The pineal gland of house sparrows was one of the first biological clocks believed to act as a circadian pacemaker. This view came from brilliant experimental studies by Menaker and coworkers (1, 2) demonstrating that removal of the pineal gland caused arrhythmic behavior and transplantation of pineal glands into pinealectomized arrhythmic birds restored locomotor rhythms. Takahashi and Menaker (3) extended this view by providing evidence for the multioscillatory nature of the house sparrow circadian system, demonstrating the presence of hypothalamic components and the necessity of coupling of oscillators to maintain rhythmicity. The interesting suggestion of two oscillators remaining in the hypothalamus of pinealectomized sparrows found experimental evidence 20 years later when rhythmic and pinealindependent clock gene expression was found in two cell groups of the house sparrow hypothalamus: the suprachiasmatic nucleus (SCN) and the lateral hypothalamic nucleus (4–6).
Mammalian Circadian Organization: A Single Pacemaker or Multiple Oscillators?
In a recent issue of PNAS, Yoo et al. (7) used a PERIOD2::LUCIFERASE fusion protein as a real-time reporter to demonstrate that cultured neural and nonneural peripheral tissues of the mouse, including SCN, pituitary, liver, lung, and kidney, exert comparable self-sustained circadian oscillations. Again, it seems that a pacemaker, this time the hypothalamic SCN of a rodent, has to resign from its “major function.” Dogmas, like the one of the SCN being the circadian pacemaker in mammals, can certainly stimulate research, but sometimes weaken scientific objectivity in the assessment of evidence. This becomes clear when looking back at how the mammalian SCN became a “pacemaker.” At about the time when Menaker and coworkers (1–3) unraveled the enigma of avian circadian organization, a small hypothalamic cell group, the SCN, was identified to be the major driving force of rhythmic behavior in mammals. As with the house sparrow pineal gland, lesions of the SCN in rodents abolished rhythms of physiology and behavior, and transplantation of SCN tissue into SCN-ablated arrhythmic animals restored these rhythms (8–11). The dogma of the circadian pacemaker was born. For many years, understanding of mammalian circadian organization was determined by the assumption that only the SCN contained autonomous circadian oscillators solely driving overt rhythmicity. The house sparrow and its multioscillatory system fell into oblivion and the mammalian SCN became the unequalled circadian “model system” (12).
The initial findings of clock gene rhythms in peripheral tissues and fibroblasts significantly extended our view of circadian organization at the whole-organism level (13–15). However, the dogma survived, and the SCN asserted its supremacy because circadian oscillations in peripheral tissues damped quickly, and cultured fibroblasts even needed a serum shock to oscillate (13–15). By making use of the central role of the Per2 gene in the mammalian circadian system (16) and further developing the “Per::Luc technique,” Yoo et al. (7) provide convincing evidence that peripheral circadian oscillations are self-sustained. Even more remarkable is the fact that circadian oscillations in peripheral tissues isolated from SCN-lesioned arrhythmic mice were still rhythmic, but their phasing was affected indicating desynchrony among tissues of individual animals and among animals. By extending the molecular approach to the whole-organism level, the results of Yoo et al. (7) suggest that the SCN does not act as a pacemaker but as an internal synchronizer, per definition a so-called “Zeitgeber,” within the mammalian circadian system, and the authors raise the interesting question of whether the supremacy of the SCN still holds. Indeed, neither the presence of a rhythm-generating molecular clockwork nor the autonomous maintenance of circadian oscillations is a unique property of the SCN, but should we replace the SCN as a model system for studying circadian mechanisms (17)? As exemplified by Yoo et al. (7), we should use neither the SCN nor fibroblasts alone as model systems, but extend the spectrum of cells and tissues that we look at. The reductionistic approach has proven to be highly effective to explain and model the intracellular clock mechanism (18). However, understanding of circadian organization at the whole-organism level requires a holistic rather than a reductionistic approach.
Coordinated Rhythmicity at the Whole-Organism Level Is the Result of Entrainment, Time Coding, and Internal Synchronization
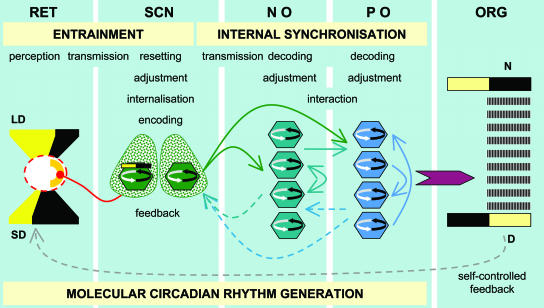
Aschoff (19, 20) worked out important formalisms and rules that describe circadian oscillations based on observations of general physiology and behavior in birds and mammals, including humans. He described circadian systems as multioscillatory and defined “internal synchronization,” i.e., the timed coordination of a multiplicity of individual oscillators without the need of an external Zeitgeber, as a central process of circadian organization at the whole-organism level (19, 20). It was the holistic view represented by pioneers such as Aschoff that formed our understanding of the general regulations of circadian rhythms. In consideration of the principles of circadian oscillations as well as recent discoveries of light entrainment mechanisms (21), the molecular circadian machinery (18), the time-coding capacity of circadian oscillators (22, 23), and the functional organization of the SCN (24) and its output signals (25), as well as the presence of self-sustained circadian oscillators in neural and nonneural peripheral tissues (7), we may be better able to sketch the essential steps of mammalian circadian organization at the whole-organism level (Fig. 1). For the maintenance of the temporal order within the organism, two processes appear central: the phase-setting effects of external Zeitgebers, i.e., entrainment, and the interaction between oscillators within the organism, i.e., internal synchronization. Entrainment is accomplished by a cascade of events that start with the perception of light by retinal neurons localized in the ganglion cell layer projecting toward the SCN and resetting the circadian clock (18, 21). Within the SCN, the light message is processed (24), and biological time adjusted to it and internalized by a yet unidentified mechanism that leads to a time-coding circadian oscillation reflecting the environmental ratio of light and dark within the 24-hr frame (22). Entrainment is completed and the process of internal synchronization starts. The SCN uses distinct types of output signals to spread the circadian message all over the brain and the body: direct and indirect neuronal projections (25) as well as the secretion of diffusable polypeptides (18). Interestingly, neuronal projections are not necessary to maintain circadian oscillations of behavior but to maintain the rhythmic and time-coding melatonin signal of the pineal gland that is essential for the regulation of annual rhythms (28). Do these two types of output signals represent two major functions that the SCN has to master, i.e., to act as a Zeitgeber coding time of day and time of year? Just as the SCN has to decode the light message originating from the retina, neural and peripheral oscillators have then to decode signals originating from the SCN and adjust their biological time, a process that may involve a variety of interaction and feedback mechanisms, as well as organ-specific synchronizers at the cell and tissue level, to complete internal synchronization. The result is a coordinated rhythmicity of general physiology and behavior of the animal reflecting the environmental light–dark cycle that feeds back on entrainment as how and when the animal is exposed to light is a consequence of the activity of the animal. This is where, at the whole-organism level, the loop closes and the circadian cycle starts into a new day. The mammalian SCN, as with the house sparrow pineal gland, has still to be regarded as being superior to other self-sustained circadian oscillators because of the capacity to code and internalize environmental time (22, 23) and its role as an internal Zeitgeber, coordinating a variety of neural and nonneural peripheral circadian oscillators to adjust biological time to the environment (7).
Fig. 1.
Mammalian circadian organization at the whole-organism level. Entrainment begins with the perception of light in the retina [RET; light input, shown as either long day (LD) or short day (SD)] and the transmission of photic information, reflecting the ratio of light and dark, to the SCN. Resetting of the clock, adjustment of biological time, internalization of environmental time, and encoding into particular output signals take place in the SCN. Internal synchronization is accomplished by spreading time-coded information to self-sustained neural oscillators (NO) and peripheral oscillators (PO). Biological time in NO and PO is adjusted, and a cascade of interaction and feedback mechanisms in NO and PO, as well as between NO and PO, and feedback signals toward the SCN result in coordinated rhythmicity of general physiology and behavior at the whole-organism level (ORG) that reflects the environmental light–dark cycle and may species-specifically be adjusted to the light (diurnal, D) or dark (nocturnal, N) phase. Molecular circadian rhythms are generated at all levels of organization. “Self-controlled feedback,” i.e., the determination of light input to the system by the activity of the animal, closes the loop, and the circadian cycle starts again.
Factors other than light can affect entrainment (18), and species-specific circadian particularities (26, 27) raise more questions that need to be answered. Hopefully, future comparative studies will elucidate how organisms at different phylogenetic levels have found distinct solutions for the common demand to cope with the solar day and whether recent findings of a close link of circadian mechanisms with early development and cell cycle regulation open fascinating new perspectives of circadian clock function (28). The molecular era of circadian research has brought tremendous progress in our understanding of circadian mechanisms and provided the basis for fascinating methodological tools such as the one presented by Yoo et al. (7). One might agree that almost any cell type may serve as a model cell for the investigation of basic molecular circadian mechanisms, but to understand the function of biological clocks at the whole-organism level we need comparative and interdisciplinary, i.e., holistic, approaches.
See companion article on page 5339 in issue 15 of volume 101.
References
- 1.Gaston, S. & Menaker, M. (1968) Science 160, 1125–1127. [DOI] [PubMed] [Google Scholar]
- 2.Zimmerman, N. H. & Menaker, M. (1979) Proc. Natl. Acad. Sci. USA 76, 999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi, J. S. & Menaker, M. (1982) J. Neurosci. 2, 815–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandstaetter, R., Abraham, U. & Albrecht, U. (2001) NeuroReport 12, 1167–1170. [DOI] [PubMed] [Google Scholar]
- 5.Abraham, U., Albrecht, U., Gwinner, E. & Brandstaetter, R. (2002) Eur. J. Neurosci. 16, 429–436. [DOI] [PubMed] [Google Scholar]
- 6.Abraham, U., Albrecht, U. & Brandstaetter, R. (2003) Chronobiol. Int. 20, 657–669. [DOI] [PubMed] [Google Scholar]
- 7.Yoo, S.-H., Yamazaki, S., Lowrey, P. L., Shimomura, K., Ko, C. H., Buhr, E. D., Siepka, S. M., Hong, H.-K., Oh, W. J., Yoo, O. J., et al. (2004) Proc. Natl. Acad. Sci. USA 101, 5339–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore, R. Y. & Eichler, V. B. (1972) Brain Res. 42, 201–206. [DOI] [PubMed] [Google Scholar]
- 9.Stephan, F. K. & Zucker, I. (1972) Proc. Natl. Acad. Sci. USA 69, 1583–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehman, M. N., Silver, R., Gladstone, W. R., Kahn, R. M., Gibson, M. & Bittman, E. L. (1987) J. Neurosci. 6, 1626–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ralph, M. R., Foster, R. G., Davis, F. C. & Menaker, M. (1990) Science 247, 975–978. [DOI] [PubMed] [Google Scholar]
- 12.Klein, D. C., Moore, R. Y. & Reppert, S. M. (1991) Suprachiasmatic Nucleus: The Mind's Clock (Oxford Univ. Press, New York).
- 13.Balsalobre, A., Damiola, F. & Schibler, U. (1998) Cell 93, 929–937. [DOI] [PubMed] [Google Scholar]
- 14.Yamazaki, S., Numano, R., Abe, M., Hida, A., Takahashi, R., Ueda, M., Block, G. D., Sakakai, Y., Menaker, M. & Tei, H. (2000) Science 288, 682–685. [DOI] [PubMed] [Google Scholar]
- 15.Abe, M., Herzog, E. D., Yamazaki, S., Straume, M., Tei, H., Sakaki, Y., Menaker, M. & Block, G. D. (2002) J. Neurosci. 22, 350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng, B., Larkin, D. W., Albrecht, U., Sun, Z. S., Sage, M., Eichele, G., Lee, C. C. & Bradley, A. (1999) Nature 400, 169–173. [DOI] [PubMed] [Google Scholar]
- 17.Rosbash, M. (1998) Cell 93, 917–919. [DOI] [PubMed] [Google Scholar]
- 18.Albrecht, U. (2004) Front. Biosci. 9, 48–55. [DOI] [PubMed] [Google Scholar]
- 19.Aschoff, J. (1960) Cold Spring Harbor Symp. Quant. Biol. 25, 11–27. [DOI] [PubMed] [Google Scholar]
- 20.Aschoff, J. (1969) Aerospace Med. 40, 844–849. [PubMed] [Google Scholar]
- 21.Berson, D. M., Dunn, D. A. & Takao, M. (2002) Science 295, 1070–1073. [DOI] [PubMed] [Google Scholar]
- 22.Mrugala, M., Zlomanczuk, P., Jagota, A. & Schwartz, W. J. (2000) Am. J. Physiol. 278, 987–994. [DOI] [PubMed] [Google Scholar]
- 23.Brandstaetter, R., Kumar, V., Abraham, U. & Gwinner, E. (2000) Proc. Natl. Acad. Sci. USA 97, 12324–12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamada, T., LeSauter, J., Venuti, J. M. & Silver, R. (2001) J. Neurosci. 21, 7742–7750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kriegsfeld, L. J., Leak, R. K., Yackulic, C. B., LeSauter, J. & Silver, R. (2004) J. Comp. Neurol. 468, 361–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oster, H., Avivi, A., Joel, A., Albrecht, U. & Nevo, E. (2002) Curr. Biol. 19, 1919–1922. [DOI] [PubMed] [Google Scholar]
- 27.Whitmore, D., Foulkes, N. S. & Sassone-Corsi, P. (2000) Nature 404, 87–91. [DOI] [PubMed] [Google Scholar]
- 28.Dekens, M. P., Santoriello, C., Vallone, D., Grasi, G., Whitmore, D. & Foulkes, N. S. (2003) Curr. Biol. 13, 2051–2057. [DOI] [PubMed] [Google Scholar]