Abstract
Background
The pleth variability index (PVI) has been demonstrated to be a useful, noninvasive indicator of continuous fluid responsiveness. Whether PVI can be used to assess the changes of intravascular volume status remains to be elucidated.
Material/Methods
Using correlation analysis and receiver operating characteristic (ROC) curves, we sought a correlation between PVI and the initial distribution volume of glucose (IDVG), evaluating PVI as an indicator of the central extracellular fluid volume after anesthesia induction in patients undergoing elective abdominal surgery.
Results
Strong negative correlations existed between IDVG and PVI (r=−0.72), IDVG, and pulse pressure variation (PPV) (r=−0.73), and between IDVG and systolic pressure variation (SPV) (r=−0.53), P<0.01. Strong positive correlations existed between PPV and PVI (r=0.66), PVI and SPV (r=0.49), and between PPV and SPV (r=0.59), P<0.01. The areas under the ROC curve of IDVG, PVI, and SPV were significantly different from the area under a reference line. The optimal cutoff values (followed by sensitivity and specificity in parentheses) comparable to PPV over 11% as the threshold of hypovolemia were IDVG 94.5 mL/kg (75%, 100%), PVI 13% (91.7%, 77.8%), and SPV 7% (41.7%, 100%).
Conclusions
Our results show that strong correlations exist among IDVG, PVI, PPV, and SPV in the evaluation of volemia. PVI can serve as a useful, noninvasive indicator of continuous central extracellular fluid volume for those patients not requiring invasive hemodynamic monitoring, but needs attention to changes in intravascular volume status for optimal fluid management.
MeSH Keywords: Fluid Therapy, Extracellular Fluid, Respiration, Artificial, Pleth Variability Index, Blood Circulation
Background
Accurate estimation of intravascular volume is very important for anesthesiologists to maintain the stability of the cardiovascular system during anesthesia, especially for unstable or hypovolemic patients. Changes in heart rate (HR), blood pressure, and central venous pressure are still most commonly used to assess the blood circulation status, but these indices have been shown to be insufficient for guiding fluid management, owing to their poor correlation with fluid responsiveness[1–3].
Recently, dynamic indices measuring respiratory variations in arterial pulse pressure, stroke volume, and the pulse oximeter plethysmographic waveform have been demonstrated to be highly predictive of fluid responsiveness in mechanically ventilated patients [4,5]. The pleth variability index (PVI), which is derived from respiratory variations in the peripheral perfusion index (PI), provides clinicians with a numerical value obtained noninvasively, automatically, and continuously [6–8]. However, whether the changes in PVI detected at the end of the finger can mirror the changes of intravascular volume or cardiac preload remains unclear.
Although the indocyanine green (ICG) dilution method has become feasible to measure plasma volume, it is not yet used in routine clinical practice. In 1993, Ishihara et al. introduced the concept of initial distribution volume of glucose (IDVG) to assess intravascular volume [9]. As opposed to the ICG dilution method, IDVG can reliably measure the extracellular fluid (ECF) volume in various pathological conditions, including those with both fluid gain and loss. The central ECF volume includes not only the plasma volume, but also the interstitial fluid volume status of highly perfused organs, making IDVG a useful tool to assess the status of intravascular volume or cardiac preload. It is especially useful in the early stages of blood or body fluid loss, when the cardiac output remains constant because of autonomic nervous system compensation and ECF redistribution [10,11].
Although IDVG has been demonstrated to assess the status of intravascular volume and predict the development of hypovolemic hypotension, repeated measurements of IDVG require intervals of at least 30 min, making it inconvenient to direct real-time fluid therapy [12–14]. The goal of this study was to explore the relationship between PVI and IDVG and to evaluate PVI as a noninvasive indicator of moment-by-moment central extracellular fluid volume in mechanically ventilated patients after anesthesia induction.
Material and Methods
After approval of the research protocol by our institutional ethics committee for human studies and personal informed consents were obtained, 31 American Society of Anesthesiologists grade I and II patients aged 20–65 years undergoing elective abdominal surgeries were included in this study. Patients with diabetes mellitus, cardiopulmonary dysfunction, liver or renal dysfunction, significant arrhythmia, or extensive peripheral arterial occlusive diseases were excluded.
All patients fasted for 6–8 h before surgery. Upon arrival in the operating room, noninvasive arterial blood pressure, electrocardiography, and pulse oximetry were monitored (MD50 monitor; Philips Healthcare, the Netherlands). A 20-G needle was inserted into the radial artery under local anesthesia for measurement of arterial pressure. An 18-G indwelling catheter was inserted into the left median cubital vein for fluid and glucose administration. PI and PVI were continuously monitored through the left index finger using a pulse oximeter wrapped with black paper to minimize light interference (Masimo Radical7; Masimo Corporation, Irvine, CA, USA).
General anesthesia was induced by intravenous injection of midazolam 0.04mg/kg, propofol 2.0–2.5 mg/kg, fentanyl 2–4 μg/kg, and succinylcholine 2 mg/kg. After tracheal intubation, all patients were ventilated in volume-controlled mode by an Ohmeda Aestiva®/5 anesthetic machine (GE Healthcare, Madison, WI, USA) with a tidal volume of 8–10 mL/kg body weight with respiratory frequency adjustment to maintain end tidal PCO2 at 3.8–4.7 kPa and zero end-expiratory pressure. Anesthesia was maintained with 1.3 MAC sevoflurane; intravenous vecuronium 0.1 mg/kg first, then 0.05 mg/kg and/or fentanyl 2 μg/kg intravenous bolus injection as needed. All patients received lactated Ringer’s solution infusion (2 mL/kg/h) during the induction period. Phenylephrine (20–40 μg) intravenous bolus was given if systolic blood pressure (SBP) was less than 90 mmHg. Patients requiring vasoactive drugs during anesthesia induction and the first 5 min after the glucose intravenous injection were excluded from the study.
Five minutes after induction, monitoring of changes of arterial pulse pressure and systolic pressure was instituted as described in the monitor manual. PPV (%) was then calculated as follows:
| (1) |
where PPmax and PPmin are the maximal and minimal values within 1 respiratory cycle [15]. Average PPV (%) was calculated 3 times within 3 consecutive respiratory cycles. The mean value of the 3 determinations was used for analysis. SPV (%) was calculated in the same way, using SBP:
| (2) |
where SBPmax and SBPmin are the maximal and minimal values within 1 respiratory cycle [15].
After the HR, PP, SBP, PI, and PVI baseline values were obtained, 10 mL of 50% glucose was injected through the left median cubital vein over 30 s to estimate IDVG. Arterial blood samples were drawn and measured immediately before and 1, 3, 5, and 60 min after glucose administration. IDVG was calculated using a one-compartment model from the increased blood glucose levels between 3 and 7 min. IDVG is calculated as follows:
| (3) |
where exp is the exponential function and Δgl is the incremental glucose level at 3 min after intravenous glucose injection [16]. The IDVG (mL/kg) is calculated based on the basal body weight before anesthesia.
PVI is an automatic measurement of the dynamic change in PI that occurs during a complete respiratory cycle, and is displayed continuously.
Numerical data are expressed as mean ± standard deviation. Comparison analysis was performed using the Student’s t-test, and correlation analysis was performed using the Pearson test. The best fit lines of IDVG, PPV, SPV, and PVI were determined using a least-squares regression technique. Receiver operating characteristic (ROC) analyses were used to calculate the comparable threshold values of IDVG, PVI, PPV, and SPV. PPV=11% or IDVG <100 mL/kg was selected as the threshold for hypovolemia. The areas under the curves (AUCs) were compared using the DeLong method [17]. Optimal cutoff values were calculated using the Youden index (calculated as: sensitivity + specificity − 100). Statistical analyses were performed using MedCalc for Windows, version 12.6.1.0 (MedCalc Software, Ostend, Belgium). The level of statistical significance was P<0.05.
Results
Thirty-one patients undergoing elective abdominal surgeries were enrolled into this study. One of the patients was excluded from the study due to a pre-injection blood glucose level over 9.0 mmol/L. The demographic characteristics and baseline parameters are shown in Table 1. No change was noted in HR and mean artery pressure (MAP) immediately before and 5 min after intravenous glucose injection (P>0.05).
Table 1.
Patient demographics and monitored parameters during surgery.
| Characteristics/parameters | Value |
|---|---|
| Number of patients | 30 |
| Age (yrs) | 51.5±13.8 |
| Gender (male/female) | 16/14 |
| Height (cm) | 164±9.0 |
| Weight (kg) | 59.57±11.98 |
| BMI (kg/m2) | 21.98±2.78 |
| ASA physical status (I/II) | 28/2 |
| Heart Rate (bmp) | |
| Pre-injection | 78.4±10.7 |
| Post-injection | 76.6±12.8 |
| MAP (mmHg) | |
| Pre-injection | 62.5±11.6 |
| Post-injection | 63.6±9.2 |
Values are presented as mean ± standard deviation or as number of patients.
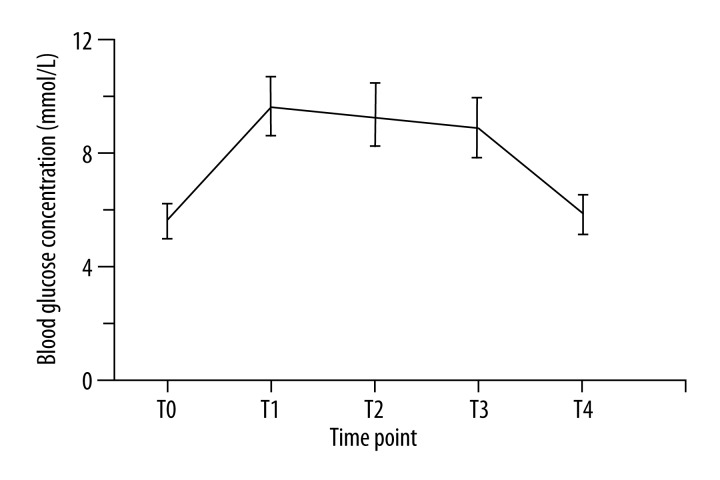
The average blood glucose levels at baseline and 1, 3, 5, and 60 min after glucose injection were 5.60, 9.69, 9.34, 8.89, and 5.85mmol/L, respectively. The blood glucose level returned to baseline level at 60 min (P>0.05) (Figure 1).
Figure 1.
Blood glucose concentrations immediately before (T0) and 1, 3, 5, and 60 min (T1, T2, T3, T4) after intravenous administration of 5 g of glucose (n=30).
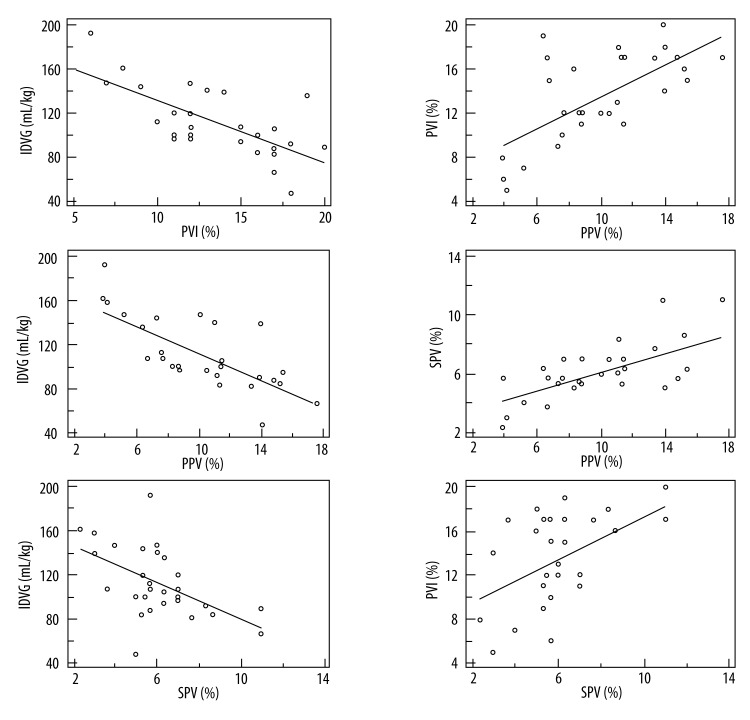
Strong negative correlations were found between IDVG and PVI, IDVG and PPV, and between IDVG and SPV. The correlation coefficients along with 95% confidence interval (CI) were −0.72 (−0.86 to −0.49), P < 0.01 (Figure 2A), −0.73 (−0.86 to −0.49), P < 0.01 (Figure 2B), and −0.53 (−0.7 to −0.21), P<0.01 (Figure 2C). Strong positive correlations existed between PPV and PVI, PPV and SPV, and between PVI and SPV. The correlation coefficients along with 95% CI were 0.66 (0.40–0.83), P<0.01 (Figure 2D), 0.59 (0.29–0.78), P<0.01 (Figure 2E), and 0.49 (0.16–0.72), P<0.01 (Figure 2F).
Figure 2.
Relationships among IDVG, PPV, SPV, and PVI. The solid lines are regression lines among IDVG, PPV, SPV, and PVI. IDVG – initial distribution volume of glucose; PPV – pulse pressure variation; SPV – systolic pressure variation; PVI – pleth variability index. r – correlation coefficient.
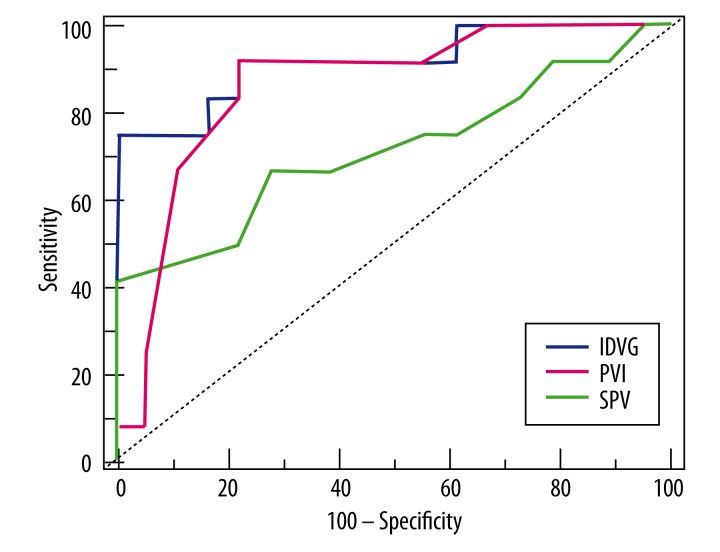
To further compare the abilities of IDVG, PVI, and SPV to detect hypovolemia, the ROC curve was drawn using MedCalc software with PPV over 11% as the threshold of hypovolemia or fluid responsiveness [18] (Figure 3). As determined by the Youden index, the optimal cutoff values (with sensitivity and specificity in parentheses) for IDVG, PVI, and SPV comparable to PPV over 11% as the threshold of hypovolemia or fluid responsiveness were 94.5 mL/kg (75%, 100%), 13% (91.7%, 77.8%), and 7% (41.7%, 100%). All individual AUCs were significantly different from the area under the reference line (area=0.5), the P values of IDVG and PVI were all less than 0.001, and the P value of SPV was 0.04. A significant difference also existed between the AUCs of IDVG and SPV (P=0.03). No difference between the AUCs of IDVG and PVI, or between PVI and SPV, were identified (Table 2).
Figure 3.
Receiver operating characteristic (ROC) analyses for initial distribution volume of glucose (IDVG, blue), systolic pressure variation (SPV, green), and the pleth variability index (PVI, red). Pulse pressure variation (PPV) over 11% was considered the threshold for hypovolemia. Dotted line – reference line.
Table 2.
The area under the ROC curve (AUC) for IDVG, PVI, and SPV.
| Parameters | AUC ± SE | 95% CI | P |
|---|---|---|---|
| IDVG | 0.92±0.06 | 0.76 to 0.99 | <0.001# |
| PVI | 0.86±0.07 | 0.68 to 0.96 | <0.001# |
| SPV | 0.72±0.11 | 0.52 to 0.86 | 0.04# |
| IDVG-PVI | 0.06±0.08 | −0.10 to 0.22 | 0.47 |
| IDVG-SPV | 0.20±0.09 | 0.02 to 0.38 | 0.03* |
| PVI-SPV | 0.14±0.13 | −0.11 to 0.39 | 0.26 |
Significant level P (Area=0.5),
significant level p<0.05 (among IDVG, PVI, and SPV).
AUC ± SE – area under the ROC curve ± Standard Error; 95% CI – 95% Confidence Interval; IDVG-PVI – AUC difference between IDVG and PVI; IDVG-SPV – AUC difference between IDVG and SPV; PVI-SPV – AUC difference between PVI and SPV.
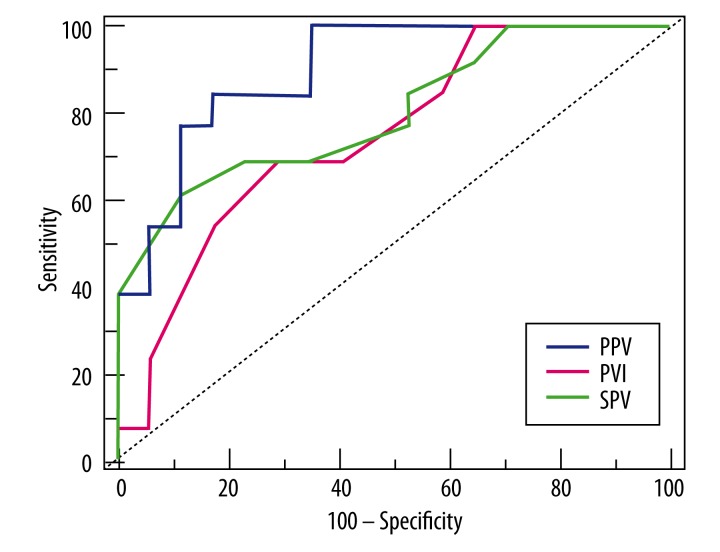
While IDVG <100mL/kg was selected as the threshold of low central ECF volume, the optimal cutoff values of PPV, PVI, and SPV (with sensitivity and specificity in parentheses) were 10.02% (84.6%, 82.4%), 14% (69.2%, 70.6%), and 6.33% (61.5%, 88.2%) (Figure 4). All individual AUCs were significantly different from the area under the reference line, the P value of PPV was less than 0.001, the P value of PVI was 0.006, and the P value of SPV was 0.03. No significant differences were found among the AUCs of PPV, SPV, and PVI (Table 3).
Figure 4.
Receiver operating characteristic (ROC) analyses for pulse pressure variation (PPV, blue), systolic pressure variation (SPV, green), and the pleth variability index (PVI, red). An initial distribution volume of glucose (IDVG) less than 100 mL/kg was considered the threshold for hypovolemia during study. Dotted line – reference line.
Table 3.
The area under the ROC curve (AUC) for PPV, PVI, and SPV.
| Parameters | AUC ± SE | 95% CI | P |
|---|---|---|---|
| PPV | 0.90±0.06 | 0.73 to 0.98 | <0.001# |
| PVI | 0.75±0.09 | 0.56 to 0.89 | 0.006# |
| SPV | 0.80±0.08 | 0.62 to 0.92 | 0.003# |
| PPV-PVI | 0.15±0.08 | −0.01 to 0.31 | 0.07 |
| PPV-SPV | 0.10±0.10 | 0.10 to 0.29 | 0.33 |
| PVI-SPV | 0.05±0.12 | −0.18 to 0.29 | 0.66 |
Significant level P (Area=0.5).
AUC ± SE – area under the ROC curve ± Standard Error; 95% CI – 95% Confidence Interval; PPV-PVI – AUC difference between PPV and PVI; PPV-SPV – AUC difference between PPV and SPV; PVI-SPV – AUC difference between PVI and SPV.
Discussion
ECF redistribution from the central to the peripheral compartment is one of the major causative factors of hypotension after anesthesia induction [13]. This relative hypovolemic hypotension is usually corrected by fast fluid infusion. However, it is hard to optimize the amount of fluid because of the lack of effective tools to assess the volume of the central or peripheral compartment or the amount of ECF shift. In this study, we found that PVI, PPV, and SPV have strong negative linear correlations with IDVG, which is simple, minimally invasive, and inexpensive measure of the central ECF volume. The optimal cutoff values for PPV, PVI, and SPV equivalent to IDVG lower than 100 mL/kg as the threshold of hypovolemia are 10.02%, 14%, and 6.33%, respectively, which are comparable to their cutoff thresholds for fluid responsiveness [6,15,19]. The AUC of PVI is lower than those of PPV and SPV, but they are all over 0.7, suggesting they are all good parameters in assessing the central ECF volume status. Although IDVG has a better correlation with cardiac output (CO) than ICG dilution, and can predict postoperative hypovolemia, repeated measurements of IDVG require at least 30-min intervals, which makes IDVG inadequate to assess individual response to a fluid challenge [10,14]. Owing to the simple, noninvasive, characteristics of PVI, our study indicates that PVI might be useful in assessing the central ECF volume status and directing fluid therapy.
PPV and SPV have been repeatedly proven to be able to predict fluid responsiveness and assess the cardiac preload or intravascular volume status. PPV is considered superior to SPV [18,20,21]. Data from the present study support these findings. When PPV over 11% is arbitrarily selected as the threshold of hypovolemia prediction based on literature reports [15,18], the optimal cutoff value of IDVG comparable to PPV in this study is 94.5 mL/kg, which is less than the lower limit of the normal IDVG range (110–130 mL/kg) [12,22]. Although PPV and SPV dynamic indices from respiratory variations strongly correlate with IDVG, the major limitations of routine clinical use of these indices are invasive arterial catheterization, expensive instruments, or specific software [18]. Stroke volume variation (SVV) can be noninvasively measured and calculated by echocardiography; however, SVV cannot be monitored continuously. There is mounting evidence that PVI is strongly correlated with PPV, and can predict fluid responsiveness in mechanically ventilated patients [8,23]. The data in our study not only confirm this strong correlation, but also demonstrate a strong correlation with IDVG. Additionally, the optimal cutoff value of PVI is about 14%, while IDVG <100 mL/kg was selected as the threshold for hypovolemia, which is outside the normal range of PVI (9–13%). The AUC is 0.75±0.09. Both parameters suggest that PVI might be used to monitor the status of central ECF volume and to predict hypovolemia.
Compared with the “gold standard” of measuring circulating blood volume [24], IDVG, as opposed to plasma volume measured by the ICG method, correlates better with CO following major abdominal surgery. More than 4000 IDVG determinations in an intensive care unit setting demonstrated that patients with an IDVG less than 100 mL/kg generally require fluid therapy [22]. Therefore, IDVG less than 100mL/kg was selected as the threshold for hypovolemia in this study.
As opposed to the measurement of IDVG, which is independent of the plasma glucose values before glucose injection, infusion of insulin, use of vasoactive drugs, and mechanical ventilation [10], the dynamic indices measure the variations induced by lung-heart interaction. Patients must be in sinus rhythm and be mechanically ventilated in a volume-controlled mode with tidal volumes >8 mL/kg [25]. Therefore, it is better to regard these dynamic indices as indicators of the position on the Frank-Starling curve to predict fluid responsiveness instead of indicators of volume status, or as markers of cardiac preload [26], despite strong correlations among IDVG, PPV, SPV, and PVI. An elevated PPV, SPV, or PVI, or an increase in these indices, implies operating on the steep portion of the Frank-Starling curve, which will remind the physician to respond to further fluid loss to avoid hemodynamic instability [20,27].
There are some limitations in our study. The gold standards of measuring circulating blood volume by 131I-labeled human serum albumin or indocyanine green are not available in our institute; therefore, it is hard to define hypovolemia. In the present study, we arbitrarily assumed an IDVG less than 100 mL/kg or a PPV over 11% as thresholds for hypovolemia according to previous reports [12,22]. PVI is based on peripheral volume and vascular tone-dependent dynamic preload variables [28,29], but we did not do a fluid challenge or manipulate vascular tone to confirm whether this linear relationship remained the same. To minimize the effects of stress on the measurement of PVI, we finished our observations before skin incision. Further experiments will be needed to explore the relationship between PVI and IDVG under different stresses or fluid challenges.
Conclusions
Strong correlations exist among IDVG, PPV, SPV, and PVI in the evaluation of volemia following anesthesia induction. PVI may be a useful indicator of moment-by-moment central ECF volume for those patients in whom invasive hemodynamic monitoring is not strictly required.
Footnotes
Source of support: This work was supported by National Natural Science Foundation of China (81271263 to J.J.Z), Shanghai Pujiang Talent Program from the Science and Technology Commission of Shanghai Municipality, China (11PJ 1408000 to J.J.Z.), and Shanghai First People’s Hospital Foundation, China (11B 05 to W.Q.L.)
References
- 1.Kumar A, Anel R, Bunnell E, et al. Pulmonary artery occlusion pressure and central venous pressure fail to predict ventricular filling volume, cardiac performance, or the response to volume infusion in normal subjects. Crit Care Med. 2004;32:691–99. doi: 10.1097/01.ccm.0000114996.68110.c9. [DOI] [PubMed] [Google Scholar]
- 2.Preisman S, Kogan S, Berkenstadt H, Perel A. Predicting fluid responsiveness in patients undergoing cardiac surgery: Functional haemodynamic parameters including the respiratory systolic variation test and static preload indicators. Br J Anaesth. 2005;95:746–55. doi: 10.1093/bja/aei262. [DOI] [PubMed] [Google Scholar]
- 3.Marik PE, Cavallazzi R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med. 2013;41:1774–81. doi: 10.1097/CCM.0b013e31828a25fd. [DOI] [PubMed] [Google Scholar]
- 4.Natalini G, Rosano A, Taranto M, et al. Arterial versus plethysmographic dynamic indices to test responsiveness for testing fluid administration in hypotensive patients: A clinical trial. Anesth Analg. 2006;103:1478–84. doi: 10.1213/01.ane.0000246811.88524.75. [DOI] [PubMed] [Google Scholar]
- 5.Cannesson M, Musard H, Desebbe O, et al. The ability of stroke volume variations obtained with vigileo/flotrac system to monitor fluid responsiveness in mechanically ventilated patients. Anesth Analg. 2009;108:513–17. doi: 10.1213/ane.0b013e318192a36b. [DOI] [PubMed] [Google Scholar]
- 6.Cannesson M, Desebbe O, Rosamel P, et al. Pleth variability index to monitor the respiratory variations in the pulse oximeter plethysmographic waveform amplitude and predict fluid responsiveness in the operating theatre. Br J Anaesth. 2008;101:200–6. doi: 10.1093/bja/aen133. [DOI] [PubMed] [Google Scholar]
- 7.Sandroni C, Cavallaro F, Marano C, et al. Accuracy of plethysmographic indices as predictors of fluid responsiveness in mechanically ventilated adults: A systematic review and meta-analysis. Intensive Care Med. 2012;38:1429–37. doi: 10.1007/s00134-012-2621-1. [DOI] [PubMed] [Google Scholar]
- 8.Vos JJ, Kalmar AF, Struys MM, et al. Comparison of arterial pressure and plethysmographic waveform-based dynamic preload variables in assessing fluid responsiveness and dynamic arterial tone in patients undergoing major hepatic resection. Br J Anaesth. 2013;110:940–46. doi: 10.1093/bja/aes508. [DOI] [PubMed] [Google Scholar]
- 9.Ishihara H, Shimodate Y, Koh H, et al. The initial distribution volume of glucose and cardiac output in the critically ill. Can J Anaesth. 1993;40:28–31. doi: 10.1007/BF03009314. [DOI] [PubMed] [Google Scholar]
- 10.Ishihara H, Suzuki A, Okawa H, et al. The initial distribution volume of glucose rather than indocyanine green derived plasma volume is correlated with cardiac output following major surgery. Intensive Care Med. 2000;26:1441–48. doi: 10.1007/s001340000653. [DOI] [PubMed] [Google Scholar]
- 11.Iwakawa T, Ishihara H, Takamura K, et al. Measurements of extracellular fluid volume in highly perfused organs and lung water in hypo- and hypervolaemic dogs. Eur J Anaesthesiol. 1998;15:414–21. doi: 10.1097/00003643-199807000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Orban JC, Blasin-Chadoutaud A, Zolfaghari P, et al. Hypovolaemic hypotension after abdominal aortic surgery is predicted by initial distribution volume of glucose. Eur J Anaesthesiol. 2010;27:364–68. doi: 10.1097/EJA.0b013e328334257c. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki A, Ishihara H, Okawa H, et al. Can initial distribution volume of glucose predict hypovolemic hypotension after radical surgery for esophageal cancer? Anesth Analg. 2001;92:1146–51. doi: 10.1097/00000539-200105000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Rose BO, Ishihara H, Okawa H, et al. Repeatability of measurements of the initial distribution volume of glucose in haemodynamically stable patients. J Clin Pharm Ther. 2004;29:317–23. doi: 10.1111/j.1365-2710.2004.00565.x. [DOI] [PubMed] [Google Scholar]
- 15.Kramer A, Zygun D, Hawes H, et al. Pulse pressure variation predicts fluid responsiveness following coronary artery bypass surgery. Chest. 2004;126:1563–68. doi: 10.1378/chest.126.5.1563. [DOI] [PubMed] [Google Scholar]
- 16.Hirota K, Ishihara H, Tsubo T, Matsuki A. Estimation of the initial distribution volume of glucose by an incremental plasma glucose level at 3 min after i.V. Glucose in humans. Br J Clin Pharmacol. 1999;47:361–64. doi: 10.1046/j.1365-2125.1999.00889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 18.He Z, Qiao H, Zhou W, et al. Assessment of cardiac preload status by pulse pressure variation in patients after anesthesia induction: Comparison with central venous pressure and initial distribution volume of glucose. J Anesth. 2011;25:812–17. doi: 10.1007/s00540-011-1225-1. [DOI] [PubMed] [Google Scholar]
- 19.Monnet X, Teboul JL. Volume responsiveness. Curr Opin Crit Care. 2007;13:549–53. doi: 10.1097/MCC.0b013e3282ec68b2. [DOI] [PubMed] [Google Scholar]
- 20.Pestel GJ, Hiltebrand LB, Fukui K, et al. Assessing intravascular volume by difference in pulse pressure in pigs submitted to graded hemorrhage. Shock. 2006;26:391–95. doi: 10.1097/01.shk.0000228792.10550.ed. [DOI] [PubMed] [Google Scholar]
- 21.Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: A systematic review of the literature. Crit Care Med. 2009;37:2642–47. doi: 10.1097/CCM.0b013e3181a590da. [DOI] [PubMed] [Google Scholar]
- 22.Hashiba E, Ishihara H, Tsubo T, et al. Use of initial distribution volume of glucose to determine fluid volume loading in pulmonary thromboembolism and right ventricular myocardial infarction. J Anesth. 2008;22:453–56. doi: 10.1007/s00540-008-0642-2. [DOI] [PubMed] [Google Scholar]
- 23.Desebbe O, Cannesson M. Using ventilation-induced plethysmographic variations to optimize patient fluid status. Curr Opin Anaesthesiol. 2008;21:772–78. doi: 10.1097/ACO.0b013e32831504ca. [DOI] [PubMed] [Google Scholar]
- 24.Haruna M, Kumon K, Yahagi N, et al. Blood volume measurement at the bedside using icg pulse spectrophotometry. Anesthesiology. 1998;89:1322–28. doi: 10.1097/00000542-199812000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Desebbe O, Boucau C, Farhat F, et al. The ability of pleth variability index to predict the hemodynamic effects of positive end-expiratory pressure in mechanically ventilated patients under general anesthesia. Anesth Analg. 2010;110:792–98. doi: 10.1213/ANE.0b013e3181cd6d06. [DOI] [PubMed] [Google Scholar]
- 26.Michard F. Changes in arterial pressure during mechanical ventilation. Anesthesiology. 2005;103:419–28. doi: 10.1097/00000542-200508000-00026. quiz 449–51. [DOI] [PubMed] [Google Scholar]
- 27.Ornstein E, Eidelman LA, Drenger B, et al. Systolic pressure variation predicts the response to acute blood loss. J Clin Anesth. 1998;10:137–40. doi: 10.1016/s0952-8180(97)00257-2. [DOI] [PubMed] [Google Scholar]
- 28.Monnet X, Guerin L, Jozwiak M, et al. Pleth variability index is a weak predictor of fluid responsiveness in patients receiving norepinephrine. Br J Anaesth. 2013;110:207–13. doi: 10.1093/bja/aes373. [DOI] [PubMed] [Google Scholar]
- 29.Takeyama M, Matsunaga A, Kakihana Y, et al. Impact of skin incision on the pleth variability index. J Clin Monit Comput. 2011;25:215–21. doi: 10.1007/s10877-011-9298-9. [DOI] [PubMed] [Google Scholar]