenin-dependent and -independent pathways. EMBO J 23: 2892–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wilson SW, Rubenstein JL (2000) Induction and dorsoventral patterning of the telencephalon. Neuron 28: 641–651. [DOI] [PubMed] [Google Scholar]
- 13. Peltopuro P, Kala K, Partanen J (2010) Distinct requirements for Ascl1 in subpopulations of midbrain GABAergic neurons. Dev Biol 343: 63–70. [DOI] [PubMed] [Google Scholar]
- 14. Mizuguchi R, Kriks S, Cordes R, Gossler A, Ma Q, et al. (2006) Ascl1 and Gsh1/2 control inhibitory and excitatory cell fate in spinal sensory interneurons. Nat Neurosci 9: 770–778. [DOI] [PubMed] [Google Scholar]
- 15. Pattyn A, Simplicio N, van Doorninck JH, Goridis C, Guillemot F, et al. (2004) Ascl1/Mash1 is required for the development of central serotonergic neurons. Nat Neurosci 7: 589–595. [DOI] [PubMed] [Google Scholar]
- 16.Jacob J, Ribes V, Moore S, Constable SC, Sasai N, et al.. (2013) Valproic Acid silencing of ascl1b/ascl1 results in the failure of serotonergic differentiation in a zebrafish model of Fetal Valproate Syndrome. Dis Model Mech. [DOI] [PMC free article] [PubMed]
- 17. Goridis C, Rohrer H (2002) Specification of catecholaminergic and serotonergic neurons. Nat Rev Neurosci 3: 531–541. [DOI] [PubMed] [Google Scholar]
- 18. Hirsch MR, Tiveron MC, Guillemot F, Brunet JF, Goridis C (1998) Control of noradrenergic differentiation and Phox2a expression by MASH1 in the central and peripheral nervous system. Development 125: 599–608. [DOI] [PubMed] [Google Scholar]
- 19. Howard MJ (2005) Mechanisms and perspectives on differentiation of autonomic neurons. Dev Biol 277: 271–286. [DOI] [PubMed] [Google Scholar]
- 20. Park CH, Kang JS, Kim JS, Chung S, Koh JY, et al. (2006) Differential actions of the proneural genes encoding Mash1 and neurogenins in Nurr1-induced dopamine neuron differentiation. J Cell Sci 119: 2310–2320. [DOI] [PubMed] [Google Scholar]
- 21. Nelson BR, Hartman BH, Ray CA, Hayashi T, Bermingham-McDonogh O, et al. (2009) Acheate-scute like 1 (Ascl1) is required for normal delta-like (Dll) gene expression and notch signaling during retinal development. Dev Dyn 238: 2163–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tomita K, Moriyoshi K, Nakanishi S, Guillemot F, Kageyama R (2000) Mammalian achaete-scute and atonal homologs regulate neuronal versus glial fate determination in the central nervous system. EMBO J 19: 5460–5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tomita K, Nakanishi S, Guillemot F, Kageyama R (1996) Mash1 promotes neuronal differentiation in the retina. Genes Cells 1: 765–774. [DOI] [PubMed] [Google Scholar]
- 24. Jasoni CL, Reh TA (1996) Temporal and spatial pattern of MASH-1 expression in the developing rat retina demonstrates progenitor cell heterogeneity. J Comp Neurol 369: 319–327. [DOI] [PubMed] [Google Scholar]
- 25. Jasoni CL, Walker MB, Morris MD, Reh TA (1994) A chicken achaete-scute homolog (CASH-1) is expressed in a temporally and spatially discrete manner in the developing nervous system. Development 120: 769–783. [DOI] [PubMed] [Google Scholar]
- 26. Marquardt T, Ashery-Padan R, Andrejewski N, Scardigli R, Guillemot F, et al. (2001) Pax6 is required for the multipotent state of retinal progenitor cells. Cell 105: 43–55. [DOI] [PubMed] [Google Scholar]
- 27. Agathocleous M, Harris WA (2009) From progenitors to differentiated cells in the vertebrate retina. Annu Rev Cell Dev Biol 25: 45–69. [DOI] [PubMed] [Google Scholar]
- 28. Hatakeyama J, Kageyama R (2004) Retinal cell fate determination and bHLH factors. Semin Cell Dev Biol 15: 83–89. [DOI] [PubMed] [Google Scholar]
- 29. Ohsawa R, Kageyama R (2008) Regulation of retinal cell fate specification by multiple transcription factors. Brain Res 1192: 90–98. [DOI] [PubMed] [Google Scholar]
- 30. Adler R, Raymond PA (2008) Have we achieved a unified model of photoreceptor cell fate specification in vertebrates? Brain Res 1192: 134–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Feng L, Xie ZH, Ding Q, Xie X, Libby RT, et al. (2010) MATH5 controls the acquisition of multiple retinal cell fates. Mol Brain 3: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mo Z, Li S, Yang X, Xiang M (2004) Role of the Barhl2 homeobox gene in the specification of glycinergic amacrine cells. Development 131: 1607–1618. [DOI] [PubMed] [Google Scholar]
- 33. Suga A, Taira M, Nakagawa S (2009) LIM family transcription factors regulate the subtype-specific morphogenesis of retinal horizontal cells at post-migratory stages. Dev Biol 330: 318–328. [DOI] [PubMed] [Google Scholar]
- 34. Zaghloul NA, Moody SA (2007) Changes in Rx1 and Pax6 activity at eye field stages differentially alter the production of amacrine neurotransmitter subtypes in Xenopus. Mol Vis 13: 86–95. [PMC free article] [PubMed] [Google Scholar]
- 35. Feng L, Xie X, Joshi PS, Yang Z, Shibasaki K, et al. (2006) Requirement for Bhlhb5 in the specification of amacrine and cone bipolar subtypes in mouse retina. Development 133: 4815–4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Duquette PM, Zhou X, Yap NL, MacLaren EJ, Lu JJ, et al. (2010) Loss of LMO4 in the retina leads to reduction of GABAergic amacrine cells and functional deficits. PLoS One 5: e13232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jiang H, Xiang M (2009) Subtype specification of GABAergic amacrine cells by the orphan nuclear receptor Nr4a2/Nurr1. J Neurosci 29: 10449–10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lang B, Zhao L, Cai L, McKie L, Forrester JV, et al. (2010) GABAergic amacrine cells and visual function are reduced in PAC1 transgenic mice. Neuropharmacology 58: 215–225. [DOI] [PubMed] [Google Scholar]
- 39. Dullin JP, Locker M, Robach M, Henningfeld KA, Parain K, et al. (2007) Ptf1a triggers GABAergic neuronal cell fates in the retina. BMC Dev Biol 7: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fujitani Y, Fujitani S, Luo H, Qiu F, Burlison J, et al. (2006) Ptf1a determines horizontal and amacrine cell fates during mouse retinal development. Development 133: 4439–4450. [DOI] [PubMed] [Google Scholar]
- 41. Jusuf PR, Almeida AD, Randlett O, Joubin K, Poggi L, et al. (2011) Origin and determination of inhibitory cell lineages in the vertebrate retina. J Neurosci 31: 2549–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lelievre EC, Lek M, Boije H, Houille-Vernes L, Brajeul V, et al. (2011) Ptf1a/Rbpj complex inhibits ganglion cell fate and drives the specification of all horizontal cell subtypes in the chick retina. Dev Biol 358: 296–308. [DOI] [PubMed] [Google Scholar]
- 43. Nakhai H, Sel S, Favor J, Mendoza-Torres L, Paulsen F, et al. (2007) Ptf1a is essential for the differentiation of GABAergic and glycinergic amacrine cells and horizontal cells in the mouse retina. Development 134: 1151–1160. [DOI] [PubMed] [Google Scholar]
- 44. Ferreiro B, Skoglund P, Bailey A, Dorsky R, Harris WA (1993) XASH1, a Xenopus homolog of achaete-scute: a proneural gene in anterior regions of the vertebrate CNS. Mech Dev 40: 25–36. [DOI] [PubMed] [Google Scholar]
- 45. Afelik S, Chen Y, Pieler T (2006) Combined ectopic expression of Pdx1 and Ptf1a/p48 results in the stable conversion of posterior endoderm into endocrine and exocrine pancreatic tissue. Genes Dev 20: 1441–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Perron M, Opdecamp K, Butler K, Harris WA, Bellefroid EJ (1999) X-ngnr-1 and Xath3 promote ectopic expression of sensory neuron markers in the neurula ectoderm and have distinct inducing properties in the retina. Proc Natl Acad Sci U S A 96: 14996–15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kanekar S, Perron M, Dorsky R, Harris WA, Jan LY, et al. (1997) Xath5 participates in a network of bHLH genes in the developing Xenopus retina. Neuron 19: 981–994. [DOI] [PubMed] [Google Scholar]
- 48. Chitnis A, Kintner C (1996) Sensitivity of proneural genes to lateral inhibition affects the pattern of primary neurons in Xenopus embryos. Development 122: 2295–2301. [DOI] [PubMed] [Google Scholar]
- 49. Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC (1994) Green fluorescent protein as a marker for gene expression. Science 263: 802–805. [DOI] [PubMed] [Google Scholar]
- 50. Talikka M, Perez SE, Zimmerman K (2002) Distinct patterns of downstream target activation are specified by the helix-loop-helix domain of proneural basic helix-loop-helix transcription factors. Dev Biol 247: 137–148. [DOI] [PubMed] [Google Scholar]
- 51. Gammill LS, Sive H (1997) Identification of otx2 target genes and restrictions in ectodermal competence during Xenopus cement gland formation. Development 124: 471–481. [DOI] [PubMed] [Google Scholar]
- 52. Nieuwkoop PD (1967) The "organization centre". 3. Segregation and pattern formation in morphogenetic fields. Acta Biotheor 17: 178–194. [DOI] [PubMed] [Google Scholar]
- 53. Parlier D, Ariza A, Christulia F, Genco F, Vanhomwegen J, et al. (2008) Xenopus zinc finger transcription factor IA1 (Insm1) expression marks anteroventral noradrenergic neuron progenitors in Xenopus embryos. Dev Dyn 237: 2147–2157. [DOI] [PubMed] [Google Scholar]
- 54. Holt CE, Garlick N, Cornel E (1990) Lipofection of cDNAs in the embryonic vertebrate central nervous system. Neuron 4: 203–214. [DOI] [PubMed] [Google Scholar]
- 55. Ohnuma S, Mann F, Boy S, Perron M, Harris WA (2002) Lipofection strategy for the study of Xenopus retinal development. Methods 28: 411–419. [DOI] [PubMed] [Google Scholar]
- 56. Li M, Sipe CW, Hoke K, August LL, Wright MA, et al. (2006) The role of early lineage in GABAergic and glutamatergic cell fate determination in Xenopus laevis. J Comp Neurol 495: 645–657. [DOI] [PubMed] [Google Scholar]
- 57. Amato MA, Boy S, Arnault E, Girard M, Della Puppa A, et al. (2005) Comparison of the expression patterns of five neural RNA binding proteins in the Xenopus retina. J Comp Neurol 481: 331–339. [DOI] [PubMed] [Google Scholar]
- 58. Souopgui J, Klisch TJ, Pieler T, Henningfeld KA (2007) Expression and regulation of Xenopus CRMP-4 in the developing nervous system. Int J Dev Biol 51: 339–343. [DOI] [PubMed] [Google Scholar]
- 59. Parain K, Mazurier N, Bronchain O, Borday C, Cabochette P, et al. (2012) A large scale screen for neural stem cell markers in Xenopus retina. Dev Neurobiol 72: 491–506. [DOI] [PubMed] [Google Scholar]
- 60. Lea R, Bonev B, Dubaissi E, Vize PD, Papalopulu N (2012) Multicolor fluorescent in situ mRNA hybridization (FISH) on whole mounts and sections. Methods Mol Biol 917: 431–444. [DOI] [PubMed] [Google Scholar]
- 61. Taelman V, Van Wayenbergh R, Solter M, Pichon B, Pieler T, et al. (2004) Sequences downstream of the bHLH domain of the Xenopus hairy-related transcription factor-1 act as an extended dimerization domain that contributes to the selection of the partners. Dev Biol 276: 47–63. [DOI] [PubMed] [Google Scholar]
- 62. Farah MH (2006) Neurogenesis and cell death in the ganglion cell layer of vertebrate retina. Brain Res Rev 52: 264–274. [DOI] [PubMed] [Google Scholar]
- 63. Wang JC, Harris WA (2005) The role of combinational coding by homeodomain and bHLH transcription factors in retinal cell fate specification. Dev Biol 285: 101–115. [DOI] [PubMed] [Google Scholar]
- 64. Lee JE, Hollenberg SM, Snider L, Turner DL, Lipnick N, et al. (1995) Conversion of Xenopus ectoderm into neurons by NeuroD, a basic helix-loop-helix protein. Science 268: 836–844. [DOI] [PubMed] [Google Scholar]
- 65. Ma Q, Kintner C, Anderson DJ (1996) Identification of neurogenin, a vertebrate neuronal determination gene. Cell 87: 43–52. [DOI] [PubMed] [Google Scholar]
- 66. Rupp RA, Snider L, Weintraub H (1994) Xenopus embryos regulate the nuclear localization of XMyoD. Genes Dev 8: 1311–1323. [DOI] [PubMed] [Google Scholar]
- 67. Turner DL, Weintraub H (1994) Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev 8: 1434–1447. [DOI] [PubMed] [Google Scholar]
- 68. Lo L, Tiveron MC, Anderson DJ (1998) MASH1 activates expression of the paired homeodomain transcription factor Phox2a, and couples pan-neuronal and subtype-specific components of autonomic neuronal identity. Development 125: 609–620. [DOI] [PubMed] [Google Scholar]
- 69. Morikawa Y, D'Autreaux F, Gershon MD, Cserjesi P (2007) Hand2 determines the noradrenergic phenotype in the mouse sympathetic nervous system. Dev Biol 307: 114–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Perron M, Kanekar S, Vetter ML, Harris WA (1998) The genetic sequence of retinal development in the ciliary margin of the Xenopus eye. Dev Biol 199: 185–200. [DOI] [PubMed] [Google Scholar]
- 71. Hatakeyama J, Tomita K, Inoue T, Kageyama R (2001) Roles of homeobox and bHLH genes in specification of a retinal cell type. Development 128: 1313–1322. [DOI] [PubMed] [Google Scholar]
- 72. Hufnagel RB, Le TT, Riesenberg AL, Brown NL (2010) Neurog2 controls the leading edge of neurogenesis in the mammalian retina. Dev Biol 340: 490–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mao W, Yan RT, Wang SZ (2009) Proneural gene ash1 promotes amacrine cell production in the chick retina. Dev Neurobiol 69: 88–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kele J, Simplicio N, Ferri AL, Mira H, Guillemot F, et al. (2006) Neurogenin 2 is required for the development of ventral midbrain dopaminergic neurons. Development 133: 495–505. [DOI] [PubMed] [Google Scholar]
- 75. Mizuguchi R, Sugimori M, Takebayashi H, Kosako H, Nagao M, et al. (2001) Combinatorial roles of olig2 and neurogenin2 in the coordinated induction of pan-neuronal and subtype-specific properties of motoneurons. Neuron 31: 757–771. [DOI] [PubMed] [Google Scholar]
- 76. Roybon L, Deierborg T, Brundin P, Li JY (2009) Involvement of Ngn2, Tbr and NeuroD proteins during postnatal olfactory bulb neurogenesis. Eur J Neurosci 29: 232–243. [DOI] [PubMed] [Google Scholar]
- 77. Henke RM, Savage TK, Meredith DM, Glasgow SM, Hori K, et al. (2009) Neurog2 is a direct downstream target of the Ptf1a-Rbpj transcription complex in dorsal spinal cord. Development 136: 2945–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zordan P, Croci L, Hawkes R, Consalez GG (2008) Comparative analysis of proneural gene expression in the embryonic cerebellum. Dev Dyn 237: 1726–1735. [DOI] [PubMed] [Google Scholar]
- 79. Gradwohl G, Fode C, Guillemot F (1996) Restricted expression of a novel murine atonal-related bHLH protein in undifferentiated neural precursors. Dev Biol 180: 227–241. [DOI] [PubMed] [Google Scholar]
- 80. Henke RM, Meredith DM, Borromeo MD, Savage TK, Johnson JE (2009) Ascl1 and Neurog2 form novel complexes and regulate Delta-like3 (Dll3) expression in the neural tube. Dev Biol 328: 529–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nakada Y, Hunsaker TL, Henke RM, Johnson JE (2004) Distinct domains within Mash1 and Math1 are required for function in neuronal differentiation versus neuronal cell-type specification. Development 131: 1319–1330. [DOI] [PubMed] [Google Scholar]
- 82. Meredith DM, Masui T, Swift GH, MacDonald RJ, Johnson JE (2009) Multiple transcriptional mechanisms control Ptf1a levels during neural development including autoregulation by the PTF1-J complex. J Neurosci 29: 11139–11148. [DOI] [PMC free article] [PubMed] [Google Scholar]