Abstract
Background
Chronic kidney disease (CKD) is a major health problem worldwide. Oxidative stress is one of the mediators of this disease. Systemic complications of oxidative stress are involved in the pathogenesis of hypertension, endothelial dysfunction, shortened erythrocyte lifespan, deformability, and nitric oxide (NO) dysfunction. L-carnosine is known as an antioxidant. In this study, our aim was to investigate the effect of carnosine on hemorheologic and cardiovascular parameters in CKD-induced rats.
Material/Methods
We used 4-month-old male Sprague-Dawley rats divided into 4 groups of 6 rats each. Three days after subtotal nephrectomy and sham operations, the surviving rats were divided into the 4 groups; 1) Sham (S), 2) Sham+Carnosine (S-C), 3) Subtotal nephrectomy (Nx), and 4) Subtotal nephrectomy + Carnosine (N-C). Carnosine was injected intraperitoneally (i.p.) (50 mg/kg) for 15 days. The control group received the same volume of physiological saline.
Results
In CKD rats, malondialdehyde (MDA) levels were increased, and NO and RBC deformability were decreased compared to Sham. Carnosine treatment decreased MDA levels, improved RBC (red blood cell) ability to deform, and increased NO levels. However, carnosine did not affect blood pressure levels in these rats.
Conclusions
We found that carnosine has beneficial effects on CKD in terms of lipid peroxidation and RBC deformability. Carnosine may have a healing effect in microcirculation level, but may not have any effect on systemic blood pressure in CKD-induced rats.
MeSH Keywords: Blood Pressure - physiology, Carnosine, Erythrocyte Deformability - physiology, Nephrectomy
Background
CKD is a major public health problem affecting millions of people worldwide [1]. Oxidative stress is a mediator of systemic complications of CKD [2]. Oxidative stress may be defined as a disturbance in regular cellular and molecular function caused by the disequilibrium between production of reactive oxygen species (ROS) and antioxidant factors [3]. ROS are normally produced during normal respiration, but in excessive production or insufficiency of natural antioxidant capacity, ROS can lead to loss of cell and tissue function; thus, oxidative stress is involved in the pathogenesis of hypertension [4], endothelial dysfunction [5], shortened erythrocyte lifespan, and deformability in CKD [6].The presence of oxidative stress in CKD is evidenced by an increased abundance of by-products of ROS [2].
One of the main effects of oxidative stress is the decrease in biological activity of NO [7]. Under normal conditions, ROS are safely converted to water and molecular oxygen. However, in the presence of excess O2, the oxidation of NO by O2 leads to functional NO deficiency and peroxynitrite formation (NO + O2 – ONOO–) [8]. Peroxynitrite is a damaging molecule that can trigger lipid peroxidation and DNA damage in the body. NO is an endothelium-derived relaxing factor (EDRF) and is a major trophic and survival factor for endothelium [9]. Also, NO contributes to the maintenance of renal vascular resistance (RVR) and renal blood flow (RBF) by regulating glomerular filtration [10,11]. Vascular endothelium is capable of modulating the tone of the underlying smooth muscle in response to local changes in shear stress, pressure, and other mechanical factors [12]. Endothelial dysfunction is characterized by a reduced synthesis of bioavailable NO [13]. Recent studies suggest a key role of the microvasculature in renal disease [14].
By promoting the ROS-mediated inactivation of NO, oxidative stress can cause endothelial dysfunction and hypertension. The remnant kidney (RK) model is widely considered to be the classic model of progressive renal disease. Inhibition of NO synthesis has been known to worsens renal disease is the RK model by hemodynamic changes [15]. It has been demonstrated that alterations in hemodynamic conditions and wall shear stress can affect NO synthesizing mechanisms in the vascular endothelium [16]. NO has been demonstrated to affect the cellular elements of blood, including RBC [17]. NO modulates RBC mechanical properties and blockade of NO synthesis results in deterioration of RBC deformability [18]. The deformability ability of RBC has crucial importance for the maintenance of microcirculation. Blood viscosity decreases at high shear stress rates through the ability of RBC deformability and altered mechanical properties of RBC were reported in hypertension [19].
L-carnosine is known to possess free radical scavenging functions [20]. In our previous study on diabetic rats, we demonstrated that carnosine has healing effects on RBC of diabetic rats, which decreased as a result of oxidative stress in diabetes mellitus [21]. In this study, our aim was to induce renal failure in rats with subtotal nephrectomy (RK model) and observe the effect of carnosine on RBC deformability and blood pressure levels of nephrectomized rats and to compare the results with sham-operated control group rats.
Material and Methods
Male Sprague-Dawley rats were used (4 months, 375±19 body weight). The animals were maintained under the conditions of 6:00–20:00 h lighting, 24±1°C temperature, and 55±5% humidity, and were given ad libitum commercial diet and water. The animals were divided into 4 groups of 6 rats each. The study was performed in accordance with the guidelines for animal welfare and was approved by the Institutional Review Board of the Faculty of Medicine, Bezmi Alem University, Turkey.
Nephrectomy and sham operations
Through a right dorsolateral incision, the right kidney was pulled out to expose the renal vessels and ureter, which were then ligated with cotton thread and cut between the hilus and the ligated portion to remove the kidney. After kidney removal, the incision was sutured. The left kidney was pulled out through a left dorsolateral incision to expose the renal vessels and ureter. The renal vessels were clipped by a clamp, and the cranial and caudal parts of the organ were cut so that ¾ of the kidney remained. Finally, the treated left kidney was returned to the abdominal cavity and the incision was sutured. Sham operations consisted in laparotomy and manipulation of the kidneys and renal pedicle without destruction of renal tissue.
3 days after surgery, the surviving ¾-nephrectomized rats were randomly divided into the 4 groups;
Sham (S, n=6),
Sham + Carnosine (S-C, n=6),
Subtotal nephrectomy (Nx, n=6),
Subtotal nephrectomy + Carnosine (N-C, n=6).
Carnosine (white powder, dissolved in physiological saline, Sigma Chemical Co, USA) was injected i.p. with 50 mg/kg in each injection every day for 15 days. The control group received the same volume of physiological saline.
Red blood cell deformability measurement
After the injections were finished at the end of the 15 days), 30 microliters of blood were obtained from a tail vein of each rat and the red blood cell deformability index was measured by a shear stress diffractometer (Rheodyn SSD, Myrenne GmbH, Germany) as previously described [22]. Briefly, 30 μl blood sample was added to 2 ml of Dextran 60 solution (viscosity 24 mPa s, osmolality 290 mOsm, pH 7.4), mixed well, and introduced to the device, where RBC suspension was shared. As a measure of deformability, RBC elongation index (EI) was measured at shear stresses from 0.3 to 60 Pa.
Arterial blood pressure and heart rate measurement
Twenty-four hours after the last treatment, mean, systolic, and diastolic blood pressure and heart rate were directly measured through the femoral artery 19 days after the operation. The animals were anesthetized with ketamine (39.35 mg/kg, i.p.) plus xylazine (4.96 mg/kg, i.p.). A polyethylene catheter (PE-50, Intramedic, Clay Adams, MD) was implanted in the femoral artery. The catheter was filled with heparinized solution of normal saline (10 IU/ml) to be connected to a pressure transducer. The transducer output was amplified, and the arterial pressure signal passed to an analog-digital converter (MP30, Biopac System), and the arterial pressures were determined from the pulse wave.
NOx and lipid peroxidation measurement
NOx and MDA levels were measured spectrophotometrically from serum samples as described before [23]. Briefly, NOx levels were measured with Griess method and the susceptibility of erythrocytes to lipid peroxidation was immediately determined by a method based on measuring the concentration of the pink chromogen compound that forms when MDA couples to thiobarbituric acid.
Statistical analyses
SPSS 15.0 and Excell were used for analyzing data. Kruskal-Wallis ANOVA, Mann-Whitney U and Student’s t tests were used for statistical analysis. Data are expressed as mean ± standard error of mean (SEM). P<0.05 was considered significant.
Results
Malondialdehyde levels
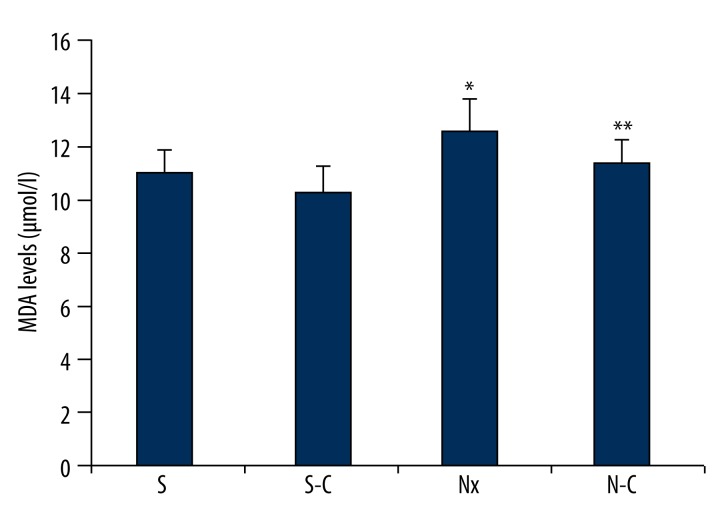
In the Nx group, MDA level in erythrocytes was significantly increased compared to the S group as we expected (p<0.05). MDA level was significantly decreased in the N-C group compared to the Nx group (p<0.05). In the S-C group, MDA level was decreased compared to the S group, but this change was not significant (p>0.05; Figure 1).
Figure 1.
Malondialdehyde levels of all groups. S – Sham-operated group, S-C – Carnosine-treated sham-operated group, Nx – Nephrectomy-operated group, N-C – Carnosine-treated nephrectomy-operated group. * p<0.05 when compared to S and ** p<0.05 when compared to N group.
NOx Levels
NOx level of the Nx group was significantly decreased compared to the S group NOx levels (p<0.05). There was no difference between Nx and N-C groups compared to S and S-C groups in terms of NOx levels (p>0.05; Figure 2).
Figure 2.
Nitric oxide levels of all groups. S – Sham-operated group, S-C – Carnosine-treated sham-operated group, Nx – Nephrectomy-operated group, N-C – Carnosine-treated nephrectomy-operated group. * Compared to S group (p<0.05)
RBC deformability levels
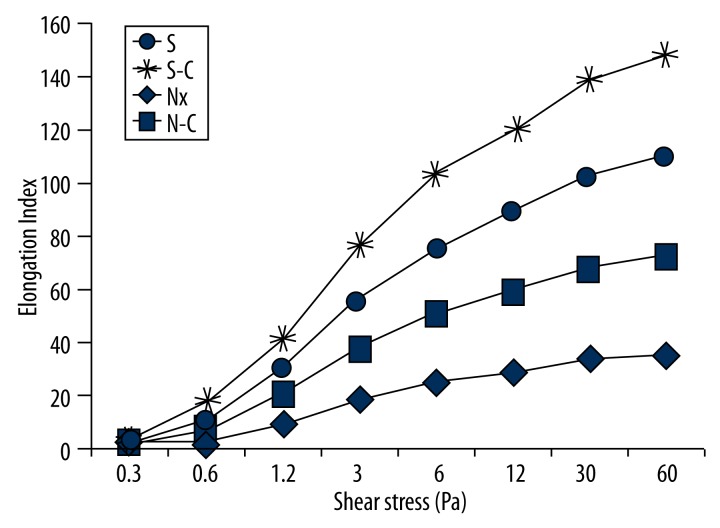
EI level was significantly decreased in the Nx group compared to the S group (p<0.05). N-C group EI level was significantly increased compared to the Nx group (p<0.05). These results show the improved effect of carnosine on nephrectomized rats’ RBC deformability indexes (Figure 3).
Figure 3.
Elongation Index in all shear stress levels of all groups. S-C – Carnosine-treated sham-operated group, Nx – Nephrectomy-operated group, N-C – Carnosine-treated nephrectomy-operated group.
We also found that S-C group EI value slightly increased compared to the S group, but this increment was not statistically significant (p>0.05). These EI values demonstrate that carnosine has this effect at all shear stress values (Figure 3).
Blood pressure and heart rate levels
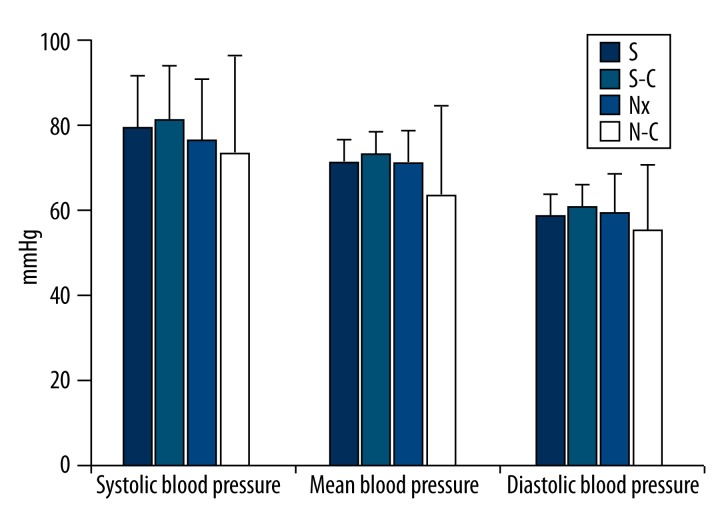
In all systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean blood pressure (MBP) measurements, there was no significant difference between S and Nx groups (p>0.05; Figure 4).
Figure 4.
Blood pressure levels of all groups. S-C – Carnosine-treated sham-operated group, Nx – Nephrectomy-operated group, N-C – Carnosine-treated nephrectomy-operated group.
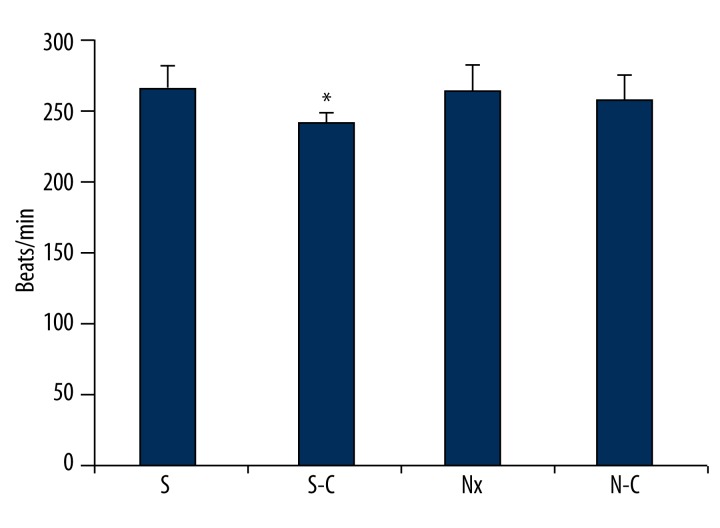
S-C group heart rate level was significantly increased compared to S group heart rate level (p<0.05). There was no important difference between other groups in terms of heart rate levels (Figure 5).
Figure 5.
Heart rate of all groups. S-C – Carnosine-treated sham-operated group, Nx – Nephrectomy-operated group, N-C – Carnosine-treated nephrectomy-operated group. * p<0.05 Compared to S group.
Discussion
CKD has emerged as a major public health problem that affects millions of patients worldwide and leads to decreased survival [1]. Reduction of the renal mass by subtotal nephrectomy in animals or by kidney disease in humans results in proteinuria, glomerulosclerosis, tubulointerstitial injury, and progressive deterioration of kidney function and structure. This process is mediated by a constellation of hemodynamic events (glomerular hypertension and hyperfiltration) and non-hemodynamic events (oxidative stress, inflammation, and apoptosis) [24]. The prevailing oxidative stress in animals and humans with chronic renal insufficiency leads to the oxidation of proteins, carbohydrates, nucleic acids, lipids, and lipoproteins, and accumulation of their harmful by-products in various tissues and body fluids. In addition, the increased generation of ROS leads to tissue injury and dysfunction by attacking, denaturing, and modifying structural and functional molecules, and by activating redox-sensitive transcription factors and the signal transduction pathway. These events, in turn, promote necrosis, apoptosis, inflammation, fibrosis, and other disorders. Oxidative stress and inflammation are constant features of advanced renal disease and play a major role in progressive deterioration of renal function and structure, as well as associated cardiovascular and numerous other complications of chronic kidney disease. It is implicated in the pathogenesis of renal injury and may be blocked by antioxidants [25]. Therefore, we used carnosine as an antioxidant to reduce oxidative stress.
Carnosine is a scavenger of hydroxyl and superoxide radicals and a strong quencher of singlet molecular oxygen [26]. It is present in various human tissues [27], but occurs in high concentrations [up to 20 mM] in skeletal muscles and the brain (where it is thought to act as a biological antioxidant), and protects these tissues against oxidative damage induced by different factors [28]. It was also reported that it protects mitochondrial membranes from free radical damage, decreases lipid peroxidation of cell membranes, and has inotropic properties. Thus, it is reasonable to propose that carnosine may protect the heart from injury and improve the functions following cardiac ischemia [29]. Carnosine has been reported to have natural ACE-inhibitor activity, serve as an oxygen free radical scavenger, and cleave advanced glycation end-products. Addition of carnosine markedly reduces the synthesis of matrix components and TGF β2 in renal cell lines [30]. In experimental studies it was shown that carnosine reduces the level of proinflammatory and profibrotic cytokines. It is suggested that carnosine is a naturally occurring anti-aging substance in humans, with a beneficial effect on the cardiovascular system [27].
Oxidative stress and inflammation play a critical part in progression of many diseases, including kidney and heart diseases. Oxidative stress is caused by a combination of increased production of ROS and impaired antioxidant capacity. Increased generation of ROS leads to tissue injury and dysfunction by attacking, denaturing, and modifying structural and functional molecules and by activating redox-sensitive transcription factors and signal transduction pathways. These events, in turn, promote necrosis, apoptosis, inflammation, fibrosis, and other disorders [31].
High oxidative stress levels, as indicated by plasma MDA concentration, was found to be significantly higher in Nx animals [31–33]. This finding is in agreement with our observation in nephrectomized rats. In the Nx group, we found significantly elevated lipid peroxidation levels, which indicates that oxidative stress is increased in these animals. In the N-C group, lipid peroxidation levels were decreased to S group lipid peroxidation levels, which indicates that carnosine prevented lipid peroxidation in Nx group rats. Several studies reported a beneficial effect of antioxidant treatment on MDA concentration or ROS in CKD. One of these studies showed that alpha-lipoic acid, an antioxidant, attenuated kidney MDA concentration in Nx rats [33]. Another study reported that sildenafil-treated Nx rats had decreased ROS levels [34].
NO also plays an important role in renal function and sodium excretion and regulates the homeostatic response to increased sodium intake [35]. In the current study, renal NOx levels were measured 2 weeks after surgery. The present study confirms that decreased renal NOx levels occurred very early after kidney damage. Decreased renal NOx levels were probably associated with MDA in the Nx animals.
Oxidative stress has many consequences in the body. It has adverse effects on many organ systems and types of cells, including RBC. RBC are susceptible to oxidant damage because of lipid contents in their membranes. Oxidative stress-related alterations may lead to changes of RBC rheologic behavior as RBC deformability [36]. It is generally accepted that RBC deformability is an important determinant of blood flow resistance, especially in the microcirculation, and thus impaired RBC deformability may contribute to tissue perfusion problems and organ damage [37]. Our results indicate that RBC deformability abilities were decreased in Nx group rats, which means oxygen consumption of tissues was diminished in these animals. In N-C group rats, we found that carnosine can increase RBC deformability ability of Nx rats, which supports the result that obtained in lipid peroxidation levels.
Blood flow, deformability, and aggregability of RBCs are the main components of hemorheology. In large blood vessels, a basic component is the flow. In microcirculation, where cells must deform to pass through narrow capillaries, deformability and aggregation of RBCs are the major determinants of resistance to flow. The ability of the entire RBC to deform is of crucial importance for performing its function in oxygen delivery and it is also a determinant of cell survival time in the circulation. Oxidative stress may decrease RBC aggregation via echinocyte formation. When the results of the current study are evaluated together, it can be seen that the alterations in RBC aggregation are far from explained by the changes in oxidative stress [38].
Prolonged ischemia, oxidative stress and inflammation-mediated alterations in erythrocyte mechanics and microvascular architecture play a major role in some pathophysiologic process such as ischemia/reperfusion injury or no-reflow phenomenon. An increase in red cell rigidity is an important rheological aspect of RBCs, which facilitates platelet aggregation with the subendothelium [39].
Impaired RBC deformability is thought to be due to increased oxidative stress. Indeed, RBCs are very sensitive to the harmful effects of free radicals. First, RBCs are constantly exposed to oxidative stress due to continuously generated oxygen radicals by the auto-oxidation of hemoglobin. Second, RBC membranes contain relatively high levels of unsaturated fatty acids, which are especially good substrates for peroxidation reactions. Studies have indicated that hypertension, as well as cardiovascular and cerebrovascular diseases, are strongly correlated with hemorheological abnormalities. Therefore, the correction of the hemorheological abnormalities may be important in the treatment of these pathologies. Oxidative stress-induced membrane damage is usually initiated by any free radical. Additionally, recent studies have shown that blood rheological parameters must be within the physiological limits for good tissue perfusion. This finding was consistent with previous studies, which showed a relationship between oxidative stress and decreased RBC deformability [40].
The increased oxidative stress reduces erythrocyte deformability. In our study, increased MDA levels and decreased plasma NOx levels manifested the role of oxidative stress in the pathogenesis of the disease. It is known that the increased MDA values and decreased microvascular flow impairs RBC deformability. Many studies have evaluated erythrocyte deformability in chronic disease, such as diabetes mellitus, chronic renal failure, and coronary artery disease [41]. In our previous study, we found that carnosine can significantly reverse erythrocyte deformability, reduce lipid peroxidation, and increase NOx levels in diabetic rats. In this study we concluded that carnosine can recover from microvascular circulation problems by increasing erythrocyte deformability and protect cells and tissues against lipid peroxidation [21].
In the present study, blood pressure levels were not significantly different between groups. Normally, high blood pressure is expected after kidney ablation surgery. These discrepancies may reflect differences in the duration of post-operative period on animals or saline loading (with food or fluid intake). First, a time-dependent increase in both systolic and diastolic blood pressure levels was observed in rats with Nx [42,43]. Secondly, high blood pressure depends on salt loading in the Nx kidney [35,44]. In this study we wanted to investigate how kidney ablation affects red cell deformability in the early phase of kidney damage without changing blood pressure. The rat model described here develops an initial modest kidney injury of uncertain cause that is independent of salt intake and lasts 2 weeks. Therefore, we chose a 2-week postoperative period after rat surgery and distilled water was also consumed by all groups. However, Lai et al. found no significant change in blood pressure with salt loading in Nx mice [45,46]. This result is in agreement with our result. High BP in response to sodium intake (salt sensitivity) is considered an important factor in the pathogenesis of human hypertension [35,47]. The other reason why we did not prefer to increase animal salt intake was that almost all physicians suggest decreased salt consumption in the diet of their patients due to kidney surgery or antihypertension treatment. Thirdly, this might relate to activation of renal afferent reflexes initiated by the renal injury and the healing process from the 2/3 Nx [46]. Thus, the absence of high blood pressure does not mean that microvascular circulation of the kidney has not been affected in these rats.
We found that only the carnosine-treated group had significantly decreased heart rate levels. We assume that carnosine increases intracellular Ca+2 levels in cardiomyocytes to increase contractility [48]; therefore, heart rate may decrease from this effect.
Understanding the mechanisms of altered RBC rheology in sepsis, and the effects on blood flow and oxygen transport, may lead to improved patient management and reductions in morbidity and mortality.
Conclusions
In conclusion, MDA levels were elevated and consequently caused a decrease in NO levels and impaired RBC deformability in Nx rats. However, blood pressure levels were not affected in these rats. We showed that MDA levels were decreased in rats treated with carnosine and this healing in MDA levels caused an increase in NO levels and RBC deformability, but we could not find any difference among groups related to blood pressure levels after carnosine treatment. We assumed that although systemic blood pressure level has not been affected in the Nx model, microcirculation might be affected due to decreased RBC deformability and NOx levels in these rats.
Footnotes
Source of support: Self financing
References
- 1.Coresh J, Astor BC, Greene T, et al. Prevalence of chronic kidney disease and decreased kidney function in the adult US population. Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41(1):1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 2.Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62(5):1524–38. doi: 10.1046/j.1523-1755.2002.00600.x. [DOI] [PubMed] [Google Scholar]
- 3.Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29(3–4):222–30. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 4.Vaziri ND, Oveisi F, Ding Y. Role of increased oxygen free radical activity in the pathogenesis of uremic hypertension. Kidney Int. 1998;53(6):1748–54. doi: 10.1046/j.1523-1755.1998.00947.x. [DOI] [PubMed] [Google Scholar]
- 5.Hasdan G, Benchetrit S, Rashid G, et al. Endothelial dysfunction and hypertension in 5/6 nephrectomized rats are mediated by vascular superoxide. Kidney Int. 2002;61(2):586–90. doi: 10.1046/j.1523-1755.2002.00166.x. [DOI] [PubMed] [Google Scholar]
- 6.Durak I, Akyol O, Basesme E, et al. Reduced erythrocyte defense mechanisms against free radical toxicity in patients with chronic renal failure. Nephron. 1994;66(1):76–80. doi: 10.1159/000187770. [DOI] [PubMed] [Google Scholar]
- 7.Costa-Hong V, Bortolotto LA, Jorgetti V, et al. Oxidative stress and endothelial dysfunction in chronic kidney disease. Arq Bras Cardiol. 2009;92(5):381–86. 398–403, 413–18. doi: 10.1590/s0066-782x2009000500013. [DOI] [PubMed] [Google Scholar]
- 8.Vaziri ND. Roles of oxidative stress and antioxidant therapy in chronic kidney disease and hypertension. Curr Opin Nephrol Hypertens. 2004;13(1):93–99. doi: 10.1097/00041552-200401000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Vessieres E, Freidja ML, Loufrani L, et al. Flow (shear stress)-mediated remodeling of resistance arteries in diabetes. Vascul Pharmacol. 2012;57(5–6):173–78. doi: 10.1016/j.vph.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Kone BC, Baylis C. Biosynthesis and homeostatic roles of nitric oxide in the normal kidney. Am J Physiol. 1997;272(5 Pt 2):F561–78. doi: 10.1152/ajprenal.1997.272.5.F561. [DOI] [PubMed] [Google Scholar]
- 11.van Koppen A, Verhaar MC, Bongartz LG, Joles JA. 5/6th nephrectomy in combination with high salt diet and nitric oxide synthase inhibition to induce chronic kidney disease in the Lewis rat. J Vis Exp. 2013;(77):e50398. doi: 10.3791/50398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walshe TE, Dela Paz NG, D’Amore PA. The Role of Shear-Induced Transforming Growth Factor-beta Signaling in the Endothelium. Arterioscler Thromb Vasc Biol. 2013;33(11):2608–17. doi: 10.1161/ATVBAHA.113.302161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broniowska KA, Diers AR, Corbett JA, Hogg N. Effect of nitric oxide on naphthoquinone toxicity in endothelial cells: role of bioenergetic dysfunction and poly (ADP-ribose) polymerase activation. Biochemistry. 2013;52(25):4364–72. doi: 10.1021/bi400342t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leung N. Hematologic manifestations of kidney disease. Semin Hematol. 2013;50(3):207–15. doi: 10.1053/j.seminhematol.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Kang DH, Nakagawa T, Feng L, Johnson RJ. Nitric oxide modulates vascular disease in the remnant kidney model. Am J Pathol. 2002;161(1):239–48. doi: 10.1016/S0002-9440(10)64175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varin R, Mulder P, Richard V, et al. Exercise improves flow-mediated vasodilatation of skeletal muscle arteries in rats with chronic heart failure. Role of nitric oxide, prostanoids, and oxidant stress. Circulation. 1999;99(22):2951–57. doi: 10.1161/01.cir.99.22.2951. [DOI] [PubMed] [Google Scholar]
- 17.Bhagat K, Vallance P. Nitric oxide 9 years on. J R Soc Med. 1996;89(12):667–73. doi: 10.1177/014107689608901204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bor-Kucukatay M, Wenby RB, Meiselman HJ, Baskurt OK. Effects of nitric oxide on red blood cell deformability. Am J Physiol Heart Circ Physiol. 2003;284(5):H1577–84. doi: 10.1152/ajpheart.00665.2002. [DOI] [PubMed] [Google Scholar]
- 19.Ajmani RS. Hypertension and hemorheology. Clin Hemorheol Microcirc. 1997;17(6):397–420. [PubMed] [Google Scholar]
- 20.Hartman PE, Hartman Z, Ault KT. Scavenging of singlet molecular oxygen by imidazole compounds: high and sustained activities of carboxy terminal histidine dipeptides and exceptional activity of imidazole-4-acetic acid. Photochem Photobiol. 1990;51(1):59–66. doi: 10.1111/j.1751-1097.1990.tb01684.x. [DOI] [PubMed] [Google Scholar]
- 21.Yapislar H, Aydogan S. Effect of carnosine on erythrocyte deformability in diabetic rats. Arch Physiol Biochem. 2012;118(5):265–72. doi: 10.3109/13813455.2012.714790. [DOI] [PubMed] [Google Scholar]
- 22.Martinez M, Vaya A, Server R, et al. Erythrocyte elongation index measured on a Rheodyn SSD laser diffractometer. Influence of the hematocrit. Clin Hemorheol Microcirc. 1998;19(3):255–57. [PubMed] [Google Scholar]
- 23.Orpana AK, Avela K, Ranta V, et al. The calcium-dependent nitric oxide production of human vascular endothelial cells in preeclampsia. Am J Obstet Gynecol. 1996;174(3):1056–60. doi: 10.1016/s0002-9378(96)70350-2. [DOI] [PubMed] [Google Scholar]
- 24.An WS, Kim HJ, Cho KH, Vaziri ND. Omega-3 fatty acid supplementation attenuates oxidative stress, inflammation, and tubulointerstitial fibrosis in the remnant kidney. Am J Physiol Renal Physiol. 2009;297(4):F895–903. doi: 10.1152/ajprenal.00217.2009. [DOI] [PubMed] [Google Scholar]
- 25.Park CH, Lee SL, Okamoto T, et al. Rokumi-jio-gan-Containing Prescriptions Attenuate Oxidative Stress, Inflammation, and Apoptosis in the Remnant Kidney. Evid Based Complement Alternat Med. 2012;2012:587902. doi: 10.1155/2012/587902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aldini G, Orioli M, Rossoni G, et al. The carbonyl scavenger carnosine ameliorates dyslipidaemia and renal function in Zucker obese rats. J Cell Mol Med. 2011;15(6):1339–54. doi: 10.1111/j.1582-4934.2010.01101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kilis-Pstrusinska K. [Carnosine, carnosinase and kidney diseases]. Postepy Hig Med Dosw (Online) 2012;66:215–21. doi: 10.5604/17322693.991600. [DOI] [PubMed] [Google Scholar]
- 28.Ozdogan K, Taskin E, Dursun N. Protective effect of carnosine on adriamycin-induced oxidative heart damage in rats. Anadolu Kardiyol Derg. 2011;11(1):3–10. doi: 10.5152/akd.2011.003. [DOI] [PubMed] [Google Scholar]
- 29.Dursun N, Taskin E, Ozturk F. Protection against adriamycin-induced cardiomyopathy by carnosine in rats: role of endogenous antioxidants. Biol Trace Elem Res. 2011;143(1):412–24. doi: 10.1007/s12011-010-8875-y. [DOI] [PubMed] [Google Scholar]
- 30.Freedman BI, Hicks PJ, Sale MM, et al. A leucine repeat in the carnosinase gene CNDP1 is associated with diabetic end-stage renal disease in European Americans. Nephrol Dial Transplant. 2007;22(4):1131–35. doi: 10.1093/ndt/gfl717. [DOI] [PubMed] [Google Scholar]
- 31.Kim HJ, Vaziri ND. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am J Physiol Renal Physiol. 2010;298(3):F662–71. doi: 10.1152/ajprenal.00421.2009. [DOI] [PubMed] [Google Scholar]
- 32.Soetikno V, Sari FR, Lakshmanan AP, et al. Curcumin alleviates oxidative stress, inflammation, and renal fibrosis in remnant kidney through the Nrf2-keap1 pathway. Mol Nutr Food Res. 2013;57(9):1649–59. doi: 10.1002/mnfr.201200540. [DOI] [PubMed] [Google Scholar]
- 33.Yu X, Liu H, Zou J, et al. Oxidative stress in 5/6 nephrectomized rat model: effect of alpha-lipoic acid. Ren Fail. 2012;34(7):907–14. doi: 10.3109/0886022X.2012.691012. [DOI] [PubMed] [Google Scholar]
- 34.Tapia E, Sanchez-Lozada LG, Soto V, et al. Sildenafil treatment prevents glomerular hypertension and hyperfiltration in rats with renal ablation. Kidney Blood Press Res. 2012;35(4):273–80. doi: 10.1159/000334952. [DOI] [PubMed] [Google Scholar]
- 35.Cruz A, Rodriguez-Gomez I, Perez-Abud R, et al. Effects of clofibrate on salt loading-induced hypertension in rats. J Biomed Biotechnol. 2011;2011:469481. doi: 10.1155/2011/469481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baskurt OK, Temiz A, Meiselman HJ. Effect of superoxide anions on red blood cell rheologic properties. Free Radic Biol Med. 1998;24(1):102–10. doi: 10.1016/s0891-5849(97)00169-x. [DOI] [PubMed] [Google Scholar]
- 37.Hinshaw LB. Sepsis/septic shock: participation of the microcirculation: an abbreviated review. Crit Care Med. 1996;24(6):1072–78. doi: 10.1097/00003246-199606000-00031. [DOI] [PubMed] [Google Scholar]
- 38.Kilic-Toprak E, Ardic F, Erken G, et al. Hemorheological responses to progressive resistance exercise training in healthy young males. Med Sci Monit. 2012;18(6):CR351–60. doi: 10.12659/MSM.882878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhavsar J, Rosenson RS. Adenosine transport, erythrocyte deformability and microvascular dysfunction: an unrecognized potential role for dipyridamole therapy. Clin Hemorheol Microcirc. 2010;44(3):193–205. doi: 10.3233/CH-2010-1274. [DOI] [PubMed] [Google Scholar]
- 40.Kucukatay V, Bor-Kucukatay M, Gundogdu G, et al. Vitamin E treatment enhances erythrocyte deformability in aged rats. Folia Biol (Praha) 2012;58(4):157–65. [PubMed] [Google Scholar]
- 41.Akman T, Akarsu M, Akpinar H, et al. Erythrocyte deformability and oxidative stress in inflammatory bowel disease. Dig Dis Sci. 2012;57(2):458–64. doi: 10.1007/s10620-011-1882-9. [DOI] [PubMed] [Google Scholar]
- 42.Moreira-Rodrigues M, Sampaio-Maia B, Pestana M. Renal dopaminergic system activity in rat remnant kidney up to twenty-six weeks after surgery. Life Sci. 2009;84(13–14):409–14. doi: 10.1016/j.lfs.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 43.Santos-Araujo C, Roncon-Albuquerque R, Jr, Moreira-Rodrigues M, et al. Local modulation of the natriuretic peptide system in the rat remnant kidney. Nephrol Dial Transplant. 2009;24(6):1774–82. doi: 10.1093/ndt/gfn719. [DOI] [PubMed] [Google Scholar]
- 44.Quelhas-Santos J, Sampaio-Maia B, Simoes-Silva L, et al. Sodium-dependent modulation of systemic and urinary renalase expression and activity in the rat remnant kidney. J Hypertens. 2013;31(3):543–52. doi: 10.1097/HJH.0b013e32835d6e34. discussion 52–53. [DOI] [PubMed] [Google Scholar]
- 45.Lai EY, Solis G, Luo Z, et al. p47(phox) is required for afferent arteriolar contractile responses to angiotensin II and perfusion pressure in mice. Hypertension. 2012;59(2):415–20. doi: 10.1161/HYPERTENSIONAHA.111.184291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lai EY, Onozato ML, Solis G, et al. Myogenic responses of mouse isolated perfused renal afferent arterioles: effects of salt intake and reduced renal mass. Hypertension. 2010;55(4):983–89. doi: 10.1161/HYPERTENSIONAHA.109.149120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Franco V, Oparil S. Salt sensitivity, a determinant of blood pressure, cardiovascular disease and survival. J Am Coll Nutr. 2006;25(3 Suppl):247S–55S. doi: 10.1080/07315724.2006.10719574. [DOI] [PubMed] [Google Scholar]
- 48.Zaloga GP, Roberts PR, Black KW, et al. Carnosine is a novel peptide modulator of intracellular calcium and contractility in cardiac cells. Am J Physiol. 1997;272(1 Pt 2):H462–68. doi: 10.1152/ajpheart.1997.272.1.H462. [DOI] [PubMed] [Google Scholar]